Abstract
Influenza A viruses (IAV) are a major cause of respiratory diseases in pigs. Invariant natural killer T (iNKT) cells are an innate-like T cell subset that contribute significantly to IAV resistance in mice. In the current work, we explored whether expanding and activating iNKT cells with the iNKT cell superagonists α-galactosylceramide (α-GalCer) would change the course of an IAV infection in pigs. In one study, α-GalCer was administered to pigs intramuscularly (i.m.) 9 days before infection, which systemically expanded iNKT cells. In another study, α-GalCer was administered intranasally (i.n.) 2 days before virus infection to activate mucosal iNKT cells. Despite a synergistic increase in iNKT cells when α-GalCer i.m. treated pigs were infected with IAV, neither approach reduced disease signs, lung pathology, or virus replication. Our results indicate that prophylactic use of iNKT cell agonists to prevent IAV infection is ineffective in pigs. This is significant because this type of approach has been considered for humans whose iNKT cell levels and IAV infections are more similar to those of pigs than mice.
Keywords: pigs, influenza, prophylactic, invariant natural killer T cells, α-galactosylceramide
1. Introduction
Influenza A viruses (IAV) are very contagious in swine and can cause acute respiratory disease that lead to significant economic losses for the swine industry (Janke, 2014; Rajao and Vincent, 2015). Influenza A viruses also reassort in pigs which give rise to novel viruses that occasionally cause human pandemics, such as the 2009 H1N1 flu pandemic (Khiabanian et al., 2009). While vaccination is an important strategy to prevent influenza in pigs, this approach often fails to prevent influenza outbreaks because antigenic shift and drift of IAV occurs at a faster rate than vaccines can be developed (Sandbulte et al., 2015). Furthermore, vaccine-mediated immune responses take days or weeks to develop during which time animals may develop disease (Lefevre et al., 2012). Another complication is that maternally derived antibodies can interfere with vaccine immunity in piglets (Pyo et al., 2015). Because of these deficiencies, there is an urgent need for alternative strategies to mitigate IAV infections in pigs, especially during periods of increased risk, such as weaning or during an influenza outbreak. One approach may be to enhance the host innate immune system in ways that increase IAV resistance. An example of this strategy is the administration of granulocyte colony-stimulating factor (Imrestor® pegbovigrastim) to dairy cows, which reduces their susceptibility to periparturient mastitis infections by increasing their circulating neutrophil concentrations (Ruiz et al., 2017).
The current study investigated whether the severity of IAV infections could be reduced in pigs by therapeutically activating and/or expanding their invariant natural killer T (iNKT) cells before infection. These cells are a distinct subset of immunoregulatory T lymphocytes that share phenotypic and functional similarities to NK cells. In contrast to conventional T cells, iNKT cells use a semi-invariant T cell receptor (TCR) to recognize glycolipid antigens presented by the non-polymorphic CD1d molecule. However, they can also be activated indirectly by pro-inflammatory cytokines, including those produced during viral infections (Brigl et al., 2011; Tyznik et al., 2008). iNKT cell effector responses appear to be important for IAV immunity in mice because iNKT cell-deficient mouse strains are highly susceptible to IAV infection (Ishikawa et al., 2010; Kok et al., 2012; Paget et al., 2012; Santo et al., 2008). Furthermore, treating mice before or during an IAV infection with iNKT cell superagonists, such as α-galactosylceramide (α-GalCer), increases survival rates and reduces early virus replication in the lung (Ho et al., 2008; Ishikawa et al., 2010; Kok et al., 2012; Lin et al., 2010). Protection is reported to arise from iNKT cell-derived IFNγ which transactivates cytolytic NK cells and CD8+ T cells and by the reduction of the immunosuppressive activity of myeloid-derived suppressor cells (MDSC) that undergo expansion during IAV infection (Santo et al., 2008). In addition, iNKT cells produce IL-22 during IAV infection that prevents IAV-triggered cell death of pulmonary epithelial cells (Paget et al., 2012).
Since swine express iNKT cells that are characteristically similar to mouse and human iNKT cells, including that they require CD1d for their development (Yang et al., 2015), and possess the canonical invariant TCR (Looringh van Beeck et al., 2009; Yang et al., 2019), a capacity to rapidly secrete cytokines upon activation (Artiaga et al., 2014; Schäfer et al., 2019; Thierry et al., 2012; Yang et al., 2017), and high expression of the transcription factor PLZF (Schäfer et al., 2019; Thierry et al., 2012), the current study evaluated whether prophylactically expanding and activating iNKT cells in pigs would increase their resistance against a subsequent IAV infection. Our goal was to determine if this approach could be used to mitigate IAV infections in pigs, an economically important livestock species of that are a natural host of IAVs. This study was also performed to establish the feasibility of harnessing iNKT cells to improve IAV resistance in humans who are much more similar to pigs than mice in regards to iNKT cells and IAV immunity.
2. Materials and methods
2.1. Pigs
Commercial Yorkshire cross bred piglets were obtained from the University of Florida swine unit. Experiments were performed in compliance with guidelines from the United States Department of Agriculture, the National Research Council’s Guide for the Care and Use of Laboratory Animals and all relevant state and federal regulations and policies. The animal care and use committee at the University of Florida approved the protocol under project number 201708209.
2.2. Experimental design
Three-week old piglets were assigned to different treatment groups after they were confirmed to be seronegative for antibodies against influenza H1N1, H3N2 and B viruses by hemagglutination inhibition assays (HAI), as previously described (WHO, 2002). In one study (Table 1), pigs were treated 9 days before infection with 100 μg/kg α-GalCer administered by intranasal (i.n.) delivery or intramuscular (i.m.) injection. Mock treated pigs received an i.m. injection of the vehicle (PBS with 2% DMSO). In another study (Table 2), 100 μg/kg of α-GalCer was administrated i.n. 2 days before virus infection. In both studies, pigs were challenged with 106 TCID50 pandemic H1N1 A/California/04/2009 (CA04) or virus-free DMEM media by the intratracheal route, as described previously (Artiaga et al., 2016a). In both studies, virus and mock infected pigs were housed in separate rooms. Each of the infected groups were separated by an empty pen to avoid close contact between pigs in different groups.
Table 1.
Setup for Experiment 1
| Treatment (Day -9)† | Challenge | Designation | N |
|---|---|---|---|
| Mock (PBS + DMSO) | Mock | Mock/Mock | 4 |
| αGalCer (i.m.) | Mock | αGC i.m./Mock | 5 |
| Mock (PBS + DMSO) | CA04 H1N1 | Mock/CA04 | 5 |
| αGalCer (i.m.) | CA04 H1N1 | αGC i.m./CA04 | 5 |
| αGalCer (i.n.) | CA04 H1N1 | αGC i.n./CA04 | 5 |
Treatments were administered 9 days before challenge
Table 2.
Setup for Experiment 2
| Treatment (day -2)† | Challenge | Designation | N |
|---|---|---|---|
| Mock (PBS + DMSO) | Mock | Mock/Mock | 4 |
| αGalCer (i.m.) | Mock | αGC i.m./Mock | 6 |
| Mock (PBS + DMSO) | CA04 H1N1 | Mock/CA04 | 6 |
| αGalCer (i.n) | CA04 H1N1 | αGC i.n./CA04 | 6 |
Treatments were was administered 2 days before virus infection.
Pigs were anesthetized with BAM™ combination drug (Butorphanol, Azaperone, Medetomidine) at a dose rate of 1.0mL per 75lbs body weight and euthanized by intravenous (i.v.) Pentobarbital Sodium injections (150 mg/kg) either 5 or 7 days after challenge. Clinical signs, including body temperature, body weight, coughing, sneezing, nasal discharge, ocular discharge and diarrhea, were monitored daily throughout the challenge period as previously described (Artiaga et al., 2016a). Nasal swabs were collected on day −1 and every day post-infection to determine virus shedding. Blood samples were collected to analyze immune cell populations. During necropsy, tissue samples from lung, trachea, bronchus, tonsil, and nasal associated lymphoid tissue (NALT) were collected to determine virus titers. Additional samples from the lung, spleen and tracheobronchial lymph nodes (TBLN) were collected for flow cytometric analysis, and histology. Bronchoalveolar lavage fluid (BALF) was collected and analyzed for viral titers and immune cell populations (Artiaga et al., 2016a).
2.3. Preparation of α-GalCer
α-Galactosylceramide purchased from Diagnocine LLC (Hackensack, NJ) was sonicated in DMSO at 2 mg/ml until fully dissolved. α-GalCer stock solutions were further dissolved in PBS for administration to pigs. Each lot of α-GalCer was tested for biological activity by measuring the upregulation of T cell costimulatory molecules on splenic dendritic cells of C57BL/6J mice as previously described (Driver et al., 2010).
2.4. Flow cytometry and antibodies
Blood was collected from the jugular vein using EDTA-containing vacutainers (BD Biosciences, San Jose, CA). BALF was centrifuged to analyze the cellular composition of the cell pellet. Lung, spleen, and TBLNs were dispersed into single cells as previously described (Artiaga et al., 2014). Leukocytes obtained from homogenized or digested tissues were treated with an ammonium chloride-based lysis buffer to eliminate residual red blood cells. Immune cell populations were characterized using either a BD Accuri C6, BD LSRFortessa (BD Bioscience) or Attune NxT (Thermofisher, Grand Island, NY) flow cytometer after cells were blocked with polyclonal rat IgG antibody and stained as previously described (Artiaga et al., 2016a). Antibodies and tetramer reagents used to characterize iNKT cells, αβ T cells, γδ T cells, and NK cells are described in Supplemental Table 1. Data were analyzed using FlowJo software v9 (Treestar, Palo Alto, CA).
2.5. Multiplex immunoassay of cytokines and chemokines
Bronchoalveolar lavage fluid (BALF) collected during necropsy was analyzed for IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, IFN-α, IFN-γ and TNF-α using a Cytokine & Chemokine 9-Plex Porcine ProcartaPlex™ array (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. The plates were read by xMAP®-based reader platform MagpixTM (Luminex Corporation, Austin, Texas). The cytokine levels were determined according to their standard curves using MilliplexTM Analyst 5.1 Flex software (VigeneTech Inc, Carlisle, MA).
2.6. Virus and viral titers
Influenza virus encoding the original consensus sequence of the pandemic H1N1 A/California/04/2009 strain (designated CA04) was generated by reverse genetics and recovered as previously described (Solórzano et al., 2005). Virus titers were calculated as the median TCID50 and viral titers expressed as TCID50/ml and TCID50/g as appropriate. TCID50 values were determined by infecting Madin Darby Canine Kidney (MDCK) cells in 96-well microtiter plates with serial dilutions of virus. MDCK cells were cultured using serum-free DMEM (ThermoFisher Scientific, Grand Island, NY) treated with 1.0 μg/ml of trypsin-TPCK (Worthington Biochemical Company, Lakewood, NJ) at 33 °C. TCID50 was calculated according to the method of Reed and Muench (Reed and Muench, 1938).
2.7. Lung histology
At necropsy, the right lung was perfused via the trachea with 10% neutral phosphate-buffered formalin at 30cm H2O pressure and processed as described previously (Artiaga et al., 2016a). Briefly, seven sections of the cranial, middle and caudal of right lung lobe that would fit in a 2.5 × 4 cm embedding mold were cut and embedded in paraffin. Sections from paraffin blocks were cut at 7 μm and were stained with hematoxylin and eosin (H&E). Each section was scored for severity of bronchiolitis and pneumonia using similar methods as previously described (Artiaga et al., 2016b). Briefly, bronchiolitis was scored according to aggregates of lymphocytes and macrophages in bronchiolar walls in focal or multifocal distribution. Each section was scored from 0 to 3; 0: no lesions, 1–3: low to high density aggregates. Pneumonia was scored from seven sections per pig according to the lesions, 0: no lesions, 1–3: small to large sized lesions. The sum total of lesion scores for seven sections for each pig are displayed.
2.8. Statistical analysis
For virus titers and lung pathology, data were analyzed using PROC GLIMMIX of SAS (v9.4, SAS Institute Inc., Cary, NC) blocking by batch. Viral titer data were log transformed in order to address the heteroscedasticity and non-normally distributed residuals of untransformed data and analyzed for repeated measures. When the F-test for a main effect or interaction was significant (P< 0.05), multiple comparisons were done using Tukey’s test. All other variables were analyzed in GraphPad Prism (version 8.4 for Macintosh, GraphPad Software Inc., La Jolla, CA). If data residuals were normally distributed, a multiple comparison Tukey’s test was conducted in two-way ANOVA. For data with residuals non-normally distributed, a non-parametric Kruskal-Wallis analysis of variance was conducted and differences between groups were analyzed by Dunn’s multiple comparisons test. A significant difference was declared at P < 0.05. Differences in iNKT cells, cytokines levels and viral titers in airway tissues were analyzed using a nonparametric Kruskal-Wallis test with GraphPad Prism (version 8.0 h for Macintosh, GraphPad Software Inc., La Jolla, CA). Differences between groups were analyzed by Dunn’s multiple comparisons test. A significant difference was declared at (P < 0.05). Lung pathology, viral titers in nasal swabs, and immune cell populations in peripheral blood (PB) and tissues were analyzed with the PROC MIXED procedure of SAS (v9.3, SAS Institute Inc., Cary, NC) for regression analysis. Groups were compared using the Turkey’s test when a main effect or interaction term was determined to be significant (P < 0.05).
3. RESULTS
3.1. α-GalCer administered systemically but not intranasally increases iNKT cells in the respiratory tract
After recognizing CD1d-bound α-GalCer, iNKT cells secrete large quantities of cytokines over a 24 h period, upon which they begin to proliferate (Crowe et al., 2003; Wilson et al., 2003). We previously published that α-GalCer injected into the neck muscle of pigs increases the frequency of iNKT cells in PB that peaks 9 days after injection [Figure 6A in (Artiaga et al., 2014)]. We also showed that α-GalCer administered by this route increases the frequency of iNKT cells in the lung tissue, BALF and TBLNs [Figure 1D in (Artiaga et al., 2016a)]. To determine whether increasing pulmonary iNKT cell levels can change the course of an IAV infection in swine, we infected pigs with 106 TCID50 of CA04 H1N1 9 days after they were intramuscularly injected with 100 μg/kg α-GalCer (αGC i.m./CA04). A separate group of pigs were administered the same amount of α-GalCer by the intranasal route (αGC i.n./CA04) to compare how iNKT cell activation at the site of infection would affect virus replication and disease. For comparison, additional groups of pigs were mock-injected with vehicle and mock-infected with virus free media (Mock/Mock), mock-treated and CA04 infected (Mock/CA04), and α-GalCer injected and mock-infected (αGC i.m./Mock).
Flow cytometry was used to assess iNKT cells in blood, BALF, and tissues using a mouse CD1d tetramer reagent loaded with the α-GalCer analog PBS57, which cross-reacts with the invariant TCR of porcine iNKT cells (Supplemental Figure 1). Analysis of PB at 8 days post-treatment found that the frequency of iNKT cells was significantly increased by i.m. but not i.n. administration of α-GalCer. Analysis of PB during the challenge period found that iNKT cells remained elevated in αGC i.m./CA04 pigs, peaking at 3 days post infection (d.p.i.) and at levels 11-fold higher than Mock/Mock pigs. In contrast, iNKT cells in αGC i.m./Mock pigs peaked at −1 d.p.i. and returned to baseline levels by the end of the challenge period (Figure 1A–C). Analysis of tissue samples at 7 d.p.i. found higher frequencies of iNKT cells in the lung, BALF, spleen, and TBLN of αGC i.m./CA04 pigs that were on average respectively 10-, 9-, 4-, and 3-fold higher than in Mock/Mock pigs (Figure 1A, D & E). Although iNKT cell frequencies tended to be higher in tissue samples from αGC i.m./Mock pigs, their levels were not significantly higher than Mock/Mock pigs. iNKT cell frequencies remained low and stable for Mock/Mock, Mock/CA04, and αGC i.n./CA04 pigs. These results indicate that i.m. application of α-GalCer and virus exposure synergistically increased iNKT cell levels in PB and tissues of pigs, and especially the lung.
Figure 1.
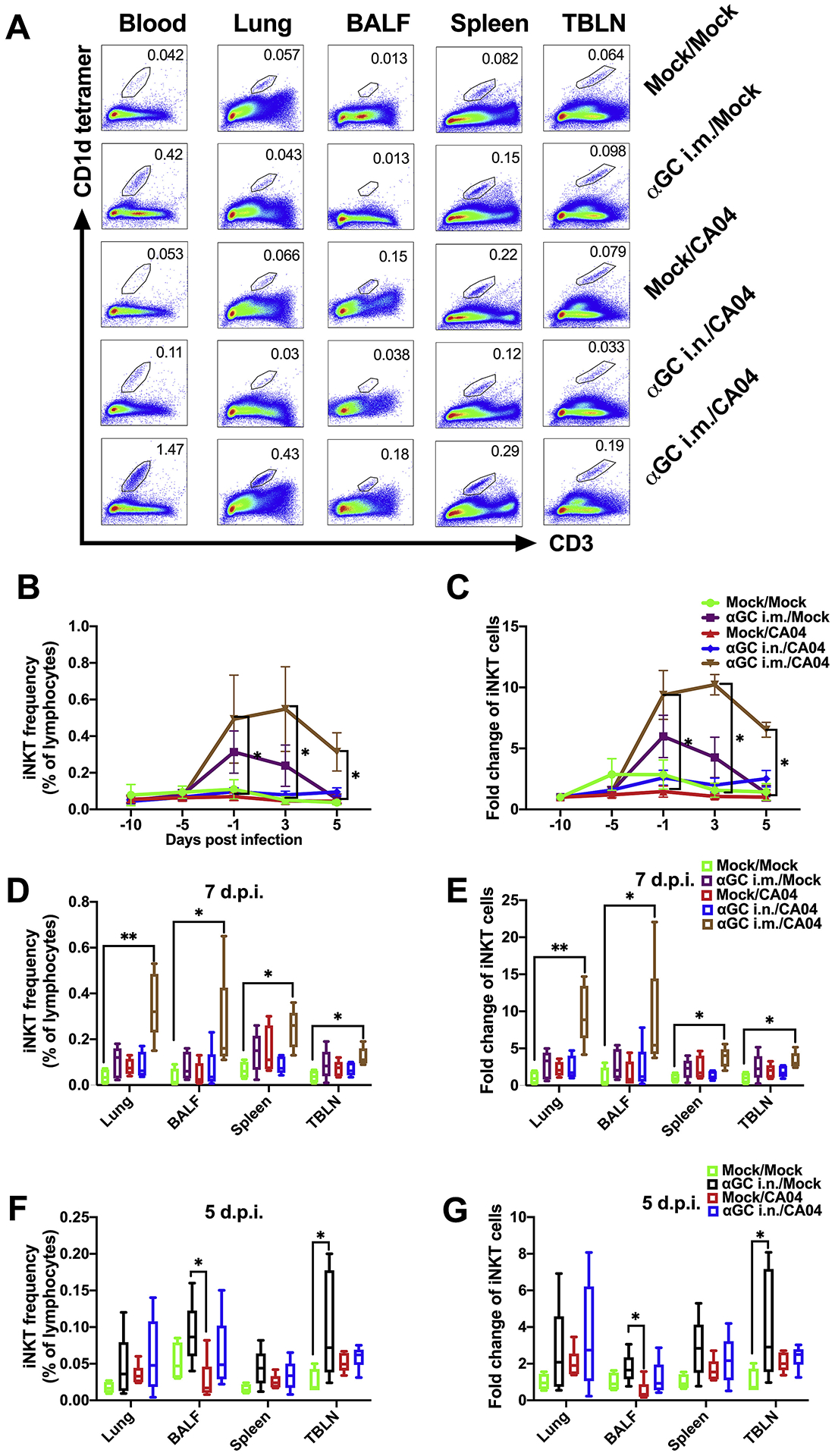
Flow cytometric analysis of iNKT cell levels. (A-E) Frequency of iNKT cells in peripheral blood (PB) and tissues in Experiment 1 when pigs were administrated α-GalCer (αGC) i.m. or i.n. 9 days prior to infection with CA04. (A) Representative flow cytometry plots showing iNKT cells in PB at 3 d.p.i. and various tissues at 7 d.p.i.. (B) Frequency of PB iNKT cells as a percentage of lymphocytes at −10, −5, −1, 3, 5 d.p.i.. (C) Fold change of iNKT cell frequency compared to the same pig at −10 d.p.i.. (D) Tissue and BALF iNKT cells as a proportion of lymphocytes at 7 d.p.i.. (E) Fold change of tissue and BALF iNKT cell frequency compared to the average iNKT cell frequency of Mock/Mock pigs. (F-G) Frequency of iNKT cells in tissues in Experiment 2 when pigs pigs were treated with α-GalCer i.n. 2 days before challenge with CA04. (F) Frequency of iNKT cells in BALF and various tissues as a proportion of total lymphocytes collected at 5 d.p.i.. (G) Fold change of tissue and BALF iNKT cells compared to the average iNKT cell frequency of Mock/Mock pigs. Data are represented as mean ± SEM. Differences in PB iNKT cell levels were analyzed using the Kruskal-Wallis test and the Dunn’s test was used to compare treatments at each time point and within each tissue. *P < 0.05.
In a separate study, we investigated whether intranasal administration of α-GalCer 2 days before CA04 inoculation would alter the course of disease. Pigs were infected with 106 TCID50 of CA04 IAV 2 days after they were administered 100 μg/kg of α-GalCer by the intranasal route (αGC i.n./CA04). Additional pigs were mock-injected and mock-infected (Mock/Mock), mock-treated and CA04 infected (Mock/CA04), and α-GalCer treated and mock-infected (αGC i.n./Mock). Peripheral blood iNKT cell frequencies remained stable for all treatment groups throughout the experiment (not shown). We observed that iNKT cells tended to be 2 to 4-fold higher in BALF and tissues of α-GalCer-treated pigs compared to Mock/Mock pigs at 5 d.p.i. (Figure 1F & G). However, the only significant differences we observed were between αGC i.n./Mock and Mock/Mock pigs in TBLNs and between αGC i.n./Mock and Mock/CA04 pigs in BALF. Thus, it appears that α-GalCer delivery via the intranasal route had only a modest effect on iNKT cell levels in the lung that did not synergize with CA04 infection.
3.2. Analysis of immune cells and cytokines
To determine whether α-GalCer or IAV infection induced downstream immune effects, we measured the frequencies of total T cells, CD4+ T cells, CD8+ T cells, CD4+CD8+ double-positive T cells, γδ T cells, and NK cells (Supplemental Figure 2) in PB, BALF, lung, spleen, and TBLN when pigs were administered α-GalCer 9 days (Supplemental Figure 3 & Supplemental Tables 2–5) and 2 days (Supplemental Figure 4 & Supplemental Tables 6–9) before infection. No significant differences in cell types were detected among treatment groups in either experiment indicating that, apart from iNKT cells, prophylactic α-GalCer administration did not significantly alter leukocyte populations in blood or tissues. We also analyzed cytokine levels in BALF using a porcine multiplex array that detects IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, IFN-α, IFN-γ, and TNF-α. We found no significant differences among treatment groups in either study with the exception of IFN-α, which was significantly increased in α-GalCer treated and infected pigs (αGC i.n./CA04) compared to mock-infected pigs in the second study (Figure 2).
Figure 2.
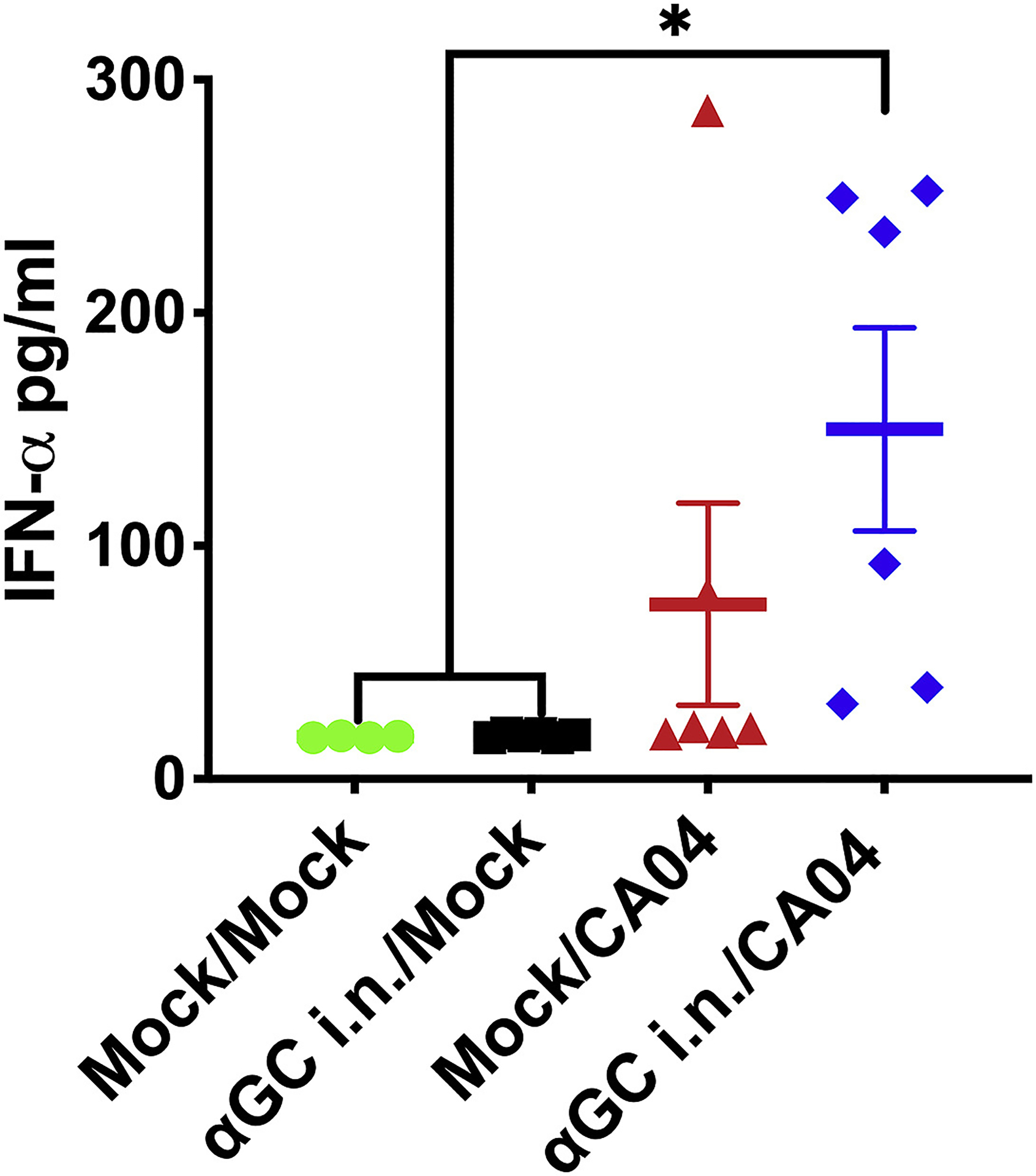
Intranasal α-GalCer (αGC) administration 2 days prior to challenge increased IFN-α concentrations in the BALF of IAV infected pigs. IFN-α levels were measured using a multiplex immunoassay at 5 d.p.i.. Data are represented as mean ± SEM. Differences between different groups were analyzed using a nonparametric Kruskal-Wallis test, *P < 0.05.
3.3. Activating NKT cells before influenza infection does not affect lung inflammation
Lung tissues were examined to determine the impact of α-GalCer administration on influenza-induced pulmonary inflammation. In the first study that administered α-GalCer i.m. or i.n. to pigs 9 days before IAV challenge, cranial, middle and caudal sections of the right lung were collected on day 7 d.p.i.. All groups of pigs that were infected with CA04 developed moderate levels of bronchiolitis and pneumonia. No significant difference in pathology scores was detected among IAV-infected pigs treated with vehicle or α-GalCer (Figure 3A & B), although pneumonia scores tended to be higher in αGC i.n./CA04 pigs than the other treated groups. There were also no differences in lung pathology score among IAV-infected pigs in the second study, where α-GalCer was administered i.n. 2 days before challenge and necropsies were performed at 5 d.p.i. (Figure 3C & D). It should be noted that some pigs in the Mock/Mock group of this second study had a low level of bronchiolitis and pneumonia due to a pre-existing bacterial infection. Collectively, these results indicate that therapeutically increasing and/or activating iNKT cells in the respiratory tract prior to infection does not reduce lung inflammation, but also does not induce immunopathological inflammation.
Figure 3.
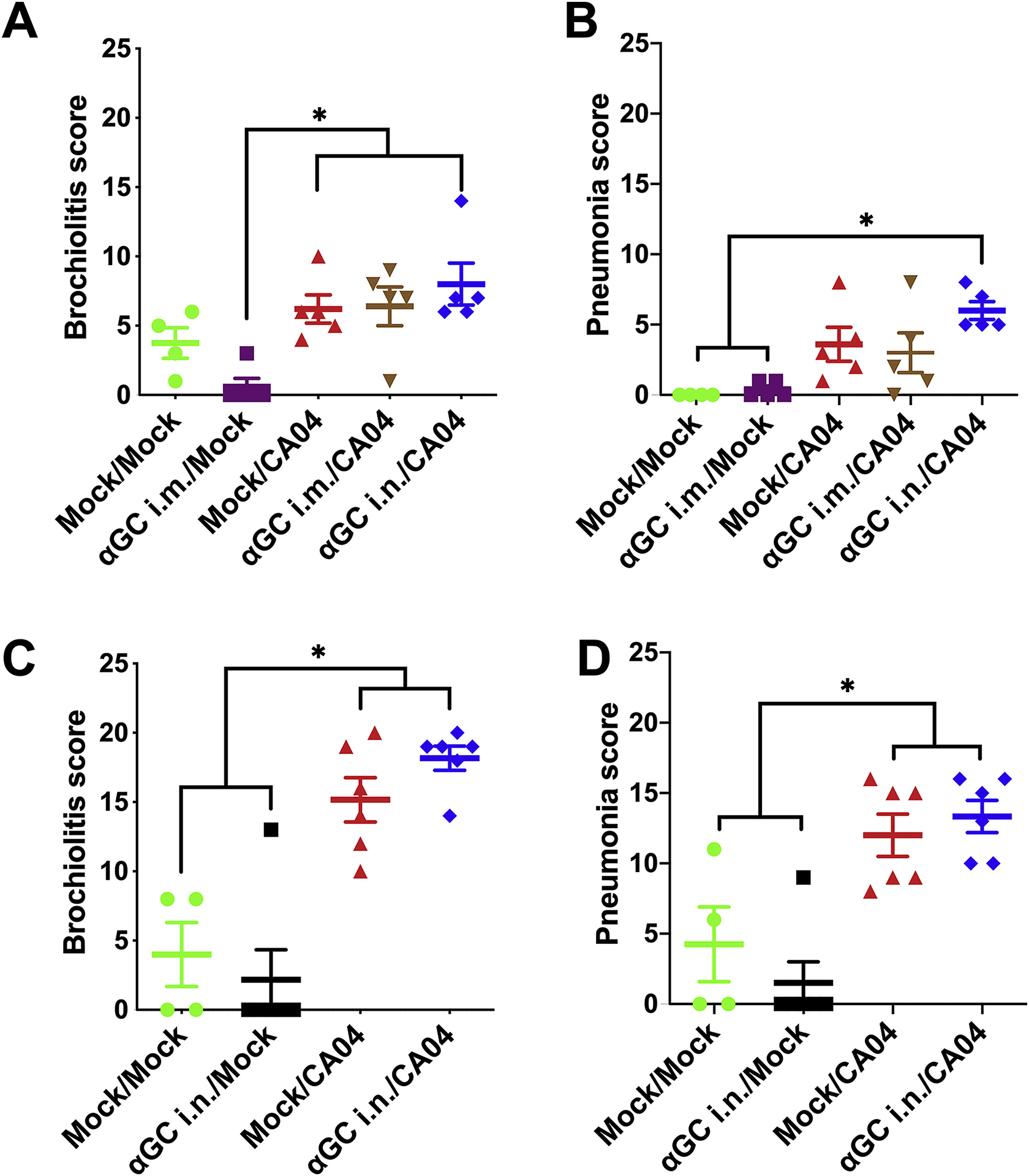
Therapeutically activating iNKT cells prior to IAV infection does not alter lung pathology. Pathology was assessed by evaluating H&E stained lung sections from pigs administered α-GalCer (αGC) i.m. or i.n. 9 days prior to infection (A & B) or i.n. 2 days prior to infection (C & D). (A & C) Bronchiolitis score; (B & D) Pneumonia score. Data are presented as mean ± SEM. Data were analyzed using the SAS PROC GLIMMIX procedure and differences between groups were analyzed using Tukey’s test, *P < 0.05.
3.4. Prophylactic α-GalCer administration does not reduce viral replication or virus shedding
We measured virus titers in nasal swabs, BALF, and various tissues throughout the respiratory tract. In the first study that administered α-GalCer i.m. or i.n. to pigs 9 days before challenge, virus was detected in nasal swabs from 1 d.p.i., and peaked at day 5 d.p.i.. However, there was no difference in viral titers between pigs treated with vehicle or α-GalCer delivered i.m. or i.n. (Figure 4A). Most pigs had undetectable levels of virus in their airway tissues by 7 d.p.i., probably because viral particles had been cleared by host immune responses at this time (data not shown). In the second study that intranasally administered α-GalCer 2 days before infection, no significant difference in virus titers was detected among any of the virus infected pigs in nasal swabs collected daily, or BALF and airway tissues collected at 5 d.p.i. (Figure 4B & C). Collectively, these results indicate that virus replication and shedding is unaffected by prior treatment with α-GalCer.
Figure 4.
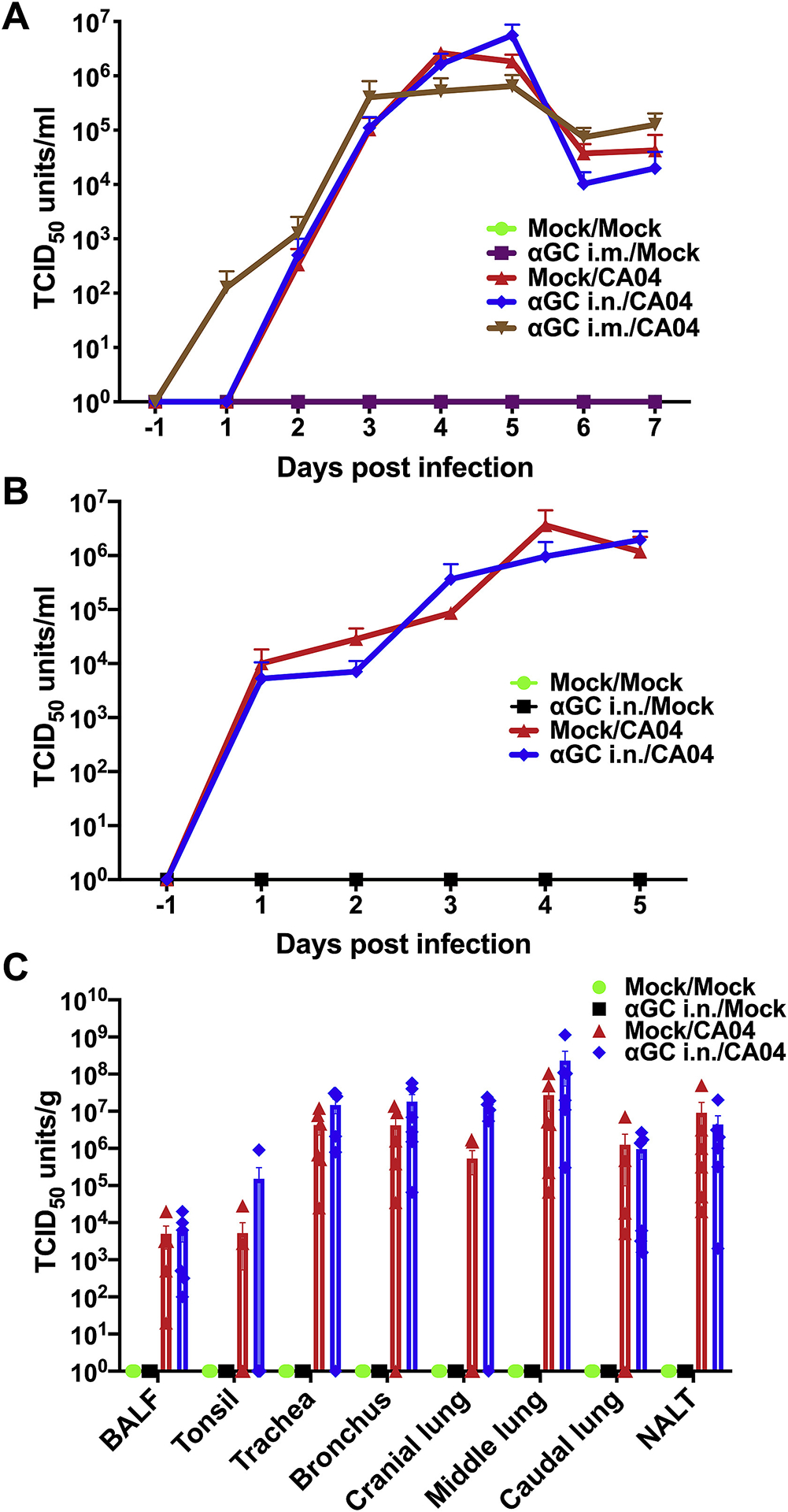
Virus titers are unaffected by prophylactically treating IAV infected pigs with α-GalCer (αGC). (A) Viral titers in nasal swabs from pigs in Experiment 1 that administrated α-GalCer (αGC) i.m. or i.n. 9 days prior to infection with CA04. (B-C) Viral titers in nasal swabs (B) and tissues 5 d.p.i. (C) from pigs in Experiment 2 that administered αGC i.n. 2 days before infection. Data are represented as mean ± SEM. Data were analyzed using the SAS PROC GLIMMIX procedure in SAS. No significant differences were detected among the infected groups.
4. Discussion
The current study evaluated whether prophylactically administering α-GalCer to pigs would increase their resistance against a subsequent influenza virus infection. Our premise was based on previous reports that iNKT cells make up a significant component of IAV immunity in mice and that therapeutically expanding and activating murine iNKT cells with α-GalCer before or during an IAV infection strongly increases influenza disease resistance in this species (Ho et al., 2008; Ishikawa et al., 2010; Kok et al., 2012; Lin et al., 2010). In addition, we previously showed that viral titers and lung pathology were significantly reduced in piglets intranasally treated with α-GalCer at the time of infection (Artiaga et al., 2016b). In our first experiment, pigs were infected with influenza 9 days after α-GalCer injection in order to synchronize virus infection with peak iNKT cell expansion (Artiaga et al., 2014). Although iNKT cell concentrations increased, including in the respiratory tract, our treatment failed to reduce virus shedding, viral replication in the airway or lung pathology. This may be because the anti-IAV effector functions of swine iNKT cells are quantitatively or qualitatively different to mouse iNKT cells or that the concentrations of iNKT cells we achieved did not reach a level sufficient to affect IAV-induced disease. Indeed, iNKT cell concentrations are approximately 10-fold lower in pigs and humans than in most inbred mouse strains (Artiaga et al., 2014; Berzins et al., 2011), while porcine iNKT cells do not expand to the same levels as murine iNKT cells after α-GalCer stimulation (Harada et al., 2004; Rampuria and Lang, 2015; Renukaradhya et al., 2011; Schäfer et al., 2019; Thierry et al., 2012; Yang et al., 2019). It is notable that a previous study has reported that only patients with high iNKT cell frequencies appeared to benefit from iNKT cell therapy (Giaccone et al., 2002). Another possibility is that our α-GalCer treatments induced iNKT cells to become anergic. This phenomenon occurs in mouse and human iNKT cells after strong activation, and is characterized by a reduced ability to produce IFNγ after re-stimulation for up to three weeks after activation (Hayakawa et al., 2004; Kojo et al., 2009; Parekh et al., 2005). In the current study, α-GalCer-expanded iNKT cells remained at least partially functional because their levels increased significantly after IAV infection, probably because of indirect stimulation by virus-induced cytokines, such as IL-12 (Taniguchi et al., 2003). Nevertheless, preexposure to α-GalCer may have diminished iNKT cell effector functions below a level necessary to impact IAV-induced disease. Indeed, we previously found less plasma IFNγ in pigs after a booster compared to a primary administration of α-GalCer (Artiaga et al., 2014).
Our second experiment delivered α-GalCer intranasally just 2 days before virus infection in order to determine whether disease could be altered in the immediate aftermath of iNKT cell activation. This treatment strategy has been reported to induce a wide range of early-innate immune responses in the pulmonary mucosa (Courtney et al., 2011), which is the site of infection (Janke, 2014). We chose this approach because i.n. administration of α-GalCer significantly reduced virus replication in pigs when delivered at the time of infection (Artiaga et al., 2016b). Also, therapeutically activating iNKT cells shortly before infection has been demonstrated to limit the severity of disease in mice infected with IAV and other diseases. They include a study where the α-GalCer analog C34 increased the survival of mice challenged with IAV one day later (Lin et al., 2010) as well as a study which found that administration of α-GalCer 30 hours prior to sepsis induction, reduced pulmonary inflammation and injury and increased survival in a mouse model of polymicrobial sepsis (Bolognese et al., 2018). Also, α-GalCer administration i.n. 24 h before Streptococcus pneumoniae infection was shown to fully protect mice from mortality and rapidly eliminated the pathogen from alveolar spaces (Ivanov et al., 2012). In our study, it was not surprising that iNKT cell levels did not increase when α-GalCer was administered i.n. 2 days before infection as iNKT cells did not increase in a previous study that used i.n. administered α-GalCer to modulate an ongoing IAV infection (Artiaga et al., 2016b). The potential reasons why our second treatment strategy did not affect disease outcome include that the effects of iNKT cell activation may have dissipated by the time the pigs became infected, as iNKT cells are known to induce potent but short-lived cytokine responses. Also, it is possible that α-GalCer may have rendered iNKT cells anergic by the time the pigs were infected, as described above.
In conclusion, therapeutically activating and/or expanding pulmonary iNKT cells before IAV-infection did not alter the course of influenza infection in pigs even though a similar approach reduced disease in mice. Our work suggests that this strategy may also be ineffective in humans because people are more similar to swine than mice for IAV infections as well as innate and adaptive immune defense systems, including iNKT cells.
Supplementary Material
Acknowledgements
We thank the National Institutes of Health Tetramer Core Facility for provision of the CD1d tetramers under the contract HHSN272201300006C.
Funding
This work was supported by U.S. Department of Agriculture Grant 2016-09448 (to J.P.D.) and National Institutes of Health Grant HD092286 (to J.P.D. & JAR).
Abbreviations
- IAV
Influenza A virus
- iNKT cell
invariant natural killer T cell
- α-GalCer
α-galactosylceramide
- TCR
T cell receptor
- MDSC
myeloid-derived suppressor cells
- CA04
A/California/04/2009
- HAI
hemagglutination inhibition assay
- i.v.
intravenous
- i.m.
intramuscular
- i.n.
intranasal
- MDCK
Madin Darby Canine Kidney
- PB
peripheral blood
- BALF
bronchoalveolar lavage fluid
- NALT
nasal associated lymphoid tissue
- TBLN
tracheobronchial lymph nodes
References
- Artiaga BL, Whitener RL, Staples CR, Driver JP, 2014. Veterinary Immunology and Immunopathology Adjuvant effects of therapeutic glycolipids administered to a cohort of NKT cell-diverse pigs. Vet. Immunol. Immunopathol 162, 1–13. 10.1016/j.vetimm.2014.09.006 [DOI] [PubMed] [Google Scholar]
- Artiaga BL, Yang G, Hackmann TJ, Liu Q, Richt JA, Salek-Ardakani S, Castleman WL, Lednicky JA, Driver JP, 2016a. α-Galactosylceramide protects swine against influenza infection when administered as a vaccine adjuvant. Sci. Rep 6, 1–13. 10.1038/srep23593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiaga BL, Yang G, Hutchinson TE, Loeb JC, Richt JA, Lednicky JA, Salek-Ardakani S, Driver JP, 2016b. Rapid control of pandemic H1N1 influenza by targeting NKT-cells. Sci. Rep 6, 2–11. 10.1038/srep37999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins SP, Smyth MJ, Baxter AG, 2011. Presumed guilty: Natural killer T cell defects and human disease. Nat. Rev. Immunol 11, 131–142. 10.1038/nri2904 [DOI] [PubMed] [Google Scholar]
- Bolognese AC, Yang W-L, Hansen LW, Sharma A, Nicastro JM, Coppa GF, Wang P, 2018. Activation of Invariant Natural Killer T Cells Redirects the Inflammatory Response in Neonatal Sepsis. Front. Immunol 9. 10.3389/fimmu.2018.00833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigl M, Tatituri RVV, Watts GFM, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB, 2011. Innate and cytokine-driven signals, rather than microbial antigens dominate in natural killer T cell activation during microbial infection. J. Exp. Med 208, 1163–1177. 10.1084/jem.20102555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney AN, Thapa P, Singh S, Wishahy AM, Zhou D, Sastry J, 2011. Intranasal but not intravenous delivery of the adjuvant α-galactosylceramide permits repeated stimulation of natural killer T cells in the lung. Eur. J. Immunol 41, 3312–3322. 10.1002/eji.201041359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJL, Hayakawa Y, Sidobre S, Keating R, Kronenberg M, Smyth MJ, Godfrey DI, 2003. Glycolipid Antigen Drives Rapid Expansion and Sustained Cytokine Production by NK T Cells. J. Immunol 171, 4020–4027. 10.4049/jimmunol.171.8.4020 [DOI] [PubMed] [Google Scholar]
- Driver JP, Scheuplein F, Chen YG, Grier AE, Wilson SB, Serreze DV, 2010. Invariant natural killer T-cell control of type 1 diabetes: A dendritic cell genetic decision of a silver bullet or Russian roulette. Diabetes 59, 423–432. 10.2337/db09-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccone G, Punt CJA, Ando Y, Ruijter R, Nishi N, Peters M, Von Blomberg BME, Scheper RJ, Van der Vliet HJJ, Van den Eertwegh AJM, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM, 2002. A phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res 8, 3702–3709. [PubMed] [Google Scholar]
- Harada M, Seino KI, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M, 2004. Down-regulation of the invariant Vα14 antigen receptor in NKT cells upon activation. Int. Immunol 16, 241–247. 10.1093/intimm/dxh023 [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Berzins SP, Crowe NY, Godfrey DI, Smyth MJ, 2004. Antigen-induced tolerance by intrathymic modulation of self-recognizing inhibitory receptors. Nat. Immunol 5, 590–596. 10.1038/ni1069 [DOI] [PubMed] [Google Scholar]
- Ho LP, Denny L, Luhn K, Teoh D, Clelland C, McMichael AJ, 2008. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur. J. Immunol 38, 1913–1922. 10.1002/eji.200738017 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Tanaka K, Kutsukake E, Fukui T, Sasaki H, Hata A, Noda S, Matsumoto T, 2010. IFN-γ production downstream of NKT cell activation in mice infected with influenza virus enhances the cytolytic activities of both NK cells and viral antigen-specific CD8+ T cells. Virology 407, 325–332. 10.1016/j.virol.2010.08.030 [DOI] [PubMed] [Google Scholar]
- Ivanov S, Fontaine J, Paget C, Fernandez M, Maele L. Van, Renneson J, Maillet I, Wolf NM, Rial A, Léger H, Ryffel B, Frisch B, Chabalgoity JA, Sirard JC, Benecke A, Faveeuw C, Trottein F, 2012. Key Role for Respiratory CD103 + Dendritic Cells, IFN- γ, and IL-17 in Protection Against Streptococcus pneumoniae Infection in Response to α -Galactosylceramide. J. Infect. Dis 206, 723–34. 10.1093/infdis/jis413 [DOI] [PubMed] [Google Scholar]
- Janke BH, 2014. Influenza A Virus Infections in Swine: Pathogenesis and Diagnosis. Vet. Pathol 51, 410–426. 10.1177/0300985813513043 [DOI] [PubMed] [Google Scholar]
- Khiabanian H, Trifonov V, Rabadan R, 2009. Reassortment Patterns in Swine Influenza Viruses. PLoS One 4. 10.1371/journal.pone.0007366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo S, Elly C, Harada Y, Langdon WY, Kronenberg M, Liu YC, 2009. Mechanisms of NKT cell anergy induction involve Cbl-b-promoted monoubiquitination of CARMA1. Proc. Natl. Acad. Sci. U. S. A 106, 17847–17851. 10.1073/pnas.0904078106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok WL, Denney L, Benam K, Cole S, Clelland C, McMichael AJ, Ho L-P, 2012. Pivotal Advance: Invariant NKT cells reduce accumulation of inflammatory monocytes in the lungs and decrease immune-pathology during severe influenza A virus infection. J. Leukoc. Biol 91, 357–368. 10.1189/jlb.0411184 [DOI] [PubMed] [Google Scholar]
- Lefevre EA, Carr BV, Inman CF, Prentice H, Brown IH, Sharon M, Garcon F, Hill ML, Iqbal M, Elderfield RA, Barclay WS, Bailey M, Charleston B, 2012. Immune Responses in Pigs Vaccinated with Adjuvanted and Non-Adjuvanted A (H1N1) pdm / 09 Influenza Vaccines Used in Human Immunization Programmes. PLoS One 7, 1–10. 10.1371/journal.pone.0032400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Liang J, Huang W, Su C, Lee Y, Jan J, Lin Y, Cheng YE, Wong C, Al LINET, 2010. In Vivo Protection Provided by a Synthetic New Alpha-Galactosyl Ceramide Analog against Bacterial and Viral Infections in Murine Models. Antimicrob. Agents Chemother 54, 4129–4136. 10.1128/AAC.00368-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looringh van Beeck FA, Reinink P, Hermsen R, Zajonc DM, Laven MJ, Fun A, Troskie M, Schoemaker NJ, Morar D, Lenstra JA, Vervelde L, Rutten VPMG, van Eden W, Van Rhijn I, 2009. Functional CD1d and/or NKT cell invariant chain transcript in horse, pig, African elephant and guinea pig, but not in ruminants. Mol. Immunol 46, 1424–1431. 10.1016/j.molimm.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, Dumoutier L, Ryffel B, Renauld JC, Gosset Philippe, Gosset Pierre, Si-Tahar M, Faveeuw C, Trottein F, 2012. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: Potential role in protection against lung epithelial damages. J. Biol. Chem 287, 8816–8829. 10.1074/jbc.M111.304758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh VV, Joyce S, Kaer L. Van, Parekh VV, Wilson MT, Olivares-villagómez D, Singh AK, Wu L, 2005. Glycolipid antigen induces long-term natural killer T cell anergy in mice Find the latest version : Glycolipid antigen induces long-term natural killer T cell anergy in mice. J. Clin. Invest 115, 2572–2583. 10.1172/JCI24762.2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo HM, Hlasny M, Zhou Y, 2015. Influence of maternally-derived antibodies on live attenuated influenza vaccine efficacy in pigs. Vaccine 33, 3667–3672. 10.1016/j.vaccine.2015.06.044 [DOI] [PubMed] [Google Scholar]
- Rajao DS, Vincent AL, 2015. Swine as a model for influenza avirus infection and immunity. ILAR J. 56, 44–52. 10.1093/ilar/ilv002 [DOI] [PubMed] [Google Scholar]
- Rampuria P, Lang ML, 2015. CD1d-dependent expansion of NKT follicular helper cells in vivo and in vitro is a product of cellular proliferation and differentiation. Int. Immunol 27, 253–263. 10.1093/intimm/dxv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H, 1938. A SIMPLE METHOD OF ESTIMATING FIFTY PER CENT ENDPOINTS. Am. J. Hyg 27, 435–469. [Google Scholar]
- Renukaradhya GJ, Manickam C, Khatri M, Rauf A, Li X, Tsuji M, Rajashekara G, Dwivedi V, 2011. Functional invariant NKT cells in pig lungs regulate the airway hyperreactivity: A potential animal model. J. Clin. Immunol 31, 228–239. 10.1007/s10875-010-9476-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz R, Tedeschi LO, Sepúlveda A, 2017. Investigation of the effect of pegbovigrastim on some periparturient immune disorders and performance in Mexican dairy herds. J. Dairy Sci 100, 3305–3317. 10.3168/jds.2016-12003 [DOI] [PubMed] [Google Scholar]
- Sandbulte MR, Spickler AR, Zaabel PK, Roth JA, 2015. Optimal Use of Vaccines for Control of Influenza A Virus in Swine. Vaccines 3, 22–73. 10.3390/vaccines3010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo C. De, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, Gröne H, Platt FM, Zambon M, Cerundolo V, 2008. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus–induced myeloid-derived suppressor cells in mice and humans. J. Clin. Invest 118, 4036–4048. 10.1172/JCI36264.tumor [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A, Hühr J, Schwaiger T, Dorhoi A, Mettenleiter TC, Blome S, Schröder C, Blohm U, 2019. Porcine invariant natural killer T cells: Functional profiling and dynamics in steady state and viral infections. Front. Immunol 10, 1–19. 10.3389/fimmu.2019.01380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solórzano A, Webby RJ, Lager KM, Janke BH, García-Sastre A, Richt JA, 2005. Mutations in the NS1 Protein of Swine Influenza Virus Impair Anti-Interferon Activity and Confer Attenuation in Pigs. J. Virol 79, 7535–7543. 10.1128/jvi.79.12.7535-7543.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Seino K, Nakayama T, 2003. The NKT cell system : bridging innate and acquired immunity. Nat. Immunol 4, 1164–1165. [DOI] [PubMed] [Google Scholar]
- Thierry A, Robin A, Giraud S, Minouflet S, Barra A, Bridoux F, Hauet T, Touchard G, Herbelin A, Gombert JM, 2012. Identification of invariant natural killer T cells in porcine peripheral blood. Vet. Immunol. Immunopathol 149, 272–279. 10.1016/j.vetimm.2012.06.023 [DOI] [PubMed] [Google Scholar]
- Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M, 2008. Cutting Edge: The Mechanism of Invariant NKT Cell Responses to Viral Danger Signals. J. Immunol 181, 4452–4456. 10.4049/jimmunol.181.7.4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2002. WHO Manual on Animal Influenza Diagnosis and Surveillance. World Health Organization. https://apps.who.int/iris/handle/10665/68026. [Google Scholar]
- Wilson MT, Johansson C, Olivares-Villagómez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, Van Kaer L, 2003. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc. Natl. Acad. Sci. U. S. A 100, 10913–10918. 10.1073/pnas.1833166100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Artiaga BL, Hackmann TJ, Samuel MS, Walters EM, Salek-Ardakani S, Driver JP, 2015. Targeted disruption of CD1d prevents NKT cell development in pigs. Mamm. Genome 26, 264–270. 10.1007/s00335-015-9564-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Artiaga BL, Lewis ST, Driver JP, 2017. Characterizing porcine invariant natural killer T cells: A comparative study with NK cells and T cells. Dev. Comp. Immunol 76, 343–351. 10.1016/j.dci.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Yang G, Artiaga BL, Lomelino CL, Jayaprakash D, Sachidanandam R, Mckenna R, Driver JP, 2019. Next Generation Sequencing of the Pig α β TCR Repertoire Identifies the Porcine Invariant NKT Cell Receptor. J. Immunol 10.4049/jimmunol.1801171 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


