Abstract
SUMMARY: We report on our experience with the intra-arterial administration of eptifibatide for thrombolysis during aneurysm-embolization procedures. In 4 cases (3 stent-assisted coiling procedures and 1 with posthemorrhagic vasospasm), we noted the formation of thrombus occluding a vessel. We administered eptifibatide (10–15 mg) through a microcatheter proximal to the thrombus. The thrombus rapidly dissolved, resulting in the recanalization of the occluded vessels with no rethrombosis or hemorrhagic complications.
Thrombus formation is a common and severe complication of neurovascular interventions. It can lead to partial or total occlusion of vascular branches and may result in transient or permanent neurologic symptoms.
Many protocols have been proposed to avoid this complication (heparinized saline drip, systemic heparinization, preoperative antiplatelet treatment, intraoperative antiplatelet intravenous administration). Despite all these measures, thrombosis can still occur, and its treatment has included mechanical clot disruption, balloon angioplasty, stent placement, intravenous antiplatelets, or intra-arterial thrombolytics.
We report on a novel treatment, the intra-arterial bolus administration of eptifibatide (Integrilin). It is a potent antiplatelet that we have used in the treatment of acute intraoperative thrombosis during aneurysm embolization.
Case Reports
In the following 4 cases of intraoperative thromboembolic complications, we administered intra-arterial eptifibatide.
The first of these 4 patients, a 54-year-old woman, had a ruptured left internal carotid artery (ICA) aneurysm clipped in another hospital 8 months before. She was admitted to our department for endovascular occlusion of an unruptured right ICA aneurysm. During the procedure, we decided to place a 4 × 15 Neuroform stent (Boston Scientific, Natick, Mass) in the right ICA to cover the wide neck of the aneurysm. We administered intravenous heparin to obtain an activated clotting time (ACT) double that of baseline and proceeded to deploy the stent. Unfortunately, the stent was not optimally placed, with its distal end protruding partially in the aneurysm sac. Two of the 4 distal markers were visualized in the artery, and the remaining 2, in the aneurysm. Despite this problem, we proceeded to embolize the aneurysm, achieving a satisfactory occlusion. Near the end of the procedure, we noticed extensive thrombus formation in the ICA all along the stent, especially at its distal end, with subsequent subtotal occlusion of the artery. Immediately, we administered an additional bolus of 2500-IU heparin, and in 3 minutes, we started slowly injecting eptifibatide from a microcatheter placed in the proximal ICA. The total dose administered was 15 mg (200 μg/kg) in 5–6 minutes. By the end of the injection, the thrombus had started to dissolve, and 10 minutes later, the ICA was again patent, with a small amount of thrombus remaining at the distal part of the stent. The patient was extubated without any neurologic symptoms and was started on our protocol of anticoagulation for stent-assisted coiling (including nadroparin 0.6 mg twice daily for 3 days, clopidogrel 75 mg daily for 3 months, and aspirin 100 mg daily for 6 months). She was discharged in excellent condition 5 days later and had no complications in the next 5 months. She is scheduled for control angiography at 12 months from the intervention.
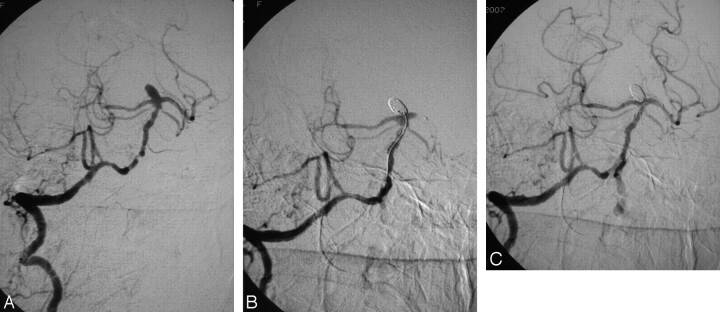
Patient 2, a 55-year-old obese woman, presented with subarachnoid hemorrhage following the rupture of a broad-based basilar tip aneurysm. Her basilar artery (BA) had multiple stenotic lesions, probably due to atherosclerosis (Fig 1A). She was intubated, and we proceeded to embolize the aneurysm. We administered IV heparin to obtain an ACT double that of baseline and placed a 3 × 15 mm Neuroform stent in the BA, extending into the left posterior cerebral artery (PCA). Catheterization and coiling of the aneurysm were uneventful until close to the completion of the intervention, when we noted that the left PCA was occluded due to thrombosis of the distal part of the stent (Fig 1C). We removed the microcatheter from the aneurysm and placed its tip just proximal to the thrombus and administered 15-mg (135 μg/kg) eptifibatide in 6–7 minutes. Three minutes later, the thrombus was dissolved and the vessel was patent again. We completed the intervention with balloon angioplasty of the most severe stenoses of the BA (Fig 1C). The patient was extubated without any neurologic symptoms and was started on our previously reported protocol for stent-assisted coiling. She was discharged in excellent condition on the 12th day after subarachnoid hemorrhage (SAH). At the 6-month follow-up, MR angiography showed persistent aneurysm occlusion with full patency of the BA and both PCAs.
Fig 1.
A, Digital subtraction angiography, right vertebral artery, working projection, showing a broad-based aneurysm of the basilar tip. The distal vertebral and the basilar arteries demonstrate areas of severe stenosis. B, Occlusion of the aneurysm with thrombosis of the distal part of the stent and total occlusion of the left PCA. C, Final result, with recanalization of the stent and of the PCA following the administration of 15-mg eptifibatide on the clot and balloon angioplasty of the stenotic lesions. Note that there are no signs of aneurysm recanalization.
The third patient, a 47-year-old woman, had been treated with coiling at another hospital for 2 right ICA aneurysms (1 at the origin of the posterior communicating artery [PcomA] and 1 at the origin of the anterior choroidal artery [AchoA]). She was admitted to our department for retreatment because the PcomA aneurysm exhibited regrowth, with distal migration of the coil mass, and the AchoA aneurysm had been partially recanalized. After administering IV heparin, we proceeded to place a 4 × 20 Neuroform stent to cover the necks of both aneurysms. First, we embolized the recanalization of the AchoA aneurysm and then the regrowth of the PcomA aneurysm without any complications. At the end of the interventions, we noticed a small thrombus formation at the origin of the middle cerebral artery (MCA), which partially obstructed its flow. We also noticed that the distal part of the stent was placed at the origin of the MCA and not at the distal part of the ICA, as we had intended. We re-inserted a microcatheter, placed its tip just proximal to the thrombus, and slowly injected 12-mg (171 μg/kg) eptifibatide for 5 minutes. Three minutes from the end of the injection, the thrombus was completely dissolved and the MCA was fully patent. The patient awoke neurologically intact and was placed on the same anticoagulation protocol. She was discharged 4 days later and is scheduled for control angiography.
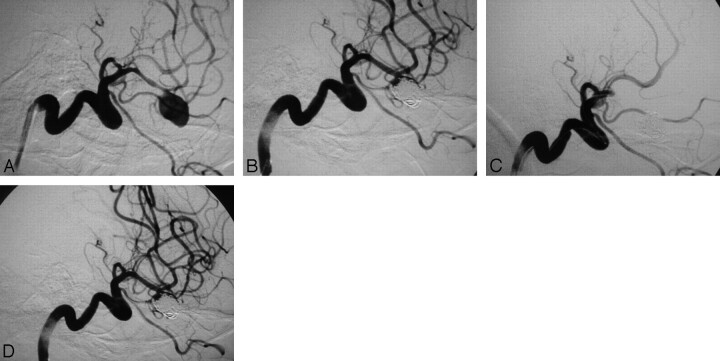
The last patient, a 55-year-old man, was referred to our department in Hunt and Hess I condition on the eighth day following a Fisher grade 3 subarachnoid hemorrhage (SAH). We immediately performed angiography, and on our discovery of a left MCA aneurysm, the patient was intubated and we proceeded to the embolization. Intense vasospasm was noted on the left MCA that harbored the aneurysm (Fig 2A), probably due to the SAH, and we added 30-mg papaverine to the drip solution. The aneurysm was embolized with difficulty because the temporal branch of the MCA originated close to its neck. Following the deployment of the first coil, 5000-IU heparin was administered intravenously. The end result was satisfactory, with preservation of the branch and also attenuation of the vasospasm due to the continuous papaverine infusion (Fig 2B). The procedure was terminated, but in the next angiography run, before retracting the microcatheter from the aneurysm, the MCA was found to be occluded (Fig 2C). We retracted the tip of the microcatheter in the origin of the MCA and administered 10-mg (105 μg/kg) eptifibatide in 5 minutes, which resulted in the recanalization of the vessel in the next 2 minutes (Fig 2D). The patient was extubated in excellent condition and was started on nadroparin 0.6 mg once a day for 3 days and aspirin 100 mg once a day for 3 months. He was discharged on the 14th post-SAH day and is scheduled for control angiography.
Fig 2.
A, Digital subtraction angiography, left ICA, working projection showing an aneurysm at the bifurcation of the MCA. The aneurysm is wide-necked and incorporates the origin of the temporal branch in its base. The MCA and its branches show signs of severe posthemorrhagic vasospasm. B, Subtotal occlusion of the aneurysm with preservation of the patency of the temporal branch of the MCA. C, Total occlusion of the MCA due to thrombus formation. The microcatheter is still in the aneurysm. D, Final result with total recanalization of the MCA following the administration of 10-mg eptifibatide on the clot. Note that there are no signs of aneurysm recanalization
Discussion
Thromboembolism is a common complication of aneurysm embolization and contributes significantly to the mortality and morbidity of the procedure.1–3 Thrombus formation is believed to be due to the presence of foreign materials in the aneurysm and in the parent artery, the electric current used for the detachment of coils, the changes in blood flow, and vessel injury.
Until recently, pharmacologic treatment included intra-arterial administration of urokinase or recombinant tissue plasminogen activator (rtPA). These fibrinolytic agents resulted in a moderate rate of vessel recanalization and were associated with a high rate of intracerebral hemorrhage, which caused significant mortality and morbidity.4-6 Following the administration of these agents, any emergency neurosurgical operation that was needed carried a significant risk of bleeding.
Several articles have proposed the intravenous administration of abciximab in the treatment of intraprocedural thrombus formation.4,7–11 Abciximab is a monoclonal antibody directed against the glycoprotein IIb/IIIa receptor complex found on platelets. Blocking of the receptor prevents platelet aggregation and thrombus propagation and contributes to the dissociation of aggregated platelets and subsequently to thrombolysis. The use of this drug in the setting of acute thrombosis appears more appropriate than the use of fibrinolytic drugs because it is very unlikely that the fresh thrombus contains a large amount of fibrin in patients under systemic heparinization and it is known to contain mainly platelet aggregates. Abciximab is mainly administered intravenously (initially as a bolus dose and then as a 12-hour infusion), but there are also reports of local intra-arterial infusion.12,13 The results of this agent appear to be better than those of urokinase/rtPA, with higher recanalization rates and lower rates of intracerebral hematoma formation.
Eptifibatide is also a glycoprotein IIb/IIIa inhibitor with a lower affinity for the receptors than abciximab. This means that larger doses of eptifibatide are necessary to achieve the same antiaggregation effect, but it is more potent in the dissociation of already-aggregated platelets.14 Eptifibatide has a longer plasma life, but this is due to the shorter receptor binding time, with more of this agent being released to the plasma.15 This shorter binding time is an advantage of eptifibatide because any operation needed can be performed without substantial risk of intraprocedural or postprocedural hemorrhage. As a result, emergency surgery can be performed almost immediately following the administration of eptifibatide but has to be postponed for 12–48 hours following abciximab treatment. Eptifibatide, as a lower molecular-weight compound than abciximab is able to penetrate the thrombus easier through the fibrin-fibrinogen network that is formed on the platelet aggregates,14 and it is responsible for thrombocytopenia less often than abciximab.16 Its antiplatelet effect is not inhibited by unfractionated heparin as is that of abciximab.17 Eptifibatide has another benefit over abciximab: it is less expensive, a factor that should also considered.18
Eptifibatide has been used in the prophylaxis against thromboembolic complications during aneurysm coiling and for the treatment of intraprocedural thrombosis but has only been administered intravenously as a bolus. Yi et al19 have used a prophylactic regimen of intravenous bolus eptifibatide (180 μg/kg) in cases they considered at high risk for thromboembolic complications (stent use, broad neck, large aneurysm, coil prolapse, retreatment, multilobulated dome, etc). Park et al6 treated distal multifocal thromboembolism with intravenous eptifibatide, whereas they treated acute proximal embolism with rtPA. We were unable to find articles reporting the intra-arterial administration of eptifibatide for acute thromboembolism during aneurysm embolization.
In the 4 cases, we present thrombus that was only a few minutes old and obviously would respond to a glycoprotein IIb/IIIa inhibitor. With all the advantages of eptifibatide over abciximab in mind when we decided to use an glycoprotein IIb/IIIa inhibitor for intra-arterial thrombolysis, we opted for the former over the latter.
Despite factors predisposing to thrombosis (stent malposition, stenoses, vasospasm), thrombolysis with the intra-arterial administration of low doses of eptifibatide (10–15 mg or 105–200 μg/kg) was successful in all 4 patients in a very short time (7–15 minutes).
The fact that very low doses of eptifibatide, similar or lower than those used intravenously for prophylaxis against thromboembolism,18 were proved sufficient for the successful thrombolysis indicates that the intra-arterial route is superior to the intravenous. It probably results in a high initial loading of the thrombus with the glycoprotein IIb/IIIa antagonist during the first pass of the agent within the blood stream. The intravenous doses required to achieve the same effect would have been considerably higher, would result in the inhibition of platelets for a longer time, and could predispose to the occurrence of hemorrhagic complications.
Achieving thrombolysis in a matter of only few minutes is extremely important. The extent of cerebral tissue damage due to ischemia is proportionate to the duration of the lack of perfusion. All 4 patients whom we describe had no new neurologic deficit despite the episodes of arterial occlusion. The reason is probably that the vessels were recanalized very early.
References
- 1.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–82 [DOI] [PubMed] [Google Scholar]
- 2.Pelz DM, Lownie SP, Fox AJ. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998;19:1541–47 [PMC free article] [PubMed] [Google Scholar]
- 3.Katsaridis V, Papagiannaki C, Violaris C. Guglielmi detachable coil versus Matrix coils: a comparison of the immediate post-treatment results of the embolization of 364 aneurysms in 307 patients—a single-center, single-surgeon experience. AJNR Am J Neuroradiol 2006;27:1841–48 [PMC free article] [PubMed] [Google Scholar]
- 4.Cronquist M, Pierrot L, Boulin A, et al. Local intraarterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracranial aneurysm: a comparison of anatomic results and clinical outcome. AJNR Am J Neuroradiol 1998;19:157–65 [PMC free article] [PubMed] [Google Scholar]
- 5.Koebbe CJ, Horowitz MB, Levy EI, et al. Intraarterial fibrinolysis for thromboemboli associated with endovascular aneurysm coiling. Intervent Neuroradiol 2002;8:151–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HK, Horowitz M, Jungreis C, et al. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2005;26:506–14 [PMC free article] [PubMed] [Google Scholar]
- 7.Lempert TE, Malek AM, Halbach VV, et al. Rescue treatment of acute parent vessel thrombosis with glycoprotein IIb/IIIa inhibitor during GDC coil embolization. Stroke 1999;30:693–95 [PubMed] [Google Scholar]
- 8.Ng PP, Phatouros CC, Khangure MS. Use of glycoprotein IIb-IIIa inhibitor for a thromboembolic complication during Guglielmi detachable coil treatment of an acutely ruptured aneurysm. AJNR Am J Neuroradiol 2001;22:1761–63 [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander MJ, Duckwiler GR, Gobin YP, et al. Management of intraprocedural arterial thrombus in cerebral aneurysm embolization with abciximab: technical case report. Neurosurgery 2002;50:899–902 [DOI] [PubMed] [Google Scholar]
- 10.Workman MJ, Cloft HJ, Tong FC, et al. Thrombus formation at the neck of cerebral aneurysms during treatment with Guglielmi detachable coils. AJNR Am J Neuroradiol 2002;23:1568–76 [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander MJ, Duckwiler GR, Gobin YP, et al. Management of intraprocedural arterial thrombus in cerebral aneurysm embolization with abciximab: technical case report. Neurosurgery 2002;50:899–901, discussion 901–02 [DOI] [PubMed] [Google Scholar]
- 12.Duncan IC, Fourie PA. Catheter-directed intra-arterial abciximab administration for acute thrombotic occlusions during neurointerventional procedures. Intervent Neuroradiol 2002;8:159–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song JK, Niimi Y, Fernandez PM, et al. Thrombus formation during intracranial aneurysm coil placement: treatment with intra-arterial abciximab. AJNR Am J Neuroradiol 2004;25:1147–53 [PMC free article] [PubMed] [Google Scholar]
- 14.Moser M, Bertram U, Peter K, et al. Abciximab, eptifibatide, and tirofiban exhibit dose-dependent potencies to dissolve platelet aggregates. J Cardiovasc Pharmacol 2003;41:586–92 [DOI] [PubMed] [Google Scholar]
- 15.Connors JJ 3rd. Pharmacologic agents in stroke prevention, acute stroke therapy, and interventional procedures. J Vasc Interv Radiol 2004;15:S87–101 [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta H, Blankenship JC, Wood GC, et al. Thrombocytopenia complicating treatment with intravenous glycoprotein IIb/IIIa receptor inhibitors: a pooled analysis. Am Heart J 2000;140:206–11 [DOI] [PubMed] [Google Scholar]
- 17.Deliargyris EN, Upadhya B, Melton LG, et al. Unfractionated heparin reduces the anti-platelet effects of abciximab but not eptifibatide during PCI. Clin Appl Thromb Hemost 2006;12:458–64 [DOI] [PubMed] [Google Scholar]
- 18.Hillegass WB, Newman AR, Raco DL. Economic issues in glycoprotein IIb/IIIa receptor therapy. Am Heart J 1999;138:S24–32 [DOI] [PubMed] [Google Scholar]
- 19.Yi HJ, Gupta R, Jovin TG, et al. Initial experience with the use of intravenous eptifibatide bolus during endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2006;27:1856–60 [PMC free article] [PubMed] [Google Scholar]