Abstract
BACKGROUND AND PURPOSE: CHARGE syndrome is a genetic disorder resulting in the association of multiple congenital anomalies. Although a high prevalence of olfactory anomalies in CHARGE syndrome has been reported in autopsy and functional studies, to our knowledge, such anomalies have not been included among the diagnostic criteria, and their radiographic prevalence has not been assessed. The purpose of this research was to determine the radiographic prevalence of olfactory anomalies in a small sample of subjects with diagnosed CHARGE syndrome.
MATERIALS AND METHODS: The medical records and high-resolution MR images (section thickness ≤3 mm and in-plane resolution ≤1 mm) in 10 patients with clinically proved CHARGE syndrome were retrospectively reviewed by 3 neuroradiologists who consensually evaluated the status of the olfactory bulbs and sulci as either normal, hypoplastic, or absent. The prevalence (p) of congenital anomalies found in the medical records and of the olfactory structures was calculated with a 95% confidence interval (CI).
RESULTS: MR imaging demonstrated olfactory anomalies in all 10 patients, including either absence or hypoplasia of the olfactory bulbs and olfactory sulci (p, 100%; CI, 0.65–1.00).
CONCLUSION: These findings suggest that olfactory abnormalities detectable on high-resolution MR imaging are among the most prevalent features of CHARGE syndrome.
CHARGE syndrome is a genetic disorder with an estimated incidence from 1/85001 to 1/12,0002 live births and has been shown to be correlated to an alteration of the CDH7 gene in 2/3 of the neonates.3 It was initially described as a nonrandom association of multiple congenital anomalies by Hall4 in 17 children with choanal atresia and was independently described by Hittner et al5 in 10 patients with coloboma. In a later study of 12 patients, Pagon et al6 united these 2 associations under the acronym CHARGE, describing the 6 diagnostic features including the following: ocular Coloboma, Heart defects, choanal Atresia, Retarded growth and/or development, Genital anomalies, and characteristic Ear anomalies.
The definition of CHARGE syndrome continues to evolve as authors update the list of diagnostic criteria.7,8 Blake et al7 added cranial nerve anomalies to the list of major criteria, emphasizing the frequency of such anomalies as facial palsy (VII), sensory neural hearing loss (VIII), and swallowing difficulties (IX, X).9 He also modified the status of characteristic ear anomalies, including them among the major criteria, and he added a host of less specific but frequent minor criteria. Verloes8 designated semicircular canal defects as a major criterion, distinguishing them from the other ear anomalies.
Congenital anomalies of the olfactory structures in patients with CHARGE syndrome have been reported,6,10,11 and a literature review indicates that olfactory dysmorphism and dysfunction are a crucial part of this malformative syndrome.10–14 However, because olfaction is difficult to evaluate in neonates and young children, this anomaly has been largely underestimated. In this study, we assessed the degree and prevalence of olfactory anomalies detected on MR imaging examinations in a small sample of subjects with diagnosed CHARGE syndrome.
Materials and Methods
Institutional review board approval (IRB #G06-12-046) was obtained for a retrospective study of all patients with clinically proved CHARGE syndrome seen at our institution between 1997 and 2007. All patients were diagnosed as having CHARGE syndrome according to the criteria of both Blake et al7 and Verloes.8 Only those patients with high-resolution MR imaging (section thickness ≤3 mm and in-plane resolution ≤1 mm) of the anterior fossa in the coronal plane were enrolled in this study, resulting in the inclusion of 10 of the initial 15 patients. Of the 10 patients in this study, 5 were female and 5, male. The cohort comprised 4 neonates (14–21 days), 2 infants (40 and 42 days), and 4 young children (3–10 years).
All MR imaging examinations were performed on a 1.5T unit. When imaging neonates or small infants, we used either a pediatric phased-array head coil or a knee coil, along with the following standard parameters: a coronal T2-weighted turbo spin-echo sequence: section thickness, 3 mm; TR, 10,000 ms; TE, 270 ms; echo-train length, 27; NEX, 2; FOV, 160 × 120 mm; matrix, 256 × 173; and a coronal T1-weighted 3D gradient-echo sequence: partition size, 1.5 mm; TR, 36 ms; TE, minimum; flip angle, 35°; FOV, 22 cm; and matrix, 256 × 192. In the 4 young children, a standard phased-array head coil was used with the following T2 parameters: coronal T2-weighted turbo spin-echo sequence: section thickness, 3 mm; TR, 5400 ms; TE, 99 ms; echo-train length, 11; NEX, 3; FOV, 290 mm; and matrix, 512. Coronal T1-weighted parameters were the same in both age groups.
The MR imaging examinations were consensually reviewed by 3 board-certified and fellowship-trained neuroradiologists (J.B., C.F.E.K., A.P.), who evaluated the status of the olfactory bulbs and sulci as either normal, hypoplastic, or absent. When consensus could not be reached, structures were classified according to a two-thirds majority decision. All other congenital anomalies found in the medical records were documented in a data base. The prevalence (p) of all congenital anomalies was calculated along with the corresponding number of patients (n) and the 2-sided confidence intervals (CI) for a proportion15 with a 95% confidence level.
Results
Clinical Findings
The prevalence, number of patients, and the 2-sided CIs, with a 95% confidence level for each of the major diagnostic criteria found in the present study, are as follows: semicircular canal hypoplasia or agenesis (p, 86%; n, 7; CI, 0.42–.99); ear defects, including characteristic external ear malformation, ossicular malformations, or chronic serous otitis (p, 90%; n, 10; CI, 0.54–.99); coloboma of the eye (p, 70%; n, 10; CI, 0.35–.92); choanal atresia (p, 60%; n, 10; CI, 0.27–.86); cranial nerve dysfunction, including facial palsy (p, 50%; n, 10; CI, 0.20–.28); sensorineural hearing loss (p, 86%; n, 7; CI, 0.42–.99); or swallowing difficulties (p, 40%; n, 10; CI, 0.14–.73).
The prevalence, number of patients, and the 2-sided CIs with a 95% confidence level for each of the minor diagnostic criteria found in the present study are as follows: genital anomalies, including ectopic testicle and/or micropenis (p, 100%; n, 5; CI, 0.46–1.00); congenital heart defects, including conotruncal and ventricular septal defects (p, 90%; n, 10; CI, 0.54–.99); retarded growth and/or development (p, 60%; n, 10; CI, 0.27–.86); and palatal-facial clefts (p, 20%; n, 10; CI, 0.035–.56).
Imaging Findings
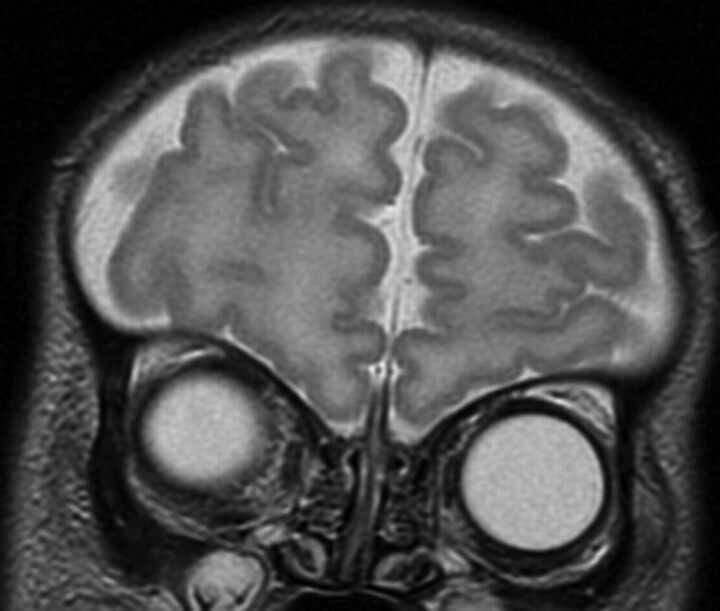
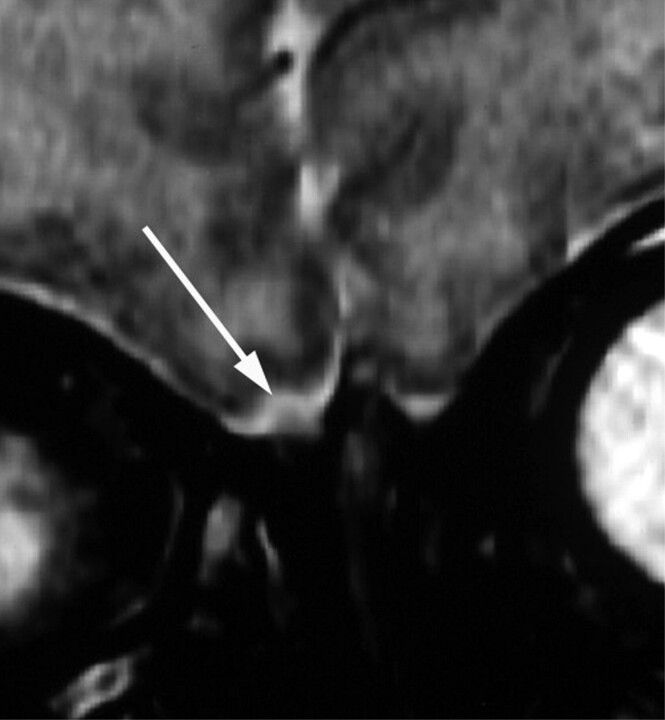
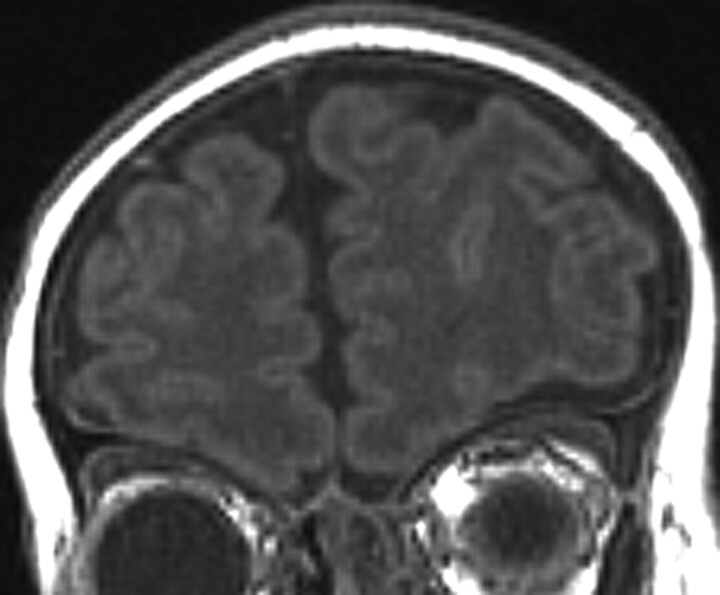
All 10 patients with CHARGE syndrome had anomalies of the olfactory bulbs and/or sulci (p, 100%; n, 10; CI, 0.65–1.00). The most common MR imaging finding was complete absence of both olfactory bulbs in 8 cases (Fig 1). There were 2 cases of asymmetrically hypoplastic and absent bulbs (Fig 2). Olfactory sulci were bilaterally absent in 6 cases (Fig 1), asymmetrically hypoplastic and absent in 2 cases (Fig 3), and normal-appearing in 2 cases.
Fig 1.
Coronal T2-weighted MR image through the anterior fossa in a 21-day-old neonate with CHARGE syndrome shows bilateral absence of olfactory bulbs and sulci.
Fig 2.
Coronal T2-weighted MR image through the anterior fossa in a 15-day-old neonate with CHARGE syndrome shows hypoplasia of the right olfactory bulb (white arrow) and absence of the left olfactory bulb.
Fig 3.
Coronal T1-weighted MR image through the anterior fossa in a 42-day-old infant with CHARGE syndrome shows asymmetrically hypoplastic right olfactory sulcus and absent left olfactory sulcus.
Discussion
MR imaging has been proved to be very sensitive in detecting normal olfactory structures in the developing fetus. In a retrospective fetal MR imaging study, the authors determined that T2-weighted coronal sequences were the most sensitive for detecting olfactory bulbs and sulci. Olfactory sulci were routinely detected starting at 30 weeks of gestation with a sensitivity of between 90.9% and 100%. MR imaging showed a posteroanterior development of these sulci, which was confirmed by neuropathologic data. Olfactory bulbs were detected between 30 and 34 weeks of gestation with a sensitivity of 80%–90.9%.16 The authors noted a discrepancy with a histopathologic study that showed the appearance of the olfactory sulcus to be at approximately the 16th week of gestation.17 This underestimation of the sensitivity of MR imaging to detect the olfactory sulcus at a younger gestational age could be attributed to the section thickness, which varied between 5 and 3 mm in the previously mentioned study. These data suggest that olfactory structures of neonates or young children should be detectible on high-resolution MR imaging; therefore, their absence or poor visibility should be regarded as pathologic. In the present study, 8 patients had bilateral absence of the olfactory bulbs and 2 patients had absence of 1 olfactory bulb associated with hypoplasia of the other bulb. Although the distinction between hypoplastic and normal bulbs may be difficult in some instances, the complete absence of olfactory bulbs on high-resolution MR imaging is unequivocal.
A review of the literature indicates that olfactory anomalies are a crucial feature of CHARGE syndrome. A postmortem study of 7 neonates with CHARGE syndrome revealed the presence of arrhinencephaly in all of the cases.11 In a postabortion study of 10 severely malformed fetuses with the CHD7 mutation characteristic of CHARGE syndrome, all were found to have arrhinencephaly.14 In a functional study, authors reported olfaction deficiency in all 14 patients with CHARGE syndrome, and imaging of all 9 patients demonstrated anomalies of the olfactory bulbs and tracts, ranging from agenesis to moderate hypoplasia.13 In a study of the hypothalamopituitary function of 18 patients with CHARGE syndrome, all were found to have central hypogonadism associated with structural anomalies of the olfactory apparatus on MR imaging.12 This led the authors to conclude that all patients with CHARGE syndrome have Kallmann syndrome as well.
In the present study, all patients had some degree of olfactory malformation. Such rare anomalies are also commonly observed in holoprosencephaly spectrum and Kallmann-De Morsier syndrome. Holoprosencephaly is a heterogeneous entity of central nervous system anomalies resulting from the impaired midline cleavage of the embryonic forebrain. Like CHARGE syndrome, holoprosencephaly may be associated with choanal atresia18–20 and extracranial anomalies thought to be of neural crest origin, including congenital heart defects.21–23 Cephalic neural crest cells have also been shown to give rise to the bones and dermal structures of the face24 and to induce forebrain development in the vertebrate embryo.25 Hengerer and Strom26 have advanced the widely accepted mesodermal flow theory of choanal atresia, in which there is excess migration of neural crest cells into the developing nasal septum and posterior choanae, interfering with their normal flow into the craniofacial region, described by Johnston27,28 and Johnston and Listgarten.29 This process may interfere with normal induction of the olfactory structures and the development of craniofacial structures seen in both entities. For example, in severe forms of holoprosencephaly, choanal atresia is associated with agenesis of the ethmoid bone, a neural crest derivative.22,23 Without the support of the ethmoid bone, the pterygoid plates collapse inwards, obstructing the choanae. Deficiency of the ethmoid bone or embryologically related structures of the anterior cranium may occur with alterations in the position of the orbits and the configuration of the nose, upper lip, philtrum, mouth, or palate.24,22 Anomalies of the midface and central nervous system in infants with CHARGE syndrome or mild forms of holoprosencephaly suggest similarities in pathogenesis. In the present study, 7 of the 10 patients presented with midfacial anomalies, including 2 cases of palatal-facial clefts and 5 cases of choanal atresia.
In the case of CHARGE syndrome, mutations have been identified in the CHD7 gene, a member of the chromodomain helicase deoxyribonucleic acid–binding gene family.30 This class of proteins is thought to play a role in embryonic development by affecting chromatin structure and gene expression.31 It has been suggested that the CHD7 protein anomaly probably interferes with the normal control of target genes expressed in mesenchymal cells derived from the cephalic neural crest during early embryonic development.12
Kallmann-De Morsier syndrome is defined as the association of hypogonadotropic hypogonadism with various degrees of deficiency in the sense of smell due to olfactory bulb aplasia or hypoplasia.32 The combination of hypogenitalism with abnormal olfactory bulb development also appears to be a common characteristic of CHARGE syndrome. In a review of 18 patients with CHARGE syndrome who had undergone functional testing of the hypothalamopituitary axis and brain MR imaging, all presented with a Kallmann-De Morsier syndrome.12 The present study confirms this finding with genital anomalies found in all male patients in our series. Kallmann syndrome is thought to result from a deficiency in fibroblast growth factor (FGF) signaling at the earliest stage of olfactory bulb morphogenesis.33 It has also been suggested that there may be a functional connection between CHD7 and FGF signaling in olfactory bulb differentiation. In particular, it is possible that CHD7 controls the transcription of 1 or several gene-encoding proteins involved in FGF signaling. Alternatively, FGF signaling through FGF receptor 1 may control the activity of CHD7 or the rate of transcription of CHD7 in the olfactory bulb primordium.12
The prevalence values of the major and minor criteria in the present study are in keeping with those that have been reported in the literature, suggesting that the diagnosis of CHARGE syndrome in this group of patients is accurate and that this group of patients is representative of CHARGE syndrome.
Although the number of patients in the present study was relatively small, the CIs for the prevalence of diagnostic criteria indicate a high and significant prevalence for olfactory anomalies in CHARGE syndrome.
Conclusion
This study confirms the high prevalence of olfactory anomalies in CHARGE syndrome, demonstrated in the high-resolution MR images of all subjects of the current series, and suggests that such anomalies are among the most prevalent features of CHARGE syndrome. Because of the clinical difficulties of assessing olfaction in neonates, infants, and young children, radiologists should systematically screen for morphologic anomalies of the olfactory structures when CHARGE syndrome is suspected, thereby playing a crucial role in the diagnosis of this syndrome.
Footnotes
Irene Netchine has been funded by a grant of the Sabbatical Leave Program from the European Society for Pediatric Endocrinology, supported by E. Lilly Co and by a grant of Serono France.
Paper previously presented at: 45th Annual Meeting of the American Society of Neuroradiology, Chicago, Ill; June 9–14, 2007.
References
- 1.Issekutz KA, Graham JM Jr, Prasad C, et al. An epidemiological analysis of CHARGE syndrome: preliminary results from a Canadian study. Am J Med Genet A 2005;133:309–17 [DOI] [PubMed] [Google Scholar]
- 2.Kallen K, Robert E, Mastroiacovo P, et al. CHARGE association in newborns: a registry-based study. Teratology 1999;60:334–43 [DOI] [PubMed] [Google Scholar]
- 3.Sanlaville D, Verloes A. CHARGE syndrome: an update. Eur J Hum Genet 2007;15:389–99 [DOI] [PubMed] [Google Scholar]
- 4.Hall BD. Choanal atresia and associated multiple anomalies. J Pediat 1979;95:395–98 [DOI] [PubMed] [Google Scholar]
- 5.Hittner HM, Hirsch NJ, Kreh GM, et al. Colobomatous microphthalmia, heart disease, hearing loss, and mental retardation: a syndrome. J Pediatr Ophthalmol Strabismus 1979;16:122–28 [DOI] [PubMed] [Google Scholar]
- 6.Pagon RA, Graham JM Jr, Zonana JY. Coloboma, congenital heart disease, and choanal atresia with multiple anomalies: CHARGE association. J Pediat 1981;99:223–27 [DOI] [PubMed] [Google Scholar]
- 7.Blake KD, Davenport SL, Hall BD, et al. CHARGE association: an update and review for the primary pediatrician. Clin Pediat 1998;37:159–73 [DOI] [PubMed] [Google Scholar]
- 8.Verloes A. Updated diagnostic criteria for CHARGE syndrome: a proposal. Am J Med Genet A 2005;133:306–08 [DOI] [PubMed] [Google Scholar]
- 9.Byerly KA, Pauli B, Pauli RM. Cranial nerve abnormalities in CHARGE. Am J Med Genet 1993;45:751–57 [DOI] [PubMed] [Google Scholar]
- 10.Lin AE, Siebert JR, Graham JM Jr. Central nervous system malformations in the CHARGE association. Am J Med Genet 1990;37:304–10 [DOI] [PubMed] [Google Scholar]
- 11.Harvey AS, Leaper PM, Bankier A. CHARGE association: clinical manifestations and developmental outcome. Am J Med Genet 1991;39:48–55 [DOI] [PubMed] [Google Scholar]
- 12.Pinto G, Abadie R, Mesnage R, et al. CHARGE syndrome includes hypogonadotropic hypogonadism and abnormal olfactory bulb development. J Clin Endocrinol Metab 2005;90:5621–26 [DOI] [PubMed] [Google Scholar]
- 13.Chalouhi C, Faulcon P, Le Bihan C, et al. Olfactory evaluation in children: application to the CHARGE syndrome. Pediatrics 2005;116:e81–88 [DOI] [PubMed] [Google Scholar]
- 14.Sanlaville D, Etchevers HC, Gonzales M, et al. Phenotypic spectrum of CHARGE syndrome in fetuses with CHD7 truncating mutations correlates with expression during human development. J Med Genet 2006;43:211–17. Epub 2005 Sep 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newcombe R. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998;17:857–72 [DOI] [PubMed] [Google Scholar]
- 16.Azoulay R, Fallet-Bianco C, Garel C, et al. MRI of the olfactory bulbs and sulci in human fetuses. Pediatr Radiol 2006;36:97–107 [DOI] [PubMed] [Google Scholar]
- 17.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol 1970;1:86–93 [DOI] [PubMed] [Google Scholar]
- 18.Siebert JR, Kokich VG, Beckwith JB, et al. The facial features of holoprosencephaly in anencephalic human specimens. II. Craniofacial anatomy. Teratology 1981;23:305–15 [DOI] [PubMed] [Google Scholar]
- 19.Siebert JR. The ethmoid bone: implications for normal and abnormal facial development. J Craniofac Genet Dev Biol 1982;1:381–89 [PubMed] [Google Scholar]
- 20.Kokich VG, Ngim CH, Siebert JR, et al. Cyclopia: an anatomic and histologic study of two specimens. Teratology 1982;26:105–13 [DOI] [PubMed] [Google Scholar]
- 21.DeMyer WE. Holoprosencephaly (cyclopia-arhinencephaly). In: Bruyn GW, Vinken PJ, eds. Congenital malformations of the brain and skull, Part I: Handbook of Clinical Neurology. Amsterdam, the Netherlands; Elsevier;1977;30:431–78 [Google Scholar]
- 22.Leech RW, Shuman RM. Holoprosencephaly and related midline cerebral anomalies: a review. J Child Neurol 1986;1:3–18 [DOI] [PubMed] [Google Scholar]
- 23.Cohen MM Jr. Perspectives on holoprosencephaly. Part I. Epidemiology, genetics, and syndromology. Teratology 1989;40:211–35 [DOI] [PubMed] [Google Scholar]
- 24.Couly G, Coltey P, Le Douarin NM. The developmental fate of cephalic mesoderm in quail-chick chimeras. Development 1992;114:1–15 [DOI] [PubMed] [Google Scholar]
- 25.Creuzet SE, Martinez S, Le Douarin NM. The cephalic neural crest exerts a critical effect on forebrain and midbrain development. Proc Natl Acad Sci U S A 2006;103:14033–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hengerer AS, Strom M. A new embryologic theory and its influence on surgical management. Laryngoscope 1982;92:913–21 [PubMed] [Google Scholar]
- 27.Johnston MC. A radioautographic study of the migration and fate of the cranial neural crest cells in the chick embryo. Anat Rec 1966;156:143–55 [DOI] [PubMed] [Google Scholar]
- 28.Johnston MC. The neural crest in abnormalities of the face and brain. In: Bergsma D, ed. Birth Defects: Original Article Series, Morphogenesis and Malformation of Face and Brain. New York: Alan R. Liss Inc;1975. :1–18 [PubMed]
- 29.Johnston MC, Listgarten MA. The migration, interaction and early differentiation of oro-facial tissues. In: Slarkin HS, Bavetta LA, eds. Developmental Aspects of Oral Biology. New York: Academic Press;1972. :55
- 30.Vissers ELM, van Ravenswaaij CMA, Admiraal R, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Gene 2004;36:955–57 [DOI] [PubMed] [Google Scholar]
- 31.Cavalli G, Paro R. Chromo-domain proteins: linking chromatin structure to epigenetic regulation. Curr Opin Cell Biol 1998;10:354–60 [DOI] [PubMed] [Google Scholar]
- 32.Kallmann FJ, Schoenfeld WA, Barrera SE. The genetic aspects of primary eunuchoidism. Am J Ment Defic 1944;48:203–06 [Google Scholar]
- 33.Dodé C, Hardelin JP. Kallmann syndrome: fibroblast growth factor signaling insufficiency? J Mol Med 2004;82:725–34. Epub 2004 Sep 8. [DOI] [PubMed] [Google Scholar]