Abstract
BACKGROUND AND PURPOSE: The occurrence of brain parenchymal signal-intensity changes within the drainage territory of developmental venous anomalies (DVAs) in the absence of cavernous malformations (CMs) has been incompletely assessed. This study was performed to evaluate the prevalence of brain parenchymal signal-intensity abnormalities subjacent to DVA, correlating with DVA morphology and location.
MATERIALS AND METHODS: One hundred sixty-four patients with brain MR imaging with contrast studies performed from July 2005 through June 2006 formed the study group. The examinations were reviewed and data were collected regarding the following: location, depth, size of draining vein, associated increased signal intensity on fluid-attenuated inversion recovery and T2-weighted images, associated CMs, and associated signal intensity on gradient recalled-echo sequences.
RESULTS: Of the 175 DVAs identified, 28 had associated signal-intensity abnormalities in the drainage territory. Seven of 28 DVAs with signal-intensity abnormalities were excluded because of significant adjacent white matter signal-intensity changes related to other pathology overlapping the drainage territory. Of the remaining DVAs imaged in this study, 21/168 (12.5%) had subjacent signal-intensity abnormalities. An adjusted prevalence rate of 9/115 (7.8%) was obtained by excluding patients with white matter disease more than minimal in degree. Periventricular location and older age were associated with DVA signal-intensity abnormality.
CONCLUSION: Signal-intensity abnormalities detectable by standard clinical MR images were identified in association with 12.5% of consecutively identified DVAs. Excluding patients with significant underlying white matter disease, we adjusted the prevalence to 7.8%. The etiology of the signal-intensity changes is unclear but may be related to edema, gliosis, or leukoaraiosis secondary to altered hemodynamics in the drainage area.
Developmental venous anomalies (DVAs) are encountered frequently on postcontrast MR imaging of the brain and are usually regarded as normal variants of venous development. The association between DVAs and cavernous malformations (CMs) has been well described.1–3 Intracranial hemorrhage in the absence of CM has been rarely reported as a complication of DVA.4 There have also been a few case reports of nonhemorrhagic presumed venous infarction in the drainage territory of the DVA.5–8 Signal-intensity abnormality on T2-weighted or fluid-attenuated inversion recovery (FLAIR) sequences has been infrequently reported in the drainage territory of DVAs and has not been thoroughly investigated. Although some of the early literature described signal-intensity abnormalities in the adjacent parenchyma, these were in small case series and appeared, in some instances, to be related to prior hemorrhage.9,10 A more recent investigation reported parenchymal alterations in up to 65% of DVAs by MR imaging and CT evaluation in a retrospectively identified patient population.11 We chose to specifically evaluate the frequency of signal-intensity abnormalities in association with DVAs in a more detailed fashion by evaluating a series of consecutive DVAs identified on MR imaging examinations during a defined time interval and correlating the presence of associated signal intensity with DVA morphology, location, size, and drainage pattern. We also attempted to assess the relationship of other white matter signal-intensity alterations not in the DVA territory to presumed DVA-associated signal-intensity changes.
Materials and Methods
From July 2005 through June 2006, brain MR imaging with contrast reports were searched for the terms “developmental venous anomaly” or “venous angioma.” This resulted in 214 consecutive patients with DVAs identified by MR imaging. On review by 2 radiologists (J.L.L., G.M.S.), 50 studies were excluded secondary to incomplete or nondiagnostic examinations. Incomplete examinations in this study were those that did not include FLAIR, proton-attenuation, or T2-weighted sequences. Nondiagnostic examinations were those that were limited by motion or other artifacts diminishing assessment of signal-intensity changes in the DVA drainage area. The remaining 164 patients formed the study group. The study was approved by the institutional review board at our medical center.
Imaging
One hundred sixty-two examinations were performed at 1.5T (Signa series; GE Healthcare, Milwaukee, Wis); 2 were performed at 0.3T (AIRIS II; Hitachi, Twinsburg, Ohio). Standard images assessed at 1.5T included an axial fast spin-echo T2-weighted sequence (TR, 3000–4000 ms; TE, 80–123.6 ms; echo-train length, 12), an axial FLAIR sequence (TR, 10,002–10,004 ms; TE, 123.4–142 ms; TI, 2200 ms), a gradient recalled-echo (GRE) sequence (TR, 650 ms; TE, 28 ms; flip angle, 25°), and an axial spin-echo (SE) T1-weighted sequence (TR, 350–400 ms; TE, 8–9 ms). Section thickness was 4 mm with no intersection gap for all patients. For the 2 examinations performed at 0.3T, imaging parameters included an axial SE T2-weighted sequence (TR, 2800 ms; TE, 90 ms), an axial proton-attenuation sequence (TR, 2800 ms; TE, 25 ms), and an axial T1-weighted sequence (TR, 500 ms; TE, 20 ms). Section thickness was 5.0 mm with an intersection gap of 1.0 mm. We administered 0.1 mmol/kg of gadolinium intravenously for the postcontrast images. Standard diffusion-weighted imaging (DWI) was performed at 1.5T, by using a b-value of 1000 and a 4.0-mm section thickness. DWI images were not performed at 0.3T.
Image Analysis
The examinations were reviewed in detail by 2 experienced radiologists. One radiologist has an added qualification in neuroradiology and 16 years experience in evaluating MR imaging, and the other radiologist has 5 years of experience interpreting MR imaging examinations and is currently enrolled in a neuroradiology fellowship. Data were collected regarding the following: DVA location (lobar, basal ganglia, brain stem, cerebellum), depth (juxtacortical, subcortical, deep, or periventricular), size of draining vein (maximal width on postcontrast images), associated increased signal intensity on FLAIR and T2-weighted images, associated CMs, and associated signal intensity on GRE sequences. The drainage territory was defined as the brain parenchyma directly adjacent to the visualized radicles of the DVA. Signal-intensity alterations adjacent to the DVA were defined as increased nonintravascular signal intensity within directly subjacent brain parenchyma. We were careful not to include signal intensity within the visible vascularity of the DVA, commonly identified on FLAIR and GRE sequences. The prevalence of signal-intensity abnormalities adjacent to the DVA was assessed and correlated with location, drainage, and size. Imaging findings were determined by consensus of the 2 radiologists.
The DVAs were classified by location as juxtacortical, subcortical, and deep, according to Lee et al.12 “Juxtacortical” (or superficial) was defined as within the gray matter or at the gray-white junction. “Subcortical” was defined as below the juxtacortical region but not adjacent to the ventricular wall. “Periventricular” (or deep) was defined as adjacent to the lateral, third, or fourth ventricle or within the center of the structure, such as the pons. The terminal or draining vein to which the caput medusae join was classified as either a deep (toward the ventricle) or superficial (toward the brain surface) draining vein.12
Assessment of venous stenosis of the draining vein was initially attempted; however, the resolution of standard clinical sequences did not allow definitive assessment, and it was not assessed further in our study.
The degree of underlying white matter disease was assessed and classified as none, minimal (≤5 foci of abnormal signal intensity), mild (6–15 foci), moderate (16–35 foci), or severe (≥36 foci or confluent abnormal signal intensity).
To assess the possibility that an inadequate sampling of DVA cases was performed by using dictated reports as the identifying mechanism, we performed a random sampling of 50 brain MR imaging examinations without and with contrast, with no mention of DVA in the report, during the same time interval as the study. These cases were reviewed for the presence or absence of DVAs by 1 author (J.L.L.). No DVAs were identified on these examinations on retrospective review.
Clinical and Imaging Findings Correlation
Clinical indications were tabulated for each case and correlated with the presence or absence of DVA-associated signal intensity. The dominant imaging findings (other than the presence of DVA) were identified in each case and also correlated with presence or absence of signal intensity in the DVA drainage territory.
Statistical Methods
The primary end point in this study was a dichotomous event, either associated signal-intensity change or no associated signal-intensity change. Major factors of interest were depth, location, direction of venous drainage, length of draining vein, caliber of draining vein, presence of GRE signal intensity, and presence of CMs. Logistical regression models were used to assess the association of signal-intensity change to these factors after adjusting for patients’ demographic characteristics. Analyses were performed under 2 scenarios, with white matter disease and its interactions with other factors being either included (adjusted) or excluded (unadjusted) in the models. All other numeric variables were summarized by median and range and compared by using a Wilcoxon rank sum test. All other categoric variables were summarized by frequency (in percentages) and compared by using a χ2 test and/or a Fisher exact test. P values < .05 were considered statistically significant. All statistical tests were performed by using SAS version 9.1 software (SAS Institute, Cary, NC).
Results
A total of 164 patients with 175 DVAs was studied, 69 men and 95 women (Table 1). Ten patients had >1 DVA, all in the group without signal-intensity change. All patients in the signal-intensity-change group had only 1 DVA. CMs were identified in the drainage territory of 6 of the 175 DVAs (3.4%). No other CMs were identified.
Table 1:
Summary of characteristics of 3 groups of patients based on signal change in the DVA territory (n = 164)
| No Signal Change (n = 136) | Signal Change (n = 28) | Excluding Other Extensive Signal Changes (n = 21)* | ||||
|---|---|---|---|---|---|---|
| Age† | 46 (17, 87) | 53‖ (23, 83) | 53‖ (23, 83) | |||
| Female‡ | 78 | 57.35% | 17 | 60.71% | 13 | 61.90% |
| White matter diseaseठ| ||||||
| None | 55 | 40.44% | 8 | 28.57% | 6 | 28.57% |
| Minimum | 51 | 37.50% | 3 | 10.71% | 3 | 14.29% |
| Mild | 14 | 10.29% | 5 | 17.86% | 5 | 23.81% |
| Moderate | 13 | 9.56% | 9 | 32.14% | 6 | 28.57% |
| Severe | 3 | 2.21% | 3 | 10.71% | 1 | 4.76% |
| No. of DVAs‡ | ||||||
| 1 | 126 | 92.65% | 28 | 100% | 21 | 100% |
| 2 | 9 | 6.62% | 0 | 0% | 0 | 0% |
| 3 | 1 | 0.74% | 0 | 0% | 0 | 0% |
Note:—White matter disease indicates degree of overall white matter signal abnormalities as outlined in the Results section.
Seven cases with very extensive additional non-DVA-associated white matter signal change were excluded from analysis.
Values in cells are medians (ranges).
Values in cells are frequency in count and percentage.
White matter disease is associated with signal change in the DVA drainage territory (P < .05 using a χ2 test).
Median in the signal-change group is different from that of no-signal-change group, with P < .05, using a Wilcoxon rank sum test.
Twenty-eight DVAs were identified with associated signal-intensity abnormalities in the DVA drainage territory. Seven of 28 DVAs with signal-intensity abnormalities were further excluded because of other adjacent pathologic white matter signal-intensity changes (due to trauma, tumor, and, in 2 cases, presumed gliosis secondary to prior CM hemorrhage) within the DVA drainage territory. Therefore, 21/168 (12.5%) DVAs imaged in this study (excluding those with other adjacent pathologic changes) had subjacent signal-intensity abnormalities. The adjusted prevalence rate, including only patients with minimal or no underlying white matter disease, was 9/115 (7.8%). Examples of DVA-associated signal intensity are given in Figs 1–3. No diffusion restriction was identified in the areas of signal-intensity abnormality in the DVA drainage territory at 1.5T.
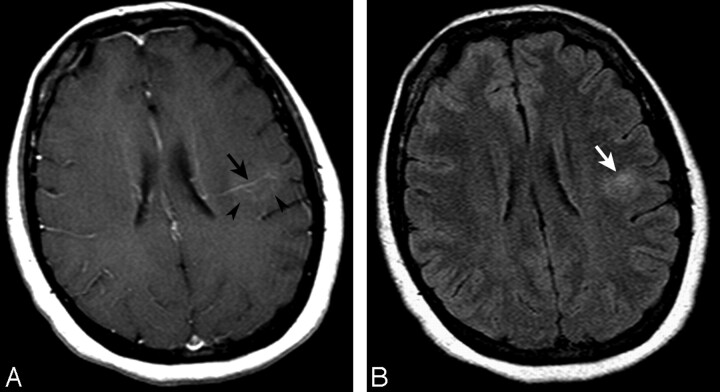
Fig 1.
Axial T1-weighted image after contrast administration (A) and a FLAIR image (B) demonstrating a left parietal subcortical DVA with deep venous drainage (black arrow, A). Some faint enhancement is seen within the DVA drainage territory (arrowheads, A). Associated signal-intensity abnormality surrounds the DVA, extending into the white matter (white arrow, B).
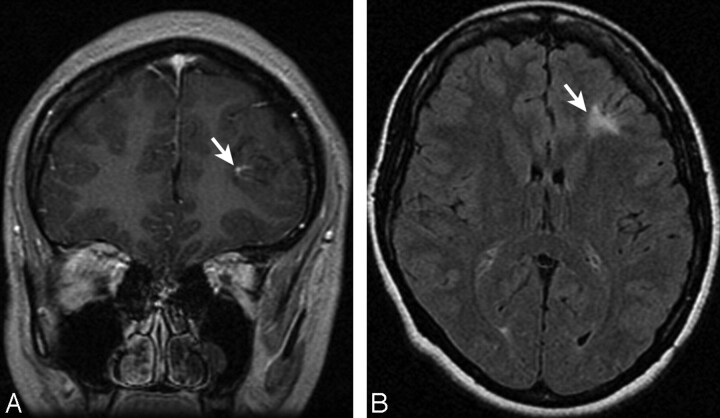
Fig 2.
Coronal T1-weighted image after contrast administration (A) and an axial FLAIR image (B) demonstrating a left frontal juxtacortical DVA (arrow, A) with marked associated signal-intensity abnormality (arrow, B). Some mild focal volume loss may also be present.
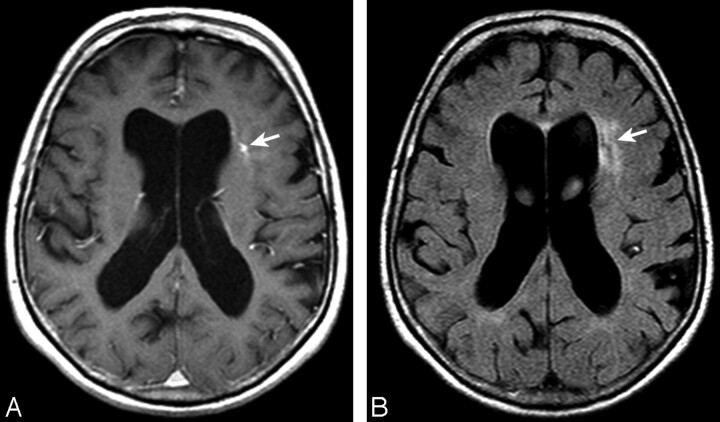
Fig 3.
Axial T1-weighted image after contrast administration (A) and a FLAIR image (B) demonstrating a left frontal subcortical DVA (arrow, A), with moderate associated signal-intensity abnormality (arrow, B) extending to the ventricular margin. Mild associated ventricular enlargement is noted.
There was no significant sex difference between patients with and without signal-intensity change. However, patients with signal-intensity change were older than those without signal-intensity change (median age, 53 years versus 46 years; P < .05).
Table 2 outlines the association of signal-intensity change with other factors, by using a total of 168 DVAs from 157 patients. The location of the DVA by depth was found to be associated with signal-intensity change. In particular, signal-intensity abnormality was more likely in association with periventricular than juxtacortical and subcortical DVAs. This achieved statistical significance, even after adjusting for underlying white matter disease. There was also some weak evidence showing the odds of signal-intensity change in the basal ganglia to be higher than that in the cerebellum (P = .08). This did not maintain borderline significance after adjusting for white matter disease. Other factors (direction of drainage, length of draining vein, GRE signal-intensity abnormality, and presence of CM) were found to have no association with the signal-intensity change. There was no difference in the caliber of the draining vein between those DVAs with associated signal-intensity change and those without (median caliber, 2 mm; range, 1–5 mm in each group).
Table 2:
Associations between signal change within DVA drainage region and other factors*
| Factor | Total | Unadjusted for WM disease† |
Adjusted for WM disease‡ |
OR (SE) | ||||
|---|---|---|---|---|---|---|---|---|
| Odds (SE) | OR (SE) | Odds (SE) | OR (SE) | Total | Odds (SE) | |||
| Depth | ||||||||
| Periventricular | 20 | 0.42 (0.21) | 1 | 0.47 (0.25) | 1 | 13 | 0.19 (0.15) | 1 |
| Juxtacortical | 91 | 0.10 (0.04) | 0.24 (0.15)§ | 0.12 (0.05) | 0.26 (0.17)§ | 67 | 0.06 (0.03) | 0.32 (0.28) |
| Subcortical | 57 | 0.09 (0.04) | 0.21 (0.15)§ | 0.12 (0.06) | 0.26 (0.18)§ | 45 | 0.06 (0.04) | 0.32 (0.32) |
| Location | ||||||||
| BG | 17 | 0.31 (0.18) | 1 | 0.29 (0.18) | 1 | 9 | 0.12 (0.12) | 1 |
| Lobar | 126 | 0.13 (0.04) | 0.42 (0.27) | 0.16 (0.05) | 0.53 (0.36) | 94 | 0.09 (0.03) | 0.75 (0.85) |
| Cerebellum | 25 | 0.04 (0.04) | 0.13 (0.15) | 0.06 (0.06) | 0.19 (0.24) | 22 | 0.00 (0.00) | 0 |
| Direction | ||||||||
| Deep | 68 | 0.16 (0.06) | 1 | 0.19 (0.07) | 1 | 48 | 0.13 (0.06) | 1 |
| Superficial | 95 | 0.10 (0.04) | 0.65 (0.32) | 0.13 (0.05) | 0.66 (0.34) | 73 | 0.04 (0.02) | 0.32 (0.23) |
| Both | 5 | 0.24 (0.28) | 1.53 (1.82) | 0.35 (0.41) | 1.78 (2.18) | 4 | 0 (0) | 0 |
| Draining vein | ||||||||
| Long | 56 | 0.15 (0.06) | 1 | 0.17 (0.07) | 1 | 36 | 0.05 (0.04) | 1 |
| Medium | 57 | 0.11 (0.05) | 0.72 (0.42) | 0.14 (0.06) | 0.86 (0.52) | 46 | 0.06 (0.04) | 1.24 (1.18) |
| Short | 53 | 0.13 (0.06) | 0.83 (0.48) | 0.17 (0.08) | 1.00 (0.60) | 43 | 0.10 (0.05) | 2.06 (1.89) |
| GRE | ||||||||
| No | 120 | 0.08 (0.03) | 1 | 0.10 (0.04) | 1 | 91 | 0.03 (0.02) | 1 |
| Yes | 4 | 0 (0) | 0 | 0 (0) | 0 | 3 | 0 (0) | 0 |
| Malformation | ||||||||
| No | 164 | 0.13 (0.03) | 1 | 0.16 (0.04) | 1 | 122 | 0.07 (0.03) | 1 |
| Yes | 4 | 0 (0) | 0 | 0 (0) | 0 | 3 | 0 (0) | 0 |
Note:—WM indicates white matter; OR, odds ratio; BG, basal ganglia; GRE, gradient recalled-echo; Malformation, associated cavernous malformation.
All DVAs (n = 168); no or minimal WM disease (n = 115).
Estimates are not adjusted for other white matter signal abnormality in logistic regression models.
Estimates are adjusted for other white matter signal abnormality in logistic regression models. The draining vein is “long” if the depth is juxtacortical and the direction is deep, or if the depth is periventricular and the direction is superficial. The draining vein is "short" if the depth is juxtacortical and the direction is superficial, or if the depth is periventricular and the direction is deep. All others are categorized as "median" draining vein length.
Significant OR with P < .05 using a logistic regression model.
The group with abnormal signal intensity in the DVA drainage territory had a higher degree of underlying white matter disease than the group without abnormal signal intensity (Table 1). Of the 21 DVAs with signal-intensity abnormalities, 10 patients had undergone a prior or subsequent examination. There was no detectable difference in the signal-intensity abnormalities between the comparison examinations and the examinations included in the study.
Clinical indications and other MR imaging findings were compared between the DVA groups with and without associated signal-intensity change (Tables 3 and 4). No significant associations were identified. There was some weak evidence showing that patients with DVA-adjacent signal intensity were more likely to have seizures and that those without adjacent signal intensity were more likely to present with headache; however, these did not reach statistical significance. Patients with signal-intensity alterations in the DVA drainage territory were more likely to have nonspecific white matter signal-intensity changes in the remainder of the brain as the most significant other imaging finding compared with those without DVA-adjacent signal intensity (47.6% versus 29.4%). This did not reach statistical significance.
Table 3:
Clinical indications for examinations in those patients with signal changes in the DVA drainage territory (signal) and those without (no signal)
| Clinical Indication | Signal | % | No Signal | % | P* |
|---|---|---|---|---|---|
| Follow-up of previous abnormality, NOS† | 0 | 0.0 | 4 | 2.9 | NS |
| Anosmia | 1 | 4.8 | 0 | 0.0 | NS |
| Assess for metastatic disease, known malignancy | 1 | 4.8 | 11 | 8.1 | NS |
| Ataxia | 0 | 0.0 | 2 | 1.5 | NS |
| Arteriovenous malformation | 0 | 0.0 | 1 | 0.7 | NS |
| Facial nerve palsy | 0 | 0.0 | 1 | 0.7 | NS |
| CM | 0 | 0.0 | 3 | 2.2 | NS |
| Dizziness, vertigo | 0 | 0.0 | 4 | 2.9 | NS |
| DVA | 1 | 4.8 | 2 | 1.5 | NS |
| Encephalitis | 0 | 0.0 | 1 | 0.7 | NS |
| Headache | 1 | 4.8 | 31 | 22.8 | .078 |
| Histoplasmosis | 0 | 0.0 | 1 | 0.7 | NS |
| Hydrocephalus | 0 | 0.0 | 2 | 1.5 | NS |
| ICH | 0 | 0.0 | 3 | 2.2 | NS |
| Intracranial tumor follow-up | 4 | 19.0 | 31 | 22.8 | NS |
| Mental status changes | 1 | 4.8 | 4 | 2.9 | NS |
| Multiple sclerosis | 1 | 4.8 | 5 | 3.7 | NS |
| Movement disorder | 0 | 0.0 | 2 | 1.5 | NS |
| Pituitary dysfunction | 0 | 0.0 | 3 | 2.2 | NS |
| Sarcoid | 1 | 4.8 | 5 | 3.7 | NS |
| Syncope | 1 | 4.8 | 0 | 0.0 | NS |
| Seizures | 6 | 28.6 | 16 | 11.8 | .083 |
| Sensorineural hearing loss | 1 | 4.8 | 4 | 2.9 | NS |
| TIA/strokelike symptoms | 2 | 9.5 | 4 | 2.9 | NS |
| Trauma | 0 | 0.0 | 3 | 2.2 | NS |
| Visual changes | 0 | 0.0 | 4 | 2.9 | NS |
Note:—NOS indicates not otherwise specified; NS, not significant; ICH, intracranial hemorrhage; TIA, transient ischemic attack.
P values are from the Fisher exact test, NS with P > .1.
Previously identified abnormality on another imaging test (prior CT or MR imaging), NOS. MR imaging performed for follow-up purposes.
Table 4:
Additional findings on brain MR imaging examinations in those patients with signal changes in the DVA drainage territory (signal) and those without (no signal)
| Other Imaging Findings | Signal | % | No Signal | % | P* |
|---|---|---|---|---|---|
| Abscess | 0 | 0.0 | 1 | 0.7 | NS |
| Aneurysm | 0 | 0.0 | 2 | 1.5 | NS |
| Atrophy | 0 | 0.0 | 1 | 0.7 | NS |
| Arteriovenous malformation | 0 | 0.0 | 1 | 0.7 | NS |
| CM | 0 | 0.0 | 3 | 2.2 | NS |
| Encephalomalacia | 0 | 0.0 | 3 | 2.2 | NS |
| Intracranial hemorrhage | 0 | 0.0 | 4 | 2.9 | NS |
| Infarct | 1 | 4.8 | 4 | 2.9 | NS |
| Lipoma | 0 | 0.0 | 1 | 0.7 | NS |
| Meningioma | 1 | 4.8 | 6 | 4.4 | NS |
| No other findings | 6 | 28.6 | 50 | 36.8 | NS |
| Postoperative changes | 2 | 9.5 | 12 | 8.8 | NS |
| Nonspecific white matter signal | 10 | 47.6 | 40 | 29.4 | NS |
| Parenchymal mass | 0 | 0.0 | 11 | 8.1 | NS |
| Sellar mass | 1 | 4.8 | 7 | 5.1 | NS |
| Tuberous sclerosis | 0 | 0.0 | 1 | 0.7 | NS |
Note:—NS indicates not significant; postoperative changes, expected findings after brain surgery for intracranial neoplasm; no other findings, no other abnormality identified on MR imaging; nonspecific white matter signal, scattered signal alterations in the white matter, not in a typical distribution for demyelinating disease.
P values are from the Fisher exact test, NS with a P > .1.
Discussion
This study focused on MR imaging–identified parenchymal abnormalities in the drainage territory of clinically detected DVAs. As in prior reports,1–3 CMs were identified in association with DVAs (3.4%). In addition, we identified nonhemorrhagic signal-intensity alterations (increased signal intensity on FLAIR sequences) in 12.5%. The high frequency of signal-intensity abnormalities in the drainage territory of DVAs in this study was surprising on the basis of our everyday clinical experience. We found a statistically significant association between signal-intensity abnormality in the DVA drainage territory and patient age (more common in older patients), degree of generalized white matter signal-intensity abnormality, and depth (periventricular), but no relationship with drainage direction (superficial or deep), size of draining vein, or location (basal ganglia, lobar, cerebellar, or brain stem).
Few previous studies have evaluated this association. In a recent study published during final preparation of our manuscript, San Millán Ruiz et al11 found signal-intensity abnormalities in the drainage territory of 28.3% of 60 DVAs evaluated by MR imaging, more than twice the frequency identified in our study. The reason for this discrepancy is unclear but may be related to selection bias and sample size. The DVAs identified in our study are based on radiology report review for key terms during a defined 1-year period. This presumably identified, as best as possible given the limitations of a clinical study, all the clinically identified DVAs in a patient population presenting for MR imaging during the time interval of the study. The inclusion criteria for the study of Ruiz et al are not as well defined because no specific mention was made of how the cases were identified and collected for evaluation. Additionally, no specific mention was made of exclusion criteria related to potential signal-intensity changes related to adjacent nonrelated pathology, and no attempt was made to control for more widespread white matter signal-intensity alterations common in the adult population. Also, we reviewed more than twice the number of DVAs by MR imaging, which is more accurate in assessing the brain parenchyma than CT. Regardless of the different prevalence noted between studies, it is clear from both that signal-intensity abnormalities in the drainage territory of DVAs may be more frequent than previously realized.
The pathologic substrate of signal-intensity changes in the drainage territory of a DVA is not definite. Some possibilities include edema, gliosis, demyelination, leukoaraiosis, or glial metaplasia. Unfortunately, none of our cases had pathologic correlation. In some early reports of a subset of vascular malformations termed “venous angiomas,” adjacent parenchymal changes including demyelination, gliosis, leukomalacia, and neuronal degeneration were reported.13 A later article reported focal scarring in the DVA drainage territory.14 Because these reports occurred before the era of cross-sectional imaging, it is not clear whether these venous angiomas would have been considered DVAs by the current consensus definition. It is now generally accepted that DVAs are variations in venous drainage of normal brain parenchyma.15 Pathologic evaluation of DVAs has been rare given their typically benign clinical course, but when undertaken, normal intervening neural tissue has usually been reported.16,17
The etiology of signal-intensity abnormalities subjacent to DVAs is also unclear. The abnormal increased FLAIR signal intensity may represent edema or gliosis related to chronic mild venous hypertension caused by anomalous venous drainage. The presence of altered hemodynamics has been suggested by reports of reduced cerebral blood flow in the drainage territory of the DVA.18,19 Stenosis of the draining vein as it crosses through the dura has been previously reported in some DVAs, postulated to result in chronic venous obstruction.20,21 Given the small size of most of the draining veins, resolution was not adequate to assess stenosis in our study. In the study by Ruiz et al,11 venous stenosis was thought to be present in 13.1%; however, it was poorly assessed because of the lack of thin section or volumetric postcontrast imaging in most cases. The relationship of venous stenosis and DVA-associated signal-intensity abnormality requires more detailed study.
There have been case reports of de novo development of CMs in the drainage territory of DVAs.22–24 It has been postulated that the abnormal vascular bed of a DVA might induce hemodynamic disturbance or might be fragile enough to result in microhemorrhage, in turn leading to angiogenic proliferation.25 This proposed mechanism has also been suggested to explain the proliferation of CMs. If substantiated, it would explain the association of CMs and venous malformations.26 The possibility that parenchymal FLAIR signal-intensity abnormality might represent an early finding in the development of CM requires further investigation. We did obtain the prior and subsequent MR imaging examinations for 10 patients in our study with abnormal signal intensity and did not detect any interval change. This does not, however, exclude the possibility that the development of abnormal signal intensity is part of a progressive process, because follow-up was at inconsistent intervals and overall covered only a several-year period.
The possibility that the subjacent signal-intensity abnormality is part of demyelinating disease or other underlying diffuse white matter diseases such as small vessel ischemia must be considered. Demyelinating disease is known to occur in a perivenular distribution, and there has been a case report of biopsy-proved demyelinating disease within the drainage territory of a DVA.27 One of the patients harboring signal-intensity abnormality in the DVA drainage territory in our study was shown to have multiple sclerosis (MS) by retrospective chart review. This patient had mild white matter signal-intensity changes. To minimize this potentially confounding variable, we excluded cases with extensive additional white matter disease in the drainage territory and in adjacent nondrained brain parenchyma from the evaluation.
However, despite these measures, the degree of underlying white matter disease was more prominent in the group with abnormal signal intensity, with 33.4% in the moderate or severe range, compared with 11.5% of the group without abnormal signal intensity. The median age of the group with abnormal signal intensity was also older (53 versus 46 years). In addition, signal-intensity change was more prevalent in the periventricular location, the most common location of small vessel ischemic changes. These results suggest that parenchymal signal-intensity abnormality due to small vessel ischemia may play a role in DVA-associated signal-intensity changes. No differences in clinical presentation were found between groups to support small vessel ischemia or MS as an etiology; however, the numbers in our study were small and clinical assessment was limited. A greater percentage of patients with nonspecific white matter changes on MR imaging as the major imaging finding had signal-intensity alterations in the DVA territory; however, this did not reach statistical significance.
Because our analysis showed that the signal-intensity abnormality was clearly within the drainage territory of the DVA, we postulate that this brain parenchyma may be more vulnerable to small vessel ischemia than brain parenchyma with nonanomalous venous drainage. If DVAs cause hemodynamic disturbance and chronic venous obstruction, chronic ischemic change would not be an unexpected finding. The potential relationship between venous pathology and leukoaraiosis has been demonstrated by Moody et al28 and Brown et al,29 by using sophisticated histologic methods. These studies demonstrated noninflammatory collagenous thickening of venous walls and venous stenosis in the periventricular regions (periventricular venous collagenosis [PVC]), which strongly correlated with the degree of leukoaraiosis on pathologic and imaging analysis. They postulated that oligodendroglial apoptosis may be related to chronic ischemia, in part due to reduced venous flow mediated by venous occlusions or stenosis in PVC.29 As also pointed out by other investigators, DVA could harbor a similar physiology in some cases and potentiate ischemic white matter lesions in their drainage territory.11
Limitations
Because cases with DVA were identified by searching the transcribed reports, DVAs that were missed on initial clinical interpretation would have been excluded from the study and the prevalence rates, accordingly, may not be entirely accurate and may potentially bias detection toward larger DVAs. To address this problem, we reviewed an additional 50 MR imaging examinations performed in the same time interval without DVAs mentioned in the report. None of these cases had DVAs identified on additional retrospective review. This suggests that our selection method was robust. Our assessment was limited to a consensus of 2 radiologists. Although great care was taken to accurately identify DVA-associated signal-intensity abnormality, it is a subjective assessment. Although we were careful in our study to exclude increased signal intensity on FLAIR sequences within the visualized venous radicles of the DVA as DVA-associated signal-intensity alterations, the possibility exists that some FLAIR signal intensity in the DVA drainage bed may be from slower flow within tiny venous radicles below the resolution of standard T1-weighted postcontrast images.
The lack of high-resolution imaging also limited our ability to assess detailed morphology of the DVA, in particular related to stenosis of the draining vein. Given the potential importance of venous stenosis and venous hypertension in the pathophysiology of DVA-associated signal-intensity change, further studies looking at venous morphology of the DVA with high-resolution contrast-enhanced MR venography or CT venography would seem warranted. Clinical correlations and detailed follow-up are limited and are beyond the scope of this imaging-based study. DVA-associated signal intensity abnormalities need to be placed in the clinical context of the patient in everyday clinical practice. Although our study population was larger than that in prior evaluations of DVA, prospective review of more cases may be necessary to confirm the prevalence of signal-intensity abnormalities in association with DVAs and to define their importance more accurately.
Conclusion
Signal-intensity abnormalities detectable by standard clinical MR images are identified in 12.5% of consecutively identified DVAs and are associated with periventricular location of the DVA. Excluding cases with additional moderate-to-severe white matter disease, we found that the adjusted prevalence is 7.8%. The etiology of the signal intensity is uncertain. Edema or gliosis related to venous stenosis or altered hemodynamics is a possible cause but cannot be completely assessed by this morphologic study. Ischemic or demyelinating disease in the drainage area of the DVA may also contribute. Further evaluations assessing DVA-associated signal-intensity abnormalities with high-resolution venographic techniques, hemodynamic techniques, and more detailed follow-up are necessary to determine their true significance and origin.
References
- 1.Wilms G, Bleus E, Demaerel P, et al. Simultaneous occurrence of DVAs and cavernous angiomas. AJNR Am J Neuroradiol 1994;15:1247–54 [PMC free article] [PubMed] [Google Scholar]
- 2.Huber G, Henkes H, Hermes M, et al. Regional association of DVAs with angiographically occult vascular malformations. Eur Radiol 1996;6:30–37 [DOI] [PubMed] [Google Scholar]
- 3.Abe T, Singer RJ, Marks MP, et al. Coexistence of occult vascular malformations and DVAs in the central nervous system: MR evaluation. AJNR Am J Neuroradiol 1998;19:51–57 [PMC free article] [PubMed] [Google Scholar]
- 4.Uchino A, Hasuo K, Matsumoto S, et al. Cerebral venous angiomas associated with hemorrhagic lesions. Clin Imaging 1996;20:157–63 [DOI] [PubMed] [Google Scholar]
- 5.Lai PH, Chem PC, Pan HB, et al. Venous infarction from a venous angioma occurring after thrombosis of a drainage vein. AJR Am J Roentgenol 1999;172:1698–99 [DOI] [PubMed] [Google Scholar]
- 6.Masson C, Godefroy O, Leclerc X, et al. Cerebral venous infarction following thrombosis of the draining vein of a venous angioma (developmental abnormality). Cerebrovasc Dis 2000;10:235–38 [DOI] [PubMed] [Google Scholar]
- 7.Hammoud D, Beauchamp N, Wityk R, et al. Ischemic complication of a cerebral developmental venous anomaly: case report and review of the literature. J Comput Assist Tomogr 2002;26:633–36 [DOI] [PubMed] [Google Scholar]
- 8.Peltier J, Toussaint P, Desenclos C, et al. Cerebral venous angioma of the pons complicated by nonhemorrhagic infarction: case report. J Neurosurg 2004;101:690–93 [DOI] [PubMed] [Google Scholar]
- 9.Cammarata C, Han JS, Haaga JR, et al. Cerebral venous angiomas imaged by MR. Radiology 1985;155:639–43 [DOI] [PubMed] [Google Scholar]
- 10.Augustyn GT, Scott JA, Olson E, et al. Cerebral venous angiomas: MR imaging. Radiology 1985;156:391–95 [DOI] [PubMed] [Google Scholar]
- 11.San Millán Ruiz DS, Delavelle J, Yilmaz H, et al. Parenchymal abnormalities associated with DVAs. Neuroradiology 2007;49:987–95. Epub 2007 Aug 17 [DOI] [PubMed] [Google Scholar]
- 12.Lee C, Pennington MA, Kenney CM III. MR evaluation of DVAs: medullary venous anatomy of venous angiomas. AJNR Am J Neuroradiol 1996;17:61–70 [PMC free article] [PubMed] [Google Scholar]
- 13.Noran HH. Intracranial vascular tumors and malformations. Arch Pathol 1945;39:403–04 [Google Scholar]
- 14.Courville CB. Morphology of small vascular malformations of the brain with particular reference to the mechanism of their drainage. J Neuropathol Exp Neurol 1963;22:274–84 [DOI] [PubMed] [Google Scholar]
- 15.Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev 1986;9:233–42 [DOI] [PubMed] [Google Scholar]
- 16.Wendling LR, Moore JS, Kieffer SA, et al. Intracerebral venous angioma. Radiology 1976;119:141–47 [DOI] [PubMed] [Google Scholar]
- 17.Cabanes J, Blasco R, Garcia M, et al. Cerebral venous angiomas. Surg Neurol 1979;11:385–89 [PubMed] [Google Scholar]
- 18.Uchida K, Tamura K, Takayama H, et al. Xenon-enhanced CT CBF measurements in intracranial vascular malformations [in Japanese]. No Shinkei Geka 1989;17:239–46 [PubMed] [Google Scholar]
- 19.Matsuda H, Terada T, Katoh M, et al. Brain perfusion SPECT in a patient with a subtle venous angioma. Clin Nucl Med 1994;19:785–88 [DOI] [PubMed] [Google Scholar]
- 20.Truwit CL. Venous angioma of the brain: history, significance, and imaging findings. AJR Am J Roentgenol 1992;159:1299–307 [DOI] [PubMed] [Google Scholar]
- 21.Dillon WP. Cryptic vascular malformations: controversies in terminology, diagnosis, pathophysiology, and treatment. AJNR Am J Neuroradiol 1997;18:1839–46 [PMC free article] [PubMed] [Google Scholar]
- 22.Maeder P, Gudinchet F, Meuli R, et al. Development of a cavernous malformation of the brain. AJNR Am J Neuroradiol 1998;19:1141–45 [PMC free article] [PubMed] [Google Scholar]
- 23.Cakirer S. De novo formation of a cavernous malformation of the brain in the presence of a developmental venous anomaly. Clin Radiol 2003;58:251–56 [DOI] [PubMed] [Google Scholar]
- 24.Campeau N, Lane J. De novo development of a lesion with the appearance of a cavernous malformation adjacent to an existing developmental venous anomaly. AJNR Am J Neuroradiol 2005;26:156–59 [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson CB. Cryptic vascular malformations. Clinl Neurosurg 1992;38:49–84 [PubMed] [Google Scholar]
- 26.Awad IA, Robinson JR Jr, Mohanty S, et al. Mixed vascular malformations of the brain: clinical and pathogenetic considerations. Neurosurgery 1993;33:179–88 [DOI] [PubMed] [Google Scholar]
- 27.Jung G, Schroder R, Lanfermann H, et al. Evidence of acute demyelination around a developmental venous anomaly: magnetic resonance imaging findings. Invest Radiol 1997;32:575–77 [DOI] [PubMed] [Google Scholar]
- 28.Moody DM, Brown WR, Challa VR, et al. Periventricular venous collagenosis: association with leukoaraiosis. Radiology 1995;194:469–76 [DOI] [PubMed] [Google Scholar]
- 29.Brown WR, Moody DM, Challa VR, et al. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci 2002;203–204:159–63 [DOI] [PubMed] [Google Scholar]