Abstract
SUMMARY: Patients with stroke on awakening are denied the potential benefit of thrombolysis on the grounds that the onset time is unknown. Relying on clinical and MR imaging to indicate the most appropriate treatment could be more rational. We report 2 cases of stroke with unknown onset time. In both cases, anamnesis and MR imaging indicated that we might still be within 6 hours from stroke onset, with salvageable tissue. Arterial recanalization was successfully performed in both cases.
Onset time is uncertain in more than one quarter of patients with acute ischemic stroke.1,2 Because the exact time of stroke onset cannot be determined, these patients are ineligible for thrombolysis using recombinant tissue plasminogen activator (rtPA). However, clinical and MR imaging data of some of the patients with awakening stroke are comparable with those of other patients with known stroke onset time, who are eligible for acute stroke therapy.1,2 Individuals who are admitted to the hospital rapidly after waking up with stroke may be appropriate candidates for thrombolysis, provided that they have favorable MR imaging characteristics.1,2 We report 2 such patients in whom information from clinical deficits combined with multiparametric MR imaging data was used to make the thrombolysis decision despite an unknown time of onset.
Case Reports
Case 1
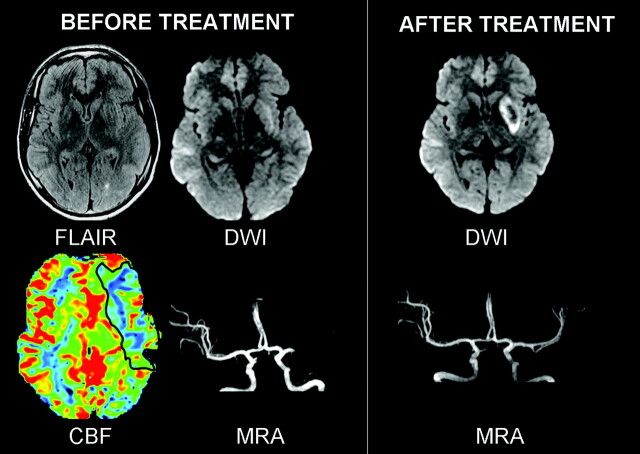
A 34-year-old man, who went to sleep at 2:00 am, woke up at 9:00 am with right hemiplegia and global aphasia, as witnessed by his wife. He reached the hospital at 11:15 am with global aphasia and right hemiplegia with a National Institutes of Health Stroke Scale (NIHSS) score of 15. No headaches or neck pain was reported, and he had no risk factors for stroke. Brain MR imaging (Signa 1.5T; GE Healthcare, Milwaukee, Wis), initiated at 11:30 am, showed a mild hyperintensity in the left deep middle cerebral artery (MCA) territory on diffusion-weighted imaging (DWI) (TR/TE = 6600/91.9 ms, b = 0\N1000 mm2/s, thickness = 6 mm, no gap, FOV = 24 × 24 cm, matrix = 128 × 128) with a decreased apparent diffusion coefficient (mean = 665 μm2/s, 75% of contralateral values). Fluid-attenuated inversion recovery (FLAIR) (parameters are unchanged except TR/TE/TI = 9800/156.9/2300 ms, matrix = 256 × 192) and T2*-weighted imaging (TR/TE = 480/13 ms, flip angle = 25°, matrix = 256 × 224) showed no parenchymal signal-intensity changes. 3D time-of-flight MR angiography (MRA) (TR/TE = 24/3.1, thickness = 1.2 mm, FOV = 20 × 17.6, matrix = 352 × 224) showed a proximal occlusion of the left MCA. First-pass gadolinium perfusion-weighted imaging (PWI) (TR/TE = 2000/60 ms, matrix = 96 × 64, 25 phases) showed prolonged mean transit time (MTT) and low cerebral blood flow (CBF) (MTT = 231%, CBF = 46% compared with contralateral hemisphere) in the superficial left MCA territory, indicating a PWI/DWI mismatch (Fig 1).
Fig. 1.
Case 1. Before treatment, FLAIR was normal and DWI showed a mildly hyperintense left putamen. Perfusion abnormalities (CBF decrease) existed in the superficial MCA territory, which was normal on DWI (PWI-DWI mismatch). MRA showed a left proximal MCA occlusion. After IA thrombolysis, the flow was restored in the left MCA. A deep MCA infarct with petechiae and a small cortical infarct in the left inferior frontal gyrus (not illustrated) were seen on 24-hour DWI follow-up.
This PWI/DWI mismatch, along with the lack of signal-intensity changes on FLAIR, indicated that the patient may still have been within the 6 hours of stroke onset. At 12:00 pm, the decision was made to proceed with intra-arterial thrombolysis. Mechanical and chemical thrombolysis (50 mg of rtPA) resulted in complete recanalization of the left proximal M1 occlusion at 1:30 pm, except for an anterior insular branch. The 24-hour MR imaging follow-up showed petechial hemorrhage in the deep MCA infarct and a small cortical infarct in the inferior frontal gyrus. The patient was discharged on day 9 with no motor deficit and mild language deficit, which slowly resolved (modified Rankin Scale score of 1 at 2 months).
Case 2
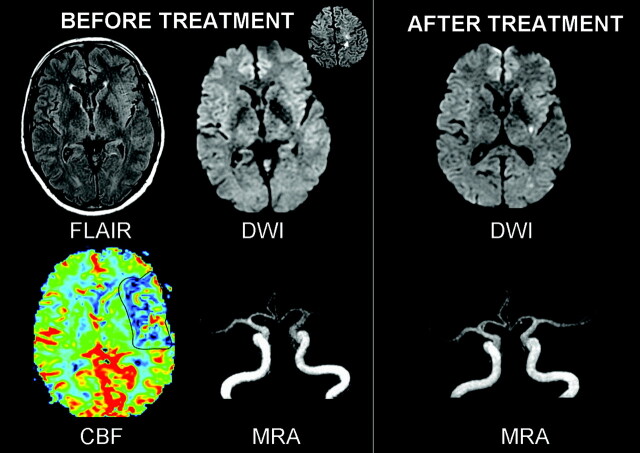
A 56-year-old man was suddenly heard breathing noisily during sleep by his wife. She immediately woke him up at 7:15 am and noticed a language and motor deficit. The patient was a former smoker, overweight, and dyslipidemic with type 2 diabetes. He had been hospitalized 5 days prior to stroke onset for an atrial fibrillation, which resolved after cardioversion. Oral anticoagulation had been initiated 2 days prior to the stroke. At admission (8:40 am), he had a global aphasia, right hemianopsia, and hemiplegia (NIHSS = 16). His heart rhythm was irregular with a serum glucose level of 9.6 mmol/L and an international normalized ratio of 1.31. Brain MR imaging, initiated at 8:50 am, showed punctuate DWI signal-intensity changes in the superficial MCA territory without FLAIR signal-intensity changes. PWI showed an abnormally perfused area (increased MTT, decreased CBF) in the superficial MCA territory (Fig 2). Hyperintense vessels were visible on FLAIR in the left Sylvian fissure, and a T2* hyposignal suggested the presence of a fresh thrombus in the left MCA, which was occluded on MR angiography (MRA). The large PWI/DWI mismatch along with the lack of brain signal-intensity changes indicated that the patient may still have been within 6 hours from stroke onset. The motor deficit partially regressed, but major language deficit persisted (NIHSS = 9).
Fig. 2.
Case 2. Before treatment, FLAIR was normal while DWI showed punctuate hyperintensities in the superficial MCA territory (inset) and a left MCA occlusion. Perfusion abnormalities existed in the superficial MCA territory, consistent with a large PWI/DWI mismatch. 24-hour MR imaging follow-up showed an additional lesion in the left putamen and MCA recanalisation after IA thrombolysis.
Despite early improvement of motor deficit, the disabling language deficit at the time of treatment decision making and the MR imaging pattern favored the decision to treat. At 11:00 am, the decision was made to proceed with intra-arterial mechanical and chemical thrombolysis (15 mg of rtPA), which resulted in complete recanalization of the left M1 embolic occlusion at 12:30 pm. The neurologic symptoms regressed rapidly (NIHSS = 4 after 2 hours, NIHSS = 2 at 24 hours). On the 24-hour follow-up, MRA and PWI findings had normalized, and a punctuate infarction was visible in the deep MCA territory. Anticoagulation was initiated and interrupted 4 days later because of a transient aphasia due to a subcortical temporal hematoma. Normal sinus heart rhythm was restored with amiodarones, and anticoagulation was reintroduced 2 weeks after thrombolysis. The patient was discharged home on day 16, free of symptoms.
Discussion
Because the exact time of onset cannot be determined, patients with stroke on awakening have been excluded from acute stroke trials and are denied the potential benefits of thrombolysis. A subset of these patients, however, may wake up soon after stroke onset and be good candidates for thrombolysis.1,2 MR imaging parameters may be valuable in the estimation of stroke-lesion age.3 However, owing to the difficulty in repeatedly imaging patients with hyperacute stroke, data on the accurate estimation of stroke age by using multiparametric MR imaging are scarce.2 Multimodal MR imaging can estimate the potential individual benefit and risk of thrombolysis. Beyond the established 3-hour time window, a substantial number of patients could be successfully treated on the basis of a penumbral pattern seen on stroke MR imaging.4 In these 2 patients, the following MR imaging pattern was similar to that seen in patients eligible for thrombolysis: MCA occlusion, small diffusion lesion, and substantial PWI/DWI mismatch. Moreover, such an MR imaging pattern has been associated with poor functional prognosis and higher risk of infarct growth, if not treated.
A few stroke centers advocate the use of imaging criteria for deciding whether to initiate thrombolysis rather than relying solely on inclusion and exclusion criteria derived from CT-based international trials.5 Neuroimaging data have been used to extend the benefit of thrombolysis to patients with seizure at stroke onset, who would otherwise have been excluded according to current rtPA guidelines.6 Recently, 2 patients with stroke on awakening have been successfully reperfused on the basis of CT perfusion findings.7 To our knowledge, this is the first report of MR imaging\Nguided decision making of thrombolysis in patients with stroke on awakening. Provided that they reach the hospital soon after awakening, a subset of patients with awakening stroke may benefit from timely multimodal brain imaging and be considered for reperfusion.
References
- 1.Serena J, Davalos A, Segura T, et al. Stroke on awakening: looking for a more rational management. Cerebrovasc Dis 2003;16:128–33 [DOI] [PubMed] [Google Scholar]
- 2.Fink JN, Kumar S, Horkan C, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke 2002;33:988–93 [DOI] [PubMed] [Google Scholar]
- 3.Lansberg MG, Thijs VN, O’Brien MW, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol 2001;22:637–44 [PMC free article] [PubMed] [Google Scholar]
- 4.Schellinger PD, Thomalla G, Fiehler J, et al. MRI-based and CT-based thrombolytic therapy in acute stroke within and beyond established time windows: an analysis of 1210 patients. Stroke 2007;38:2640–45 [DOI] [PubMed] [Google Scholar]
- 5.Schellinger PD, Fiebach JB, Hacke W. Imaging-based decision making in thrombolytic therapy for ischemic stroke: present status. Stroke 2003;34:575–83 [PubMed] [Google Scholar]
- 6.Sylaja PN, Dzialowski I, Krol A, et al. Role of CT angiography in thrombolysis decision-making for patients with presumed seizure at stroke onset. Stroke 2006;37:915–17 [DOI] [PubMed] [Google Scholar]
- 7.Hellier KD, Hampton JL, Guadagno JV, et al. Perfusion CT helps decision making for thrombolysis when there is no clear time of onset. J Neurol Neurosurg Psychiatry 2006;77:417–19 [DOI] [PMC free article] [PubMed] [Google Scholar]