Abstract
BACKGROUND AND PURPOSE: Intracranial aneurysms with a wide-neck or an unfavorable dome-to-neck ratio may be difficult to treat properly and safely. Our aim was to evaluate the TriSpan neck-bridge device to assist coiling of wide-neck bifurcation aneurysms in the anterior circulation.
MATERIALS AND METHODS: In 14 patients, we performed 16 TriSpan-assisted coil embolizations with wide-neck bifurcation aneurysms of the anterior circulation. Eleven procedures were indicated for acutely ruptured aneurysms. Five were performed electively for the following: recurrent aneurysm after coil only (n = 1) or after TriSpan-assisted embolization (n = 2), aneurysm remnant after clipping (n = 1), and aneurysm incidentally found (n = 1). Procedural and clinical complications were recorded. Follow-up angiography was performed, and clinical outcomes were assessed by using the modified Rankin Scale score.
RESULTS: TriSpan-assisted embolization was successful in 15/16 (93.8%) procedures, with complete occlusion in 2/16 (12.5%), near-complete occlusion in 10/16 (62.5%), and incomplete occlusion in 3/16 (18.75%). There were 6 (37.5%) intraprocedural complications: thrombus formation (n = 3), protrusion of a TriSpan loop in the parent artery (n = 1), TriSpan displacement in the aneurysm (n = 1), and tangling of a coil loop in the device (n = 1). Three patients died in the hospital (21.4%). Follow-up angiography or MR angiography was available in 8 (57.1%) patients and showed complete (n = 2), near-complete (n = 2), and incomplete occlusion (n = 4). Long-term clinical outcome was no (n = 4) or minor symptoms (n = 1) and moderate (n = 2), moderately severe (n = 2), or severe handicap (n = 2).
CONCLUSION: The use of the TriSpan device is feasible in the anterior circulation and can assist treatment of difficult wide-neck bifurcation aneurysms.
The endovascular occlusion of intracranial aneurysms by using electrolytically detachable platinum coils has developed into a widely used and popular technique.1 Microcatheterization and filling of saccular aneurysms with coils has proved adequate to exclude the aneurysm effectively from the circulation but is limited by the configuration of some of these aneurysms. Aneurysms with a wide (≥4 mm) neck or an unfavorable dome-to-neck ratio (≥1.5) may be difficult to treat properly and safely.2 For wide-neck sidewall aneurysms, 3D coils,3 temporary balloon remodelling, and stent placement are valuable options. For wide-neck bifurcation aneurysms (for instance basilar tip aneurysms), bilateral balloon remodelling4 or retrograde remodelling5 may permit the successful obliteration. However, in many bifurcation aneurysms, geometry may not allow these techniques to be applied effectively and safely. For many technical reasons, these options may be reserved for operators with considerable experience in the endovascular treatment of aneurysms.5
The TriSpan (Target Therapeutics/Boston Scientific, Fremont, Calif) is a detachable device that has been designed to address the issue of wide-neck (≥4 mm) bifurcation aneurysms. Once this device is placed at the neck of a wide-neck aneurysm, platinum coils are inserted through a second microcatheter.6 The TriSpan allows coils to be positioned and deployed in the aneurysm lumen with reduced risk of coil herniation into the parent artery.7–9 The TriSpan was originally designed for basilar bifurcation aneurysms. Although experimental work was very promising,10 only a few investigators have published clinical results on the usefulness of the TriSpan for wide-neck basilar aneurysms.6 In bifurcation aneurysms of the anterior circulation, to our knowledge, experience with the TriSpan device is even more limited. Therefore, we evaluated the feasibility of placement of a TriSpan coil in the ostium of wide-neck bifurcation aneurysms of the anterior circulation to assist in the placement of detachable platinum coils.
Materials
Patient Population
From February 2002 to October 2006, we treated 7 women and 7 men, (37–83 years of age; mean, 55.9 year of age) with anterior circulation aneurysms showing a wide-neck aneurysm and a bifurcation configuration. They were treated with Guglielmi detachable coils (GDC; Boston Scientific, Natick, Mass; n = 11), Matrix detachable coils (Boston Scientific; n = 7), the MicroCoil System (Micrus Endovascular, San Jose, Calif; n = 1), or HydroCoils (MicroVention, Aliso Viejo, Calif; n = 1), with the assistance of the TriSpan neck-bridge device.
Eleven procedures were indicated for acutely ruptured aneurysms. Five coilings were performed on an elective basis for the following conditions: recurrent aneurysm after coil-only embolization (n = 1), recurrent aneurysm after previous TriSpan-assisted coil embolization (n = 2), aneurysm remnant after 2 clippings (n = 1), and aneurysm incidentally found after investigation for headache (n = 1). Patients with acutely ruptured aneurysms were clinically assessed according to the Hunt and Hess (HH) scale at the time of treatment in combination with the Glasgow Coma Scale.11–12 Of 11 patients with acutely ruptured aneurysms, 5 aneurysms were HH grade I, 3 were grade III, and 3 were grade V.
Aneurysm Characteristics
Aneurysms were most frequently located at the bifurcation of the middle cerebral artery (MCA, n = 6) and anterior communicating artery (AcomA, n = 6). Other sites included the carotid siphon (n = 1) and carotid bifurcation (n = 1). Scale-independent measurements on the angiographic tracings displaying the best view of the neck were adopted from Parlea et al.13 Neck width ranged from 4.5 to 12.0 mm (mean, 7.0 mm). The dome height should be greater than the neck width to securely engage the loops or petals of the TriSpan coil in the aneurysm. Therefore, according to the manufacturer, it is not recommended to use the TriSpan device when the height to neck (H/n) ratio is smaller than or equal to 1. In our series, mean H/n ratio was 1.7. Despite an H/n ratio < 1 in 2 patients (patients 8 and 12), a TriSpan-assisted coil embolization could, nevertheless, be performed successfully. In both cases, an adjusted H/n ratio (maximal aneurysm height not perpendicular to the neck/neck width) was >1. Maximal aneurysm diameter ranged from 6.5 to 21.3 mm (mean, 13.8 mm).
Procedure
Device.
The TriSpan neck-bridge device is made of 3 polyxylylene (Para Tech Coating, Middletown, Conn)–coated nitinol loops or “petals”; the proximal ends of these petals are fixed together by coiled platinum wire to form a “stem.” This stem is attached to a stainless steel GDC delivery wire, measuring 0.014-inch in diameter. To increase radiopacity under fluoroscopy, a portion of each petal is wrapped with a platinum wire. During introduction, the TriSpan neck-bridge device is compressed in an 0.018-inch 2.6F microcatheter (minimal inner lumen diameter of 0.48 mm × 0.019 inch) with 2 tip markers. When the device is positioned at the neck of the aneurysm, the nitinol loops of the TriSpan device open like petals on a flower to protect the parent vessel and assist in the placement of the coils, which are introduced through a second microcatheter. As the aneurysm is progressively packed with coils, the TriSpan neck-bridge device becomes fixed inside the lesion so that it can be detached at the end of the procedure. The device is detachable from the pusher wire with the use of a standard GDC detachment system.
Technique.
All patients were treated while they were under general anesthesia. Bilateral femoral approaches involving the use of 5F-6F introducing sheaths permitted placement of the appropriate guiding catheters. Two guiding catheters were positioned in or just beneath the proximal internal carotid artery. In 2 patients (patients 9 and 11), a single 8F guiding catheter allowed the introduction of both microcatheters. All procedures were performed by using a single-plane angiographic unit with 3D reconstruction. 3D angiography was performed to choose a working position of a 2D plane and to perform scale-independent measurements. The first microcatheter was inserted into the aneurysm to place the TriSpan device at the neck. When the neck-bridge device was deployed inside the aneurysm, the carrying microcatheter (Excelsior; Target Therapeutics) was slightly withdrawn until a stable position was ensured. The second microcatheter was then introduced through 1 of the loops to the aneurysm. An appropriate coil was then introduced but not detached. The position and stability of this initial construction were then assessed angiographically. The first coil (3D or 2D) was then detached. Coils of decreasing sizes were introduced to pack the aneurysm as completely as possible. The neck-bridge device was electrolytically detached from the pusher wire at the end of the procedure, after retrieval of the coiling microcatheter. After endovascular treatment, patients were transferred to the intensive care unit where fluid balance, neurologic status, and blood pressure were carefully monitored.
Anticoagulation.
In our institution, all elective procedures (patients 1, 7, 8, 10, and 14) were performed with full heparin anticoagulation. In procedures indicated for acutely ruptured aneurysms, full heparin anticoagulation was only started during or at the end of the procedure in case of technical difficulties or complications (patients 1–4, 7, 12, and 13).
Indications.
Indication for treatment resulted from a consensus between the neurosurgeon and interventional neuroradiologist. Endovascular therapy was the first-intention treatment. Ruptured aneurysms were treated as soon as possible in conjunction with diagnostic cerebral angiography, to prevent aneurysmal rebleeding. For unruptured aneurysms, the aim was to prevent bleeding or improve neurologic symptoms due to mass effect. All patients and/or their families received complete information before therapy, and an informed consent was obtained.
Methods
Technical Outcome
Aneurysm occlusion after coil placement was scored in the procedural working position and labeled as complete (98%–100% occlusion), near-complete (90%–98%), and incomplete (<90% occlusion).14 Incomplete occlusion at any point in time was considered an indication for further therapy.
Procedural Complications
All technical complications were recorded, whether or not they were symptomatic. All clinical changes were noted as well as their relationship with the technique and device used.
Clinical and Angiographic Outcome
The modified Rankin Scale (mRS) score was used to evaluate the clinical outcome of patients at the time of hospital discharge and at subsequent follow-up. Imaging follow-up was performed by digital subtraction angiography (DSA) or MR angiography (MRA).
Results
Technical Success
TriSpan-assisted coil packing was successfully performed in 15 of 16 procedures, with complete occlusion in 2/16 (12.50%), near-complete occlusion in 10/16 (62.50%), and incomplete occlusion in 3/16 (18.75%) procedures. Technical failure occurred in patient 5 (6.25%) because the TriSpan coil could not bridge the neck due to aneurysm asymmetry. The neck-bridge devices used were the TriSpan-6 (n = 1), TriSpan-8 (n = 1), TriSpan-10 (n = 6), TriSpan-12 (n = 5), TriSpan-14 (n = 1), and TriSpan-16 (n = 1). In 5 procedures (patients 3, 6–8, and 10), we had to exchange the first TriSpan coil because it could not be deployed adequately. In 2 procedures, the exchanged TriSpan coil was the one recommended by the company. In the 3 other cases, the neck-bridge device was chosen at our own discretion. The total coil length per procedure ranged from 46 to 420 cm (mean, 150 cm). We used a 3D or 2D coil as a first coil in 10/16 (62.5%) and 4/16 (25%) procedures, respectively.
Procedural and Clinical Complications
Of 16 procedures, 6 (37.5%) were complicated. Intraprocedural thrombus formation occurred in 3 (18.75%), protrusion of a TriSpan loop in the parent artery in 1 (6.25%), and displacement of the TriSpan in the aneurysm in 1 (6.25%). In 1 case (6.25%), the first 3D coil loop tangled and got trapped in the TriSpan device. Complications occurred in 4 of 11 ruptured aneurysms (36.4%) (patients 3, 4, 11, and 12) and in 2 of 5 unruptured aneurysms (40%) (patients 8 and 10). Thrombotic complications were treated with intra-arterial thrombolysis or with intravenous glycoprotein IIb/IIIa receptor inhibitor. In patient 8, thrombus formation at the carotid bifurcation resulted in paresthesis of the lip and tongue, with excellent neurologic recovery. In patient 12, thrombus formation in an MCA branch could also be treated by using intra-arterial thrombolysis (urokinase), but this treatment may have caused severe postoperative bleeding, with a residual plegia of the right arm and dysphasia. In patient 4, thrombus formation in an opercular branch was treated with full heparinization, but unfortunately, this patient died after 3 days due to intracranial vasospasm. Protrusion of a TriSpan loop in the AcomA (patient 3, HH I) and TriSpan displacement in the aneurysm (patient 11, HH V) were not corrected because we judged the situation as not critical. Prophylactically, a platelet inhibitor was prescribed. Nevertheless, clinical outcome was poor because in both patients, we encountered a disease-related death. In patient 10, entrapment of the first 3D coil loop in the TriSpan was treated by withdrawal of both devices and microcatheters. This maneuver was without clinical consequence, so we started the procedure with a new TriSpan device.
Long-Term Clinical Outcome
In summary, we encountered 1 (1/16, 6.25%) severe procedure-related complication. Severe postcoiling bleeding, necessitating urgent decompression, was encountered in patient 12. Because coiling was complete, it is likely that the hemorrhage was related to local fibrinolysis for a major MCA branch occlusion. Unfortunately, the patient had a severe motor deficit and dysphasia with little recovery (mRS 5). In all other patients with acutely ruptured aneurysms, disease-related clinical outcomes were the following: mRS 0 in 1 (patient 1), mRS 1 in 1 (patient 6), mRS 3 in 2 (patients 5 and 7), mRS 4 in 2 (patients 2 and 9), mRS 5 in 1 (patient 10), and mRS 7 in 3 (patients 3, 4, and 11). We did not encounter deaths related to the procedure.
Long-Term Angiographic Outcome
Late aneurysmal outcome, assessed by MRA or angiography at 1–39 months (mean, 12.9 months) posttreatment, was available in 8 patients (57.1%) and showed complete occlusion in 2 (14.3%) (patients 5 and 14), near-complete occlusion in 2 (14.3%) (patient 1 after the second procedure and 8), and incomplete occlusion by coil compaction in 4 (28.6%) (patients 6, 7 after the second procedure, 10, and 13). In 5 patients (patients 1, 6, 7, 10, and 13), initial TriSpan-assisted coil embolization resulted in an incomplete occlusion, and this was, therefore, considered as an indication for further therapy. Repeat treatment (assisted again with a TriSpan neck-bridge device in patients 1 and 7) resulted in complete occlusion in patient 13, near-complete occlusion in patient 1, and incomplete occlusion in patient 7. Neurosurgical clipping was proposed for a residual MCA aneurysm in patient 6. In patients 7 and 10, 2-year follow-up DSA showed residual aneurysm, but the recanalization was limited. Because both patients had disabling neurologic deficits post-subarachnoid hemorrhage, we considered the indication for (repeat) recoiling as relative and, with consent of the family, did not take further action.
Discussion
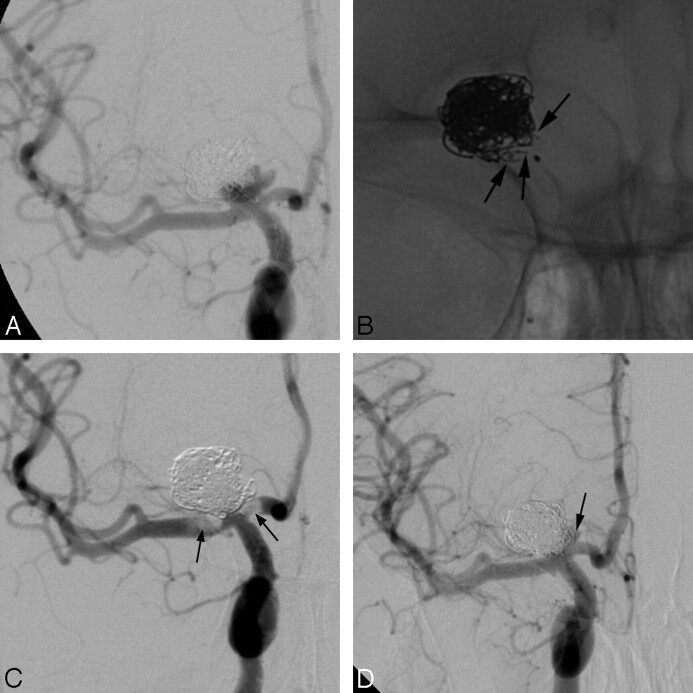
Although experimental10–15 and preliminary human work6 with the TriSpan neck-bridge device was promising, its clinical application remained mostly limited to basilar tip aneurysms. On 25 wide-neck lesions, Raymond et al6 treated 19 basilar tip aneurysms with this neck-bridging device. Although 3 other aneurysms were also located in the posterior circulation, it was the aneurysms in the anterior circulation (ophthalmic, n = 2; carotid bifurcation, n = 1) treated with the TriSpan device that did not escape our attention. In the anterior circulation, bifurcation aneurysms similar to the basilar tip aneurysm can be encountered at the bifurcation of the internal carotid artery into the MCA and anterior cerebral artery (carotid tip aneurysms, Fig 1A–D). More distally, at the MCA, bifurcation aneurysms are often observed when the long axis of the distal M1 cuts the aneurysm into approximately equal parts on at least 2 projections or on the 3D angiogram (Fig 2A–E). These observations have induced us to use the TriSpan device in the anterior circulation to bridge challenging wide necks of bifurcation aneurysms.
Fig 1.
A, Patient 8: right carotid DSA shows a carotid bifurcation aneurysm, partially recanalized due to coil compaction after coil-only embolization 6 months before. B, Plain digital image shows the compacted coils and, partially, the 3 nitinol petals of the TriSpan-10 device (arrows). C, Right carotid DSA shows thrombus (arrows) at the bifurcation in front of the neck. D, Right carotid DSA 1 day after treatment with intra-arterial urokinase and intravenous glycoprotein IIb/ IIIa receptor inhibitor demonstrates resolution of the thrombus and a “dog ear” at the medial site of the neck (arrow).
Fig 2.
A, Patient 1: left carotid DSA shows a wide-neck MCA aneurysm with temporal and parietal branches (arrows) originating from the base. The TriSpan-12 is already in position. B, Plain digital image of a TriSpan configuration. The 3 nitinol “petals” are partially marked with platinum (arrowheads) and fixed by a coiled platinum “stem” wire (arrow). Markers of the coiling microcatheter (with the first coil inside) are indicated by asterisks. The proximal marker of the TriSpan-carrying microcatheter is indicated by a white arrow. C, Control left carotid DSA after embolization shows a “dog ear” (arrow) at the neck-parent vessel junction. D, Left carotid DSA at 8 months shows recurrence at the neck (asterisk) by coil compaction. E, Left carotid DSA after recoiling again shows a “dog ear” (arrow) at the neck-parent vessel junction.
In our study of wide-neck anterior circulation bifurcation aneurysms, we observed a primary complete occlusion rate of 12.5%, a near-complete occlusion rate of 62.5%, and a residual aneurysm or incomplete occlusion rate of 18.75%, which is very similar to the results of Raymond et al.6 In view of the mean neck diameter of 7 mm and the complexity of the aneurysms, we think that this is an acceptable occlusion rate. Maybe we had fewer residual aneurysms because we often used 1 or more 3D coils as initial coils after TriSpan positioning, whereas Raymond et al worked only with helical coils. Whether this makes the difference is unclear because in both series, only 1 early rebleeding occurred, and in the long term, recurrence was frequent in both series. In contrast to small- or medium-sized (<10 mm) aneurysms, compaction is unlikely if 24% or more of the aneurysm volume could be packed with coils. In larger aneurysms, a high filling rate is difficult to achieve, and hence compaction is difficult to avoid.14,16
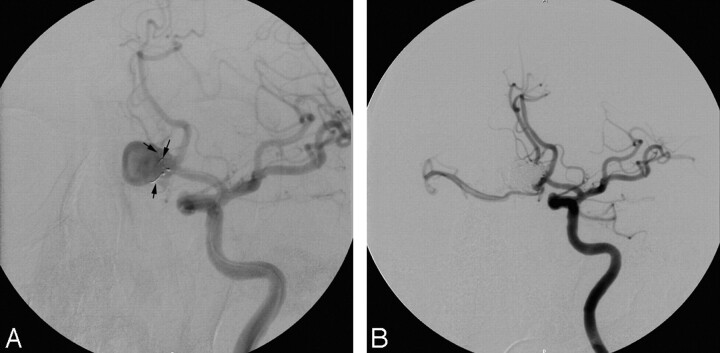
In our study, the only failure to deploy the TriSpan was seen in a giant supraophthalmic aneurysm for the same reason as that in the failure case of Raymond et al.6 Here too, the angle of positioning was too acute for an adequate bridging effect. However, we learned that perfect “symmetry” of the aneurysm is not mandatory to deploy the TriSpan device effectively (Fig 3A). The device could be used to protect the central part of the neck and 1 side of the bifurcation to allow a stable coil mesh. We observed that in such cases, at the opposite site of the bifurcation, and thus eventually beyond the TriSpan petals, accumulating coil loops may provide safe packing (Fig 3B). Moreover, the device allowed slight correction of the coil position by changing its degree of deployment and/or by slightly moving the microcatheter. Such maneuvers were only safe at the initial stage of the coiling. Moreover, it required a very stable position of the carrying microcatheter, which was checked carefully before coil deployment. Gradually, we realized that the restrictions imposed by the company sizing chart are relative.10 In 5 of 16 procedures, the TriSpan had to be smaller than what was recommended by the manufacturer's sizing table to optimize the position and minimize protrusion. It seemed that only the absolute height of the dome determined whether a TriSpan could be deployed. Even when the neck width was larger than the height of the sac (H/n ratio smaller or equal to 1 in patients 8 and 12), a TriSpan coil could still be securely placed when the maximal height (not perpendicular to neck) to neck ratio was >1 (“adjusted” H/n 1.8 and 1.2 in patients 8 and 12, respectively).
Fig 3.
A, Patient 14: left carotid DSA shows the asymmetric placement of the 3 TriSpan nitinol loops (arrows) at the neck of the aneurysm. B, Left carotid DSA shows complete occlusion of the aneurysm.
In the series of Raymond et al6 and in ours, thromboembolic complications were frequent (3/23 [13%] and 3/16 [18.75%], respectively). Full anticoagulation with heparin during and for 12–24 hours after the procedure was consequently applied in both series, but a short-term antiplatelet regime was only started on specific indication in our series. However, we noted no late thromboembolic complications. Technical complications, such as tangling of the first 3D coil in the TriSpan or protrusion of a TriSpan loop in the parent artery, could be managed by subtle handling and never had clinical consequences. Pros and cons of repositioning or retrieving maneuvers should be weighed carefully. Because TriSpan stem or loop protrusion in the bifurcation or native vessel is a risk factor for thromboembolism, we empirically recommended that patients receive postprocedural single antiplatelet therapy (acetylsalicylic acid, 160 mg for 3 months). We did not consequently use an antiplatelet drug regimen in patients in whom we thought the TriSpan stem was placed in the aneurysm and in the absence of TriSpan loop protrusion. On a similar basis, we recommended a 3-month antiplatelet regimen in case of complete or near-complete occlusion of the wide-neck aneurysm after TriSpan-assisted coil embolization. In this way, we hoped to reduce platelet aggregation, which might be activated by the extensive exposure of coil material to blood in the native vessel.
Whether in our series of wide-neck bifurcation aneurysms, remodelling balloons or stents could have worked as well is unknown because we primarily decided on the TriSpan device. With the remodelling technique of Moret et al,17 a noncompliant balloon was used to facilitate coiling of sidewall aneurysms. Using this technique in bifurcation aneurysms implies the placement of 2 balloons, 1 to protect each ostium, and the introduction of a third microcatheter for coil delivery.18 Although this might have served for some of our patients, the risks of triple catheterization and double temporary occlusion are substantial.18 Sluzewski et al19 calculated from a large series of 71 single balloon-assisted coilings that the risk of death or dependency was as high as 14%. Although the concept of a permanent reconstruction of a terminal (T-configured) bifurcation with the use of a double stent is attractive,20 its feasibility and safety have not been tested in larger series. Also the complete apposition of the stents to the wall of the acute branching bifurcation vessels might be very difficult to achieve. This poor apposition is a well-known risk factor for thromboembolic complications.21 More ingenious for neck protection seems to be a new compliant balloon, which, during inflation, adapts its form to the vascular anatomy. By expanding into the base of the aneurysm, this balloon may protect both ostia simultaneously.4
Preliminary experience with small wide-neck aneurysms is promising.22 Whether these results can be extrapolated to large wide-neck aneurysms has to be investigated. Although we do not refrain from using remodelling balloons, we learned that catheterization of native vessels across the wide neck of a large aneurysm can be very laborious. Often these vessels turn off at sharp angles, which leaves them accessible only from inside the aneurysmal sac. Therefore, as attractive and promising as they are, these alternative supporting approaches require much more expertise than placing a catheter proximally in the aneurysmal sac for TriSpan delivery.
Conclusion
The TriSpan neck-bridge device is sometimes helpful, and its use is feasible to assist coiling of wide-neck bifurcation aneurysms of the anterior circulation.
References
- 1.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 2.Fernandez Zubillaga A, Guglielmi G, Vinuela F, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol 1994;15:815–20 [PMC free article] [PubMed] [Google Scholar]
- 3.Cloft HJ, Joseph GJ, Tong FC, et al. Use of three-dimensional Guglielmi detachable coils in the treatment of wide-necked cerebral aneurysms. AJNR Am J Neuroradiol 2000;21:1312–14 [PMC free article] [PubMed] [Google Scholar]
- 4.Baldi S, Mounayer C, Piotin M, et al. Balloon-assisted coil placement in wide-neck bifurcation aneurysms by use of a new, compliant balloon microcatheter. AJNR Am J Neuroradiol 2003;24:1222–25 [PMC free article] [PubMed] [Google Scholar]
- 5.Moret J, Ross IB, Weill A, et al. The retrograde approach: a consideration for the endovascular treatment of aneurysms. AJNR Am J Neuroradiol 2000;21:262–68 [PMC free article] [PubMed] [Google Scholar]
- 6.Raymond J, Guilbert F, Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: initial experience. Radiology 2001;221:318–26 [DOI] [PubMed] [Google Scholar]
- 7.Pelz DM, Lownie SP, Fox AJ. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998;19:1541–47 [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter BW, Rosso D, Lownie SP. Double microcatheter technique for detachable coil treatment of large, wide-necked intracranial aneurysms. AJNR Am J Neuroradiol 1998;19:1176–78 [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders WP, Burke TH, Mehta BA. Embolization of intracranial aneurysms with Guglielmi detachable coils augmented by microballoons. AJNR Am J Neuroradiol 1998;19:917–20 [PMC free article] [PubMed] [Google Scholar]
- 10.Turk AS, Rappe AH, Villar F, et al. Evaluation of the TriSpan neck bridge device for the treatment of wide-necked aneurysms: an experimental study in canines. Stroke 2001;32:492–97 [DOI] [PubMed] [Google Scholar]
- 11.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14–20 [DOI] [PubMed] [Google Scholar]
- 12.Cavanagh SJ, Gordon VL. Grading scales used in the management of aneurysmal subarachnoid hemorrhage: a critical review. J Neurosci Nurs 2002;34:288–95 [DOI] [PubMed] [Google Scholar]
- 13.Parlea L, Fahrig R, Holdsworth DW, et al. An analysis of the geometry of saccular intracranial aneurysms. AJNR Am J Neuroradiol 1999;20:1079–89 [PMC free article] [PubMed] [Google Scholar]
- 14.Sluzewski M, van Rooij WJ, Rinkel GJ, et al. Endovascular treatment of ruptured intracranial aneurysms with detachable coils: long-term clinical and serial angiographic results. Radiology 2003;227:720–24 [DOI] [PubMed] [Google Scholar]
- 15.Raymond J, Salazkin I, Georganos S, et al. Endovascular treatment of experimental wide neck aneurysms: comparison of results using coils or cyanoacrylate with the assistance of an aneurysm neck bridge device. AJNR Am J Neuroradiol 2002;23:1710–16 [PMC free article] [PubMed] [Google Scholar]
- 16.Sluzewski M, van Rooij WJ, Slob MJ, et al. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology 2004;231:653–58 [DOI] [PubMed] [Google Scholar]
- 17.Moret J, Cognard C, Weill A, et al. Reconstruction technic in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results—apropos of 56 cases [in French]. J Neuroradiol 1997;24:30–44 [PubMed] [Google Scholar]
- 18.Arat A, Cil B. Double-balloon remodeling of wide-necked aneurysms distal to the circle of Willis. AJNR Am J Neuroradiol 2005;26:1768–71 [PMC free article] [PubMed] [Google Scholar]
- 19.Sluzewski M, van Rooij WJ, Beute GN, et al. Balloon-assisted coil embolization of intracranial aneurysms: incidence, complications, and angiography results. J Neurosurg 2006;105:396–99 [DOI] [PubMed] [Google Scholar]
- 20.Akpek S, Morsi H, Benndorf G, et al. Reconstruction of the basilar tip with T stent configuration for treatment of a wide-neck aneurysm. J Vasc Interv Radiol 2004;15:1024–26 [DOI] [PubMed] [Google Scholar]
- 21.Brisman JL, Song JK, Niimi Y, et al. Treatment options for wide-necked intracranial aneurysms using a self-expandable hydrophilic coil and a self-expandable stent combination. AJNR Am J Neuroradiol 2005;26:1237–40 [PMC free article] [PubMed] [Google Scholar]
- 22.Lubicz B, Leclerc X, Gauvrit JY, et al. HyperForm remodeling-balloon for endovascular treatment of wide-neck intracranial aneurysms. AJNR Am J Neuroradiol 2004;25:1381–83 [PMC free article] [PubMed] [Google Scholar]