Abstract
BACKGROUND AND PURPOSE: The presence of cervical lymph node metastases is an important prognostic factor for oral tongue cancer. The accurate preoperative assessment is essential for treatment. Several studies have suggested that histologic tumor thickness is related to the metastases. The aim of this study was to determine whether MR images of oral tongue tumor have the potential to predict cervical lymph node metastases.
MATERIALS AND METHODS: A total of 43 patients with squamous cell carcinoma of the oral tongue were investigated. Tumor thickness, sublingual distance between tumor and sublingual space, and paralingual distance between tumor and paralingual space, as determined from coronal MR imaging, were preoperatively estimated. Logistic regression analysis was used to identify independent predictors of lymph node metastases.
RESULTS: Univariate logistic regression analysis showed that T classification, N classification, and 3 measured MR imaging distances (millimeters) were significantly associated with lymph node metastases. Multivariate logistic regression analysis showed that tumor thickness (odds ratio, 1.34; 95% confidence interval [CI], 1.11–1.63; P < .005) and paralingual distance (odds ratio, 0.53; 95% CI, 0.35–0.82; P < .005) were significant predictors for lymph node metastases. The probability of metastases was estimated with these models. The preoperative decision (20% probability) as to whether to perform neck dissection could be based on tumor thickness of >9.7 mm and paralingual distance of <5.2 mm.
CONCLUSION: MR images provide satisfactory accuracy for the preoperative estimation of the tumor thickness and the paralingual distance, which are valuable for predicting cervical lymph node metastases.
Squamous cell carcinoma (SCC) of the oral tongue has a relatively high propensity for cervical lymph node metastases, which ranges 37%–58%.1,2 The presence of cervical lymph node metastases is the most important prognostic factor for survival.3–5 Clinical assessment of the neck is an essential part of the examination. Advances in imaging techniques such as CT, MR imaging, and sonography have improved the accuracy of detection of cervical lymph node metastases, but patients with N0 classification may still harbor occult metastases. The incidence of occult metastases varies from 20% to 50%,1,2,6–10 and the management of the clinically negative (N0) neck remains a controversial issue. Several studies have suggested that histologic tumor thickness is related to cervical metastases of oral tongue cancer.2,10–18 This finding indicates that presurgical determination of tumor thickness might be useful for neck treatment planning. More recent studies have demonstrated that tumor thickness on MR imaging directly correlates with histologic thickness.19–21 MR imaging thickness in patients with metastatic lymph nodes tended to be greater than that in metastases-free patients, though the difference was not significant between patients with and without metastasis.21 Tongue carcinoma varies in the tumor shape (reductive or expansive) and in the growth pattern (endophytic or exophytic). Therefore, how far tumor cells invade and which structures these cells infiltrate, rather than tumor thickness, may be important. The surface epithelium on the lateral side of the tongue is supported by submucosa; underlying the submucosa are intrinsic tongue muscles. The sublingual space is below the intrinsic tongue muscles and contains the sublingual gland. The genioglossus muscle lies medial to the sublingual space and the paralingual space, between the genioglossus and the intrinsic tongue muscles. Therefore, the distances between these spatia and tumor might be a more reliable predictor for lymph node metastases than tumor thickness.
We performed a retrospective study of the ability of preoperative MR images to estimate the tumor thickness and the distances between the tumor and the sublingual/paralingual space, and we assessed the relationship between these variables and cervical lymph node metastases.
Materials and Methods
The study population included patients with SCC of the oral tongue who had an MR imaging before their treatment and underwent surgery without preoperative radiation therapy or chemotherapy. From 1998, we treated 104 consecutive patients with previously untreated SCC of the oral tongue. Among them, 70 patients had an MR imaging before their treatment. We excluded 12 patients who received induction chemotherapy,22 preoperative external radiation therapy (linear accelerator), interstitial brachytherapy with iridium-192,10 or concomitant chemoradiotherapy. Five patients who underwent intraoral excision alone without neck dissection were excluded because their follow-up duration was less than 1 year. We reviewed the clinical records and MR images of 53 consecutive patients with SCC of the tongue. Ten (19%) of the 53 patients had tongue MR images that could not be interpreted because of the interference of artifacts. A total of 43 patients with SCC of the oral tongue were investigated for this study. Patients included 29 men and 14 women, with a median age of 58 years (range, 22–83 years). According to the TNM classification of the International Union Against Cancer, 11 (26%) patients had clinical T1 classification, 23 (53%) patients had T2, 3 (7%) had T3, and 6 (14%) had T4. Thirty-two (74%) had clinical N0 classification, 5 (12%) had N1, 3 (7%) had N2b, 1 (2%) had N2c, and 2 (5%) had N3. Ten (23%) had well-differentiated SCC, 28 (65%) had moderately differentiated SCC, and 5 (12%) had poorly differentiated SCC.
All 43 patients underwent surgery with no preoperative radiation therapy or chemotherapy. Four patients received local immunotherapy consisting of peritumoral injection of OK-432 (Picibanil; Chugai Pharmaceutical, Tokyo, Japan), lyophilized powder prepared from a penicillin G-treated Su strain of type III avirulent human strain of group A Streptococcus pyogenes (OK-432) just 1 week before the surgery.23 Twenty-five (58%) patients underwent partial glossectomy, 15 (35%) patients underwent hemiglossectomy, and 3 (7%) underwent subtotal glossectomy. Therapeutic radical/modified radical neck dissection was performed in 11 patients with N1–3 disease, and elective neck dissection was in performed 11 (6 supraomohyoid and 5 suprahyoid neck dissections). Twenty-one (49%) patients had no neck dissection, and lymph node metastases did not develop during an average follow-up duration of 44 months (range, 12–101 months).
MR images were obtained with a 1.5T superconducting magnet scanner (Signa Horizon or Signa LX; GE Healthcare, Milwaukee, Wis) equipped with a quadrature head/neck/vascular coil. The scanning protocol included T1-weighted (TR/TE/NEX, 500 ms/9 ms/1) spin-echo (SE) sequences with the fat suppression (FS) technique and T2-weighted (TR/TE/NEX, 3500 ms/98 ms/1) SE sequences. Gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany) was administered, and T1-weighted SE sequences with the FS technique were repeated. The FOV was 25 × 25 cm. The matrix size was 512 × 256, and section thickness was 5 mm with 1-mm intersection spacing. Tumor was measured on coronal T1-weighted with FS images.
The MR imaging tumor thickness was determined as described by Lam et al19 and illustrated in Figs 1–4. A vertical line joining the 2 tumor-mucosa junctions was drawn as a reference line. The tumor thickness was determined by drawing perpendicular lines from the reference line to the point of maximal tumor projection and invasion. The coronal T1-weighted MR imaging shows that a high-intensity area extends from the medial border of the sublingual space to the deep lingual artery along the genioglossus. The thin high-intensity area has an intensity similar to that of the sublingual space, indicating the paralingual space. The sublingual distance was determined by measuring the distance between the tumor and the sublingual space. For lesions in which the tumor invasion exceeds the sublingual space, the sublingual distance was determined as 0, because the tumor has an intensity similar to that of the sublingual gland. The paralingual distance was determined by measuring the distance between the paralingual space and the tumor. For lesions in which the tumor invasion extended beyond the space, the paralingual distance was determined as a minus.
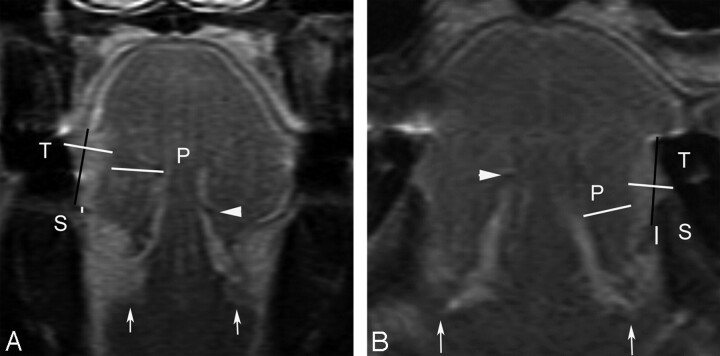
Fig 1.
Coronal contrast-enhanced T1-weighted images show measured tumor thickness (T), sublingual distance (S), and paralingual distance (P). White arrows show sublingual glands, and a white arrowhead shows the contralateral deep lingual artery. A, MR image of a 51-year-old woman with T2N0 disease shows a vertical black line connecting 2 tumor-mucosa junctions as a reference line. A horizontal white line drawn perpendicular to the reference line represents radiologically determined tumor thickness (T) of 8.7 mm. White line (S) between the tumor and the sublingual space demonstrates the sublingual distance of 0.6 mm. The line (P) between the tumor and the paralingual space demonstrates the paralingual distance of 8.9 mm. B, A 73-year-old man with T1N0 disease (T = 6.2 mm, S = 2.8 mm, P = 6.6 mm). Both patients had no evidence of lymph node metastases for their follow-up duration of >1 year.
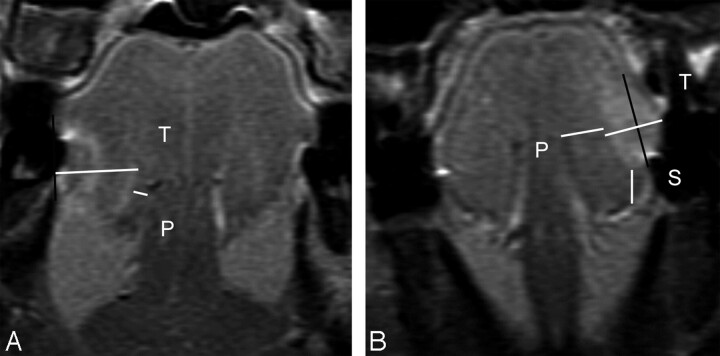
Fig 4.
A, MR image of a 74-year-old man with T4N2b disease, which invaded the mandible, demonstrates a tumor thickness (T) of 19.0 mm, sublingual distance of 0 mm, and paralingual distance (P) of −5.8 mm. Therapeutic neck dissection revealed 9 metastatic nodes in levels I-V. B, MR image of a 61-year-old man with T4N1 disease demonstrates tumor thickness (T) of 27.2 mm, sublingual distance of 0 mm, and paralingual distance (P) of −3.1 mm. The T is the sum of both of these horizontal white lines perpendicular to the reference line. Therapeutic neck dissection revealed 1 metastatic node in level I.
A parametric unpaired t test or a nonparametric Mann-Whitney U test was used for continuous variables. Normality was tested by using the Kolmogorov-Smirnov test. Comparisons between dichotomous variables were performed by using the χ2 test with Yates correction. To evaluate the crude association between each factor and the likelihood of having lymph node metastasis, we initially conducted univariate logistic regression analysis for each predictor variable. The following variables were considered as possible predictors of lymph node metastasis: sex, age (years/10), Eastern Cooperative Group performance status (<2 or ≥2), T classification (T1–2 or T3–4), N classification (N0 or N1–3), tumor differentiation (well-moderate or poor), tumor thickness (millimeters), sublingual distance (millimeters), and paralingual distance (millimeters). To determine the magnitude of the collinearity of the independent variables, we used the Pearson correlation. Variables that had a P value of <.20 in univariate logistic regression analysis were entered into a multivariate logistic regression analysis model. A backward stepwise elimination process was used to remove variables that had a Wald χ2 test P value >.20. A P value <.05 was considered to indicate statistical significance. Odds ratios and their 95% confidence intervals (CI) were calculated. The StatView statistical package (Version 5.0; SAS Institute, Cary, NC) was used for the previously mentioned statistical analyses.
Results
Eleven (26%) patients were thought to have positive nodes preoperatively, and the nodes of 32 (74%) were considered negative on the basis of the MR images. Therapeutic neck dissection was performed in the 11 patients, 8 of whom were verified as having pathologically positive nodes. Of the 32 patients with N0 disease, elective neck dissection was performed in 11 patients, 3 of whom were verified as having pathologically positive nodes. Of the remaining 21 patients who had partial glossectomy alone with observations of their necks, late cervical lymph node metastases developed in 4 (2, 4, 8, and 12 months after the glossectomy). The median time to the development of the late metastases was 6 months. In total, 15 (35%) patients had cervical lymph node metastases. The MR imaging had a 53% sensitivity rate for the neck, an 89% specificity rate, and a 77% overall accuracy. The negative and positive predictive values were calculated as 78% and 73%, respectively.
Parametric or nonparametric statistical analysis revealed that sex, age, performance status, or tumor differentiation was not significantly different between patients with and without lymph node metastases, but T classification (P < .01), N classification (P < .01), and 3 measured MR imaging distances were associated with lymph node metastases (P < .005, Table 1). Similarly, univariate logistic regression analysis showed statistically significant association between lymph node metastases and T, N, and measured MR imaging distances (Table 2). If 2 of the independent variables are highly correlated with one another, then collinearity occurs and may create highly unstable estimated regression coefficients.24 Pearson correlation analysis demonstrated tumor thickness had a very strong negative association with paralingual distance (correlation coefficient = −0.86). Sublingual distance weakly correlated with tumor thickness (−0.57) and paralingual distance (0.69). Therefore, the significant variables in the univariate analysis except for paralingual distance were entered into multivariate logistic regression model 1, and tumor thickness instead of paralingual distance was excluded from model 2 (Table 3). In model 1, multivariate logistic regression analysis showed that tumor thickness emerged as an independent parameter contributing to the model predicting lymph node metastasis. The P values of all variables except for tumor thickness were >0.20 in the stepwise backward logistic regression procedure. The final result was equivalent to the univariate analysis (Table 2). The odds ratio of tumor thickness was 1.36 (95% CI, 1.12–1.65; P < .005). In model 2, paralingual distance was identified as a significant variable. The probability of the Wald statistic for the variables, T classification, N classification, and sublingual distance, was >0.20, indicating that these 3 variables would not be significant for the model. These variables were removed from the model by stepwise selection procedure. The odds ratio of paralingual distance was 0.53 (95% CI, 0.34–0.80; P < .005).
Table 1:
Patient characteristics and associations between lymph node metastases and variables
| Patient Characteristics | Lymph Node Metastases |
|||
|---|---|---|---|---|
| Absent (n = 28) | Present (n = 15) | P value | ||
| Sex, N (%) | ||||
| Male | 29 (67) | 20 (69) | 9 (31) | .67 |
| Female | 14 (33) | 8 (57) | 6 (43) | |
| Age (mean ± SD) | 58 ± 15 | 57 ± 14 | 59 ± 16 | .48 |
| Performance status | ||||
| <2 | 35 (81) | 23 (68) | 12 (32) | >.99 |
| ≥2 | 8 (19) | 5 (63) | 3 (38) | |
| T classification, N (%) | ||||
| T1–2 | 34 (79) | 26 (76) | 8 (24) | <.01 |
| T3–4 | 9 (21) | 2 (22) | 7 (78) | |
| N classification, N (%) | ||||
| N0 | 32 (74) | 25 (78) | 7 (22) | <.01 |
| N1–3 | 11 (26) | 3 (27) | 8 (72) | |
| Differentiation, N(%) | ||||
| Well-moderate | 38 (88) | 25 (66) | 13 (34) | >.99 |
| Poor | 5 (12) | 3 (60) | 2 (40) | |
| Tumor thickness (mm) (mean ± SD) | 11.7 ± 7.3 | 8.5 ± 4.5 | 17.8 ± 7.8 | <.0001 |
| Sublingual distance (mm) (mean ± SD) | 4.0 ± 4.4 | 5.5 ± 4.4 | 1.1 ± 2.4 | <.005 |
| Paralingual distance (mm) (mean ± SD) | 4.7 ± 5.1 | 7.2 ± 3.3 | 0 ± 4.5 | <.0001 |
Table 2:
Univariate logistic regression analysis for lymph node metastasis
| Parameter | β Coefficient | SE | Odds Ratio (95% CI) | P |
|---|---|---|---|---|
| Sex, male | −0.51 | 0.67 | 0.60 (0.16–2.24) | .60 |
| Age (years/10) | 0.07 | 0.22 | 1.07 (0.69–1.65) | .77 |
| Performance status, ≥2 | 0.14 | 0.81 | 1.15 (0.23–5.65 | .86 |
| T classification, T3–4 | 2.43 | 0.90 | 11.38 (1.96–66.13) | <.01 |
| N classification, N1–3 | 2.25 | 0.80 | 9.52 (1.98–45.76) | <.005 |
| Differentiation, poor | 0.25 | 0.98 | 1.28 (0.19–8.67) | .80 |
| Tumor thickness (mm) | 0.31 | 0.10 | 1.36 (1.12–1.65) | <.005 |
| Sublingual distance (mm) | −0.34 | 0.12 | 0.71 (0.56–0.91) | <.01 |
| Paralingual distance (mm) | −0.64 | 0.22 | 0.53 (0.34–0.80) | <.005 |
Note:—SE indicates standard error.
Table 3:
Multivariate logistic regression analysis for lymph node metastasis
| Parameter | β Coefficient | SE | Odds Ratio (95% CI) | P |
|---|---|---|---|---|
| Model 1 | ||||
| T classification, T3–4 | 1.89 | 1.40 | 6.60 (0.43–101.90) | .18 |
| N classification, N1–3 | −2.23 | 1.75 | 0.11 (0.01–3.30) | .20 |
| Tumor thickness | 0.36 | 0.16 | 1.43 (1.05–1.96) | <.05 |
| Sublingual distance | −0.13 | 0.13 | 0.88 (0.68–1.14) | .32 |
| Model 2 | ||||
| T classification, T3–4 | 0.25 | 1.69 | 1.28 (0.05–35.15) | .88 |
| N classification, N1–3 | −1.37 | 1.39 | 0.25 (0.02–3.89) | .32 |
| Sublingual distance | 0.06 | 0.18 | 1.07 (0.75–1.50) | .72 |
| Paralingual distance | −0.84 | 0.37 | 0.43 (0.21–0.89) | <.05 |
The mean of MR imaging tumor thickness was 11.7 mm (Table 1). All 3 patients with a tumor thickness of >22.5 mm had positive nodes, and all 15 patients with tumor thickness of <8.3 mm had no neck involvement (Table 4). The mean of MR imaging paralingual distance was 4.7 mm (Table 1). All 6 patients with the paralingual distance of <0 mm had positive lymph nodes, and all 20 patients with the distance of >5.3 mm had no neck involvement (Table 4). Tumor cells invaded sublingual space in 19 (44%) patients; 11 (58%) patients had lymph node metastases, and 8 (42%) had no neck node involvement (Table 4).
Table 4:
Correlation between measured MR imaging distance and cervical lymph node metastasis
| MR Imaging Distance (mm) | No. of Patients (%) by Presence of Metastases |
|
|---|---|---|
| Absent (n = 28) | Present (n = 15) | |
| Tumor thickness | ||
| <8.3 | 15 (100) | 0 (0) |
| 8.3–22.5 | 13 (52) | 12 (48) |
| >22.5 | 0 (0) | 3 (100) |
| Sublingual distance | ||
| 0 | 8 (42) | 11 (58) |
| 0–8.5 | 10 (71) | 4 (29) |
| >8.5 | 10 (100) | 0 (0) |
| Paralingual distance | ||
| <0 | 0 (0) | 6 (100) |
| 0–5.3 | 8 (47) | 9 (53) |
| >5.3 | 20 (100) | 0 (0) |
By means of the 2 logistic regression models, the estimated probabilities (PM) of lymph node metastases are the following: PM = 1 / [1 + exp(4.40–0.31 × tumor thickness)] and PM = 1 / [1 + exp(−1.93 + 0.64 × paralingual distance)]. [Exponential function, exp(x) = ex, where e is the base of the natural logarithm.] If PM = 0.2, paralingual distance is 5.2 mm and tumor thickness is 9.7 mm.
Discussion
Numerous studies2,10–18 have reported that histologic tumor thickness correlates closely with the presence of lymph node metastases; however, accurate preoperative assessment of the histologic tumor thickness is no easy task. In MR imaging, the structures of normal tongue architecture and the oral tongue carcinoma can be clearly shown, and coronal MR imaging can also be used to satisfactorily measure the thickness of oral tongue carcinoma.19 Preda et al21 found that tumor thickness of tongue carcinoma on MR imaging correlated directly with the histologic tumor thickness, and mean tumor thickness in patients with lymph node metastases was greater than that of those without metastases; but the difference did not reach significance in 33 patients. In our 43 patients, the tumor thickness was significantly (P < .05) associated with lymph node metastases, and the thickness was a statistically significant predictor of lymph node metastases in multivariate logistic regression analysis. In this model, the probability of lymph node metastases was estimated with tumor thickness. In a published meta-analysis, elective neck treatment was recommended when the risk of occult metastases is estimated to be >20%.25 The preoperative decision as to whether to perform elective neck dissection can, therefore, be based on tumor thickness of >9.7 mm.
Paralingual distance was another reliable predictor for lymph node metastases. When tumor cells invade beyond the paralingual space and into genioglossus muscle, paralingual distance is determined as a minus. All patients with the minus paralingual distance had lymph node metastases (Table 4). In the TNM classification of the International Union Against Cancer and the cancer staging of the American Joint Committee on Cancer, tumor invading into the genioglossus muscle is defined as T4. Preoperative MR imaging is, therefore, valuable for predicting lymph node metastases and for clarifying primary tumor staging. MR imaging for neck status provided a reasonable overall accuracy (77%), and N classification (N0 or N1–3) was significantly associated with lymph node metastases in univariate logistic regression analysis (odds ratio, 9.52; 95% CI, 1.98–45.76; P < .005). However, the N classification became insignificant in multivariate analysis. Hence, tumor thickness and paralingual distance were more reliable predictors for lymph node metastases than the N classification. The preoperative decision as to whether to perform elective neck dissection can be based on paralingual distance of <5.2 mm.
Sublingual distance does not seem useful for prediction of lymph node metastases. The sublingual space was infiltrated by the tumor in 19 (44%) patients; 11 (58%) had lymph node metastases, and 8 (42%) had no neck node involvement (Table 4). The incidence of neck metastases is very similar to that of SCC of the floor of mouth reported by Steinhart and Kleinnsasser.26 They demonstrated that in SCC of the floor of the mouth, the sublingual gland was histologically infiltrated by the tumor in nearly all cases and cervical metastases were found in 54%.
Rouviere27 described the existence of lateral and median lingual lymph nodes. Groups of the lateral lingual lymph nodes are located either on the lateral aspect of the genioglossus muscle or on the hyoglossus muscle along the lingual artery and vein. This location is the paralingual space. Metastatic spread in the lingual lymph nodes has been reported,28,29 though this finding is rarely seen. In the rabbit tongue, the deep lingual artery has collecting lymphatic vessels.30 Therefore, the drainage to the lymphatic capillaries in the paralingual space may have a key role in cervical lymph node metastases.
Survival probability analysis has not been included in the present study because the series is small (3 patients died of disease, 2 are alive with distant metastases, and the remaining patients are alive without the disease). The paralingual distances of all the 3 deceased patients and the one with a distant lesion were <0 mm; their tumor thicknesses were >17.8 mm. The paralingual distance of another patient with pulmonary metastases was 2.7 mm, and the tumor thickness was 13.8 mm. Tumor thickness and paralingual distance might have an influence on survival, but further studies are required to confirm this important prognostic parameter.
Conclusion
We conclude that MR imaging provides an accurate method for the preoperative estimation of the tumor thickness and the paralingual distance, which can predict cervical lymph node metastasis and has a role in treatment planning.
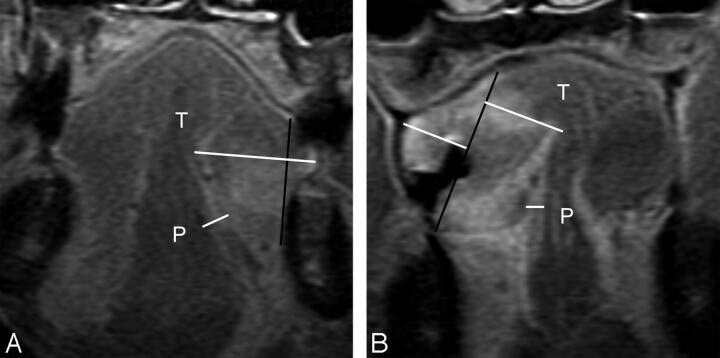
Fig 2.
A, MR image of a 54-year-old man with T2N0 disease shows a vertical black line connecting 2 tumor-mucosa junctions as a reference line. Horizontal white lines are drawn perpendicular to the reference line. Tumor thickness (T) is the sum of both of these horizontal lines and is determined as 10.1 mm (sublingual distance = 0 mm, paralingual distance [P] = 3.8 mm). The elective dissected neck specimen revealed no pathologically positive lymph node. B, MR image of a 41-year-old woman with T3N0 disease demonstrates T of 15.5 mm, sublingual distance of 0 mm, and P of 0.8 mm. Elective dissected neck specimen revealed 1 metastatic node in level III.
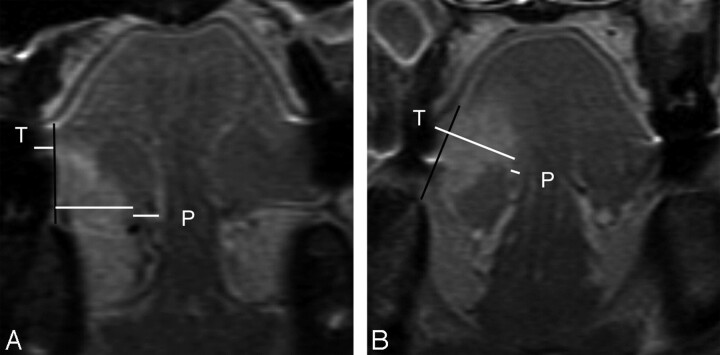
Fig 3.
A, MR image of a 22-year-old man with T2N0 disease and tumor thickness (T) of 13.8 mm, sublingual distance of 0 mm, and paralingual distance (P) of 2.7 mm. Elective dissected neck specimen revealed 1 pathologically positive node in level I. B, MR image of a 33-year-old woman with T2N0 disease demonstrates T of 8.4 mm, S of 4.4 mm, and P of 5.3 mm. Late lymph node metastasis developed 2 months after glossectomy, and 2 pathologically positive nodes (level II and III) were verified with neck dissection.
References
- 1.Franceschi D, Gupta R, Spiro RH, et al. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg 1993;166:360–65 [DOI] [PubMed] [Google Scholar]
- 2.Byers RM, El-Naggar AK, Lee YY, et al. Can we detect or predict the presence of occult nodal metastases in patients with squamous carcinoma of the oral tongue? Head Neck 1998;20:138–44 [DOI] [PubMed] [Google Scholar]
- 3.Shah JP, Medina JE, Shaha AR, et al. Cervical lymph node metastasis. Curr Probl Surg 1993;30:1–335 [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi Y, Okura M. Prognostic significance of perioperative blood transfusion in oral cavity squamous cell carcinoma. Head Neck 2003;25:931–36 [DOI] [PubMed] [Google Scholar]
- 5.Okura M. Lymph node metastasis. In: Pandalai SG, ed. Recent Research Developments in Cancer. 4 . Part 1. Trivandrum, India; Transworld Research Network;2002. :331–37 [Google Scholar]
- 6.Results of a prospective trial on elective modified radical classical versus supraomohyoid neck dissection in the management of oral squamous carcinoma: Brazilian Head and Neck Cancer Study Group. Am J Surg 1998;176:422–27 [DOI] [PubMed] [Google Scholar]
- 7.Ferlito A, Rinaldo A, Silver CE, et al. Elective and therapeutic selective neck dissection. Oral Oncol 2006;42:14–25 [DOI] [PubMed] [Google Scholar]
- 8.Takes RP, Righi P, Meeuwis CA, et al. The value of ultrasound with ultrasound-guided fine-needle aspiration biopsy compared to computed tomography in the detection of regional metastases in the clinically negative neck. Int J Radiat Oncol Biol Phys 1998;40:1027–32 [DOI] [PubMed] [Google Scholar]
- 9.Bourgier C, Coche-Dequeant B, Fournier C, et al. Exclusive low-dose-rate brachytherapy in 279 patients with T2N0 mobile tongue carcinoma. Int J Radiat Oncol Biol Phys 2005;63:434–40 [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki H, Inoue T, Yoshida K, et al. Lymph node metastasis of early oral tongue cancer after interstitial radiotherapy. Int J Radiat Oncol Biol Phys 2004;58:139–46 [DOI] [PubMed] [Google Scholar]
- 11.Asakage T, Yokose T, Mukai K, et al. Tumor thickness predicts cervical metastasis in patients with stage I/II carcinoma of the tongue. Cancer 1998;82:1443–48 [DOI] [PubMed] [Google Scholar]
- 12.Fukano H, Matsuura H, Hasegawa Y, et al. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck 1997;19:205–10 [DOI] [PubMed] [Google Scholar]
- 13.Lim SC, Zhang S, Ishii G, et al. Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin Cancer Res 2004;10:166–72 [DOI] [PubMed] [Google Scholar]
- 14.O-charoenrat P, Pillai G, Patel S, et al. Tumour thickness predicts cervical nodal metastases and survival in early oral tongue cancer. Oral Oncol 2003;39:386–90 [DOI] [PubMed] [Google Scholar]
- 15.Po Wing Yuen A, Lam KY, Lam LK, et al. Prognostic factors of clinically stage I and II oral tongue carcinoma: a comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck 2002;24:513–20 [DOI] [PubMed] [Google Scholar]
- 16.Spiro RH, Huvos AG, Wong GY, et al. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg 1986;152:345–50 [DOI] [PubMed] [Google Scholar]
- 17.Woolgar JA. T2 carcinoma of the tongue: the histopathologist's perspective. Br J Oral Maxillofac Surg 1999;37:187–93 [DOI] [PubMed] [Google Scholar]
- 18.Yuen AP, Lam KY, Wei WI, et al. A comparison of the prognostic significance of tumor diameter, length, width, thickness, area, volume, and clinicopathological features of oral tongue carcinoma. Am J Surg 2000;180:139–43 [DOI] [PubMed] [Google Scholar]
- 19.Lam P, Au-Yeung KM, Cheng PW, et al. Correlating MRI and histologic tumor thickness in the assessment of oral tongue cancer. AJR Am J Roentgenol 2004;182:803–08 [DOI] [PubMed] [Google Scholar]
- 20.Iwai H, Kyomoto R, Ha-Kawa SK, et al. Magnetic resonance determination of tumor thickness as predictive factor of cervical metastasis in oral tongue carcinoma. Laryngoscope 2002;112:457–61 [DOI] [PubMed] [Google Scholar]
- 21.Preda L, Chiesa F, Calabrese L, et al. Relationship between histologic thickness of tongue carcinoma and thickness estimated from preoperative MRI. Eur Radiol 2006;16:2242–48 [DOI] [PubMed] [Google Scholar]
- 22.Okura M, Hiranuma T, Adachi T, et al. Induction chemotherapy is associated with an increase in the incidence of locoregional recurrence in patients with carcinoma of the oral cavity: results from a single institution. Cancer 1998;82:804–15 [PubMed] [Google Scholar]
- 23.Kitahara S, Ikeda M, Inouye T, et al. Inhibition of head and neck metastatic and/or recurrent cancer by local administration of multi-cytokine inducer OK-432. J Laryngol Otol 1996;110:449–53 [DOI] [PubMed] [Google Scholar]
- 24.Moss M, Wellman DA, Cotsonis GA. An appraisal of multivariable logistic models in the pulmonary and critical care literature. Chest 2003;123:923–28 [DOI] [PubMed] [Google Scholar]
- 25.Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg 1994;120:699–702 [DOI] [PubMed] [Google Scholar]
- 26.Steinhart H, Kleinsasser O. Growth and spread of squamous cell carcinoma of the floor of the mouth. Eur Arch Otorhinolaryngol 1993;250:358–61 [DOI] [PubMed] [Google Scholar]
- 27.Rouviere H. Anatomy of the Human Lymphatic System. Tobies MJ, trans-ed. Ann Arbor, Mich: Edwards Brothers;1938. :44
- 28.Ozeki S, Tashiro H, Okamoto M, et al. Metastasis to the lingual lymph node in carcinoma of the tongue. J Maxillofac Surg 1985;13:277–81 [DOI] [PubMed] [Google Scholar]
- 29.Woolgar JA. Histological distribution of cervical lymph node metastases from intraoral/oropharyngeal squamous cell carcinomas. Br J Oral Maxillofac Surg 1999;37:175–80 [DOI] [PubMed] [Google Scholar]
- 30.Fujimura A, Seki S, Liao MY, et al. Three dimensional architecture of lymphatic vessels in the tongue. Lymphology 2003;36:120–27 [PubMed] [Google Scholar]