Abstract
We analyzed reports on safety and efficacy of JAK-inhibitors in patients with coronavirus infectious disease-2019 (COVID-19) published between January 1st and March 6th 2021 using the Newcastle-Ottawa and Jadad scales for quality assessment. We used disease severity as a proxy for time when JAK-inhibitor therapy was started. We identified 6 cohort studies and 5 clinical trials involving 2367 subjects treated with ruxolitinib (N = 3) or baricitinib 45 (N = 8). Use of JAK-inhibitors decreased use of invasive mechanical ventilation (RR = 0.63; [95% Confidence Interval (CI), 0.47, 0.84]; P = 0.002) and had borderline impact on rates of intensive care unit (ICU) admission (RR = 0.24 [0.06, 1.02]; P = 0.05) and acute respiratory distress syndrome (ARDS; RR = 0.50 [0.19, 1.33]; P = 0.16). JAK-inhibitors did not decrease length of hospitalization (mean difference (MD) –0.18 [–4.54, 4.18]; P = 0.94). Relative risks of death for both drugs were 0.42 [0.30, 0.59] (P < 0.001), for ruxolitinib, RR = 0.33 (0.13, 0.88; P = 0.03) and for baricitinib RR = 0.44 (0.31, 0.63; P < 0.001). Timing of JAK-inhibitor treatment during the course of COVID-19 treatment may be important in determining impact on outcome. However, these data are not consistently reported.
Subject terms: Infectious diseases, Public health
Introduction
Infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causes coronavirus disease-2019 (COVID-19), an important feature of which is dysregulated immune responses resulting in so-called cytokine release syndrome (CRS). There are several reports of using JAK-inhibitors in persons with COVID-19 [1–3]. These data are from small uncontrolled, non-randomized, open-label trials which mostly conclude JAK-inhibitors are safe and effective. However, with appropriate controls these conclusions are unconvincing. We analyzed 11 studies of safety and efficacy of ruxolitinib and baricitinib in persons with COVID-19. We found these drugs decreased the use of invasive mechanical ventilation, had borderline effects on rates of intensive care unit (ICU) admission and acute respiratory distress syndrome (ARDS) and did not decrease interval of hospitalization. The risk of death was decreased, most convincingly for baricitinib.
Methods
Search strategy and selection criteria
We conducted a systematic review and meta-analysis focused on effects of JAK-inhibitors on diverse outcomes in persons with SARS-CoV-2-infection and/or COVID-19. We searched on PubMed, Web of Science and Medline with search terms using the Boolean operators including coronavirus OR COVID-19 OR 2019-nCoV OR SARS-CoV-2 AND ruxolitinib OR baricitinib OR Janus kinase OR JAK. Inclusion dates were 1st January 2020 to 6th March 2021. Two investigators independently reviewed the identified abstracts and selected articles for full reviewing. Discordances were resolved by a 3rd reviewer. Review Manager 5.4 was used for the meta-analysis and modified Newcastle-Ottawa scale (NOS) and Jadad scale for quality assessment. Our focus was on clinically relevant outcomes including rates of ICU admission, ARDS and invasive mechanical ventilation, interval of hospital stay, and death. We also considered relevant laboratory co-variates including blood C-reactive protein (CRP) procalcitonin concentrations, pulse oxygen saturation (SPO2) and PaO2/FiO2.
We identified 823 articles excluding 516 duplicates. Next, we identified 96 relevant articles by reviewing the title and abstract. After further review 11 studies were included in the meta-analysis [1–11] (Supplementary Fig. 1).
Inclusion and exclusion criteria
We included all English language clinical trials and observational dataset of JAK-inhibitors used singly or with other therapies in persons with COVID-19. We excluded reviews and case reports. Studies had to report outcomes including data of survival, ICU admission, or invasive mechanical ventilation.
Data extraction
Two authors independently reviewed identified abstracts and selected articles for full review and a third reviewer resolved discordances. For each selected article we extracted baseline and study co-variates including 1st author, publication year, country, number subjects, subject co-variates, and therapy details (Table 1, Supplementary Table 1). Outcomes measures included rates of ICU admission, ARDS and use of invasive mechanical ventilation, interval of hospitalization, and survival. We used anonymized, published data without a need of Ethics Committee approval.
Table 1.
Studies included.
| Ref. | Study-type | Jadad scale/NOS scale | Drug | Deaths |
|---|---|---|---|---|
| 1 | Observational | NOS scale: 9 | Baricitinib | 1/20 vs. 25/56 |
| 2 | Clinical trial | Jadad scale: 1 | Baricitinib | NE |
| 3 | Observational | NOS scale: 7 | Baricitinib | 5/117 vs. 11/270 |
| 4 | Observational | NOS scale: 9 | Baricitinib | 0/113 vs. 5/78 |
| 7 | RCT | Jadad scale: 7 | Baricitinib | 24/515 vs. 37/518 |
| 9 | Observational | NOS scale: 7 | Baricitinib | 5/40 vs. 65/275 |
| 10 | Observational | NOS scale: 8 | Baricitinib | 2/12 vs. 6/17 |
| 11 | Observational | NOS scale:7 | Baricitinib | 1/37 vs. 47/142 |
| 5 | RCT | Jadad scale: 7 | Ruxolitinib | 0/20 vs. 3/21 |
| 6 | Clinical trial | Jadad scale: 1 | Ruxolitinib | 1/7 vs. 1/10 |
| 8 | Clinical trial | Jadad scale: 1 | Ruxolitinib | 3/32 vs. 13/43 |
RCT randomized controlled trial, NOS Newcastle-Ottawa scale.
Risk of bias assessment
The risk of bias was assessed according to the Jadad scale in the domains of random sequence generation, allocation concealment, blinding of participants and personnel, and completed withdrawals and dropouts. The methodological quality of retrospective studies was assessed by the modified NOS consisting of subject selection, comparability of the study groups, and assessment of outcome. A score of 0–9 was allocated to each observational study. Studies with ≥6 score were judged high quality.
Statistical analyses
We pooled data and used relative risks (RRs) and confidence intervals (CIs) to describe dichotomized outcomes including rates of ICU admission and ARDS, use of invasive mechanical ventilation, and death. We used mean difference (MD) and 95% CIs for continuous outcomes including interval of hospitalization, blood CRP and procalcitonin concentrations, SPO2, and PaO2/FiO2. A fixed-effect model was used if there the heterogeneity test statistic between studies was I2 ≤ 50% and a random-effects model was used if I2 statistic was >50%. Funnel plots were used to screen for publication bias. Statistical analyses were carried out with Review Manager 5.4 (The Cochrane Collaboration).
Results
We included 6 observational studies and 5 clinical trials comprising 2367 subjects [1–11]. Studies were conducted in Italy [1, 2, 4, 6, 8, 9], China [5], USA [7], UK [11], and Spain [3, 10]. NOS scores of the observational studies ranged from 7 to 9. Jadad scales of the clinical trials were 1, 1, 1, 7, and 7.
Surrogate outcomes
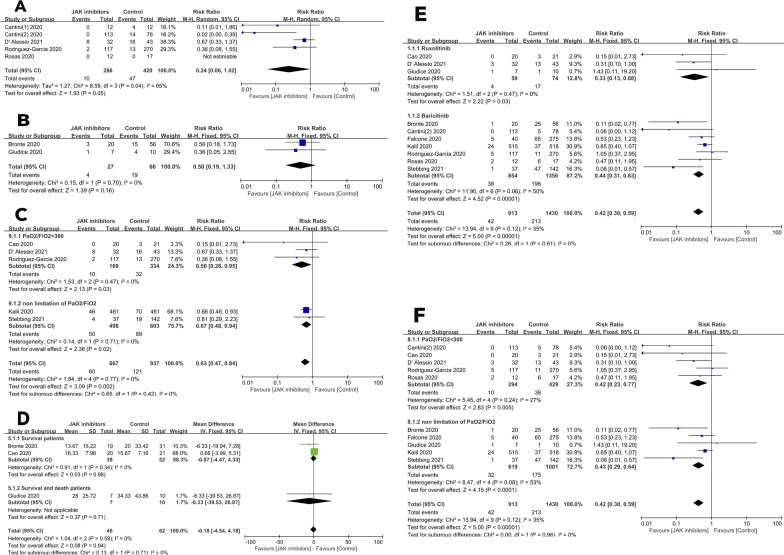
To test the impact of JAK-inhibitors on ICU admission, we included 5 studies of 706 subjects [2–4, 8, 10]. RR of ICU admissions = 0.24 (95% CI, 0.06, 1.02; P = 0.05; I2 = 65%; Fig. 1A). To test the impact of JAK-inhibitors on the rate of ARDS, we included 2 studies of 93 subjects [1, 6]. RR = 0.50 (0.19, 1.33; P = 0.16; I2 = 0%; Fig. 1B). To test the impact of JAK-inhibitors on the rate of invasive mechanical ventilation, we included 5 studies of 1604 subjects [3, 5, 7, 8, 11]. RR = 0.63 (0.47, 0.84; P = 0.002; I2 = 0). RRs for PaO2/FiO2 < 300 mmHg and any PaO2/FiO2 based on the study protocols were RR = 0.50 (0.26, 0.95, P = 0.03; I2 = 0) and RR = 0.67 (0.48, 0.94, P = 0.02; I2 = 0; Fig. 1C). To test the impact of JAK-inhibitors on hospitalization interval, we included 3 studies of 108 subjects [1, 5, 6]. MD was −0.18 (−4.54, 4.18, P = 0.94; I2 = 0). In subjects discharged from hospital, MD was −0.07 (−4.47, 4.33; P = 0.98; I2 = 0; Fig. 1D).
Fig. 1. The effect of JAK-inhibitors on COVID-19 related outcomes.
Risks of A ICU admission; B ARDS; C invasive mechanical ventilation; D interval of hospitalization; E risk of death grouped by agents; F risk of death grouped by the study protocol.
Survival
To test the impact of JAK-inhibitors on survival we included 10 studies of 2343 subjects [1, 3–11]. RR in a fixed-effects model = 0.42 (0.30, 0.59; P < 0.001; I2 = 35%). RRs for ruxolitinib and baricitinib were RR = 0.33, (0.13, 0.88; P = 0.03; I2 = 0%) and RR = 0.44 (0.31, 0.63; P < 0.001; I2 = 50%; Fig. 1E). RRs for survival for PaO2/FiO2 < 300 mmHg and any PaO2/FiO2 based on the study protocol were RR = 0.42 (0.23, 0.77, P = 0.005; I2 = 27) and RR = 0.43 (0.29, 0.64, P < 0.001; I2 = 53; Fig. 1F).
Secondary endpoints
To test the impact of JAK-inhibitors on CRP concentration we used 4 studies of 293 subjects [1, 2, 4, 10]. MD was −25 (−49, −1; P < 0.001; I2 = 96%). To test the impacts of JAK-inhibitors on procalcitonin concentration, SPO2, and PaO2/FiO2, we included 2 studies of 215 subjects [2, 4]. MD = −0.06 (−0.24, 0.12; P = 0.54; I2 = 0%), 2 studies of 215 subjects [2, 4] MD = 5.49 (4.25, 6.72; P < 0.001; I2 = 0%) and 5 studies of 393 subjects [1–4, 6] MD = 81 (15, 147; P = 0.02; I2 = 86%; Supplementary Table 2).
Publication bias
We found no convincing evidence of publication bias in survival studies in subjects receiving or not receiving JAK-inhibitors with no study falling outside the 95% CI of the funnel plot (Supplementary Fig. 2).
Discussion
The use of JAK-inhibitors in persons with COVID-19 decreased the use of invasive mechanical ventilation and increased survival, most convincingly for baricitinib. Estimates of RRs of surrogate endpoints of ICU admission and ARDS favored a substantial reduction but the 95% CI included 1. Changes in other surrogate co-variates were not significantly altered by JAK-inhibitor therapy except PaO2/FiO2.
JAK-inhibitors target JAK1, JAK2, JAK3, and TYK2 whose inhibition should downregulate the JAK/STAT signaling pathway decreasing cytokine concentrations and thereby reducing CRS. Given this hypothesis, it’s not surprising that CRP concentrations were decreased.
When persons with COVID-19 receive JAK-inhibitors may be important in determining safety and efficacy. Some data suggest a start time for JAK-inhibitors based on an estimation of inflammation severity. For example, La Roseé et al. based the decision to start JAK-inhibitors on a COVID-19 inflammation score [12, 13]. Other data support this suggestion [14, 15]. There were no data on a score or measure of inflammation in the studies we analyzed. As a surrogate, we performed subgroup analyses of disease severity based on PaO2/FiO2 data. Our data suggest JAK-inhibitors may be more effective in subjects with a PaO2/FiO2 < 300 mmHg compared efficacy in all subjects. Because we used a surrogate for inflammation severity this conclusion should be viewed cautiously.
Our study has limitations. First, although there are >2000 subjects in the combined analyses, some co-variate analyses had substantially fewer subjects. Second, some studies we analyzed were observational and subject to selection biases. Third, JAK-inhibitor therapy was likely confounded by other interventions. Fourth, there was substantial heterogeneity between studies but no evidence of publication bias. Fifth, one large study gave concurrent remdesivir confounding our evaluation of baricitinib. Lastly, we could not analyze data on diverse blood cytokine concentrations, presumptive target of JAK-inhibitors.
In conclusion, we found a potential role for JAK-inhibitors in reducing the risk of death in persons with COVID-19. The mechanism(s) underlying this benefit is unknown and when therapy is begun may be important. Because the available dataset was limited and our conclusion should be viewed cautiously.
Supplementary information
Acknowledgements
YL supported in part, by Sun Yat-sen University Cancer Center Start-Up Funding (No. 201603), and the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2017ZT07S096). RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme. Prof. Andreas Hochhaus (Jena Univ.) provided helpful comments.
Author contributions
YL, RPG, and CXC designed study. CXC and JJW searched databases and processed analysis. CXC, JJW, HL, LTY, YL, and RPG drafted the typescript. YL, RPG, CXC, JJW, and LTY revised the final typescript. YL and RPG are responsible for the paper.
Compliance with ethical standards
Conflict of interest
RPG is a consultant to BeiGene Ltd, Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Parmaceuticals Inc., and CStone Pharmaceuticals. Advisor: Antegene Biotech LLC, Medical Director: FFF Enterprises Inc. Partner: AZACA Inc. Board of Directors: RakFond Foundation for Cancer Research Support. Scientific Advisory Board, StemRad Ltd. All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chong-xiang Chen, Jiao-jiao Wang, Huan Li
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-021-01266-6.
References
- 1.Bronte V, Ugel S, Tinazzi E, Vella A, De Sanctis F, Canè S, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Investig. 2020;130:6409–16. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020;81:318–56. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, Garcia-Gomez C, Jimenez-Vizuete JM, Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology. 2021;60:399–407. doi: 10.1093/rheumatology/keaa587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantini F, Niccoli L, Nannini C, Matarrese D, Natale MED, Lotti P, et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. 2020;81:647–79. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137–46.e133. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giudice V, Pagliano P, Vatrella A, Masullo A, Poto S, Polverino BM, et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front Pharmacol. 2020;11:857. doi: 10.3389/fphar.2020.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alessio A, Del Poggio P, Bracchi F, Cesana G, Sertori N, Di Mauro D, et al. Low-dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia. Leukemia. 2021;35:635–8. doi: 10.1038/s41375-020-01087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falcone M, Tiseo G, Barbieri G, Galfo V, Russo A, Virdis A, et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7:ofaa563. doi: 10.1093/ofid/ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosas J, Liaño FP, Cantó ML, Barea JMC, Beser AR, Rabasa JTA, et al. Experience with the use of baricitinib and tocilizumab monotherapy or combined, in patients with interstitial pneumonia secondary to coronavirus COVID19: a real-world study. Reumatol Clin. 2020;S1699-258X:30271-0. [DOI] [PMC free article] [PubMed]
- 11.Stebbing J, Sánchez Nievas G, Falcone M, Youhanna S, Richardson P, Ottaviani S, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7:eabe4724. [DOI] [PMC free article] [PubMed]
- 12.La Rosée F, Bremer HC, Gehrke I, Kehr A, Hochhaus A, Birndt S, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34:1805–15. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidel F, Hochhaus A. Holding CoVID in check through JAK? The MPN-approved compound ruxolitinib as a potential strategy to treat SARS-CoV-2 induced systemic hyperinflammation. Leukemia. 2020;34:1723–5. doi: 10.1038/s41375-020-0898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gozzetti A, Capochiani E, Bocchia M. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19. Leukemia. 2020;34:2815–6. doi: 10.1038/s41375-020-01038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neubauer A, Wiesmann T, Vogelmeier CF, Mack E, Skevaki C, Gaik C, et al. Ruxolitinib for the treatment of SARS-CoV-2 induced acute respiratory distress syndrome (ARDS) Leukemia. 2020;34:2276–8. doi: 10.1038/s41375-020-0907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.