Abstract
Hexokinases catalyze glucose phosphorylation at the first step in glycolysis in eukaryotes. In the budding yeast Saccharomyces cerevisiae , three enzymes for glucose phosphorylation have long been known: Hxk1, Hxk2, and Glk1. In this study, we focus on Emi2, a previously uncharacterized hexokinase-like protein of S. cerevisiae . Our data show that the recombinant Emi2 protein (rEmi2), expressed in Escherichia coli , possesses glucose-phosphorylating activity in the presence of ATP and Mg 2+ . It was also found that rEmi2 phosphorylates not only glucose but also fructose, mannose and glucosamine in vitro . In addition, we examined changes in the level of endogenous Emi2 protein in S. cerevisiae in the presence or absence of glucose and a non-fermentable carbon source. We found that the expression of Emi2 protein is tightly suppressed during proliferation in high glucose, while it is strongly upregulated in response to glucose limitation and the presence of a non-fermentable carbon source. Our data suggest that the expression of the endogenous Emi2 protein in S. cerevisiae is regulated under the control of Hxk2 in response to glucose availability in the environment.
Keywords: hexokinase, glycolysis, fermentation, glucose repression, yeast
Abbreviations
G6P, glucose 6-phosphate (G6P); G6PDH, glucose 6-phosphate dehydrogenase; LDH, lactate dehydrogenase; PK, pyruvate kinase; rEmi2, recombinant Emi2 protein.
INTRODUCTION
Hexokinases (ATP: D-hexose 6-phosphotransferase, EC 2.7.1.1) catalyze the reaction of ATP-dependent phosphorylation of glucose into glucose 6-phosphate (G6P) at the first rate-limiting step in the glycolytic pathway. These enzymes transfer the gamma-phosphoryl group of an ATP molecule onto the oxygen at the C-6 of hexose to generate a hexose 6-phosphate and an ADP. The hexokinase-catalyzed reaction is important both for driving energy metabolism and for controlling the metabolic flux in eukaryotic cells. It has been suggested that glycolytic intermediates, including G6P, act as signaling molecules to regulate intracellular pathways to adjust to the presence of varying amounts of carbon sources in a very complex but highly controlled way. 1) 2) In the budding yeast Saccharomyces cerevisiae , the well-known regulatory system to control the catabolic pathways for different carbon sources in response to glucose availability is “glucose repression”. That is, in the presence of high glucose concentrations, the glycolytic pathway is highly activated, whereas alternative-metabolic pathways for respiration, gluconeogenesis and utilization of non-fermentable carbon sources are suppressed at the transcriptional level. 1) 2) It has been proposed that budding yeast utilizes fermentation through the rapid glycolysis, prior to aerobic respiration by means of glucose repression, when enough glucose is available. 1) 2)
It has long been known that S. cerevisiae possesses three enzymes for glucose phosphorylation in glycolysis: Hxk1 (hexokinase PI or A), Hxk2 (hexokinase PII or B), and Glk1 (glucokinase). 3) Since it was reported that yeast mutant cells lacking the three corresponding genes ( HXK1, HXK2 , and GLK1 ) do not grow on either of two fermentable sugars, i.e., glucose or fructose, these three enzymes were suggested to be indispensable for growth on fermentable sugars. 4) Northern analyses of the mRNAs of HXK1, HXK2 , and GLK1 have demonstrated that glucose-responsive transcriptional changes in these genes: in the presence of high glucose, the transcription of HXK2 is strongly expressed, whereas that of HXK1 and GLK1 is decreased by glucose repression. 5) In contrast, in the presence of non-fermentable sugars, the transcription of HXK2 is suppressed whereas the transcriptions of HXK1 and GLK1 are increased. 5) Accordingly, Hxk2 is considered to be a predominant hexokinase for glucose phosphorylation in proliferating cells under a high glucose condition. In addition, it has been established that Hxk2 has dual functions. In the cytoplasm, Hxk2 functions as a glucose-phosphorylating enzyme for glycolysis. A portion of Hxk2 also partially be translocated into the nucleus to play a regulatory role in the glucose repression of HXK1 and GLK1 , as well as in the abilities of GAL1 and SUC1 to utilize non-fermentable galactose and sucrose, respectively. 2) 6) The regulatory function of Hxk2 has been extensively studied, and has been clarified that the nuclear Hxk2 forms a DNA-protein complex with Mig1, a key transcriptional repressor, on the promoter of genes to suppress their transcription. 2) 6) Thus, in S. cerevisiae , the glucose-responsive regulatory function of Hxk2, the most predominant hexokinase in cells grown under a high glucose condition, has been a subject of particular focus and well-studied by yeast genetic approaches. In contrast, very few studies have been focused on the role of the other enzymes induced by non-fermentable carbon sources—i.e., Hxk1 and Glk1. In addition, there have been few analyses of the individual enzymatic activities of these hexokinases in vitro by using each of the isolated enzymes.
Analysis of the whole genome sequences of S. cerevisiae has revealed another hexokinase-like gene, YDR516C , with the standard name EMI2 . 7) However, there is no experimental evidence that the Emi2 protein possesses the enzyme activity for hexose phosphorylation. EMI2 was first identified as one of the genes required for the E arly M eiotic-specific transcription I nduction ( EMI ) by a large-scale genomic screening from yeast knockout strain collections. 8) In this study, we further characterized Emi2 as the fourth hexokinase in S. cerevisiae . We investigated the in vitro enzymatic activity of Emi2 for hexose phosphorylation by using the recombinant Emi2 protein expressed in Escherichia coli . In addition, we examined the changes in expression of the endogenous Emi2 protein in S. cerevisiae in response to glucose availability.
MATERIALS AND METHODS
Preparation of the recombinant Emi2.
Full length DNA fragment of the ORF region of the EMI2 gene was amplified by PCR using the genomic DNA of the BY4741 strain of S. cerevisiae as a template. The forward (5ʹ- ATTTATGGTACCATGTCATTTGAAAATTTACATAAAGTCAATGCTGAGGC-3ʹ) and reverse (5ʹ-ATTTATCTCGAGTTATGCCACCAGAGCACACAAAGC-3ʹ) primers were designed from nucleotides 1-38 and 1479–1503, respectively. The amplified DNA fragment was treated with Kpn I and Xho I and ligated into the corresponding sites of a pCold II vector (Takara Bio, Japan) to be fused with a His 6 -tag at N-terminus. The nucleotide sequence of the resultant plasmid pColdII-His 6 -Emi2 was confirmed by sequencing. To express the recombinant protein, E. coli BL21 (DE3) was transformed with pColdII-His 6 -Emi2. The transformed BL21 (DE3) was cultured in LB liquid medium containing 100 µg/mL ampicillin at 37 °C until the optical density at 600 nm was reached to 0.4–0.5. Then, the culture medium was cooled rapidly in ice water for 30 min. After 0.5 mM isopropyl β-D-1-thiogalactopyranoside was added to the culture medium, cells were cultured at 15 °C for 23 h to express the recombinant Emi2 protein (rEmi2). The cells were harvested and sonicated on ice in 50 mM Tris-HCl buffer (pH 8.0) containing 150 mM NaCl. The supernatant was applied to a Bio-Scale Mini Profinity IMAC cartridge (Bio-Rad Laboratories, CA, USA) and the protein was purified according to the manufacturer’s protocol. The purified protein solution was immediately desalted with 50 mM Tris-HCl (pH 8.0). The purified protein solution was concentrated with ultrafiltration and the protein concentration was measured using Protein Assay CBB solution (Nacalai Tesque, Japan). The purity of the protein was monitored by SDS-PAGE.
Enzyme assay.
The enzyme activity of rEmi2 for glucose phosphorylation was assessed by the coupling reaction of the glucose 6-phosphate dehydrogenase (G6PDH) from Leuconostoc mesenteroides (Oriental Yeast, Japan) with the conversion of NAD + into NADH. 9) 10) The assay mixture comprised 5 mM glucose, 1 mM ATP, 2 mM MgCl 2 , 2 mM NAD + , 50 mM Tris-HCl (pH 7.8), 1.6 U/mL G6PDH and an appropriate amount of rEmi2. The reaction at 30 °C was started by addition of rEmi2 and followed by monitoring the increase in absorbance at 340 nm for NADH production. The kinetic parameters were determined using seven different concentrations (0.078 mM to 5 mM) of glucose as a sugar substrate and by a nonlinear regression analysis with Origin software (OriginLab Co., Northampton, MA, USA). To assess the effect of divalent metal ions, MgCl 2 was substituted to CoCl 2 , MnCl 2 , ZnCl 2 , CaCl 2 or EDTA at the same concentration (2 mM). Before these assessments, we confirmed that the maximum enzyme activity was obtained around pH 8.0 using Tris-HCl buffer.
The substrate specificity of rEmi2 for different sugars was determined by means of utilizing the coupling reaction of pyruvate kinase (PK) and lactate dehydrogenase (LDH), as established previously. 9) 10) In this assay, the amount of ADP formed by sugar phosphorylation can be monitored spectrophotometrically: PK utilizes ADP to convert phosphoenolpyruvate into pyruvate, and the coupled LDH converts pyruvate into lactate with the conversion of NADH to NAD + , which can be monitored by the decrease in absorbance at 340 nm. The assay mixture comprised 10 mM hexose (either of glucose, fructose, 2-deoxyglucose, mannose, galactose, N -acetylglucosamine or L-fucose), 1 mM ATP, 2 mM MgCl 2 , 4 mM phosphoenolpyruvate, 0.3 mM NADH, 10 mM KCl, 0.1 M Tris-HCl (pH 8.0), 18 U/mL PK from rabbit muscle (Oriental Yeast), 20 U/mL LDH from chicken heart (Oriental Yeast) and appropriate amount of rEmi2. The reaction at 30 °C was started by the addition of rEmi2 and followed by monitoring the decrease in absorbance at 340 nm.
Immunoblot analysis for endogenous Emi2-GFP expression in Saccharomyces cerevisiae
The S. cerevisiae strains used in the present study were derived from BY4741 (Matα his3 leu2 met15 ura3 ). We obtained the BY4741 strain expressing Emi2-GFP driven by its own promoter ( By4741 Emi2-GFP::HIS3 ) from yeast GFP clone collection (Invitrogen, CA, USA). We created the strain lacking Hxk2 ( By4741 Emi2-GFP::HIS3 hxk2::kanMX ) by a standard PCR-based method. 11) These two strains were grown at 30 °C in YP medium (1 % yeast extract and 2 % peptone) containing either of carbon source (2 % glucose, 2 % galactose or 3 % glycerol), after precultured until the growth was saturated in the same medium. For glucose restriction, cells were grown in YP medium containing 2 % glucose until the early stage of growth, before shifted to YP medium containing 0.05 % glucose for 5 h. At the indicated time points, cells were collected and total protein extracts were precipitated with trichloroacetic acid-acetone method as we described previously. 12) 13) The protein amount of Emi2-GFP was monitored by immunoblotting using anti-GFP antibody (sc-9996) (Santa Cruz Biotechnology, TX, USA). The endogenous tubulin was monitored as a loading control using anti-alpha Tubulin antibody (ab-184970) (Abcam, CB, UK). Immunoreactive bands were visualized using a Chemi-Lumi One L Kit (Nacalai Tesque) and images were captured using an ImageQuant LAS500 (GE Healthcare, IL, USA). The independent experiment was repeated at least twice and the reproducibility was confirmed.
RESULTS
EMI2 is a hexokinase-like gene.
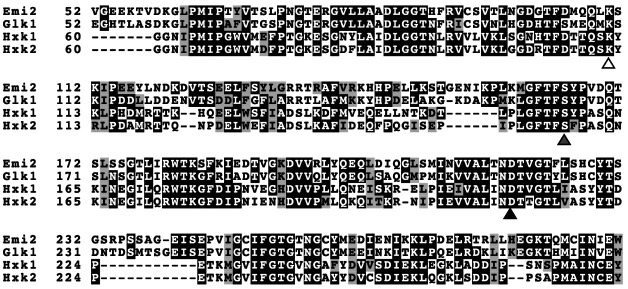
Since 1983, when it was reported that an S. cerevisiae knockout strain lacking the genes for Hxk1, Hxk2, and Glk1 did not grow in glucose, it has been known that S. cerevisiae utilizes these three hexokinases for glycolysis. 4) However, there is another hexokinase-like gene, YDR516C , with the standard name EMI2 , in the S. cerevisiae genome. The ORF of EMI2 comprises 1,500 bp and encodes a protein of 500 amino acids. For comparison, we carried out the multiple alignment of the protein sequences of Emi2, Hxk1, Hxk2, and Glk1, comprising 500, 485, 486, and 500 amino acids, respectively ( Fig. 1 ). We found that the Emi2 protein sequence is 72 % identical to Glk1 and 37 % identical to Hxk1 and Hxk2. Moreover, three key residues of hexokinases, which are predicted to be important for catalysis and for binding to glucose and ATP, are conserved in Emi2 as Asp-211, Ser-165, and Lys-110, respectively. In addition, six amino acids for ATP binding, Asp-Gly-Ser-Gly-Val-Gly, at positions 487 to 492 are conserved in the sequence of Emi2 (UniProtKB-Q04409). These findings suggest that the translational product of the EMI2 gene may possess an enzymatic activity of hexokinase.
Fig. 1. Multiple alignment of partial amino acid sequences including three key residues of Emi2 and other reported hexokinases from S. cerevisiae .
Multiple sequence alignment of Emi2 and the other characterized hexokinases, Hxk1, Hxk2, and Glk1, from S. cerevisiae . The sequences were aligned using Clustal W. 20) Strictly conserved residues are highlighted in black. The similar residues are shown in gray. Three conserved key residues, Asp-211, Ser-165, and Lys-110 of Emi2, predicted to be important for catalysis and for binding to glucose and ATP, respectively, are indicated by black, gray and white triangles below the sequences. The figure was created using BoxShade software.
The recombinant Emi2 protein possesses hexokinase activity.
To assess whether the Emi2 protein has hexokinase enzyme activity, we cloned the EMI2 gene from S. cerevisiae to express the N-terminally His 6 -tagged Emi2 protein in E. coli BL21 (DE3). As a result, the recombinant Emi2 protein (rEmi2) was successfully expressed at 15 °C in the presence of 0.5 mM isopropyl β-D-1-thiogalactopyranoside and purified from cell-free extract by the affinity column chromatography ( Fig. 2 ). The molecular size of rEmi2 was estimated at approximately 50 kDa by SDS-PAGE ( Fig. 2 ).
Fig. 2. SDS-PAGE of rEmi2 expressed in E. coli .
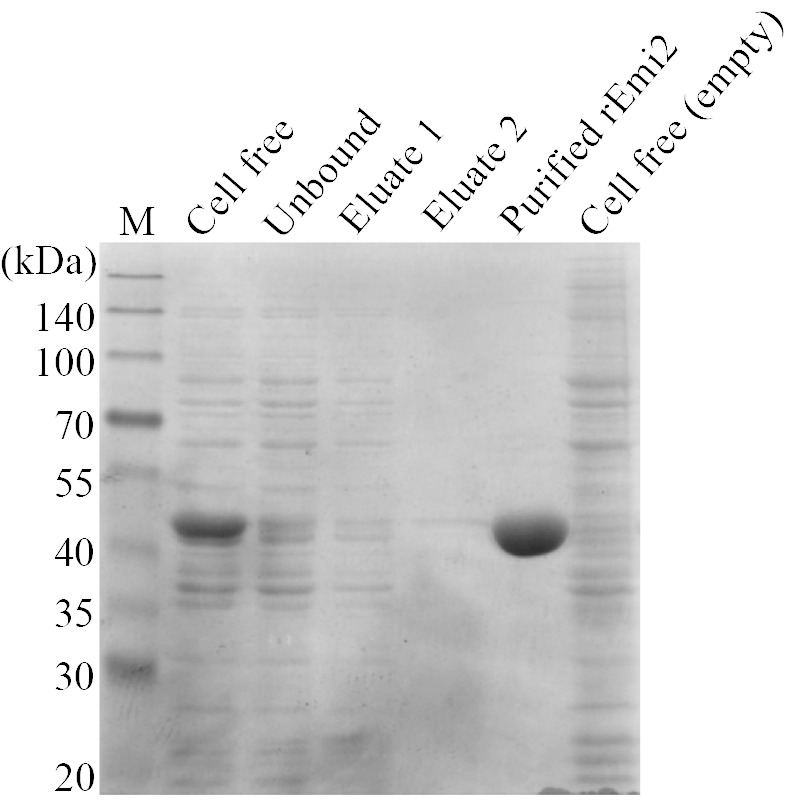
M, molecular mass markers; Cell free, the cell free extract of BL21(DE3) expressing rEmi2; Unbound, the unbound fraction eluted from the column; Eluate 1 and 2 , the fraction eluted from the column by imidazole at 5 and 10 mM, respectively; Purified rEmi2 , the eluate from the column by imidazole at 250 mM; Cell free (empty), the cell free extract of BL21(DE3) carrying the empty pCold II vector.
We assessed the enzymatic activity of the purified rEmi2 for glucose phosphorylation in the presence of ATP by using the G6PDH coupling method. The results showed that the rEmi2 protein exhibited glucose-phosphorylating activity. Under our assay condition, the specific activity of rEmi2 for glucose phosphorylation was determined to be 0.044 U/mg and the kinetic parameters were determined as follows: the k cat value was 0.042 s -1 and the K m value for glucose was 0.13 mM ( Fig. 3 ). Accordingly, these data indicate that rEmi2 possesses the enzyme activity for glucose phosphorylation, although its turnover rate is low.
Fig. 3. Kinetic analysis of glucose phosphorylation by rEmi2.
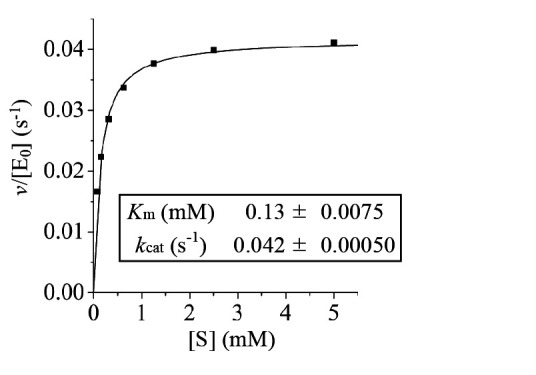
The kinetic parameters of rEmi2 for glucose phosphorylation were determined by a nonlinear regression analysis. The assay was repeated three times and the reproducibility was confirmed.
Next, we examined the effect of divalent cations on the enzyme activity of rEmi2 by using the G6PDH-coupling method. In common, hexokinases require divalent cations such as Mg 2+ , since an ATP molecule can be reacted as the phosphoryl donor by forming a complex with a divalent cation. 10) 14) To compare the effects of various divalent cations on the Emi2 activity of glucose phosphorylation, either of MgCl 2 , CoCl 2 , MnCl 2 , ZnCl 2 , CaCl 2 , or EDTA was added to the reaction mixture at 2 mM and the specific activities were compared. It was found that the rEmi2 activity was mostly diminished in the absence of divalent cations ( Fig. 4 ). In contrast, rEmi2 showed 100, 108, and 39 % relative activity in the presence of Mg 2+ , Co 2+ , and Mn 2+ , respectively. The enzyme activity was drastically reduced in the presence of Zn 2+ or Ca 2+ . These results demonstrate that rEmi2 requires divalent cations such as Mg 2+ and Co 2+ for catalysis, like other hexokinases.
Fig. 4. rEmi2 requires divalent metal ions such as Mg 2+ and Co 2+ for the glucose phosphorylation activity.
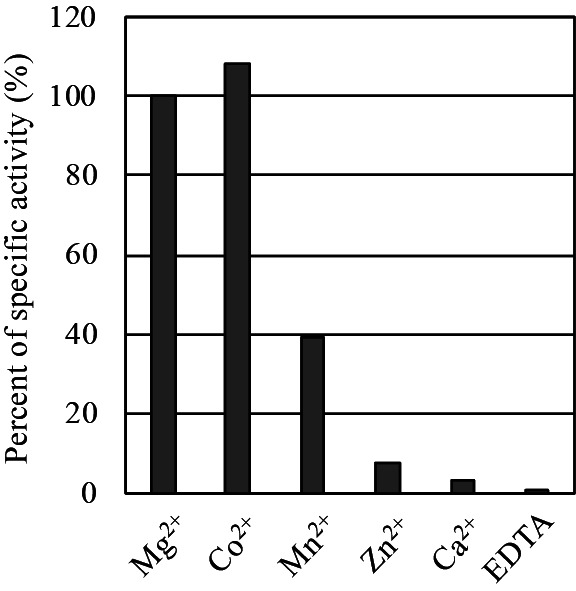
The specific activity of rEmi2 for glucose phosphorylation in the presence of either of metal ions, Mg 2+ , Co 2+ , Mn 2+ , Zn 2+ , or Ca 2+ . None , metal ions were replaced by EDTA at 2 mM. Values were normalized to that of Mg 2+ as 100 %. The assay was repeated twice and the average of relative specific activities are indicated.
The recombinant Emi2 phosphorylates not only glucose but also fructose and mannose in vitro.
We assessed the substrate specificity of rEmi2 for different sugars by monitoring the amount of ADP formed by the ATP-dependent sugar phosphorylation, with the use of the PK-LDH coupled method. The results showed that the relative activities of rEmi2 for each sugar phosphorylation at 10 mM in the presence of 2 mM Mg 2+ were 100, 120, 99, 62, and 43 % for glucose, fructose, 2-deoxyglucose, glucosamine and mannose, respectively ( Table 1 ). On the other hand, the phosphorylation activities for galactose, N -acetylglucosamine and L-fucose were marginal under this condition. From these results, we concluded that rEmi2 can phosphorylate different sugar substrates other than glucose in vitro , such as fructose, mannose and glucosamine as well as the glucose analogue 2-deoxyglucose. For comparison, the relative specific activities of Glk1 are 100, 0.4, 45, 9, and 20 % for glucose, fructose, 2-deoxyglucose, glucosamine and mannose, respectively, as reported previously by using the partially purified enzymes from yeast mutant cells ( hxk1 hxk2 ) lacking the HXK1 and HXK2 genes. 9) These results suggest that the substrate specificity of Emi2 is broader than that of Glk1, although the protein sequences of these two enzymes are very similar ( Fig. 1 ).
Table 1.
Substrate specificity of rEmi2 for different hexoses.
| Substrate | Relative specific activity (%) |
|---|---|
| Glucose | 100 |
| Fructose | 120 ± 7.2 |
| 2-Deoxyglucose | 99 ± 1.5 |
| Glucosamine | 62 ± 1.9 |
| Mannose | 43 ± 8.0 |
| N -acetylglucosamine | N.D. |
| Galactose | N.D. |
| L-Fucose | N.D. |
The specific activities for glucose, fructose, 2-deoxyglucose, glucosamine, mannose, N -acetylglucosamine, galactose and L-fucose were measured. Values were normalized to that of glucose as 100 %. The assay was repeated three times and the standard deviation (±) was calculated from each value of relative specific activities. N.D. , not determined under this condition.
The endogenous Emi2 protein in Saccharomyces cerevisiae is expressed under glucose limitation.
In S. cerevisiae , Hxk2 is known to be a predominant hexokinase when cells are exponentially growing in the presence of a high concentration of glucose. On the other hand, Hxk1 and Glk1 are suppressed at the transcriptional level in the presence of glucose under the control of Mig1-Hxk2. 5) In contrast, when cells are growing in non-fermentable carbon sources such as galactose, glycerol and ethanol, the transcription of the HXK1 and GLK1 genes is significantly increased while that of the HXK2 gene is significantly decreased. 5) Previously, Lutfiyya et al. searched for and identified several genes containing Mig1-binding sites on their promoter regions. 15) They carried out a reporter gene assay to show that the transcription of the YDR516 gene (later named EMI2 by Enyenihi and Saunders 8) ) is suppressed under a high-glucose condition, whereas it is increased in medium containing glycerol as a carbon source. However, it has not been clarified how the Emi2 expression is changed at the protein level in response to exogenous carbon sources.
We monitored the amount of change in the level of the endogenous Emi2 protein in yeast cells growing in the presence of different carbon sources. For this purpose, we utilized a yeast strain expressing Emi2 chromosomally tagged with GFP at the C-terminus (Emi2-GFP), driven by its own promoter. It was found that the expression of the endogenous Emi2-GFP protein was hardly detected in the wild-type cells that were growing exponentially in the presence of a high concentration of glucose ( Fig. 5A, lane 1 ). In contrast, the level of Emi2-GFP was greatly increased when the cells were grown in non-fermentable galactose ( Fig. 5A, lane 2 ). The amount of Emi2-GFP was also clearly higher in the presence of glycerol than in glucose, although the Emi2-GFP level was still lower in the presence of glycerol than in galactose under our experimental conditions ( Fig. 5A, lane 3 ). These data indicate that the translation of the EMI2 gene was suppressed in cells grown on glucose.
Fig. 5. The endogenous Emi2 protein of S. cerevisiae is expressed under the low glucose or non-fermentable carbon sources.
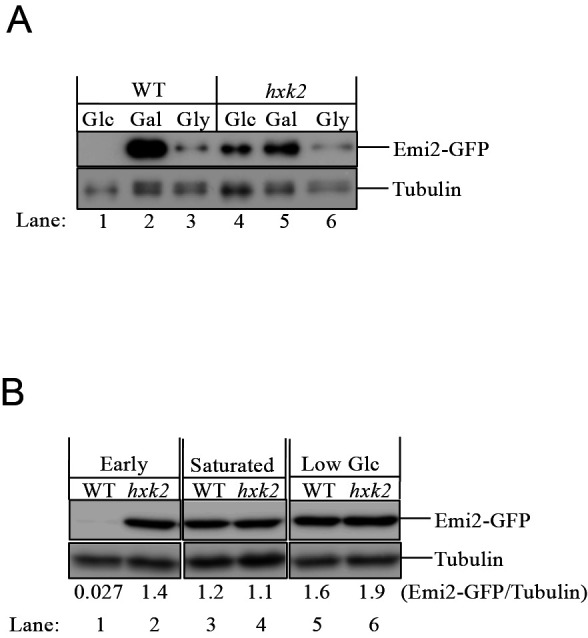
The amount of endogenous Emi2-GFP in the wild-type (WT) and the hxk2 deletion ( hxk2 ) yeast cells was monitored by immunoblot. Tubulin , a loading control. A, Cells grown in glucose (Glc), galactose (Gal) or glycerol (Gly). B, Cells were grown in glucose. Early , the cells at the early stage of growth (OD 600 , approx. 1.0); Saturated , the cells at the saturated stage of growth (OD 600 , approx. 14); Low Glc , the cells subjected to glucose restriction for 5 h. The ratio of the signal of Emi2-GFP/Tubulin was quantified and the values are indicated below the panel.
Expression of the endogenous Emi2 protein is suppressed by Hxk2.
Next, we examined whether the glucose repression of Emi2 was Hxk2-dependent. We created a HXK2 -knockout strain based on the BY4741 strain expressing the chromosomally tagged Emi2-GFP. Our results showed that the expression of Emi2-GFP was greatly increased in the glucose-grown cells by deletion of the HXK2 gene, and the level of Emi2-GFP was comparable to that in the galactose-grown cells ( Fig. 5A, lanes 1, 4 and 5 ). As to the glycerol-grown cells, the amount of Emi2-GFP protein appeared not to be affected by the deletion of HXK2 , suggesting that Hxk2 does not play a regulatory role for Emi2 expression in the presence of glycerol ( Fig. 5A, lanes 3 and 6 ).
When glucose becomes limiting, yeast cells enter a diauxic shift characterized by decreased growth rate and by a switch in metabolism from anaerobic glycolysis/fermentation to aerobic utilization of non-fermentable carbon sources. 16) When no other carbon source is available, cells enter the quiescent or stationary phase G 0 , in which the cell cycle is arrested. Next, therefore, we evaluated how the glucose limitation along with the alteration of the growth phase affects the translation of the Emi2 protein in yeast. For this purpose, we monitored the expression level of the endogenous Emi2 protein in glucose-grown cells at the different growth phases, the early stage or the saturated-stage of growth. As a result, in glucose-grown cells, the expression of the Emi2 protein increased strikingly at the saturated stage of growth, while the expression was hardly detected at the early stage of growth ( Fig. 5B, lanes 1 and 3 ). Next, to examine whether glucose restriction affects the Emi2 expression, we monitored the protein amount in cells shifted to a low-glucose condition (0.05 % glucose) for 5 h after grown under a high-glucose condition (2 %) until the early stage of growth. We found that the level of the Emi2 protein was significantly increased by glucose restriction compared to the level in cells at the early stage of growth ( Fig. 5B, lanes 1 and 5 ). In contrast, in cells lacking Hxk2, the Emi2 protein was strongly expressed irrespective of changes in the growth phase and/or the glucose limitation. These results suggested that the expression of the endogenous Emi2 protein is tightly suppressed by Hxk2 when cells are proliferating rapidly by glycolysis/fermentation in the presence of a high concentration of glucose. In contrast, Emi2 expression is greatly increased in response to glucose limitation as the metabolic shift, due to the release from the Hxk2-dependent glucose repression.
DISCUSSION
In this study, we show that the Emi2 protein possesses hexokinase activity in vitro , by the enzymatic assessment of the recombinant Emi2 protein purified from E. coli . Our data suggested that, unlike Glk1, Emi2 can phosphorylate not only glucose but also other hexoses such as fructose, mannose and glucosamine. Further, we show that the expression of the endogenous Emi2 protein is tightly suppressed by Hxk2 when cells are grown in the presence of a high concentration of glucose. On the other hand, the Emi2 expression is increased prominently upon glucose limitation.
In S. cerevisiae , it has long been known that there are three glucose-phosphorylating enzymes, Hxk1, Hxk2 and Glk1. The complete genome information of S. cerevisiae was released in the 1990s, and thereby the existence of another hexokinase-like gene, YDR516C (later given the standard name EMI2 ), was revealed. 7) Until now, however, there has been no experimental evidence that the translational product of EMI2 possesses hexokinase enzyme activity. It was reported that the yeast triple mutant strain ( hxk1 hxk2 glk1 ) does not grow on glucose, 4) suggesting that Emi2 cannot take the place of the other three enzymes for driving cell growth on glucose. In contrast, we observed that the yeast hxk2 mutant was grown in high glucose at a rate comparable to the wild-type, suggesting that Hxk1 and Glk1 can be substituted for Hxk2 ( Fig. 5 ). In our enzymatic assay, the k cat and the K m values of rEmi2 for glucose phosphorylation were 0.042 s -1 and 0.13 mM, respectively ( Fig. 3 ). It was reported that the k cat and the K m values of glucose phosphorylation by other three enzymes were 10 s -1 and 0.15 mM for Hxk1, 63 s -1 and 0.20 mM for Hxk2 and 0.072 s -1 and 0.011 mM for Glk1. 17) Therefore, the turnover rate of Emi2 for glucose phosphorylation is much lower than Hxk2 and Hxk1. In addition, the present data show that the expression of endogenous Emi2 was hardly detectable in the presence of a high concentration of glucose ( Fig. 5A , B ). From these findings, we speculate that Emi2, unlike the other three enzymes, does not function in the rapid glycolysis during fermentative growth on glucose, due to its low activity and low expression.
On the other hand, the expression of the endogenous Emi2 protein was substantially increased when glucose was limited, suggesting that Emi2 may play a role in the metabolism of alternative carbon sources. Recently, we have revealed that a cytosolic mannosidase in S. cerevisiae , that is responsible for glycan-mannoside degradation is upregulated drastically upon glucose limitation. 18) Our present data show that Emi2 possesses phosphorylation activity for mannose ( Table 1 ). One possibility would be that Emi2 is involved in mannose metabolism under the glucose-restricted conditions.
Yeast cells obtain ATP by means of anaerobic glycolysis and fermentation, when grown in the presence of a sufficient amount of glucose, regardless of whether oxygen is present. 1) Glycolysis/fermentation seems to be a relatively inefficient way of generating energy, since this pathway yields fewer ATP equivalents per mole of sugar than respiration. However, in yeast, the anaerobic glycolysis to fermentation pathway proceeds at much higher rates than respiration, since the glycolytic enzymes are expressed at a high level in the presence of glucose. 1) On the other hand, the expression of most of the enzymes that are involved in the respiratory pathway via the pyruvate metabolism and the citric acid cycle, is subjected to glucose repression under this condition. Recently, it was reported that the rate of hexokinase catalyzed-glucose phosphorylation at the first step of glycolysis is a decisive factor for controlling the metabolic shift of glucose fermentation and respiration with non-fermentable carbon. 19) In our experiments, the turnover rate of rEmi2 for glucose phosphorylation was very low. We speculate that the Emi2-catalyzed phosphorylation may delay the glycolytic flux, resulting in the metabolic shift from fermentation to respiration under glucose- limited conditions.
A large-scale genomic analysis in S. cerevisiae revealed that the EMI2 gene is one of the genes required for the sporulation, which is induced under the glucose-restricted condition. 8) In our recent study, it was suggested that the glucose starvation-responsive pathways are tuned to the pathways for cell cycle arrest, although the detailed mechanism has yet to be clarified. 13) Further studies will be needed to examine whether the hexokinase activity of Emi2 is involved in the cellular control of sporulation as well as the cell cycle arrest in response to glucose limitation in the environment.
CONFLICTS OF INTEREST
The authors declare no conflict of interests.
Acknowledgments
We thank Dr. Hideo Miyake (Mie University) for helpful discussions. This work was financially supported by JSPS KAKENHI No.19K15806 and in part by the Sugiyama Chemical & Industrial Laboratory (Japan).
References
- 1).Rolland F., Winderickx J., and Thevelein J.M.: Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res., 2, 183–201 (2002). [DOI] [PubMed] [Google Scholar]
- 2).Gancedo J.M.: The early steps of glucose signaling in yeast. FEMS Microbiol Rev., 32, 673–704 (2008). [DOI] [PubMed] [Google Scholar]
- 3).Walsh R.B., Kawasaki G., and Fraenkel D.G.: Cloning of genes that complement yeast hexokinase and glucokinase mutants. J. Bacteriol., 154, 1002–1004 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Bisson L.F. and Fraenkel D.G.: Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. USA., 80, 1730–1734 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Rodríguez A., De La Cera T., Herrero P., and Moreno F.: The hexokinase 2 protein regulates the expression of the GLK1 , HXK1 and HXK2 genes of Saccharomyces cerevisiae. Biochem J., 355 (Pt 3): 625–631 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Moreno F. and Herrero P.: The hexokinase 2-dependent glucose signal transduction pathway of Saccharomyces cerevisiae. FEMS Microbiol. Rev., 26, 83–90 (2002). [DOI] [PubMed] [Google Scholar]
- 7).Goffeau A., Barrell B.G., Bussey H., Davis R.W., Dujon B., Feldmann H., Galibert F., Hoheisel J.D., Jacq C., Johnston M., Louis E.J., Mewes H.W., Murakami Y., Philippsen P., Tettelin H., Oliver S.G.: Life with 6000 genes. Science, 274, 546, 563–567. [DOI] [PubMed] [Google Scholar]
- 8).Enyenihi A.H. and Saunders W.S.: Large-scale functional genomic analysis of sporulation and meiosis in Saccharomyces cerevisiae. Genetics, 163, 47–54 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Maitra P.K.: A glucokinase from Saccharomyces cerevisiae. J. Biol. Chem., 245, 2423–2431 (1970). [PubMed] [Google Scholar]
- 10).Nishimasu H., Fushinobu S., Shoun H., and Wakagi T.: Identification and characterization of an ATP-dependent hexokinase with broad substrate specificity from the hyperthermophilic archaeon Sulfolobus tokodaii. J. Bacteriol., 188, 2014–2019 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Gueldener U., J. Heinisch, Koehler G.J., Voss D., and Hegemann J.H.: A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic. Acids. Res., 30, e23 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Umekawa M., Ujihara M., Nakai D., Takematsu H., and Wakayama M.: Ecm33 is a novel factor involved in efficient glucose uptake for nutrition-responsive TORC1 signaling in yeast. FEBS Lett., 591, 3721–3729 (2017). [DOI] [PubMed] [Google Scholar]
- 13).Umekawa M., Shiraishi D., Fuwa M., Sawaguchi K., Mashima Y., Katayama T., and Karita S.: Mitotic cyclin Clb4 is required for the intracellular adaptation to glucose starvation in Saccharomyces cerevisiae. FEBS Lett., 594, 1329–1338 (2020). [DOI] [PubMed] [Google Scholar]
- 14).Purich D.L. and Fromm H.J.: Activation of brain hexokinase by magnesium ions and by magnesium ion-adenosine triphosphate complex. Biochem. J., 130, 63–69 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Lutfiyya L.L., Iyer V.R., DeRisi J., DeVit M.J., Brown P.O., and Johnston M.: Characterization of three related glucose repressors and genes they regulate in Saccharomyces cerevisiae. Genetics., 150, 1377–1391 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Galdieri L., Mehrotra S., Yu S., and Vancura A.: Transcriptional regulation in yeast during diauxic shift and stationary phase. OMICS: J. Integ. Biol., 14, 629–638 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Smallbone K., Messiha H.L., Carroll K.M., Winder C.L., Malys N., Dunn W.B., and Murabito E.. et al.: A model of yeast glycolysis based on a consistent kinetic characterization of all its enzymes. FEBS Lett., 587, 2832–2841 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Umekawa M., Ujihara M., Makishima K., Yamamoto S., Takematsu H., and Wakayama M.: The signaling pathways underlying starvation-induced upregulation of α-mannosidase Ams1 in Saccharomyces cerevisiae. Biochim. Biophys. Acta, 1860, 1192–1201 (2016). [DOI] [PubMed] [Google Scholar]
- 19).Lane S., Xu H., Oh E.J., Kim H., Lesmana A., Jeong D., Zhang G., Tsai C.S., Jin Y.S., and Kim S.R.: Glucose repression can be alleviated by reducing glucose phosphorylation rate in Saccharomyces cerevisiae. Sci Rep., 8, 2613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Thompson J.D., Higgins D.G., and Gibson T.J.: CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic. Acids. Res., 22, 4673–4680 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]