Abstract
BACKGROUND AND PURPOSE: Onyx was recently approved for the treatment of pial arteriovenous malformations, but its use to treat dural arteriovenous fistulas (DAVFs) is not yet well established. We now report on the treatment of intracranial DAVFs using this nonadhesive liquid embolic agent.
MATERIALS AND METHODS: We performed a retrospective analysis of 12 consecutive patients with intracranial DAVFs who were treated with Onyx as the single treatment technique at our institution between March 2006 and February 2007.
RESULTS: A total of 17 procedures were performed in 12 patients. In all of the cases, transarterial microcatheterization was performed, and Onyx-18 or a combination of Onyx-18/Onyx-34 was used. Eight patients were men. The mean age was 56 ± 12 years. Nine patients were symptomatic. There was an average of 5 feeders per DAVF (range, 1–9). Cortical venous reflux was present in all of the cases except for 1 of the symptomatic patients. Complete resolution of the DAVF on immediate posttreatment angiography was achieved in 10 patients. The remaining 2 patients had only minimal residual shunting postembolization, 1 of whom appeared cured on a follow-up angiogram 8 weeks later. The other patient has not yet had angiographic follow-up. Follow-up angiography (mean, 4.4 months) is currently available in 9 patients. There was 1 angiographic recurrence (asymptomatic), which was subsequently re-embolized with complete occlusion of the fistula and its draining vein. There was no significant morbidity or mortality.
CONCLUSION: In our experience, the endovascular treatment of intracranial DAVFs with Onyx is feasible, safe, and highly effective with a small recurrence rate in the short-term follow-up.
Dural arteriovenous fistulas (DAVFs) are acquired abnormal arteriovenous connections within the dura that account for 10%–15% of all intracranial arteriovenous malformations (AVMs).1 The origin of these malformations is not entirely understood but has been associated with several conditions including venous thrombosis, intracranial surgery, tumor, puerperium, and trauma.2 DAVFs may be asymptomatic or present with symptoms that range from tinnitus to intracranial hemorrhage (ICH) and severe neurologic deficits. Their behavior is fundamentally determined by the venous drainage pattern. Retrograde leptomeningeal or cortical venous drainage has a strong correlation with adverse clinical events: such patients are thought to have an annual risk of aggressive neurologic presentation of 15% resulting in an annual mortality of 10.4%.3 Moreover, rebleeding rates may be as high as 35% over the first 2 weeks after the initial hemorrhage.4 Thus, the DAVFs that have these features require treatment.
The current management of DAVFs includes endovascular, surgical, and radiosurgical treatments, either alone or in combination. Endovascular therapy is typically performed with cyanoacrylate, ethyl alcohol, coils, and/or particles. Onyx (ev3, Irvine, Calif), an ethylene vinyl alcohol copolymer preparation, was recently approved for the treatment of pial AVMs, but its use to treat DAVFs is not yet well established. We report our preliminary experience with the endovascular treatment of intracranial DAVFs using this nonadhesive liquid embolic agent.
Materials and Methods
Patients
From March 2006 to February 2007, 12 consecutive patients with intracranial DAVFs were treated with Onyx embolization at our institution as the single treatment modality. The mean age of these patients was 56 ± 12 years (median, 59 years; range, 31–73 years). There were 8 men and 4 women. Clinical presentation included ICH in 4 patients (33%), tinnitus in 2 patients (17%), superficial siderosis in 1 patient (8%), trigeminal neuralgia in 1 patient (8%), and severe sudden headache in 1 patient (8%). Three patients (25%) were asymptomatic; however, they all had significant cortical venous drainage on angiography. No causative process could be identified for 9 of the cases. One DAVF was discovered on angiographic follow-up after resection of an ipsilateral tentorial DAVF; 1 was associated with severe ipsilateral stenosis at the transverse-sigmoid transition, as well as internal jugular occlusion; and 1 was associated with sigmoid sinus occlusion. It was not possible to determine whether the findings in the 2 later cases were cause or consequence of the DAVF.
Embolization Procedure
All of the procedures were performed with the patient under general anesthesia on a biplane angiographic unit (Neurostar and Axion Artis; Siemens Medical Solutions, Erlangen, Germany). Informed consent was obtained. Catheterization was performed via a transfemoral approach using standard coaxial techniques and with the catheter subject to continuous flushing with heparinized saline (4000 U of heparin per liter). All of the patients were treated exclusively via a transarterial approach. After the diagnostic angiography was concluded, a 5F MPC Envoy guide catheter (Cordis Endovascular, Miami Lakes, Fla) was placed in the external carotid artery (ECA).
A Marathon microcatheter (ev3) was subsequently navigated over an X-Pedion-10 microwire (ev3) to reach the distal aspect of the pedicle supplying the DAVF. Microcatheter angiography was then performed to confirm optimal position. The microcatheter was flushed with 5 mL of normal saline. Onyx and dimethyl-sulfoxide (DMSO) were drawn into 2 separate 1-mL syringes. The dead space of the microcatheter was subsequently filled with 0.25 mL of DMSO, and the microcatheter hub was bathed with DMSO forming a meniscus. Onyx was injected over 90 seconds to fill the microcatheter and replace the DMSO in the dead space. Under continuous fluoroscopic guidance, Onyx injection was then slowly carried out using a “thumb-tapping” technique. A small amount of initial reflux was allowed, as it typically leads to the formation of a “plug,” which may subsequently facilitate antegrade flow of the embolic agent. Excessive reflux, however, was avoided, since it may lead to the occlusion of normal vessels or make the microcatheter removal more difficult. Single injections of Onyx were carried out for as long as 90 minutes. Injection pauses of 30–120 seconds were performed whenever unwanted reflux or flow into nontarget areas was observed. This seems to allow for precipitation of the Onyx at those areas, which results in diversion of the newly injected Onyx to other areas. Angiography was occasionally performed through the guide catheter during these injection pauses to evaluate for residual flow to the DAVF or nontarget embolization.
Results
Anatomic Results
There was an average of 5 ± 2 arterial feeders for each DAVF (median, 4.5; range, 1–9). Six lesions (50%) were classified as Cognard IV/Borden 3; 4 lesions (33%) as Cognard III/Borden 3; 1 lesion (8%) as Cognard IIa + IIb/Borden 2; and 1 lesion (8%) as Cognard IIa/Borden 1. Four DAVFs (33%) were located at the transverse/sigmoid sinus region, 4 (33%) were located at the tentorial region, 3 (25%) were located at the midline/superior sagittal sinus region, and 1 (8%) was located at the torcular/transverse sinus region.
Immediate and Follow-up Embolization Results
A total of 17 procedures were performed in 12 patients. Complete angiographic cure on immediate posttreatment angiography was achieved in 10 patients. This was accomplished after only 1 procedure in 8 patients and after embolization from a single pedicle in 5 patients. The remaining 2 patients had minimal residual shunting postembolization. We believed that there was a reasonable likelihood that these would go onto cure without further embolization. As such, we did not attempt further embolization for cure in the initial sitting. In fact, 1 of the lesions did appear cured on follow-up angiogram performed 8 weeks later. The other patient has not yet had angiographic follow-up. Patency of the venous sinuses was maintained in all of the cases except 1 in which the sigmoid sinus was purposefully occluded with Onyx. In that patient, the sigmoid sinus was nonfunctional with ipsilateral jugular vein occlusion and severe stenosis at the transverse-sigmoid junction (Fig 1). There was 1 technical complication (asymptomatic extracranial vertebral artery dissection managed with antiplatelet therapy). There was no significant morbidity or mortality.
Fig 1.
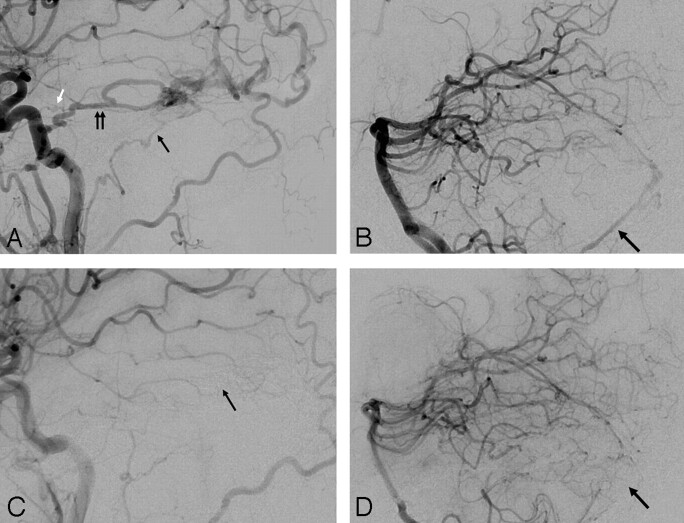
A, Left common carotid angiography (lateral view). B, Left vertebral angiography (anteroposterior view). C, Postembolization left common carotid angiography (lateral view). The black arrow points to the subtracted Onyx cast. D, Postembolization left vertebral angiography (AP view, native image). The double white arrow points to the Onyx cast. The single white arrow points to Onyx reflux into the distal vertebral dural branch.
Follow-up angiography is currently available in 9 patients at a mean of 4.4 months (median, 4.4 months; range, 1.8–6.5 months). There was no evidence of residual or recurrent DAVF in 8 patients. One of the initially “cured” patients had evidence of a small recurrent DAVF on follow-up. This patient was asymptomatic and was subsequently embolized with Onyx with apparent cure on the immediate posttreatment angiogram. In this patient, dense penetration of Onyx into the draining vein was only accomplished during the latest treatment (Fig 2).
Fig 2.
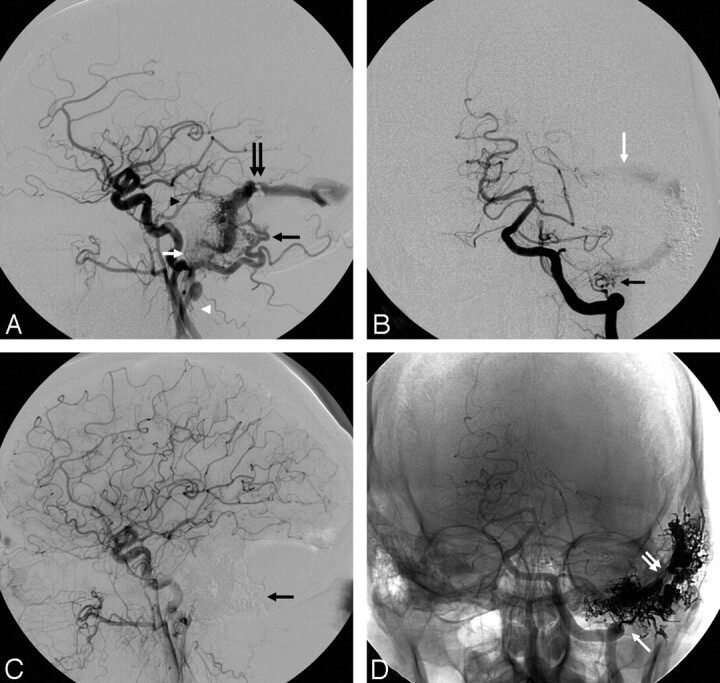
A, Simultaneous bilateral external carotid angiography (AP view). B, Left external carotid angiography (lateral view). C, Right external carotid angiography 4.6 months after stage II embolization. Right upper corner shows microcatheter angiography before stage III Onyx embolization (arrowhead, microcatheter tip; arrow, previous Onyx cast; circle, final target). D, Left common carotid angiography 4.6 months after stage II embolization (lateral view). E–F, Poststage III embolization right (E, AP view late venous phase) and left (F, lateral view arterial phase) common carotid angiography show complete resolution of the fistula with preservation of the venous sinuses and obliteration of the left PCA (F, double white arrow), middle meningeal (F, black arrowhead), and occipital supply (F, black arrow). E, double white arrow, Subtracted Onyx cast; E, black arrowhead, Onyx in the transverse sinus leaflet. G and H, (AP view-native images) Onyx cast before (G) and after (H) the third and final embolization. Note that the target area showed on G has been completely filled with the embolic agent on H (white circle).
Illustrative Cases
Case 1.
A 66-year-old man presented with left-sided tinnitus in the setting of a Cognard IIa + IIb DAVF fed by the left occipital artery (Fig 1A, black arrow) and the left middle meningeal artery (Fig 1A, black arrowhead), as well as the left ascending pharyngeal and posterior auricular arteries (Fig 1A, white arrow) and a dural branch off the distal left vertebral artery (Fig 1B, black arrow). The fistula drained into the left sigmoid sinus with retrograde filling of the transverse sinus (Fig 1B, white arrow). There was severe focal stenosis at the transverse-sigmoid junction (Fig 1A, double black arrow) and complete occlusion of the left internal jugular vein (Fig 1A, white arrowhead). Three pedicles off the left occipital artery were embolized at different stages resulting in complete occlusion of the sigmoid sinus with resolution of the DAVF (Fig 1C, -D). Prompt recognition of retrograde flow of Onyx from the sigmoid sinus to the dural branch arising from the vertebral artery prevented inadvertent embolization of the left vertebral artery (Fig 1D, white arrow).
Case 2.
A 63-year-old woman presented with sudden onset of severe headache and right homonymous hemianopsia in the setting of a left occipital ICH related to a Cognard IV DAVF fed by the left occipital artery (Figs 2A, -B, black arrows) and a branch of the left calcarine artery. In addition, there was mild contribution from the right middle meningeal (Fig 2A, double black arrow) and right occipital (Fig 2A, white arrow) arteries. Venous drainage was into a partially thrombosed ectatic cortical vein (Figs 2A, -B, double white arrows) and then into the left transverse sinus. Embolization via a left occipital pedicle in the acute phase resulted in near complete occlusion of the DAVF with mild residual filling via the left posterior cerebral artery (PCA). Angiography performed 8 weeks later showed significant interval enlargement of the right middle meningeal artery supply and less markedly so of the right occipital artery supply. There was stable residual filling of the fistula via the left PCA but no residual left occipital artery supply. Stage II Onyx embolization was then performed via a right middle meningeal pedicle. Immediate postprocedural angiogram showed no residual arteriovenous shunting; however, follow-up angiography performed 4.6 months later showed recurrence of the DAVF with supply via the right occipital artery (Fig 2C, white arrow) and left PCA (Fig 2D, double white arrow), as well as interval recruitment of the left middle meningeal artery (Fig 2D, black arrowheads). There was persistent occlusion of the right middle meningeal (Fig 2C, double black arrow) and left occipital supply (Fig 2D, black arrow). Eight weeks later, a third embolization was performed via the right occipital artery. During this procedure, a guide catheter was placed in the right ECA to allow microcatheter access to the right occipital artery supply to the fistula. A second 5F catheter was then placed in the left common carotid artery in order to allow for angiographic confirmation of occlusion of the left PCA and ECA supply during the Onyx injection, as well as to monitor for possible nontarget artery-to-artery embolization. This resulted in complete obliteration of the fistula and draining vein with complete preservation of the transverse sinus (Fig 2E, -F).
Case 3.
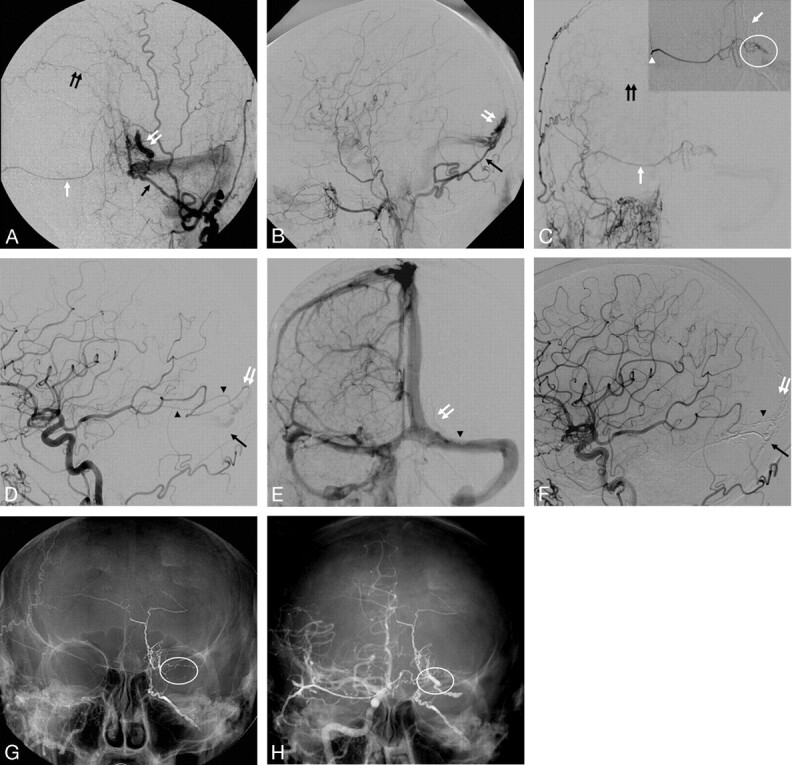
A 73-year-old man was found to have a Cognard III DAVF during an angiogram performed for work-up of superficial siderosis. The DAVF (Fig 3A, -B, white arrowheads) was supplied by the right middle meningeal artery (Fig 3A, -B, black arrows) and drained into the vein of Trolard (Fig 3A, -B, white arrows). The pedicle that supplied the DAVF was successfully catheterized, but navigation into its more distal aspect was not possible because of marked tortuosity. Embolization was carried out from the proximal portion of the pedicle (Fig 3B, black arrow) with optimal penetration into the fistula (Fig 3C, arrowheads) resulting in angiographic cure and return of antegrade flow in the vein of Trolard (Fig 3D, white arrow).
Fig 3.
A, Right external carotid angiography (lateral view). B, Right middle menigeal microcatether angiography (lateral view). C, Native image demonstrating a dense Onyx cast in the right middle meningeal artery (black arrow) extending into the fistula (arrowheads). D, Postembolization right common carotid angiography (lateral view-late venous phase).
Case 4.
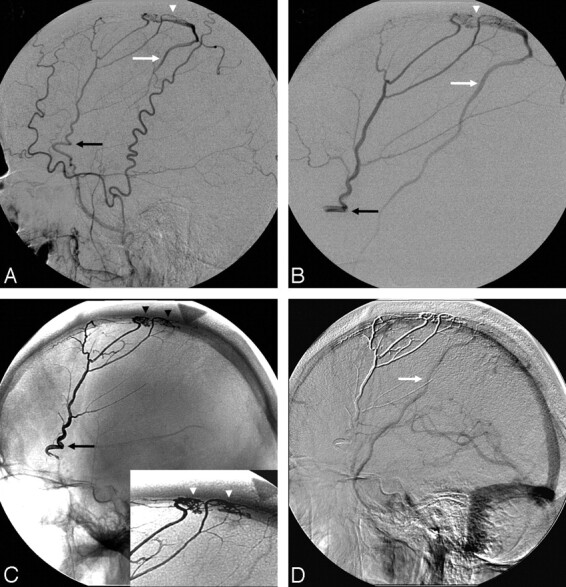
A 59-year-old woman presented with sudden onset of severe headache and speech difficulties in the setting of a left temporoparietal ICH related to a large Cognard IV DAVF fed by the bilateral middle meningeal arteries (Fig 4A, -B, white arrows), bilateral occipital arteries (Fig 4A, -B, white arrowheads), angular branch of the left middle cerebral artery (MCA; Fig 4C, arrowhead), right recurrent meningeal artery, left PCA (Fig 4D, white arrowhead), right posterior meningeal artery (Fig 4D, black arrow), and left artery of the falx cerebelli (Fig 4D, white arrow). The venous drainage was into multiple ectatic cortical veins and then into both superficial (Fig 4A, -B, black arrowheads) and deep venous systems (Fig 4A, -B, black arrows). Embolization of a left middle meningeal artery pedicle with 12 mL of Onyx-18 resulted in near complete occlusion of the DAVF with minimal residual filling via the left PCA of a small dural vein that drained into the DAVF. However, there was complete occlusion of the fistula itself. Follow-up angiogram performed 8 weeks later revealed interval thrombosis of the small draining vein with no residual fistula (Fig 4E–H).
Fig 4.
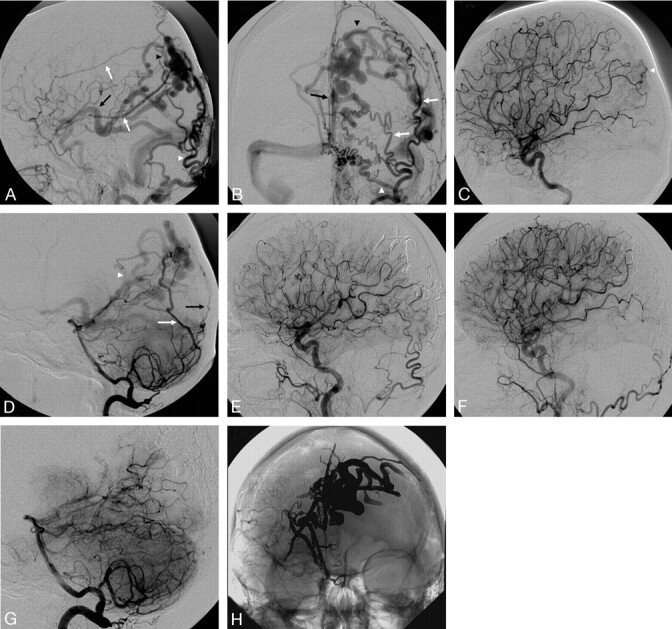
A, Right external carotid angiography (lateral view). B, Left external carotid angiography (AP view). C, Left internal carotid angiography (lateral view). D, Left vertebral artery angiography (lateral view). E, Postembolization right common carotid angiography (lateral view). F, Postembolization left common carotid angiography (lateral view). G, Postembolization left vertebral angiography (lateral view). H, Postembolization native image, Onyx cast (AP view).
Case 5.
A 51-year-old man was found to have a left tentorial Cognard IV DAVF during work-up of the left trigeminal neuralgia. The fistula was fed by the left middle meningeal artery (Fig 5A, double black arrow), left ascending pharyngeal artery (Fig 5A, black arrow), lateral tentorial branch of the left internal carotid artery (ICA) (Fig 5A, white arrow), and the right artery of the falx cerebelli (Fig 5B, black arrow). Embolization was performed via the left middle meningeal artery and the right artery of the falx cerebelli. On both occasions, Onyx-34 was initially injected and early reflux was purposely sought with the intent of building a dense plug that would subsequently facilitate antegrade deposition of Onyx. Later injections were performed with Onyx-18. Complete occlusion of the DAVF was accomplished (Fig 5C, -D, black arrows).
Fig 5.
A, Left common carotid angiography (lateral view). B, Left vertebral angiography (lateral view). C, Postembolization left common carotid angiography (lateral view). D, Postembolization left vertebral angiography (lateral view).
Discussion
The main goal of endovascular treatment of aggressive DAVFs is to eliminate the cortical venous drainage and the resulting risk of ICH. Different endovascular approaches can be used in the treatment of these lesions. The transvenous approach consists of the retrograde catheterization and sacrifice of the involved venous structure (usually a dural sinus). This approach has high rates of cure and complete occlusion of the fistula; however, it may be associated with severe complications, including vessel rupture or perforation, venous infarct, and hemorrhage.5 Hemorrhage, when not related to vessel injury, is usually related to the sacrifice of a dural sinus that was functionally draining normal cerebral or cerebellar parenchyma.2 Transvenous treatment of DAVFs with angioplasty and stent deployment within the involved dural sinus has also been proposed.6 The transarterial approach consists of superselective catheterization of the dural branches supplying the DAVF to deliver the embolic agent. Polyvinyl alcohol (PVA) particles can be used to reduce the shunt flow; however, permanent cure is difficult to achieve with this method alone. Therefore, PVA embolization is usually not recommended as sole therapy for aggressive lesions. The use of n-butyl-2-cyanoacrylate (n-BCA) has been applied to treat DAVF with variable success. Complete and lasting fistula obliteration can be achieved, particularly when the embolic material reaches the fistulous connection.7 The major disadvantage of this technique is the somewhat unpredictable nature of n-BCA polymerization, often leading to inadequate penetration into the fistula. Another potential disadvantage is the fact that n-BCA is an adhesive agent and risks permanent retention of the microcatheter.
Ethylene-vinyl alcohol copolymer (Onyx) is a nonadhesive liquid embolic agent with a lava-like flow pattern. Onyx is dissolved in the organic solvent DMSO at a concentration of 6% (Onyx-18), 6.5% (Onyx-20), or 8% (Onyx-34). Micronized tantalum powder is added to the mixture for radiopacity. Onyx must be shaken for at least 20 minutes before its injection to achieve homogeneous radiopacity. When this mixture contacts aqueous media, such as blood, DMSO rapidly diffuses away from the mixture, causing in-situ precipitation and solidification of the polymer, with formation of a spongy embolus.8 This solidification occurs more slowly than that of cyanoacrylates, and since Onyx is nonadherent to the walls of the vessel or microcatheter, this material allows prolonged injection times while decreasing the chances of permanent microcatheter retention.8 If proximal microcatheter reflux is observed or unfavorable distal filling occurs, the injection is stopped and resumed after 30 seconds to 2 minutes (reflux-hold-reinjection technique).9 Due to its properties and increased predictability, the use of Onyx may allow for more controlled injections with better distal nidus or fistula penetration as compared to cyanoacrylate. Histopathologic and angiographic studies have shown no evidence of short-term recanalization after Onyx embolization.8,10 In addition, Onyx appears to have better surgical handling characteristics than cyanoacrylate.11 Several series have now established a high degree of safety and efficacy of Onyx in the treatment of intracranial AVMs.10,12–14
Our preliminary results with the use of Onyx for the embolization of intracranial DAVFs appear very promising. Complete angiographic cure was seen in 11 of the 12 patients (91.7%), and minor residual supply was left in 1 patient. The aforementioned results were accomplished after only 1 procedure in most patients despite the high degree of complexity of the treated fistulas, which had an average of 5 arterial feeders per lesion. There were no major complications, and none of our patients required surgery or radiosurgery. We believe that Onyx is potentially more predictable and controllable than other embolic materials. Moreover, the techniques of Onyx use appear to be more easily understood and used, quickening the learning curve of becoming technically facile with this embolic agent relative to n-BCA.
There have been only a few sporadic reports of Onyx embolization for the treatment of intracranial DAVF.7,9,15 Arat and Inci15 described a case of superior sagittal sinus DAVF supplied by multiple dural as well as pial branches. Transarterial injection of 2 dural pedicles resulted in complete obliteration of the dural supply with residual pial supply via anterior cerebral artery and MCA branches. However, follow-up angiography performed 10 months later showed complete resolution of the DAVF. Rezende et al7 reported a Cognard type IV DAVF of the lesser sphenoid wing region supplied by ECA and ICA branches that was successfully treated with embolization of a single middle meningeal artery pedicle. Temporary balloon occlusion of the ophthalmic segment of the ICA was performed in order to avoid inadvertent reflux of Onyx into the ICA via the dural branches. These same authors reported this and another 5 cases of DAVFs in the French literature.16 In all of the cases, a single Onyx injection after one unique feeder catheterization led to complete anatomic exclusion of the fistula without any clinical complications. Finally, Suzuki et al9 reported 3 cases of dural carotid cavernous fistulas that were completely obliterated with transvenous embolization using a combination of detachable coils and Onyx.
When DAVFs are predominantly supplied by branches of the ECA, significant amounts of Onyx reflux do not preclude safe and effective transarterial embolization with Onyx. Full awareness of the relevant anatomy, including vascular supply to the cranial nerves, anatomy of the dura, the vascular collaterals, as well as the potentially dangerous anastomosis, is critical. One particularly dangerous feature of Onyx is a propensity to retrogradely fill other arterial feeders.12 In cerebral AVMs, this behavior of the material may be associated with cerebral infarction. This phenomenon was witnessed in several of the cases in this series (eg, case 1), though none resulted in complications. With ECA branch embolization, the possibility of anastomosis to the intracranial circulation either through the DAVF itself or along pre-existing collateral vessels (eg, C1 occipital to vertebral artery), must be considered, even if not visualized in the initial angiogram, since small collaterals may only become apparent after the embolization is initiated and changes in hemodynamics occur.
We have identified some helpful techniques and some pitfalls for the use of Onyx in DAVFs. As demonstrated in case 1, Onyx offers the possibility of venous sinus packing/occlusion from an arterial side approach, which may be quite helpful in cases with previous occlusion of the draining venous structures. We have seen recurrence of the DAVF after apparent angiographic cure on the immediate postprocedural angiography (eg, case 2). Long-term follow-up angiography is, therefore, required. Not unexpectedly, likelihood of “recurrence” increases with poor or no penetration of Onyx through the fistulous segment into the draining vein. Presumably, this allows for recruitment of nonvisualized arterial supply into the low-pressure system. Case 2 further illustrates that a relatively long interval between treatments may allow for enlargement of a pedicle that was initially not large enough to allow for catheterization/embolization. This strategy may be especially useful in cases where partial embolization of the fistula results in minimal or no cortical venous reflux and the residual pedicles are too small to be embolized. Case 2 highlights the use of the dual catheter technique in cases of multiterritorial supply to the DAVF. This technique allows for the angiographic confirmation of complete occlusion of the fistula during the Onyx injection. Furthermore, it better defines adjacent normal territory and, thus, helps in preventing possible nontarget artery-to-artery embolization.
In our experience, Onyx appears to allow for embolization from more proximal locations than cyanoacrylate. As shown in case 3, this may be accomplished without compromising the Onyx penetration into the fistula. We have used this technique in cases where unfavorable vascular anatomy prevents more distal microcatheterization. Another potential advantage of Onyx over cyanoacrylate is that Onyx embolization may allow for the occlusion of several different vessel feeders from a single pedicle as exemplified by case 4. This avoids the time and repeated risk of subsequent catheterizations that may be required with n-BCA.
We have found it extremely helpful to allow the early formation of a dense plug proximal to the microcatheter tip by purposefully avoiding the antegrade deposition/refluxing the embolic agent during the initial phases of the injection. This can be more easily accomplished with the initial use Onyx-34, since the higher concentration of ethylene-vinyl alcohol copolymer increases viscosity, and the lower concentration of DMSO leads to a faster precipitation. In our experience, this technique has resulted in a more rapid creation of a dense plug that has subsequently facilitated the antegrade deposition of Onyx-18. This technique is illustrated in case 5.
Despite its advantages, adverse events, such as catheter rupture or entrapment, and angiotoxicity have been associated with Onyx embolization.16 Potential causes for catheter rupture or entrapment are the pressure required to move the Onyx plug forward, the length or density of reflux, the tortuosity of feeding vessel, and the injection time.14 However, such events are uncommon as shown in a recent series of AVM embolization where microcatheters were glued to the nidus in only 2 of 138 injections.12 Angiotoxicity with vasospasm or angionecrosis has also been associated with Onyx embolization due to use of DMSO.10 The DMSO angiotoxicity is dependent on the volume injected and time in contact with the endothelium.8 Murayama et al8 demonstrated that the injection of 0.3 mL for 40 seconds was safe with no histopathologic evidence of angiotoxicity. Another potential disadvantage of Onyx is the amount of radiation exposure required for prolonged injections. However, this did not appear to be an issue in a recent study,12 and one needs to counterbalance the amount of radiation required for these long injections against the fluoroscopy time required for the several catheterizations and microcatheterizations that are frequently required with transarterial embolization with cyanoacrylate, as well as for transvenous coil occlusion of the dural sinuses.
Conclusion
In our experience, the endovascular treatment of intracranial DAVFs with Onyx is feasible, safe, and highly effective, with a small recurrence rate in the short-term follow-up. Further studies are needed to evaluate the long-term efficacy of this treatment and to compare it with the more traditional endovascular therapies. The current data suggest that the improved safety and efficacy profile of Onyx in this setting appears to outweigh its known risks.
Footnotes
This work has been presented at: American Society of Neuroradiology, June 9–14, 2007; Chicago, Ill.
References
- 1.Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology 1969;93:1071–78 [DOI] [PubMed] [Google Scholar]
- 2.Sarma D, ter Brugge K. Management of intracranial dural arteriovenous shunts in adults. Eur J Radiol 2003;46:206–20 [DOI] [PubMed] [Google Scholar]
- 3.van Dijk JM, terBrugge KG, Willinsky RA, et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 2002;33:1233–36 [DOI] [PubMed] [Google Scholar]
- 4.Duffau H, Lopes M, Janosevic V, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg 1999;90:78–84 [DOI] [PubMed] [Google Scholar]
- 5.Kiyosue H, Hori Y, Okahara M, et al. Treatment of intracranial dural arteriovenous fistulas: current strategies based on location and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics 2004;24:1637–53 [DOI] [PubMed] [Google Scholar]
- 6.Liebig T, Henkes H, Brew S, et al. Reconstructive treatment of dural arteriovenous fistulas of the transverse and sigmoid sinus: transvenous angioplasty and stent deployment. Neuroradiology 2005;47:543–51 [DOI] [PubMed] [Google Scholar]
- 7.Rezende MT, Piotin M, Mounayer C, et al. Dural arteriovenous fistula of the lesser sphenoid wing region treated with Onyx: technical note. Neuroradiology 2006;48:130–34 [DOI] [PubMed] [Google Scholar]
- 8.Murayama Y, Vinuela F, Ulhoa A, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery 1998;43:1164–75 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Lee DW, Jahan R, et al. Transvenous treatment of spontaneous dural carotid-cavernous fistulas using a combination of detachable coils and Onyx. AJNR Am J Neuroradiol 2006;27:1346–49 [PMC free article] [PubMed] [Google Scholar]
- 10.Jahan R, Murayama Y, Gobin YP, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery 2001;48:984–95; discussion 995–97 [DOI] [PubMed] [Google Scholar]
- 11.Akin ED, Perkins E, Ross IB. Surgical handling characteristics of an ethylene vinyl alcohol copolymer compared with N-butyl cyanoacrylate used for embolization of vessels in an arteriovenous malformation resection model in swine. J Neurosurg 2003;98:366–70 [DOI] [PubMed] [Google Scholar]
- 12.van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. AJNR Am J Neuroradiol 2007;28:172–77; discussion 178 [PMC free article] [PubMed] [Google Scholar]
- 13.Weber W, Kis B, Siekmann R, et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx: technical aspects. AJNR Am J Neuroradiol 2007;28:371–77 [PMC free article] [PubMed] [Google Scholar]
- 14.Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol 2007;28:518–23 [PMC free article] [PubMed] [Google Scholar]
- 15.Arat A, Inci S. Treatment of a superior sagittal sinus dural arteriovenous fistula with Onyx: technical case report. Neurosurgery 2006;59:169–70 [DOI] [PubMed] [Google Scholar]
- 16.Toulgoat F, Mounayer C, Tulio Salles Rezende M, et al. Transarterial embolisation of intracranial dural arteriovenous malformations with ethylene vinyl alcohol copolymer (Onyx18). J Neuroradiol 2006;33:105–14 [DOI] [PubMed] [Google Scholar]