Abstract
Background:
The association between the SLCO1B1 rs4149056 variant and statin-associated muscle symptoms (SAMS) is well validated, but the clinical utility of its implementation in patient care is unknown.
Design:
The Integrating Pharmacogenetics in Clinical Care (I-PICC) Study is a pseudo-cluster randomized controlled trial of SLCO1B1 genotyping among statin-naïve primary care and women’s health patients across the Veteran Affairs Boston Healthcare System. Eligible patients of enrolled primary care providers are aged 40-75 and have elevated risk of cardiovascular disease by American College of Cardiology/American Heart Association (ACC/AHA) guidelines. Patients give consent by telephone in advance of an upcoming appointment, but they are enrolled only if and when their provider co-signs an order for SLCO1B1 testing, performed on a blood sample already collected in clinical care. Enrolled patients are randomly allocated to have their providers receive results through the electronic health record at baseline (PGx+ arm) versus after 12 months (PGx− arm). The primary outcome is the change in low-density lipoprotein cholesterol (LDL-C) after one year. Secondary outcomes are concordance with Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for simvastatin prescribing, concordance with ACC/AHA guidelines for statin use, and incidence of SAMS. With 408 patients, the study has >80% power to exclude a between-group LDL-C difference of 10 mg/dL (non-inferiority design) and to detect between-group differences of 15% in CPIC guideline concordance (superiority design).
Conclusion:
The outcomes of the I-PICC Study will inform the clinical utility of preemptive SLCO1B1 testing in the routine practice of medicine, including its proposed benefits and unforeseen risks.
Keywords: pharmacogenetics, precision medicine, cardiovascular disease, statin-associated muscle symptoms
I. Introduction
Despite the rapid pace of discovery in pharmacogenetic associations for drug safety and efficacy, the clinical implementation of pharmacogenetic testing in patient care lags behind2-4. Professional consensus is emerging on how certain pharmacogenetic findings should be used to inform drug therapy5-7, but there are few empiric data on the patient outcomes after testing for many of these well validated pharmacogenetic associations8,9. This clinical utility evidence gap contributes to the reluctance of some healthcare providers, systems, and payers to incorporate pharmacogenetic testing broadly into their patient care activities9-12. Demonstrations of improved patient outcomes from pharmacogenetic testing will help close this gap.
The well validated pharmacogenetic association between the solute carrier organic anion transporter family member 1B1 (SLCO1B1) gene and statin-associated muscle symptoms (SAMS) exemplifies this state of the science and clinical practice. Statins, or 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, are widely used cholesterol-lowering medications with proven efficacy in the primary and secondary prevention of atherosclerotic cardiovascular disease (CVD)13. Although statins are generally well tolerated, up to 20% of patients taking statins experience SAMS, ranging in severity from mild muscle aches (incidence ≤200/100,000 person-years) to life-threatening rhabdomyolysis (incidence ≤8/100,000 person-years)14-16. A decade ago, a genome-wide association study identified an association between the common c.521T>C variant in SLCO1B1 (rs4149056) and severe simvastatin-related myopathy17, later also found to be associated with the milder phenotype of statin intolerance18-20. The rs4149056 variant results in a functional, nonsynonymous valine to alanine substitution (p.V174A) in the SLCO1B1 transporter and is contained within the SLCO1B1*5, *15, and *17 haplotypes, all associated with decreased transporter function21. The rs4149056 C variant is common: approximately 20-30% of individuals of European ancestry carry at least one copy, while this proportion ranges from 2-10%, 15-25%, and 15-25% among African, Hispanic, and Asian ancestries, respectively21. The association between this variant and SAMS appears strongest for simvastatin, may or may not be present with atorvastatin, and probably does not occur with pravastatin or rosuvastatin19,21,22. Based on the high validity of the SLCO1B1-SAMS association, the Clinical Pharmacogenetics Implementation Consortium (CPIC) has developed recommendations for simvastatin prescribing and dosing when an individual’s SLCO1B1 genotype is known 21.
Although genotyping for the SLCO1B1 variant is commercially available and has been adopted by selected medical centers for clinical care, a recent systematic review found few studies demonstrating that such testing improves patient outcomes23. The proposed clinical utility of SLCO1B1 testing in patient care is that it will help guide safer medication prescribing for patients who would benefit from statin therapy for CVD risk reduction. However, statin therapy is already complicated by great variability in providers’ prescribing patterns and patients’ adherence24-26. Adding pharmacogenetic results to this clinical context might bring unintended consequences, such as compromising ongoing efforts in CVD risk reduction, if, for example, rs4149056 carriers are undertreated with statins for their level of CVD risk. On the other hand, SLCO1B1 results might improve statin adherence among non-carriers, if they are reassured by their lower genetic risk for SAMS.
Here we describe the design of the Integrating Pharmacogenetics in Clinical Care (I-PICC) Study, a randomized controlled trial examining the impact of SLCO1B1 testing on patient outcomes in primary care. The I-PICC Study is testing the hypotheses that the clinical integration of SLCO1B1 testing reduces the risk of SAMS in a primary care patient population without concomitantly deteriorating CVD prevention.
II. Material and methods
A. Setting
The Veterans Health Administration (VA) is a large integrated health system caring for more than 8 million military veterans across the United States27. Veterans may receive healthcare in the VA system if they meet certain requirements related to military service, disability, and income. About 90% of VA patients are men, although the number of female patients is expected to double to 20% by 204028. As a group, veterans have a greater number of physical and mental health comorbidities than the average US population29. About 60% of patients have cardiovascular disease, half have hypertension, and one-quarter have diabetes mellitus. One-third have a substance use or other mental health disorder30.
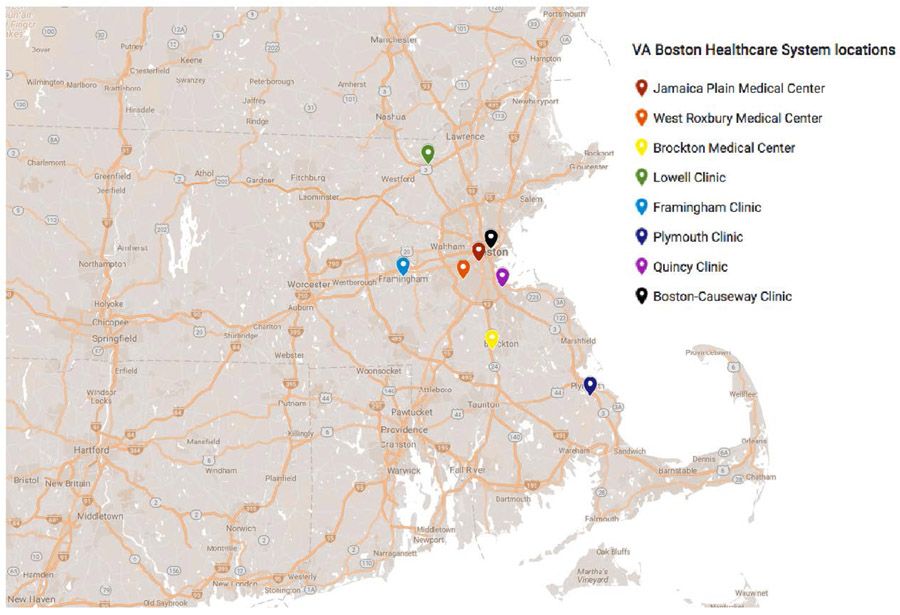
Within the Veterans Health Administration, the VA Boston Healthcare System (VABHS) spans three large medical centers and five community clinics across the greater metropolitan area of Boston, Massachusetts (Figure 1). In 2016, 51,428 unique patients had a least one clinical encounter across the eight VABHS locations. All locations provide primary care and women’s health services, whereas certain specialty care services are provided only at the large medical centers. All locations provide phlebotomy and basic laboratory services, but specimens for specialized laboratory tests are sent to the large West Roxbury medical center for analysis or send-out to external reference laboratories. Many primary care and women’s health providers practice at multiple VABHS locations. The VABHS locations all use the same instance of the national VA electronic health record (EHR) system, the Computerized Patient Record System (CPRS), which can be customized locally for clinical decision support, templates for notes and patient letters, and the ordering and reporting of laboratory tests31-36. Patients may also communicate with their care teams and view their personal health records through the online patient portal, My HealtheVet37.
Figure 1:
Map of VA Boston Healthcare System locations
B. Study population
The I-PICC Study is enrolling both primary care providers and patients as research participants.
1. Provider eligibility
Providers in primary care and women’s health clinics across the eight VABHS locations are eligible to participate in the trial, including physicians, nurse practitioners, and physician assistants. Resident physicians from the three affiliated internal medicine residency programs who practice primary care at VA Boston are not eligible to enroll as participating providers.
2. Patient eligibility
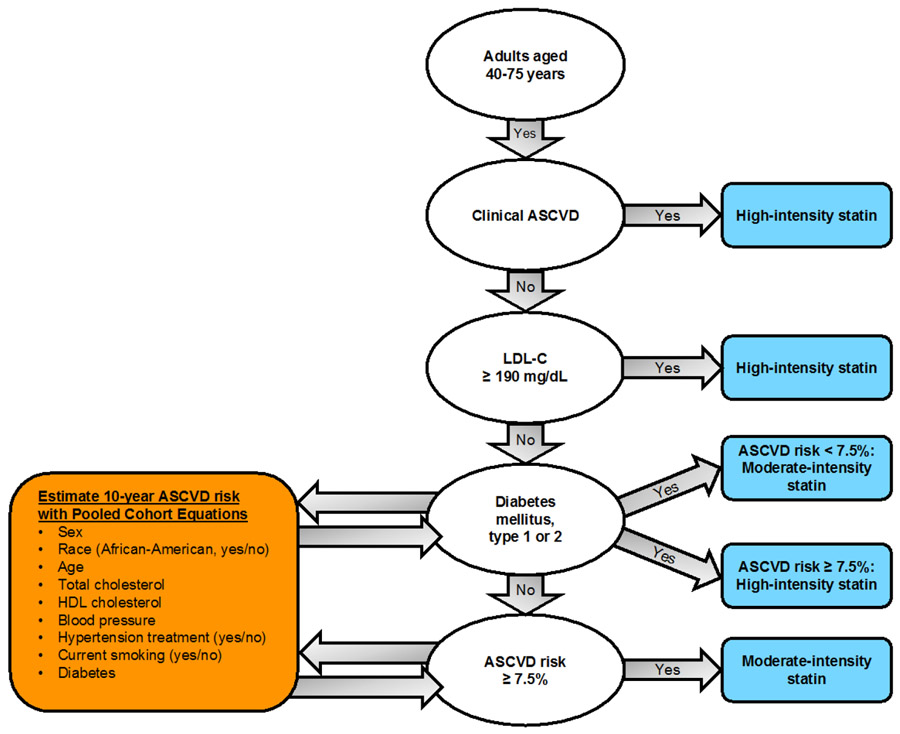
Patient eligibility criteria were chosen to model preemptive pharmacogenetic testing before the initiation of treatment with statins. That is, eligible patients have no prior history of statin use but meet American College of Cardiology/American Heart Association (ACC/AHA) recommendations for consideration of statin therapy for the primary or secondary prevention of atherosclerotic cardiovascular disease (Figure 2). Table 1 shows the patient eligibility criteria and ascertainment methods. Study staff perform Structured Query Language (SQL) queries of the VA Corporate Data Warehouse (CDW)38 to generate candidate and eligibility tables. Initially, candidate patients associated with consented providers are extracted from CDW every 30 days for study eligibility screening. Using this candidate table, study staff perform a daily SQL query that identifies potentially eligible patients and enters them into the study database. The query algorithm first confirms that the patient is alive and meets age criteria. To ensure that participants are established patients of the health system, eligible patients must have had at least one encounter at VABHS between six months and two years before the query. The algorithm queries the most recent low-density lipoprotein cholesterol (LDL-C) value and all available ICD-9 and ICD-10 codes for CVD and diabetes from outpatient encounters. The algorithm then queries CDW pharmacy data and structured data documenting medications obtained outside the VA and excludes any patient with an active or prior statin prescription since 1999. Patients meeting the inclusion criteria for existing CVD, diabetes, or LDL-C ≥190 mg/dL (Table 1) are then stored in a table of potentially eligible patients. For patients not meeting one of these three inclusion criteria, an additional algorithm uses the ACC/AHA pooled risk equations1 to estimate 10-year CVD risk. To improve algorithm processing time, 10-year CVD risk is calculated daily on only a subset of candidate patients, stratified by date of birth: each day, CVD risk is calculated for those candidate patients for whom the parity of birth quarter matches the parity of the current month and whose day of birth (e.g. the 15th of the month) matches the current day. Patients with 10-year CVD risk ≥7.5% are added to the table of potentially eligible patients to be contacted for recruitment.
Figure 2: Major recommendations from the 2013 ACC/AHA guidelines (adapted from 1).
ASCVD, atherosclerotic cardiovascular disease; HDL, high-density lipoprotein; LDL-C, low-density lipoprotein cholesterol.
Table 1:
Patient eligibility criteria for the I-PICC Study
| Eligibility criterion | Ascertainment method(s) |
|---|---|
| 1. Treated by an enrolled provider | CDW: Primary care provider assignments in RPCMM table |
| 2. Age 40-75 years | CDW: Age 40-74 at date of eligibility screen, to minimize occurrences of aging out of eligibility |
| 3. At least ONE of the following CVD risk factors: | |
| A. Existing CVD | CDW: ICD-9 and IC-10 codes* |
| B. Diabetes | CDW: ICD-9 and ICD-10 codes* |
| C. LDL-C ≥190 mg/dL | CDW: Most recent LDL-C value in laboratory tables |
| D. 10-year CVD risk ≥7.5% | CDW: Race, sex, age, and most recent values of total cholesterol, HDL-C, smoking status, blood pressure and blood pressure treatment. CVD risk calculated per ACC/AHA pooled risk equations37 |
| 4. No history of statin use | CDW: VA and non-VA medications tables, queried for adjudicated list of statin medications Informed consent call: Study staff verbally confirm absence of prior or current statin use |
See Supplementary Material for list of ICD codes. Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; CDW, Corporate Data Warehouse; CVD, cardiovascular disease; HDL-C, high-density lipoprotein; ICD, International Classification of Disease; LDL-C, low-density lipoprotein; RPCMM, Reengineered Primary Care Management Module
C. Recruitment, consent, and enrollment
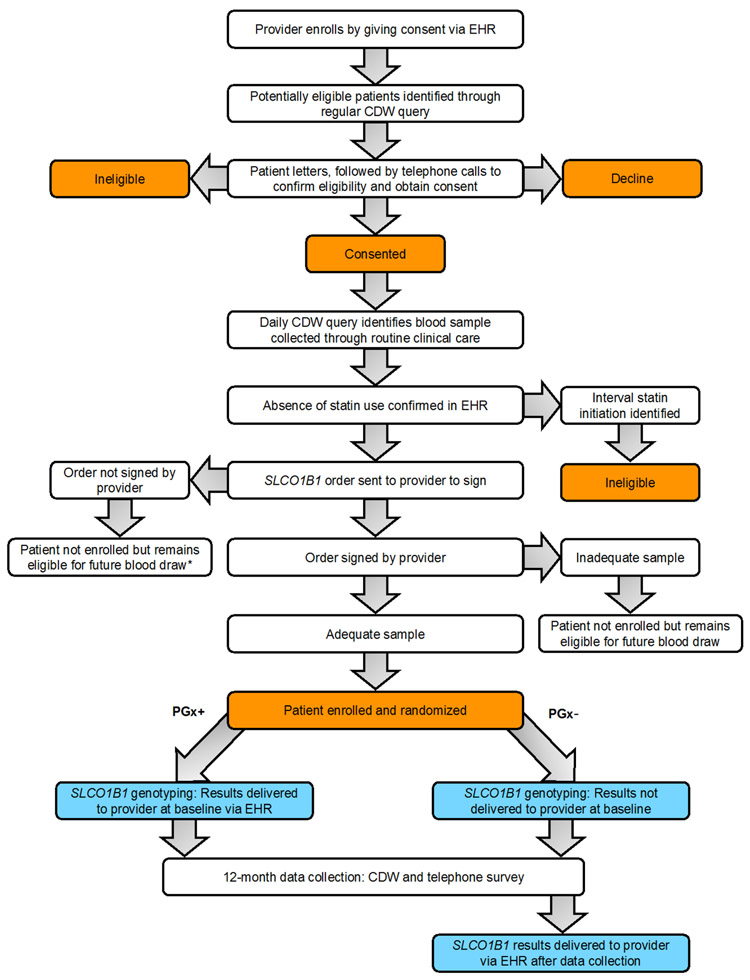
Figure 3 illustrates the design of the I-PICC Study.
Fig. 3. Design of the I-PICC Study.
Abbreviations: CDW, Corporate Data Warehouse; EHR, electronic health record.
* Provider may request that patient be removed from eligible pool.
1. Providers
Study staff inform providers about the study through presentations at staff meetings, e-mails, and individual outreach. An educational slide presentation briefly summarizes the evidence supporting the association between SLCO1B1 genotype and SAMS, CPIC recommendations for the use of SLCO1B1 genotype in simvastatin prescribing, and the study procedures (Supplementary Material). Providers may then enroll in the trial by signing either an electronic copy of the informed consent form (Figure 4), forwarded to them by study staff directly through the EHR, or a paper copy of the same form. Study staff send periodic emails reminding unenrolled providers about the opportunity to enroll in the study. Upon enrollment of the 100th, 200th, and 300th patients, study staff send all primary care and women’s health providers, regardless of enrollment status, an email newsletter that includes study updates, web links to relevant pharmacogenetics articles or resources, and an invitation to enroll in the study if not already enrolled.
Figure 4:
Examples of two study-related clinical alerts delivered to providers through the electronic health record in the I-PICC Study: 1) a provider informed consent note allowing the provider to sign and enroll in the study and 2) a SLCO1B1 genotyping order for the provider to sign for a specific consented patient when a clinical blood sample is available, enrolling the patient in the study.
2. Patient recruitment and consent
Using the candidate table described above, study staff mail letters in batches to potentially eligible patients, targeting those who have primary care appointments or laboratory test orders scheduled in the next six months. These letters explain the purpose and procedures of the study and include all required elements of an informed consent form (Supplementary Material). Study staff follow each letter with a telephone call to the potential participant to confirm absence of prior or current statin use, review the study details, answer any questions, and obtain verbal consent to participate. Patients who give telephone consent to participate are then designated as consented in the study database. As described below, patient consent at this stage does not equate to patient enrollment.
3. Patient enrollment
A pragmatic clinical trial, the I-PICC Study does not require a research blood draw, instead taking advantage of blood samples collected through routine clinical care. Study staff perform a daily CDW query that cross-references the database of consented patients with all laboratory samples collected across VABHS in the prior three days, identifying any blood sample on which SLCO1B1 genotyping can be performed (complete blood count or hemoglobin A1c). When the query identifies an eligible blood sample from a consented patient, study staff create a laboratory order for SLCO1B1 genotyping in the EHR and forward the order as a clinical alert to the enrolled primary care provider for signature (Figure 4). The provider’s signature of the laboratory order enrolls the patient in the study, provided the clinical sample has at least 300 μL of remaining blood and is thus adequate for SLCO1B1 genotyping. If the sample is inadequate, the patient is not enrolled at that time but remains eligible for enrollment at a future blood draw. If the provider does not sign the order within three days, the study staff send an email reminder. If the provider discontinues the order or does not sign the order within the seven-day timeframe that the laboratory saves clinical samples, the patient is not enrolled. For every discontinued order, the study staff emails the provider to inquire whether they would ever consider enrolling the patient in the future or whether the study staff should send no future laboratory orders for that patient.
D. Randomization
The I-PICC Study uses pseudo-cluster randomization to balance the risks of contamination and referral bias, as described by Pence39. Blinding is not possible for interventions such as the reporting of pharmacogenetic results, and contamination bias occurs in a trial when a provider’s experience treating patients in the intervention arm influences their treatment of patients in the control arm. Some studies minimize the risk of contamination bias with randomization at the level of the provider or practice, but this cluster design brings the risk of referral bias, whereby providers assigned to the control arm are less likely to refer patients to the study39. In the I-PICC Study, provider-level randomization might result in few signed SLCO1B1 orders from providers assigned to the control arm. Instead, the I-PICC Study uses pseudo-cluster randomization, in which enrolled providers are randomly allocated in a 1:1 ratio to either having 80% of their enrolled patients in the intervention arm and 20% of their enrolled patients in the control arm (80/20) or having 20% of their enrolled patients in the intervention arm and 80% of their enrolled patients in the control arm (20/80). Enrolled providers are blinded to their 80/20 or 20/80 allocation. A random number generator was used to produce a sequence of 56 provider allocations (80/20 or 20/80), to which study staff sequentially assign providers upon enrollment. Each provider is then sequentially assigned to one of four patient randomization tables, each with a sequence of 100 patient allocations, also generated by a random number generator, in the appropriate intervention/control ratio for that provider. After a provider signs a SLCO1B1 order and the laboratory confirms that the blood sample is adequate for genotyping, study staff enroll the patient and sequentially assign a randomization status from that provider’s patient allocation table. The SLCO1B1 results of patients in the intervention (PGx+) arm are reported immediately, while the SLCO1B1 results of patients in the control (PGx−) arm are reported after 12 months, at the end of the study.
E. Intervention: SLCO1B1 genotyping and reporting
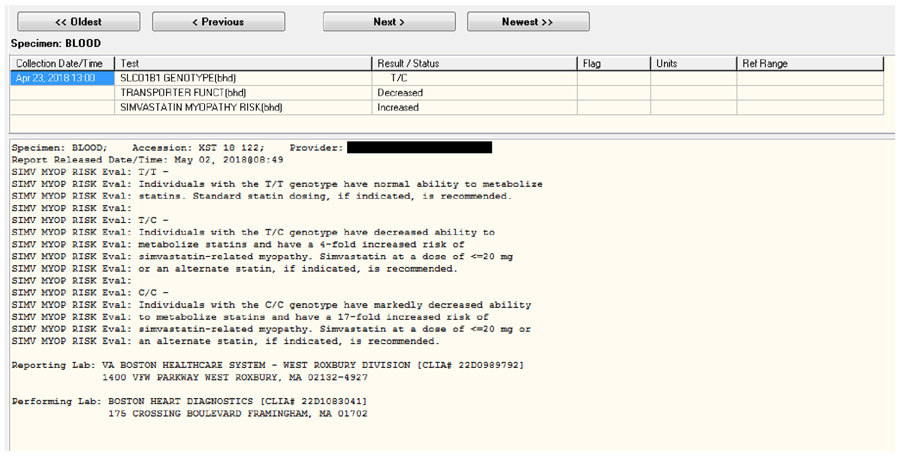
Study staff notify the VA clinical laboratory staff of each patient enrollment. Laboratory staff then send each sample (PGx+ and PGx−) via a common carrier delivery service and chain of custody to Boston Heart Diagnostics (BHD) in Framingham, Massachusetts, for SLCO1B1 rs4149056 genotyping, using its Clinical Laboratory Improvement Amendments (CLIA)-certified and College of American Pathologists (CAP)-accredited polymerase chain reaction assay. The BHD laboratory staff send SLCO1B1 results for both arms to study staff via secure fax. Study staff then send the SLCO1B1 result for each PGx+ patient via encrypted email to the VA clinical laboratory staff for immediate upload into the EHR. The SLCO1B1 result appears as a clinical alert for the ordering primary care provider the next time they log onto the EHR (Figure 5). The SLCO1B1 results screen in the EHR conforms to recommendations that molecular test reports include results, interpretation, and management guidance40 (Figure 5 and Table 2), adapted specifically from CPIC guidelines for the use of simvastatin when SLCO1B1 genotype is known21. Standardized terms for transporter function phenotype (normal, decreased, or poor) are also used41.
Figure 5:
Example of SLCO1B1 results in EHR of an enrolled patient in the I-PICC Study. A clinical alert notifies the enrolled primary care provider that the result is available, but all other members of patient care team may view the results in the laboratory section of the EHR.
Table 2:
Possible SLCO1B1 results reported to primary care providers in the I-PICC Study
|
SLCO1B1 rs4149056 genotype |
Transporter function |
Simvastatin myopathy risk |
Interpretation |
|---|---|---|---|
| T/T | Normal | Typical | Individuals with the T/T genotype have normal ability to metabolize statins. Standard statin dosing, if indicated, is recommended. |
| T/C | Decreased | Increased | Individuals with the T/C genotype have decreased ability to metabolize statins and have a 4-fold increased risk of simvastatin-related myopathy. Simvastatin at a dose of ≤20 mg or an alternate statin, if indicated is recommended. |
| C/C | Poor | Markedly increased | Individuals with the C/C genotype have markedly decreased ability to metabolize statins and have a 17-fold increased risk of simvastatin-related myopathy. Simvastatin at a dose of ≤20 mg or an alternate statin, if indicated, is recommended. |
The first time an enrolled provider receives a SLCO1B1 result alert in the EHR, study staff also send them an email reminding them about the study and notifying them that a new result has been entered into the EHR. The study staff do not send the SLCO1B1 results directly to patient participants during the observation period but instead encourage providers to communicate the results as they see fit (e.g. by telephone, letter, or message through the patient portal). A standardized SLCO1B1 results letter template is available in the EHR for providers to use if they choose (Supplementary Material). After the 12-month observation period and after the end-of-study telephone survey is complete, the study staff mail the patient participants (PGx+ and PGx−) standardized results letters. Copies of these end-of-study letters are also emailed to the participating primary care provider.
F. Data collection
Study data in addition to SLCO1B1 genotype derive primarily from three sources.
1. Corporate Data Warehouse
The VA CDW is a health data warehouse that consolidates data from disparate sources into a single logical data model to enable clinical care, business management, and research38. Twelve-month outcomes data collected from the CDW include cholesterol testing dates and values; medication prescriptions including doses, start dates, and, as applicable, dates of discontinuation or dosage change; pharmacy records of filled prescriptions; and structured medication allergy data.
2. Chart review
Study staff review the EHR for each enrolled patient 12 months after enrollment to abstract data including provider documentation of discussions about CVD risk, statin therapy, pharmacogenetic testing or results, and adverse drug effects. In the VA EHR, such documentation takes the form of office visit notes, telephone notes, patient letters, and messages sent between patient and provider in the patient portal.
3. End-of-study telephone survey
Twelve months after a patient’s enrollment date, study staff blinded to the patient’s randomization status call the patient to administer a brief, telephone survey (Supplementary Material). The survey assesses the patient’s use of statins or other cholesterol-lowering medications in the prior 12 months, self-reported SAMS, and recall of genetic testing and test results. It also assesses patients’ perceived necessity of and concerns about their medications using two items adapted from the validated Beliefs about Medicines Questionnaire42, a widely used instrument associated with medication adherence43.
G. Outcomes
1. Primary outcome
The primary outcome of the I-PICC Study is change in LDL-C, defined as the most recent LDL-C value on or prior to the enrollment date subtracted from the LDL-C value 12 months after enrollment, without regard to fasting status. The study protocol does not mandate end-of-study LDL-C testing; all data derive from clinical care. Thus, baseline LDL-C values will be carried forward for any patient who does not undergo updated LDL-C testing during the study period. This biomarker outcome serves as a surrogate for CVD risk reduction, as the 5-year risk of major CVD event decreases by 5% for each 10-mg/dL reduction in LDL-C44.
2. Secondary outcomes
The secondary outcomes examine additional potential benefits and risks of integrating preemptive SLCO1B1 genotyping in clinical care.
a. Concordance with CPIC guidelines
Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines recommend specific simvastatin doses when a patient's SLCO1B1 genotype is known21. Each participant’s SLCO1B1 genotype and statin type and dose at the end of the 12-month observation period are compared to CPIC guidelines for safe simvastatin prescribing; potentially unsafe simvastatin dosing includes 80 mg daily for any person and 40 mg daily for any person with a CT or CC genotype. All other combinations are considered potentially safe, including no simvastatin prescription or use of a statin other than simvastatin. This scheme generates a two-level safety outcome (potentially safe vs. potentially unsafe simvastatin prescription) for each patient.
b. Concordance with ACC/AHA guidelines
In 2013, the ACC/AHA endorsed guidelines recommending statin therapy of specific intensities (moderate or high) for patients with specific CVD risk profiles (Table 3). In a similar manner to CPIC guideline concordance, for each patient a two-level ACC/AHA guideline concordance outcome (concordant vs. non-concordant) indicates whether his or her statin therapy at 12 months, including absence of therapy, is adequate for the level of CVD risk, as assessed at baseline as a part of the eligibility determination.
Table 3:
Intensity of specific daily statin therapy regimens.
| High-intensity | Moderate-intensity | Low-intensity |
|---|---|---|
| Atorvastatin 40-80 mg | Atorvastatin 10-20 mg | Simvastatin 10 mg |
| Rosuvastatin 20-40 mg | Rosuvastatin 5-10 mg | Pravastatin 10-20 mg |
| Simvastatin 80 mg* | Simvastatin 20-40 mg | Lovastatin 20 mg |
| Pravastatin 40-80 mg | Fluvastatin 20-40 mg | |
| Lovastatin 40 mg | Pitavastatin 1 mg | |
| Fluvastatin XL 80 mg | ||
| Fluvastatin 40 mg bid | ||
| Pitavastatin 2-4 mg |
Initiation of simvastatin 80 mg not recommended by US Food & Drug Administration due to increased myopathy risk.
c. Statin-associated muscle symptoms
Chart review of all patient notes from the 12 months after enrollment determines the proportion of patients in each arm with clinical documentation of SAMS during the observation period. This outcome includes any mention of a clinical suspicion of SAMS, with or without laboratory testing such as creatinine kinase.
3. Exploratory outcomes
Exploratory outcomes derived from the CDW include initiation of and changes to statin therapy during the 12-month observation period; patient adherence to statin therapy, measured from pharmacy data with medication possession ratios45,46; and entry of a medication allergy to statins. Exploratory outcomes from the end-of-study telephone survey include self-reported SAMS, genetic testing recall, and perceived necessity of and concerns about medications.
H. Hypotheses and statistical analyses
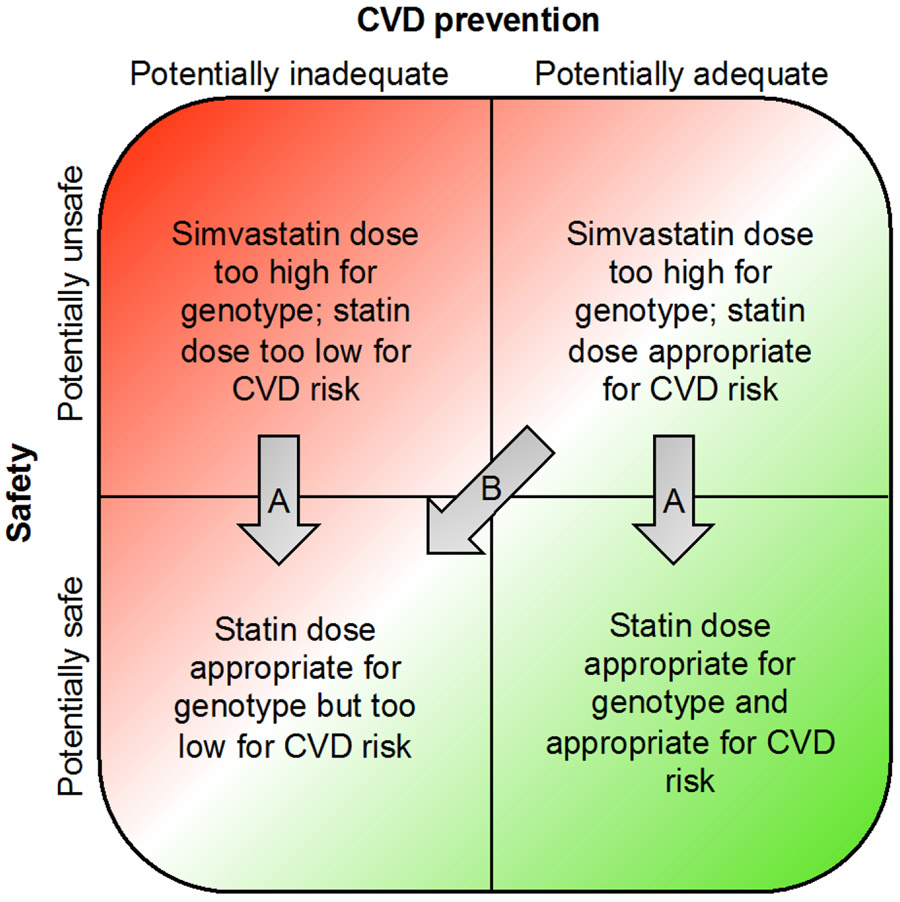
Statin therapy, a product of both prescriber and patient behaviors, mediates the impact of SLCO1B1 results on patient outcomes. Testing might improve statin safety by reducing the proportion of patients receiving simvastatin doses higher than what is safe for their genotype (Figure 6, Arrows A). However, SLCO1B1 testing may result in an inappropriate under-dosing of statin for a patient’s level of CVD risk, resulting in higher LDL-C levels (Figure 6, Arrow B). The hypotheses of the I-PICC Study reflect the desired outcome of the clinical integration of SLCO1B1 testing: a reduction in the risk of SAMS without a concomitant deterioration of established CVD prevention.
Figure 6:
Possible categories of statin safety and CVD prevention among patients and the potential impact of SLCO1B1 testing on these outcomes (see text).
For the primary outcome of 12-month change in LDL-C, generalized estimating equations (GEE) with an identity link function, accounting for clustering by provider, will test the null hypothesis that SLCO1B1 testing diminishes 12-month LDL-C reductions by more than 10 mg/dL (non-inferiority design). The alternative hypothesis is that SLCO1B1 testing is non-inferior to standard of care with no testing, within the non-inferiority limit of a 10-mg/dL change in LDL-C. Indeed, SLCO1B1 testing might increase provider and patient utilization of statin therapy and thereby actually improve LDL-C values in the PGx+ arm compared to the PGx- arm.
For the secondary outcomes of CPIC guideline concordance and documentation of SAMS, GEE with a logit link function, accounting for clustering by physician, will test the null hypothesis that the proportion of patients with each outcome 12 months after enrollment does not differ between the two arms (superiority design). Using a non-inferiority design, GEE will also test the null hypothesis that the proportion of PGx− patients whose prescriptions at 12 months meet ACC/AHA guidelines for CVD prevention is better by 15% than that proportion in the PGx+ arm. Although the primary and secondary analyses will preserve randomization by comparing the PGx+ and PGx− arms, exploratory analyses, stratified by genotype, will examine whether outcomes differ within the CC/CT and TT subgroups.
I. Power calculations:
Under the assumption of a common standard deviation of 30 mg/dL in the primary outcome of change in LDL-C, 112 patients in each arm (224 total) are needed to have 80% power at a one-sided α=0.05 to exclude a between-group difference of 10 mg/dL 12 months after enrollment47. Addition of a design effect of 1.36 to account for clustering by provider (cluster size of 10 patients/provider and an intracluster correlation coefficient of 0.04)48 increases this requirement to 304 total patients. Participant attrition is assumed to be null. The I-PICC Study is enrolling 408 total patients to enable at least 80% power at a 2-sided α=0.05 to detect between-group differences of 15% in the secondary outcomes of CPIC guideline concordance and documented SAMS49, again with a design effect of 1.36. This sample size also enables >80% power at a one-sided α=0.05 to reject the null hypothesis that ACC/AHA guideline concordance is better by 15% in the PGx− arm compared to the PGx+ arm50.
III. Discussion
Despite the robust association between SLCO1B1 genotype and SAMS, particularly with simvastatin, evidence of improved patient outcomes after SLCO1B1 pharmacogenetic testing remains one critical gap in the widespread promotion of its use in clinical care8,23,51. Because the management of hypercholesterolemia and CVD risk falls squarely in the domain of primary care, the SLCO1B1-SAMS association provides an opportunity to examine one example of integrating genomic medicine in general practice. The I-PICC Study is a randomized controlled trial (RCT) generating rigorous outcomes data on the clinical utility of SLCO1B1 testing in a real-world clinical setting.
The I-PICC Study is examining what we term a timely preemptive model of pharmacogenetic testing, on the continuum between preemptive testing well before a relevant medication is being considered for a given patient and reactive testing at the time of initiation or continuation of a relevant medication52,53. In one recent RCT of reactive testing, delivery of SLCO1B1 results to previously statin-intolerant patients resulted in more new statin prescriptions and lower LDL-C values at 3 months, compared to patients not receiving results54. The I-PICC Study models the clinical context when a provider has the opportunity to order SLCO1B1 testing for a patient at elevated CVD risk in anticipation of statin initiation in the near future, thus seeking just-in-time pharmacogenetic information at a clinically relevant moment. Some expect that preemptive pharmacogenetic testing will become more common as patients increasingly undergo genotyping through clinical care, participation in biobanks or other research projects, or even direct-to-consumer products6. In the preemptive model, automated clinical decision support (CDS) triggers at the moment a relevant medication is ordered in the EHR, guiding prescribers towards safer or more efficacious pharmacotherapy. Many of the institutions participating in the Electronic Medical Records and Genomics (eMERGE)-PGx Consortium55, the Pharmacogenomics Research Network Translational Pharmacogenetics Program (TPP)56, and the Implementing Genomics in Practice (IGNITE) Consortium57 in the US are integrating such automated CDS triggered by preemptive SLCO1B1 results in their EHR53.
However, there are presently several barriers to the widespread adoption of preemptive pharmacogenetics systems53,58. Only a fraction of patients in the U.S. and internationally receive care in health systems with laboratory and EHR systems equipped to incorporate structured pharmacogenetic data from varying sources and guide pharmacotherapy at the relevant clinical moments4,59,60, which occur potentially decades after genotyping. Instead, the I-PICC Study models the scenario where the provider may order pharmacogenetic testing in anticipation of near-term utility, similar to most other laboratory tests in clinical medicine. Nonetheless, the evidence generated by the I-PICC Study on the downstream impact of SLCO1B1 testing on patient outcomes will be informative for systems using automated CDS for such results. Indeed, automated CDS can be subsequently added to delivery models similar to that of the I-PICC Study, such that providers order timely preemptive SLCO1B1 testing for near-term use and structured results are then stored in the EHR to interface with future CDS alerts with other providers in the healthcare system.
The RCT design of the I-PICC Study is rare among studies of genomic medicine interventions. Although some have argued that RCTs are not necessary to support the clinical implementation of pharmacogenetics61,62, professional organizations, payers, and other stakeholders often look for such rigorous outcomes data in making decisions about clinical guidelines and coverage10,11,12. The I-PICC Study aims to collect empiric data on the clinical utility of SLCO1B1 testing, which encompasses improvements in patient outcomes such as drug effectiveness or adverse drug events or more proximal mediators of these outcomes, such as changes in medication prescriptions and adherence. The I-PICC Study is designed to exclude the possibility that SLCO1B1 testing results in a clinically meaningful deterioration in an important patient outcome, change in LDL-C, an established biomarker for CVD risk44,63,64. The trial is not powered to detect between-group differences in severe but rare SAMS such as rhabdomyolysis65-67, but concordance with CPIC guidelines for simvastatin serves as a surrogate outcome for safe statin prescribing. Exploratory data from chart review and patient surveys are examining the more common occurrence of SAMS of any severity, which occurs in 5-20% of patients taking statins14,15 and is a common reason for non-adherence14,24,25,68-70 and resulting poorer CVD outcomes70,71. Together, the outcomes of the I-PICC Study will inform the clinical utility of SLCO1B1 testing in the routine practice of medicine, including its proposed benefits and unforeseen risks.
Supplementary Material
Acknowledgements
This work was supported by Career Development Award IK2 CX001262 from the United States Department of Veterans Affairs (VA) Clinical Sciences Research and Development Service. Boston Heart Diagnostics provided genotyping for this study but had no role in its design or analysis and publication of study data. The authors thank the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) for additional study support and Sophie Ludin for assistance with manuscript preparation. This work does not reflect the views of the VA or the United States government.
References
- 1.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. [DOI] [PubMed] [Google Scholar]
- 2.Selkirk CG, Weissman SM, Anderson A, Hulick PJ. Physicians' preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genetic testing and molecular biomarkers. 2013;17(3):219–225. [DOI] [PubMed] [Google Scholar]
- 3.Stanek EJ, Sanders CL, Taber KA, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharmacol Ther. 2012;91(3):450–458. [DOI] [PubMed] [Google Scholar]
- 4.Klein ME, Parvez MM, Shin JG. Clinical Implementation of Pharmacogenomics for Personalized Precision Medicine: Barriers and Solutions. J Pharm Sci. 2017;106(9):2368–2379. [DOI] [PubMed] [Google Scholar]
- 5.Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Current Drug Metabolism. 2014;15(2):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swen JJ, Nijenhuis M, van Rhenen M, et al. Pharmacogenetic Information in Clinical Guidelines: The European Perspective. Clin Pharmacol Ther. 2018;103(5):795–801. [DOI] [PubMed] [Google Scholar]
- 8.Jansen ME, Rigter T, Rodenburg W, et al. Review of the Reported Measures of Clinical Validity and Clinical Utility as Arguments for the Implementation of Pharmacogenetic Testing: A Case Study of Statin-Induced Muscle Toxicity. Front Pharmacol. 2017;8:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassy JL, Stone A, Callaghan JT, et al. Pharmacogenetic testing in the Veterans Health Administration: Policy recommendations from the VHA Clinical Pharmacogenetics Subcommittee. Genet Med. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel HN, Ursan ID, Zueger PM, Cavallari LH, Pickard AS. Stakeholder views on pharmacogenomic testing. Pharmacotherapy. 2014;34(2):151–165. [DOI] [PubMed] [Google Scholar]
- 11.Keeling NJ, Rosenthal MM, West-Strum D, Patel AS, Haidar CE, Hoffman JM. Preemptive pharmacogenetic testing: exploring the knowledge and perspectives of US payers. Genet Med. 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hess GP, Fonseca E, Scott R, Fagerness J. Pharmacogenomic and pharmacogenetic-guided therapy as a tool in precision medicine: current state and factors impacting acceptance by stakeholders. Genetics Research. 2015;97:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. Health, United States, 2013: With Special Feature on Prescription Drugs. 2014; http://www.cdc.gov/nchs/data/hus/hus13.pdf-listfigures. Accessed February 2, 2018. [PubMed] [Google Scholar]
- 14.Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23(8):1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfirevic A, Neely D, Armitage J, et al. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther. 2014;96(4):470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008;359(8):789–799. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly LA, Doney AS, Tavendale R, et al. Common nonsynonymous substitutions in SLCO1B1 predispose to statin intolerance in routinely treated individuals with type 2 diabetes: a go-DARTS study. Clin Pharmacol Ther. 2011;89(2):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puccetti L, Ciani F, Auteri A. Genetic involvement in statins induced myopathy. Preliminary data from an observational case-control study. Atherosclerosis. 2010;211(1):28–29. [DOI] [PubMed] [Google Scholar]
- 20.de Keyser CE, Peters BJ, Becker ML, et al. The SLCO1B1 c.521T>C polymorphism is associated with dose decrease or switching during statin therapy in the Rotterdam Study. Pharmacogenet Genomics. 2014;24(1):43–51. [DOI] [PubMed] [Google Scholar]
- 21.Ramsey LB, Johnson SG, Caudle KE, et al. The Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin Pharmacol Ther. 2014;96(4):423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunham LR, Lansberg PJ, Zhang L, et al. Differential effect of the rs4149056 variant in SLCO1B1 on myopathy associated with simvastatin and atorvastatin. Pharmacogenomics J. 2012;12(3):233–237. [DOI] [PubMed] [Google Scholar]
- 23.Vassy JL, Chun S, Advani S, Ludin SA, Smith JG, Alligood EC. Impact of SLCO1B1 pharmacogenetic testing on patient and healthcare outcomes: A systematic review. Clin Pharmacol Ther. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113(2):203–212. [DOI] [PubMed] [Google Scholar]
- 25.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med. 2004;19(6):638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson TA, Khan A, Maki KC, Brinton EA, Cohen JD. Provider recommendations for patient-reported muscle symptoms on statin therapy: Insights from the Understanding Statin Use in America and Gaps in Patient Education survey. J Clin Lipidol. 2018;12(1):78–88. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Veterans Affairs VHA. Health Benefits: Veterans Eligibility. http://www.va.gov/HEALTHBENEFITS/apply/veterans.asp. Accessed April 16th, 2015. [Google Scholar]
- 28.National Center for Veterans Analysis and Statistics. 2014 Minority Veterans Report. 2016; www.va.gov/vetdata/docs/SpecialReports/Minority_Veterans_2014.pdf. Accessed October 16, 2017. [Google Scholar]
- 29.Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252–3257. [DOI] [PubMed] [Google Scholar]
- 30.Veterans Health Administration Office of Health Equity. National Veteran Health Equity Report–FY2013. Washington, DC: 2016. [Google Scholar]
- 31.Shelton JB, Ochotorena L, Bennett C, et al. Reducing PSA-Based Prostate Cancer Screening in Men Aged 75 Years and Older with the Use of Highly Specific Computerized Clinical Decision Support. J Gen Intern Med. 2015;30(8):1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried TR, Niehoff KM, Street RL, et al. Effect of the Tool to Reduce Inappropriate Medications on Medication Communication and Deprescribing. J Am Geriatr Soc. 2017;65(10):2265–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filice GA, Drekonja DM, Thurn JR, et al. Use of a computer decision support system and antimicrobial therapy appropriateness. Infect Control Hosp Epidemiol. 2013;34(6):558–565. [DOI] [PubMed] [Google Scholar]
- 34.Saleem JJ, Haggstrom DA, Militello LG, et al. Redesign of a computerized clinical reminder for colorectal cancer screening: a human-computer interaction evaluation. BMC Med Inform Decis Mak. 2011;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheuner MT, Hamilton AB, Peredo J, et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet Med. 2014;16(1):60–69. [DOI] [PubMed] [Google Scholar]
- 36.Sperber NR, Carpenter JS, Cavallari LH, et al. Challenges and strategies for implementing genomic services in diverse settings: experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med Genomics. 2017;10(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nazi KM, Woods SS. MyHealtheVet PHR: a description of users and patient portal use. AMIA Annual Symposium Proceedings. 2008:1182. [PubMed] [Google Scholar]
- 38.Price LE, Shea K, Gephart S. The Veterans Affairs's Corporate Data Warehouse: Uses and Implications for Nursing Research and Practice. Nurs Adm Q. 2015;39(4):311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pence BW, Gaynes BN, Thielman NM, et al. Balancing Contamination and Referral Bias in a Randomized Clinical Trial: An Application of Pseudo-Cluster Randomization. Am J Epidemiol. 2015;182(12):1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheuner MT, Hilborne L, Brown J, Lubin IM. A report template for molecular genetic tests designed to improve communication between the clinician and laboratory. Genetic Testing and Molecular Biomarkers. 2012;16(7):761–769. [DOI] [PubMed] [Google Scholar]
- 41.Caudle KE, Dunnenberger HM, Freimuth RR, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med. 2017;19(2):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horne R, Weinman J, Hankins M. The Beliefs about Medicines Questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. [Google Scholar]
- 43.Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients' adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One. 2013;8(12):e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. [DOI] [PubMed] [Google Scholar]
- 45.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. [DOI] [PubMed] [Google Scholar]
- 46.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. [DOI] [PubMed] [Google Scholar]
- 47.Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23(12):1921–1986. [DOI] [PubMed] [Google Scholar]
- 48.Glynn RJ, Brookhart MA, Stedman M, Avorn J, Solomon DH. Design of cluster-randomized trials of quality improvement interventions aimed at medical care providers. Med Care. 2007;45(10 Supl 2):S38–43. [DOI] [PubMed] [Google Scholar]
- 49.Pocock SJ. Clinical Trials: A Practical Approach. New York: John Wiley & Sons; 1983. [Google Scholar]
- 50.Blackwelder WC. "Proving the null hypothesis" in clinical trials. Control Clin Trials. 1982;3(4):345–353. [DOI] [PubMed] [Google Scholar]
- 51.Pezalla EJ. Payer view of personalized medicine. Am J Health Syst Pharm. 2016;73(23):2007–2012. [DOI] [PubMed] [Google Scholar]
- 52.Schildcrout JS, Denny JC, Bowton E, et al. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther. 2012;92(2):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunnenberger HM, Crews KR, Hoffman JM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu Rev Pharmacol Toxicol. 2015;55:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peyser B, Perry EP, Singh K, et al. Effects of delivering SLCO1B1 pharmacogenetic information in randomized trial and observational settings. Circulation: Genomic and Precision Medicine. 2018;11(9):e002228. [DOI] [PubMed] [Google Scholar]
- 55.Rasmussen-Torvik LJ, Stallings SC, Gordon AS, et al. Design and anticipated outcomes of the eMERGE-PGx project: a multicenter pilot for preemptive pharmacogenomics in electronic health record systems. Clinical Pharmacol Ther. 2014;96(4):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luzum JA, Pakyz RE, Elsey AR, et al. The pharmacogenomics research network translational pharmacogenetics program: outcomes and metrics of pharmacogenetic implementations across diverse healthcare systems. Clinical Pharmacol Ther. 2017;102(3):502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weitzel KW, Alexander M, Bernhardt BA, et al. The IGNITE network: a model for genomic medicine implementation and research. BMC Medical Genomics. 2016;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526(7573):343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shirts BH, Salama JS, Aronson SJ, et al. CSER and eMERGE: current and potential state of the display of genetic information in the electronic health record. J Am Med Inform Assoc. 2015;22(6):1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caraballo PJ, Sutton JA, Moyer AM, et al. Technical Challenges and Opportunities when Implementing Pharmacogenomics Decision Support Integrated in the Electronic Health Record. Stud Health Technol Inform. 2017;245:1255. [PubMed] [Google Scholar]
- 61.Relling MV, Veenstra DL. Implementation of pharmacogenomics: Evidence needs. Institute of Medicine Roundtable on Translating Genomic-Based Research for Health; 2015; Washington, DC. [Google Scholar]
- 62.Caudle KE, Gammal RS, Whirl-Carrillo M, Hoffman JM, Relling MV, Klein TE. Evidence and resources to implement Pharmacogenetic Knowledge for Precision Medicine. American Journal of Health-System Pharmacy. 2016;73(23):1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. [DOI] [PubMed] [Google Scholar]
- 64.National Heart Lung and Blood Institute. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). National Institutes of Health; September 2002. 2002. NIH Publication Number 02–5215. [Google Scholar]
- 65.Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther. 2006;28(1):26–35. [DOI] [PubMed] [Google Scholar]
- 66.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C–60C. [DOI] [PubMed] [Google Scholar]
- 67.Armitage J The safety of statins in clinical practice. Lancet. 2007;370(9601):1781–1790. [DOI] [PubMed] [Google Scholar]
- 68.Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–779. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe JH, Bounthavong M, Chen T, Ney JP Association of Polypharmacy and Statin New-User Adherence in a Veterans Health Administration Population A Retrospective Cohort Study. Ann Pharmacother. 2013;47(10):1253–1259. [DOI] [PubMed] [Google Scholar]
- 70.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186. [DOI] [PubMed] [Google Scholar]
- 71.Shalev V, Chodick G, Silber H, Kokia E, Jan J, Heymann AD. Continuation of statin treatment and all-cause mortality: a population-based cohort study. Arch Intern Med. 2009;169(3):260–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.