Abstract
Opioid use disorder (OUD) causes the death of nearly 130 Americans daily. It is evident then that new avenues for treatment are needed. To this end, studies have reported that ‘satiety’ agents such as the glucagon-like peptide-1 receptor (GLP-1R) agonist, exendin-4 (Ex-4), decreases responding for addictive drugs such as cocaine, nicotine, alcohol, and oxycodone, but no work has been done with heroin. In this study, we used a reward devaluation model in which rats avoid ingesting a saccharin solution that predicts drug availability to test the effects of 2.4 μg/kg Ex-4 on responding for a natural reward cue (i.e., saccharin) and on cue- and drug-induced heroin seeking. The results showed that treatment with Ex-4 during the 16-day abstinence period and on the test day decreased cue-induced heroin seeking. Drug-induced reinstatement of heroin seeking also was reduced by Ex-4, but only when using a 1h, but not a 6h, pretreatment time. Treatment with Ex-4 did not alter intake of the saccharin cue when the drug was on board, but a history of treatment with Ex-4 did increase acceptance of the saccharin cue in later extinction trials. Finally, treatment with Ex-4 did not alter body weight, but was associated with an increase in Orexin 1 receptor (OX1) mRNA expression in the nucleus accumbens shell. Taken together, these findings are the first to show that treatment with a GLP-1R agonist can reduce both cue-induced heroin seeking and drug-induced reinstatement of heroin seeking. As such, a GLP-1R agonist may serve as an effective treatment for OUD in humans.
Keywords: Addiction, Individual Differences, Opioid use disorder, Relapse, Satiety, Treatment
INTRODUCTION
Drug addiction is a chronic disease that is difficult to treat due to its relapsing nature (Leshner & Koob, 1999). Deaths related to drug overdose tripled from 1999 to 2014 and, in 2017, around 70% of the drug overdose deaths involved opioid use (Rudd, Seth, David, & Scholl, 2016). Indeed, misuse of prescription pain relievers, heroin, and synthetic opioids causes the death of more than 130 people a day in the United States alone, a tragedy of epidemic proportion and a major concern for the Centers for Disease Control and Prevention (CDC) (Centers for Disease control and Prevention, 2017). As a consequence, it is imperative that we find effective treatments for drug addiction that can mitigate the craving and withdrawal that precipitates relapse and, thereby, increases vulnerability to opioid overdose (Binswanger et al., 2007).
Addiction is recognized as the pathological usurpation of neural systems associated with reward-related learning (Edwards & Koob, 2013; Hyman, Malenka, & Nestler, 2006). In this context, the drug becomes the prominent goal, and in turn, natural rewards such as sex, food, work and money are devalued. This shift in motivation can be observed in individuals with a substance use disorder who fail to provide sufficient care to their children (Nair et al., 1997), show loss of productivity in the workplace (Jones, Casswell, & Zhang, 1995), and decreased sensitivity to monetary rewards (Goldstein et al., 2007). Reward devaluation also has been observed in animal models, as female rats exposed to cocaine show greater preference for drug-associated stimuli than for their own pups (Seip & Morrell, 2007) and hungry and thirsty rats will avoid intake of a palatable solution when it predicts the availability of a drug of abuse (Grigson & Twining, 2002; Twining, Bolan, & Grigson, 2009; Twining et al., 2016).
In this latter case, we have hypothesized that rats avoid intake of a drug-paired saccharin cue because the taste cue elicits the onset of an aversive state of conditioned withdrawal and conditioned withdrawal invokes the ‘need’ for drug (Twining et al., 2009). In accordance, like conditioned withdrawal (McDonald, Parker, & Siegel, 1997; Nozaki, 1976; Nunez, Földes, Laorden, Milanes, & Kovács, 2007; Shaham & Stewart, 1995), avoidance of a drug-paired taste cue (anhedonia) also is associated with blunted levels of dopamine in the nucleus accumbens (Grigson & Hajnal, 2007)(Nyland & Grigson, 2013). Further, greater avoidance of (or aversion to) the drug-paired cue also predicts greater drug-seeking, greater drug-taking, a strong willingness to work for the drug, and greater susceptibility to cue- and drug-induced relapse (Colechio, Imperio, & Grigson, 2014; Grigson & Twining, 2002; Imperio & Grigson, 2015; Twining et al., 2009; Wheeler et al., 2008; Wise, Yokel, & DeWit, 1976). Responding for drug, then, may be driven by not only liking and wanting (Berridge, Robinson, & Aldridge, 2009), but also by needing.
In recent years, scientists have shown that hormones that modulate hunger and satiety also can modulate responding for drugs of abuse (Engel & Jerlhag, 2014; Kenny, 2011). One of the most studied has been Glucagon-like peptide-1 (GLP-1), an incretin hormone (Novak, Wilks, Buell, & McEwen, 1987) essential for regulation of food intake in both animals and humans. GLP-1 also is a neurohormone released by neurons located in the nucleus tractus solitarius (NTS) that project to different brain regions, including key structures in the reward pathway such as the ventral tegmental area (VTA) and the nucleus accumbens (NAc) and hypothalamic nuclei involved in homeostasis and motivated behavior (Merchenthaler, Lane, & Shughrue, 1999). Exendin-4 (Ex-4) is a natural GLP-1 receptor (GLP-1R) agonist (Eng, Kleinman, Singh, Singh, & Raufman, 1992) and, due to its incretin and appetite suppressant action, has been used to treat type 2 diabetes mellitus and obesity (Lovshin & Drucker, 2009; Shukla, Buniak, & Aronne, 2015). Moreover, treatment with Ex-4 also has been reported to reduce conditioned place preference for a drug of abuse, accumbens dopamine release induced by nicotine, cocaine and amphetamine, cocaine self-administration and seeking in rats (Egecioglu, Engel, & Jerlhag, 2013a, 2013b; Hernandez et al., 2018; Sorensen et al., 2015), and relapse to ethanol drinking in mice (Thomsen et al., 2017). Thus far, one study found Ex-4 not effective in reducing abuse-related effects of remifentanil in mice (Bornebusch, Fink-Jensen, Wortwein, Seeley, & Thomsen, 2019); while a second study reported that Ex-4 was effective in reducing oxycodone seeking and self-administration in rats (Zhang et al., 2020).
The present study sought to further our understanding of the effects of Ex-4 on opioid addiction, using the taste-drug model described above. Specifically, we tested the effect of treatment with Ex-4 on acceptance of the drug-paired saccharin cue and on both cue-induced heroin seeking and drug-induced reinstatement of heroin seeking in rats. Here, Ex-4 was administered daily throughout a 16-day abstinence and prior to test in an effort to model such a treatment regimen in humans. We also analyzed mRNA expression in the NAc shell (NAcS), a crucial structure in reward modulation, to assess long-lasting changes in receptors associated with homeostatic regulation and reward. We hypothesized that daily treatment with Ex-4 during abstinence and at test will facilitate recovery of responding for the natural reward cue and will reduce cue- and drug-induced heroin-seeking following a period of abstinence in rats.
MATERIALS AND METHODS
The subjects were 55 outbred male Sprague-Dawley rats delivered from Charles River (Wilmington, MA) at approximately 90 days of age, weighing between 300 – 400g at the start of the experiment. Because prior access to sweet can be more protective against drug self-administration in female rats (Cason & Grigson, 2013), we performed the present study in male rats. All subjects were housed individually in standard, suspended, stainless steel cages. The environment in the animal colony room had controlled humidity and temperature (21 °C), with a 12/12 hour light/dark cycle, and lights on at 7:00 am. All experimental manipulations were conducted starting 2 h into the light phase of the cycle. Following one week of acclimation to their home cages, rats were habituated to experimenter handling by daily weighing. Food and water were available ad libitum, except where noted otherwise. All studies were approved by the Pennsylvania State University College of Medicine, Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health specifications outlined in their Guide for the Care and Use of Laboratory Animals.
Self-Administration Catheter
Jugular Catheter Implantation Surgery
Rats were anesthetized with intramuscular (im) ketamine (70 mg/kg) and xylazine (14 mg/kg) and then implanted with custom-made intravenous jugular catheters as described previously (Grigson & Twining, 2002). Following surgery, rats received two days of post-op care consisting of a daily subcutaneous (sc) injection of the NSAID, carpofen, and a full week to recover. Carprofen treatment was continued when indicated. Maintenance of catheter patency included daily flushing of catheters using heparinized saline (0.2 ml of 30 IU/ml heparin). Catheter patency was verified at the end of each week of drug self-administration and before each test day using 0.3 ml of propofol (Diprivan 1%).
Phase 1: Acquisition of Heroin Self-administration Behavior
Habituation
The animals experienced three days of habituation to the self-administration chamber to learn the behavioral task (spout licking). One day before the first habituation session, ad-lib water was removed. The animals then had a 5 min habituation session on each of 3 days, starting 2 h into the light phase. During this 5 min period, water was available in one of the three spouts, varying the location each day (left, middle, right). The center spout was the future “inactive” spout on which responding led to no consequence, and the rightmost spout was the future “active” spout where completion of a given number of contacts lead to an intravenous (iv) infusion of drug or saline. In order to maintain proper hydration, each day rats had overnight access to 20 ml filtered water at the front of the home cage beginning at 5pm. After the third day of habituation ad-lib water was restored.
Taste-Drug Pairings
Two hours into the light cycle, rats were placed in the self-administration chambers (MED Associates, Inc., St. Albans, VT) where they had 5 min access to 0.15% saccharin via the leftmost spout. Thereafter, the saccharin spout retracted, the house light was turned off and the middle and right empty spouts advanced. Licking on the inactive spout (middle spout) had no consequence. The availability of the empty active, rightmost spout was signaled by a cue light located above, and completion of a fixed ratio of 10 (FR=10) contacts with this spout led to a 6-second iv infusion of either saline (n=15) or 0.06 mg/infusion heroin (n=40) for a period of 6 h. Each infusion was followed by a 20-second time-out period in which the cue light turned off, the house light turned on, the empty spouts retracted and the sound of a tone signaled the time out period. Rats were trained using this protocol 5 days a week for 15 trials (Figure 1). Due to lack of catheter patency, seven animals in the saccharin-heroin group were excluded from the experiment after Phase 1.
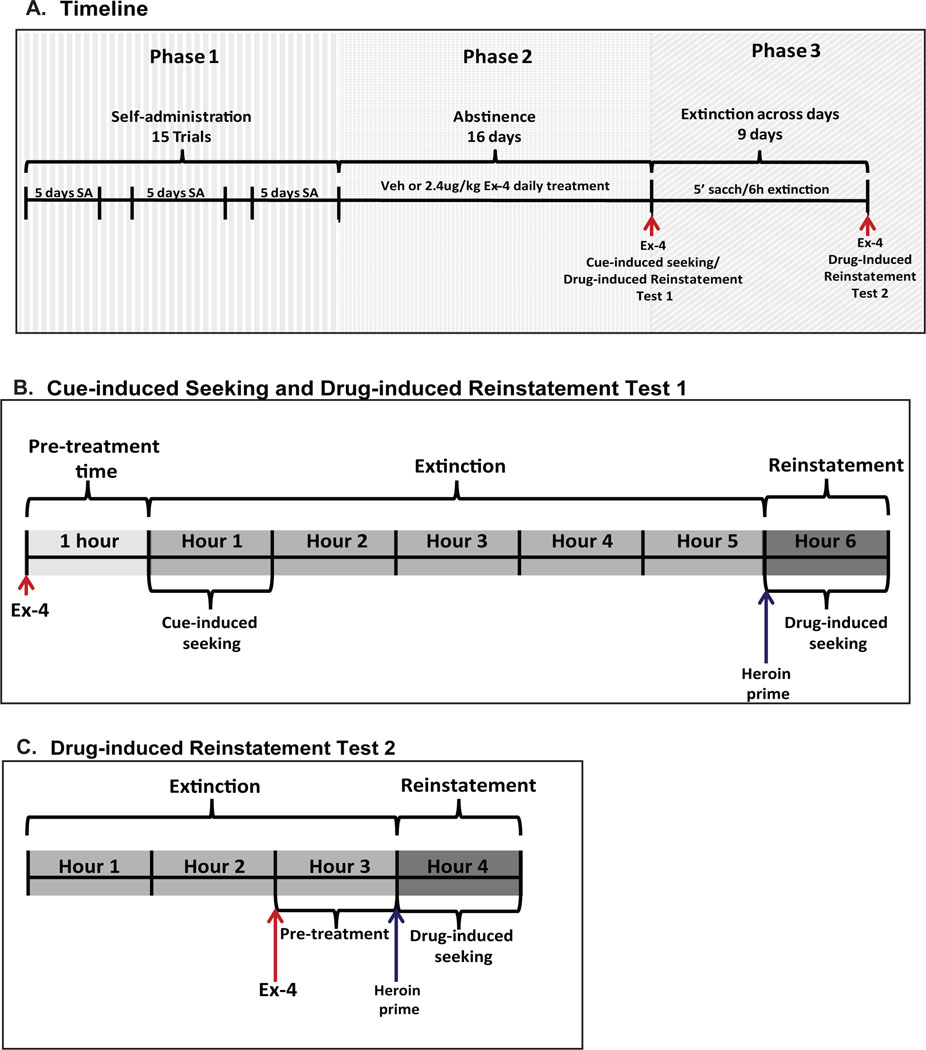
Figure 1.
Timeline of the study and test design. A. The study consisted of 3 phases. During Phase 1, rats had 5 min access to a saccharin cue followed by the opportunity to self-administer either heroin or saline 5 days a week for 15 trials. Phase 2 consisted of a 16-day period of home cage abstinence followed by Cue-induced seeking/Drug-induced Reinstatement Test 1. During this period rats were treated with a daily ip injection of either Veh (saline) or 2.4 μg/kg Exendin-4 (Ex-4). On phase 3, rats were subjected to a daily pairing of saccharin with a 6-h extinction period for 9 days followed by Drug-induced Reinstatement Test 2. B. During Cue-induced Seeking and Drug-induced Reinstatement Test 1 rats received an injection of either Veh or Ex-4. One hour later, they were given 5-min access to saccharin followed by a 5-hour period of extinction. After the 5th hour, an infusion of either heroin or saline was automatically delivered and reinstatement of heroin seeking behavior (Drug-induced seeking) was recorded for one hour. C. During Drug-induced Reinstatement Test 2, the rats were given four hours of extinction. Then, they were injected with Veh or Ex-4 at the end of h 2, the iv heroin prime injection was infused at the end of h 3, and reinstatement of heroin seeking behavior was examined for 1 h thereafter.
Behavioral Stratification
It has been established that, in this model, rats in the saccharin-drug group show individual differences when responding for saccharin. Some rats greatly avoid the drug-paired saccharin cue and greater avoidance is associated with greater drug-seeking and taking (Grigson & Twining, 2002). Consequently, terminal saccharin intake (i.e., average saccharin licks emitted on trials 14 and 15) was used to separate the heroin self-administration group into large and small suppressors (Grigson & Twining, 2002; Imperio & Grigson, 2015). Specifically, using 200 licks/5min as the cutoff, Large suppressors (LS) emitted <200 licks/5min of the saccharin cue (n=19), while small suppressors (SS) emitted >200 licks/5min (n=21) of the saccharin cue. Saccharin-saline (Sac-sal) controls were not stratified.
Phase 2: Abstinence, Treatment and Drug-Seeking Tests
Abstinence
A 16-day home cage abstinence period followed immediately after the last taste-drug pairing. During this abstinence period, rats remained in their home cage with ad-libitum access to water and food and were injected intraperitoneally (ip) once daily with either vehicle (n=23) or 2.4μg/kg of Ex-4 (n=25) one hour into the light cycle.
Cue-induced Seeking and Drug-induced Reinstatement Test 1
Twenty-four h later, rats received their daily injection of vehicle (Veh) or Ex-4 one hour prior to placement in the self-administration chamber. They were then given 5 min access to saccharin followed by a 5-hour extinction test during which all cues associated with the drug (cue light, tone, etc.) were presented as usual, but contacts on the active spout did not deliver an infusion of saline or drug. Immediately after the fifth hour, a computer-controlled non-contingent iv infusion of saline or heroin (0.06 mg/infusion) was delivered and subsequent contacts with the spouts were recorded to assess drug-induced reinstatement of heroin seeking behavior. Due to lack of catheter patency, five additional rats in the saccharin-heroin group were excluded from the experiment after Phase 2. In addition, one rat in the Sac-sal control group developed an infection and was euthanized.
Phase 3: Extinction across Days and Drug-induced Reinstatement Test 2
As described, in Phase 2, our standard protocol led to a 6-hour delay between Ex-4 treatment and the drug prime. Since Ex-4 has a half-life of approximately 150 minutes (Parkes et al., 2001), by the time the drug prime was delivered the levels of Ex-4 would have been low. In order to more effectively assess the impact of Ex-4 on drug-induced reinstatement, the same rats were subjected to a protocol of extinction as described below to reduce cue-induced seeking, followed by a 2nd drug-induced reinstatement test with a 1 h Ex-4 pretreatment.
Extinction across Days
Two hours into the light cycle rats were placed in the chamber and given 5 minutes access to saccharin, followed by a 6-hour extinction test in which all the cues were present as usual, but contacts with the active spout were without consequence. During this 9-day period, no Ex-4 or Veh was administered.
Drug-induced Reinstatement Test 2
On day 10, and two hours into the light phase, rats had 5 minutes access to saccharin. Because cue-induced seeking was not fully extinguished across the 9-day period, rats were exposed to 3 hours of within session extinction on Day 10. At the beginning of hour 2, rats were injected ip with either Veh or Ex-4. One hour thereafter, rats received a non-contingent iv infusion of saline or heroin (0.06 mg/infusion) and reinstatement of heroin seeking was examined across the next hour.
Sacrifice, Dissection and Molecular Analysis
One hour after the last test, all rats were sacrificed by live decapitation, the skull was removed and the brain dissected. The harvested right NAcS was flash frozen and stored at −80 °C until mRNA was extracted and analyzed by real time quantitative polymerase chain reaction (RT-qPCR). Briefly, NAc shell was homogenized and RNA was isolated using the Allprep DNA/RNA kit (Qiagen). Complimentary DNA (cDNA) was generated from the isolated RNA using Random hexamer primers (Invitrogen) and Superscript III Reverse Transcriptase (Invitrogen). Messenger RNA expression was analyzed using real time quantitative polymerase chain reaction (RT-qPCR) Quantstudio 102k flex. The 2−ΔΔCT method was employed to assess relative gene quantification with beta actin as the endogenous control. Quantitative PCR analysis of targets of interest was performed using standard laboratory methods, with 384-well optical plates, TaqMan Assay-On-Demand (Applied Biosystems, Foster City, CA, USA) gene-specific primers/probe assays and a 7900HT Sequence Detection System (Applied Biosystems). Gene expression assays included Orexin Receptor 1 (Hcrtr1, Rn0056995_m1), dopamine D2 receptor (d2r, Rn00561126_m1), Leptin receptor (Lepr, Rn00565158_m1) and GLP-1 receptor (glp-1r, Rn00562406_m).
Data Analysis
Subjects that lost catheter patency were removed from the subsequent phase of the experiment. All data were analyzed using Prism version 8.00, GraphPad Software (La Jolla California USA). Mixed factorial Analysis of Variance (ANOVAs), followed by Tukey’s post hoc tests, were used to compare different groups. In addition, Student-t tests were used when comparing only two means, with alpha set at 0.05. Log latency was analyzed using ANOVA, while raw latency data was analyzed using the Kaplan-Meier survival curves and the Log-rank (Mantel-Cox) test (see supplemental information).
RESULTS
Phase 1: Acquisition of Heroin Self-administration Behavior
Saccharin Intake
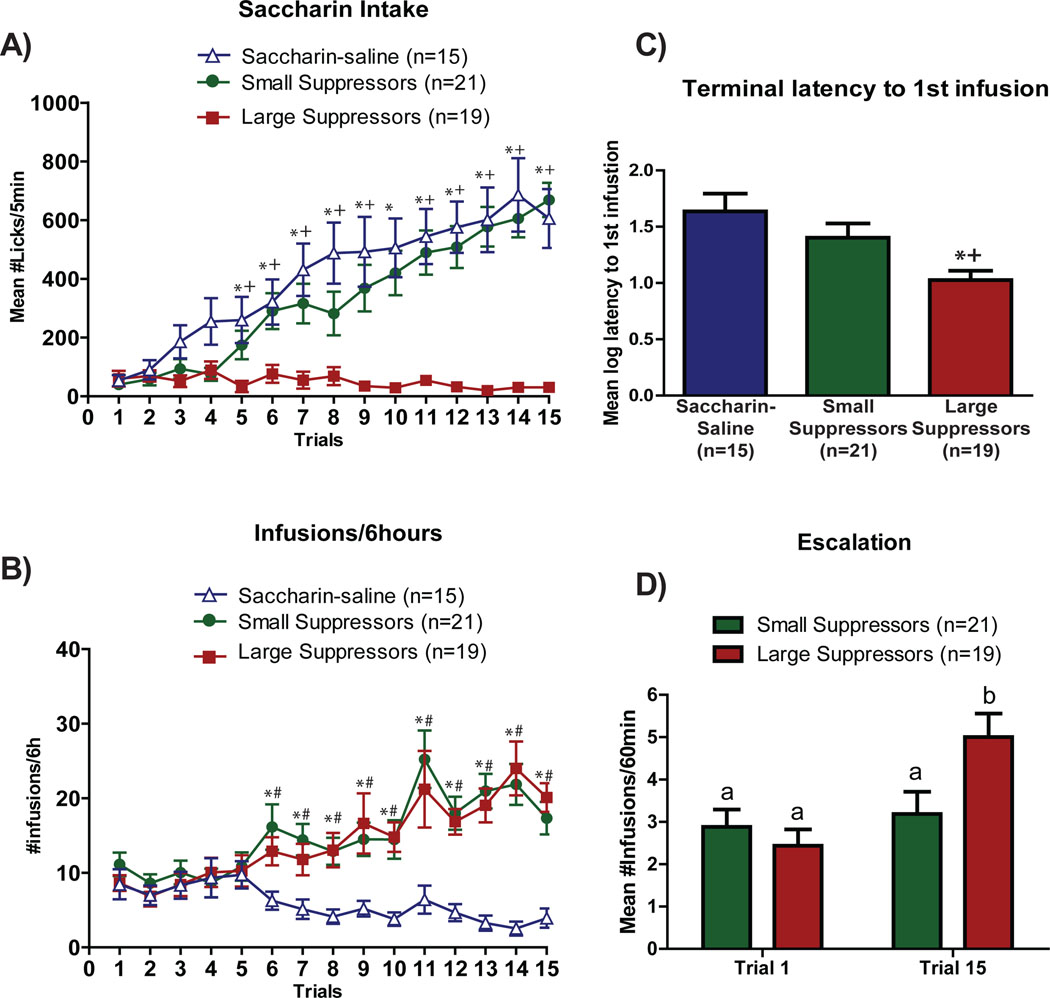
As shown in Figure 2A, the number of saccharin licks/5min increased across trials in both Sac-sal and SS, but not in LS. Support for this conclusion was provided by post-hoc (Tukey’s) assessment of a significant interaction of a 3 × 15 mixed factorial ANOVA varying group (Sac-sal, SS and LS) and trials (1–15) (F28,728=10.52, p<0.0001) showing a significant reduction in intake of the saccharin cue on trials 5 – 15 for the LS vs. both the SS and Sac-sal (ps<0.05).
Figure 2.
Acquisition of drug-taking behavior. A) Saccharin intake. Mean ± SEM number of licks/5min of 0.15% saccharin across trials 1 – 15 for rats in the saccharin-saline, small suppressor or large suppressor group. B) Self-administration. Mean ± SEM number of saline or heroin infusions/6h across trials 1 – 15 for rats in the saccharin-saline, small suppressor, or large suppressor groups. C) Latency to 1st infusion. Mean± SEM terminal log latency to 1st heroin infusion for saccharin-saline, small suppressor, or large suppressor groups. D) Escalation. Mean ± SEM infusions during the 1st hour of self-administration in trials 1 and 15 (saccharin-saline controls: n=15, small suppressors: n=21, large suppressors: n=19). +Significant difference between large suppressors and small suppressors; *Significant difference between large suppressors and saccharin-saline controls. # Significant difference between small suppressors and saccharin-saline controls. Different letters indicate significant differences.
Heroin Self-administration
There were no differences in the mean number of heroin infusions/6 hours across the 15 acquisition trials between SS and LS, and both groups self-administered more infusions than Sac-sal (Figure 2B). This conclusion was supported by a 3 × 15 mixed factorial ANOVA showing a significant group × trial interaction (F28,728=4.53, p<0.0001). Post-hoc tests revealed a greater number of infusions by both SS and LS compared to Sac-sal beginning on trial 6 (ps<0.05). In addition, when averaged across trials 14 and 15, LS evidenced a shorter terminal latency to obtain the first infusion compared with SS and Sac-sal (see Figure 2C and Supplemental Figure 2). This was confirmed by post-hoc analysis of a significant one-way ANOVA (F2,51=5.78, p<.005, p<0.05). Finally, post hoc tests of a significant interaction of a two-way ANOVA (F1,30=8.13, p<0.01, ps<0.01) showed, on the last trial, that LS obtained more infusions during the 1st hour than SS (Figure 2D). Moreover, 1st hour intake was found to increase significantly from trial 1 to trial 15 (i.e., to escalate across trials) for the LS (ps<0.05), but not for the SS (ps>0.05) (Figure 2D).
Phase 2: Abstinence, Treatment, and Drug-seeking Test 1
Saccharin Intake after Abstinence
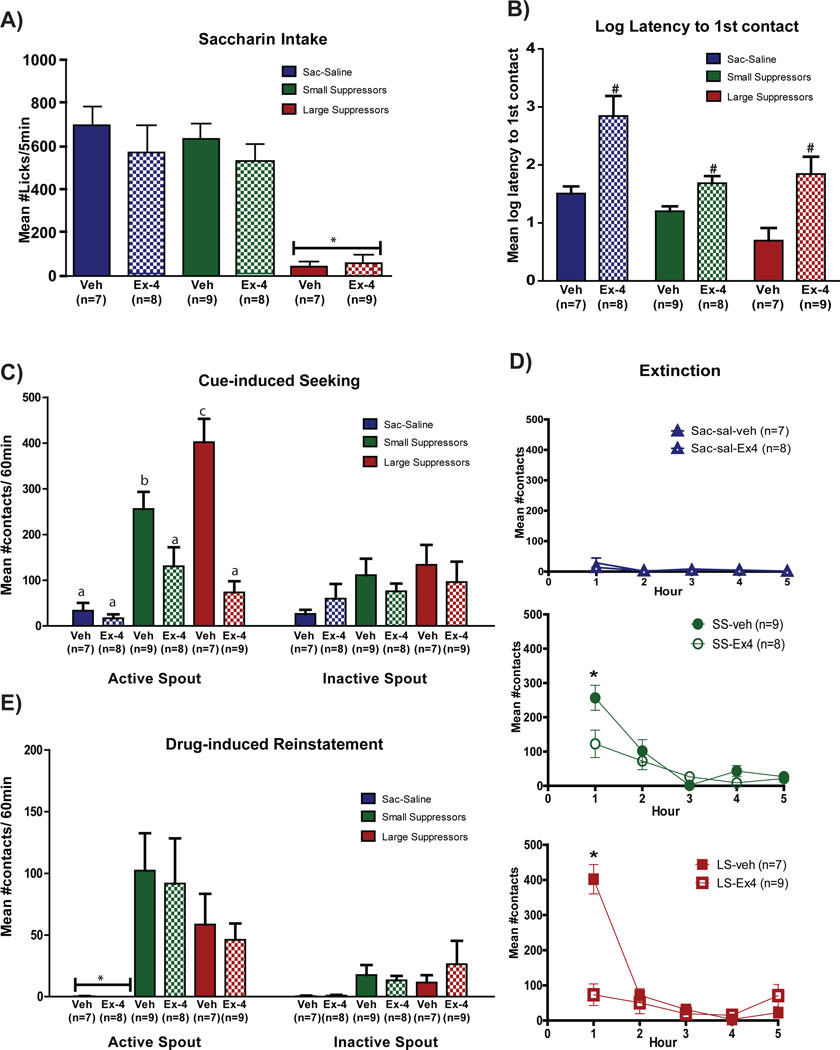
Ex-4 treatment during abstinence did not affect body weight (see Supplemental Figure 1) or saccharin intake during Test 1 (Figure 3A). Thus, the number of saccharin licks/5min during Test 1 was analyzed using a 3 × 2 ANOVA varying group (Sac-sal, SS and LS) and treatment (Veh vs. Ex-4). The results revealed a significant main effect of group (F2,22=26.98, p<0.0001), with the LS continuing to emit fewer saccharin licks/5min than SS or Sac-sal overall (p<0.0001). Neither the main effect of treatment (F1,18=1.3, p=0.27) nor the group × treatment interaction (F<1.0) was significant.
Figure 3.
Effect of Ex-4 treatment on cue- and drug-induced reinstatement. A) Saccharin intake on test day. Mean ± SEM number of licks/5min of 0.15% saccharin for rats in the saccharin-saline controls, small suppressors and large suppressors groups treated with Veh or Ex-4 on cue- and drug-induced reinstatement test 1. B) Latency to 1st contact. Mean ± SEM log latency to 1st contact with the active spout for saccharin-saline, small and large suppressor groups treated with Veh or Ex-4. C) Cue-induced seeking. Mean ± SEM number active (left) and inactive (right) spout contacts/60min for saccharin-saline, small and large suppressors groups treated with Veh or Ex-4. D) Extinction. Mean ± SEM number of contacts with active and inactive spouts/6h of extinction for saccharin-saline (top panel), small suppressors (middle panel) and large suppressors (bottom panel) treated with Veh or Ex-4. E) Drug-induced reinstatement test 1. Mean ± SEM number of active (left) and inactive (right) spouts contacts during the 60 minutes after the drug prime for saccharin-saline, small and large suppressors groups treated with Veh or Ex-4 six hours prior to the drug prime (Sac-sal-veh: n=7, Sac-sal-Ex-4: n=8, SS-veh: n=9, SS-Ex-4: n=8, LS-veh: n=7, LS-Ex-4: n=9). *Significant difference between groups. #Significant difference between Veh and Ex-4 treatment. Different letters indicate significant differences.
Cue-induced Seeking
Figure 3B shows that treatment with Ex-4 increased the latency to the first contact with the active spout overall (see also Supplemental Figure 3). In support, the 3 × 2 ANOVA varying group and treatment found a significant main effect of treatment (F1,42=29.3, p<0.0001) and group (F2,42=10.42, p<0.001), but not group × treatment interaction (F2,42=1.935, p=0.16). Post-hoc analysis of the significant main effect of group (ps<0.005) confirmed that groups SS and LS exhibited a shorter latency to make first contact with the active spout than group Sac-sal (ps<0.05).
When examined during the 1st hour of the 5-hour extinction session, treatment with Ex-4 significantly reduced the number of contacts with the active spout in both SS and LS (Figure 3C). Support for this conclusion was provided by a mixed factorial ANOVA showing a significant group × treatment × spout interaction (F2,40=5.9, p<0.01) and confirmed by post-hoc analyses (ps<0.05). No effect on inactive spout responding was observed. Finally, when examined across the 5-hour extinction trial, the data show that active spout contacts extinguished after the first hour for all groups (Figure 3D). Thus, post hoc analysis of a significant group × treatment × hour interaction (F8,160=8.072, p<0.0001) confirmed a reduction in contacts with the active spout following the 1st hour for both the Veh-treated SS (Figure 3D, middle panel) and the Veh-treated LS (Figure 3D, bottom panel; ps<0.05). Responding by the Ex-4 treated rats, on the other hand, was low in the 1st hour and remained so thereafter (ps<0.05).
Drug-induced Reinstatement Test 1
Treatment with Ex-4 six hours earlier had no effect on drug-induced heroin seeking (Figure 3E). While both Sac-sal groups did not interact with the active spout, Veh- and Ex-4-treated SS and LS showed a higher number of contacts than Sac-sal in the hour following the drug prime. In support, a 3 × 2 × 2 ANOVA varying group (Sac-sal, SS, and LS), treatment (Veh vs. Ex-4), and spout (Active vs. Inactive) revealed a significant group × spout interaction (F2,40=7.56, p<0.001) and a post-hoc analysis showed significantly greater active spout contacts by both SS and LS vs. Sac-sal (p<0.05). Neither the treatment × spout (F<1.0) nor the group × treatment × spout (F<1.0) interaction was significant.
Phase 3: Extinction across Days and Drug-induced Reinstatement Test 2
Saccharin Intake across Extinction Days
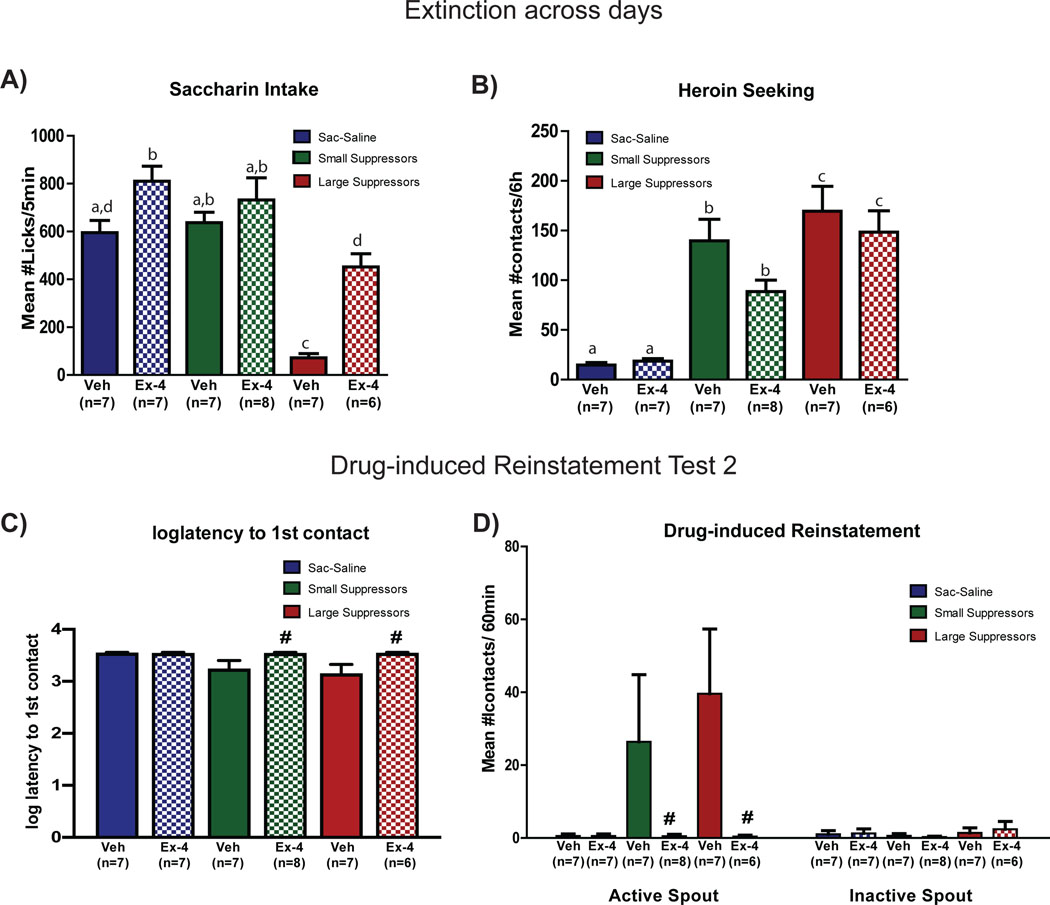
A history of treatment with Ex-4 during abstinence significantly increased the average number of saccharin licks/5min emitted across the 9 days of extinction training by the LS (Figure 4A). In support, a 2 × 3 ANOVA revealed a significant group × treatment interaction (F2,44=5.33, p<0.0001) and post-hoc tests confirmed a significant increase in saccharin intake by the LS rats with a history of Ex-4 treatment (ps<0.05), and a smaller, but still significant, increase in saccharin intake by the Sac-sal rats with a history of Ex-4 treatment (ps<0.05). No effect of prior Ex-4 treatment was observed on saccharin intake in the SS group (ps>0.05).
Figure 4.
Extinction across days and drug-induced reinstatement Test 2. A) Saccharin intake during extinction. Mean ± SEM number of licks/5min of 0.15% saccharin averaged across the 9 days of extinction training for rats in the saccharin-saline, small suppressors or large suppressors groups with history of treatment with Veh or Ex-4. B) Heroin seeking during extinction. Mean ± SEM number of infusion attempts/6h averaged across 9 days of extinction testing for saccharin-saline, small or large suppressors with a history of treatment with Veh or Ex-4. C) Latency to 1st contact. Mean ± SEM log latency to 1st contact with the active spout during drug-induced reinstatement Test 2 for saccharin-saline, small and large suppressor groups with a history of treatment with Veh or Ex-4. D) Drug-induced reinstatement test 2. Mean ± SEM number of active (left) and inactive (right) spout contacts/60min for saccharin-saline, small and large suppressors treated with Veh or Ex-4 one hour prior to the drug prime (Sac-sal-veh: n=7, Sac-sal-Ex-4: n=7, SS-veh: n=7, SS-Ex-4: n=8, LS-veh: n=7, LS-Ex-4: n=6). #Significant difference between Veh and Ex-4 treatment. Different letters indicate significant differences.
Drug Seeking across Extinction Days
Small suppressors and LS exhibited greater seeking (i.e., average number of contacts with active spout) during extinction training than Sac-sal and, while a history of Ex-4 treatment tended to reduce seeking in SS, this trend did not attain statistical significance (Figure 4B). This conclusion is confirmed by a 3 × 2 ANOVA showing a significant main effect of group (F2,44=37.15, p<0.0001) and no significant main effect of treatment (F1.44=2.39, p=0.13) or group × treatment interaction (F2,44=1.05, p=0.36).
Drug-induced Reinstatement Test 2
Figure 4C shows that acute pre-treatment with Ex-4 one hour prior the drug prime significantly increased the latency to make the 1st contact with the active spout in both the SS and the LS (see also Supplemental Figure 4). This conclusion was supported by a 3 × 2 ANOVA that revealed a significant main effect of treatment (F1,24=10.36, p<0.005, ps<0.05), mainly carried by SS and LS. Group Sac-sal emitted no contacts with the active spout. Figure 4D shows that acute-pretreatment with Ex-4 completely abolished heroin seeking during Drug-induced Reinstatement Test 2. Veh-treated SS and LS showed an increased number of contacts with the active spout, while Ex-4-treated SS and LS groups did not contact the active spout at all. This conclusion was supported by a mixed factorial 3 × 2 × 2 ANOVA varying group, treatment, and spout showing a significant main effect of treatment (F1,27=5.21, p<0.05) and a significant treatment × spout interaction (F1,27=5.19, p<0.05). Neither the group × spout (F2,27=1.73, p=0.19), nor the group × treatment × spout interaction (F2,27=1.47, p=0.25) was significant.
Molecular Analysis
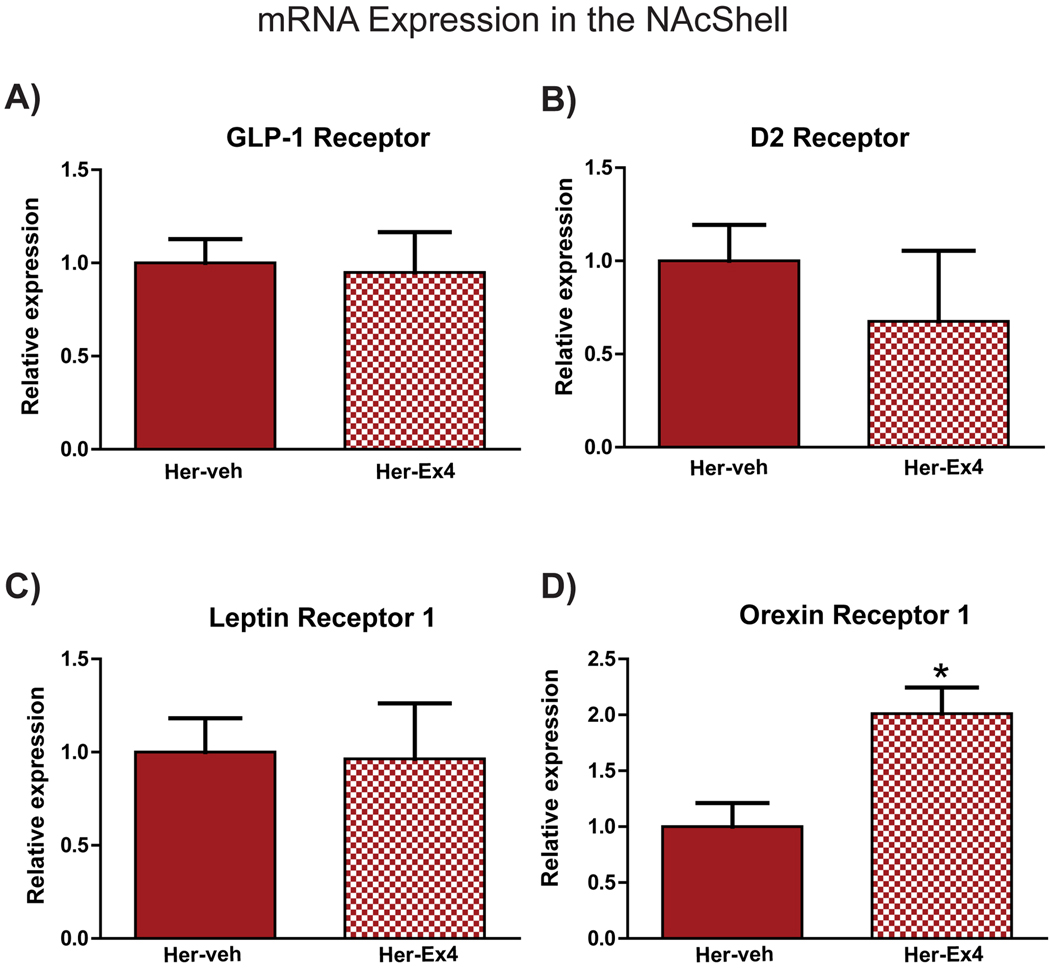
To further explore the effects of Ex-4, mRNA expression of select genes known to be associated with reward and feeding behaviors was examined in the NAcS. Figure 5 shows the relative expression of 4 such genes in rats that self-administered heroin and were treated with Veh or Ex-4. Compared to Veh-treated controls, no significant difference in fold change expression was observed in GLP-1R (Figure 5A), D2R (Figure 5B), or leptin receptors (Figure 5C). There was, however, a significant increase in the expression of Orexin receptor 1 (OX1) in heroin self-administering rats with a history of Ex-4 treatment compared to vehicle-treated controls (t=3.16; p=0.006, Figure 5D).
Figure 5.
Relative mRNA expression of GLP-1 receptor (A), dopamine D2 receptor (B) leptin receptor 1 (C) and orexin receptor 1 (D) in rats with a history of heroin self-administration and treatment with Veh or Ex-4 throughout the abstinence period and test (Heroin-veh: n=10, herion-Ex-4: n=9). *Significant difference between groups.
DISCUSSION
This study examined the effects of Ex-4 on heroin seeking in rats. Specifically, we showed that systemic treatment with Ex-4 significantly attenuated heroin seeking when the rats were re-exposed to the heroin-associated cues, without affecting body weight. Importantly, treatment with Ex-4 also abolished drug-induced reinstatement of heroin seeking behavior when administered 1 hour, but not 6 hours, prior to the drug challenge. As such, we can conclude that the protective effects of this dose of Ex-4 on cue- and drug-induced reinstatement are related to the acute, rather than the chronic, effects of this relatively short-acting GLP-1R agonist. Although treatment with Ex-4 did not attenuate suppression of intake of the heroin-paired saccharin cue, a history of treatment with Ex-4 was associated with increased acceptance of the heroin-paired saccharin cue, particularly in the most vulnerable LS population. Finally, reduced reinstatement of heroin seeking by Ex-4 treated rats was accompanied by an increase in the expression of the OX1 mRNA in the NAcS.
Drug-induced Suppression of Saccharin Intake
In Phase 1, we observed two subpopulations within the heroin group. Using terminal intake and a median split (Grigson & Twining, 2002), we identified a group of rats, referred to as LS, that greatly avoided intake of the saccharin cue compared to the other group, referred to as the SS, that did not. Such avoidance of the drug-paired taste cue has been interpreted as a conditioned taste aversion (CTA), akin to a lithium chloride CTA (Nachman & Ashe, 1973). This phenomenon was later re-interpreted as a reward comparison effect, reflecting drug-induced devaluation of the lesser valued saccharin solution (Grigson, 1997). More recent data, however, suggest that devaluation of the saccharin cue (i.e., reduced intake) may be indicative of anhedonia, a symptom associated with the onset of a conditioned aversive state of withdrawal (Nyland & Grigson, 2013; Wheeler et al., 2008).
Effects of Ex-4 on Drug-paired Saccharin Intake
As stated in the Introduction, part of our hypothesis was that treatment with Ex-4 would increase responding for the natural reward cue. This hypothesis was partially correct. Treatment with Ex-4 did not alter intake of the saccharin cue in SS or LS during the 1st cue-induced seeking test – i.e., when Ex-4 was on board, rats ingested (SS), or avoided (LS), the drug-paired saccharin cue as they did during acquisition. That said, a history of daily treatment with Ex-4 during the abstinence period was associated with an increase in acceptance of the saccharin solution by the LS in later extinction training. The overall increase in responding for saccharin in the LS is a promising finding, as natural rewards can be highly protective against addiction (Lenoir, Serre, Cantin, & Ahmed, 2007; Venniro, Russell, Zhang, & Shaham, 2019). Future studies will need to determine whether extended treatment with Ex-4 increases intake of the saccharin cue due to an increase in the perceived palatability of the saccharin cue, due to a dissociation of the saccharin cue from the conditioned aversive state of withdrawal, or by a direct reduction in withdrawal itself, as GLP-1 analogs have been shown to reduce ethanol withdrawal-induced anxiety (Sharma, Pise, Sharma, & Shukla, 2015) and aversive taste reactivity behavior to a gustatory cue paired with naloxone-induced withdrawal (Olsen et al., in preparation).
Avoidance of the Drug-paired Taste Cue and Drug-taking
It has been shown that rats that most greatly avoid the taste cue take less time to obtain the first infusion, take more drug, work hard to get the drug, and exhibit the greatest cue-induced drug seeking and drug-induced reinstatement of drug seeking behavior (Grigson & Twining, 2002; Imperio & Grigson, 2015; Twining et al., 2009). That said, here, during acquisition, both the SS and LS actually self-administered the same amount of heroin across trials. Greater saccharin suppression in the LS group, however, was associated with a shorter latency to obtain the first infusion of heroin, with greater 1st hour drug taking, and with escalation of drug-taking across trials – a cardinal feature of addiction (Edwards & Koob, 2013). This pattern of behavior is consistent with a greater conditioned-withdrawal state elicited by the saccharin cue in the LS subpopulation.
The Effect of Ex-4 on Cue-induced seeking and Drug-induced Reinstatement
In Phase 2 of this study, vehicle-treated rats exhibited clear cue-induced heroin seeking. Treatment with 2.4 μg/kg of Ex-4, on the other hand, increased the latency to seek heroin and reduced cue-induced heroin seeking during the 1st h of the extinction test in both the SS and LS groups. That said, Ex-4 treatment also led to an increase in the latency to ‘seek’ saline in the Sac-Sal controls. This finding raises the possibility that Ex-4 may have an inhibitory effect on motivated responding per se, rather than a specific effect on heroin seeking. There are, however, a number of arguments against this interpretation. First, while Ex-4 can lead to locomotor depression (Sorensen et al., 2015), the use of spout-licking as the operant task presents an advantage over lever-pressing, as it is a more natural behavior for the rats that does not require high locomotor effort. Second, if locomotor depression were to have mediated these effects, the rats should have begun seeking the drug once Ex-4 was cleared from the system (i.e., during hours 3, 4 and 5 of extinction testing). This, however, was not the case. Third, sedative effects, or a general lack of motivation, are unlikely to account for the data because this dose of Ex-4 failed to diminish saccharin licking on the test day in either the SS or the Sac-Sal controls. Indeed, these Ex-4-treated rats made approximately 600 licks of saccharin/5 min (the equivalent of 60 infusions on a FR 10 schedule of reinforcement) and, importantly, the latency to initiate the first contact with the saccharin spout was quite short for both Ex-4 treated Sac-sal controls and SS (0.087 sec and 0.64 sec respectively). Finally, this finding may be an anomaly, as Ex-4 failed to increase the latency to ‘seek’ saline compared to the vehicle treated Sac-Sal controls during Drug-induced Reinstatement Test 2. A general disruption in motivation, per se, and/or increased locomotor depression, then, are unlikely to account for the decrease in heroin seeking by the Ex-4 treated groups.
In contrast to the Ex-4-induced reduction in cue-induced heroin seeking, Ex-4 did not reduce heroin-seeking during drug-induced reinstatement of heroin seeking in Test 1. As mentioned, in this case, Ex-4 was administered 1 hour before rats were placed in the chamber. As such, there was a 6-hour (i.e., more than twice the half-life of the drug) delay between Ex-4 administration and the heroin prime. Thus, while we intended a chronic treatment regimen, the protective properties of this dose of the shorter acting GLP-1R agonist clearly did not carry across even the entire 6-hour test session. For this reason, in Phase 3, the effects of Ex-4 were tested directly on drug-induced reinstatement by reducing the pre-treatment time to one hour. Using this regimen, Ex-4 completely abolished heroin-induced reinstatement of heroin-seeking behavior (i.e., ‘relapse’) in both SS and LS rats. It should be noted, however, that the reinstatement behavior observed in the vehicle treated rats was not as robust as expected (this group, showed an average of 20 to 40 active spout responses, approximately 2 – 4 infusion attempts over the 60 min test period). Even so, confidence is gained by observing nearly zero contacts with the inactive spout, showing clear goal-directed behavior. Thus, when using an appropriate pretreatment time, the short-acting GLP-1R agonist, Ex-4, effectively reduced both cue- and drug-induced heroin seeking in rats.
The effects of Ex-4 on the rewarding properties of opioids have only been studied twice, yielding opposite results. Bornebusch et al. studied several opioid-associated behaviors in mice and showed that Ex-4 had no effect on morphine-induced conditioned place preference, naloxone-precipitated morphine withdrawal, or locomotor activity (Bornebusch, Fink-Jensen, Wortwein, Seeley, & Thomsen, 2019). The effect of Ex-4 on opioid-seeking was not tested, but Bornebusch et al. did show a tendency for Ex-4 to increase remifentanil intake. Here, a firm conclusion was hampered by large variability and a small sample size. In contrast, Zhang et al. used rats as a model and showed that Ex-4 can robustly and dose-dependently reduce oxycodone self-administration (Zhang et al., 2020). In the present study we did not examine the effects of Ex-4 on heroin self-administration, but our model is more akin to that used by Zhang et al. Similar to that study, we found that Ex-4 also can reduce cue- and drug-induced heroin seeking. Further research needs to be done to elucidate the discrepancies across these reports, but differences in species (i.e., rats vs. mice) and drug (oxycodone and heroin vs. remifentanil) should be explored.
Molecular Data
In the molecular analysis, treatment with Ex-4 increased mRNA expression for OX1 in the NAcS, but did not change the expression for GLP-1R, D2R or LepR1. The integrative nature of NAc, receiving inputs from different parts of the brain, including regions that control reward and feeding-motivated behaviors (Noori, Spanagel, & Hansson, 2012; Park et al., 2019), makes it a candidate area to look for long-term changes produced by Ex-4 treatment. Indeed, GLP-1 producing neurons in the NTS project to the NAc (Alhadeff, Rupprecht, & Hayes, 2012). Moreover, systemic administration of Ex-4 reaches the NAc, and infusion of a GLP-1R agonist directly into the NAcS dose-dependently reduces cocaine seeking (Hernandez, O’Donovan, Ortinski, & Schmidt, 2019). It was therefore expected that prolonged, daily treatment with Ex-4 may affect the expression of GLP-1R in the NAcS. This, however, was not observed by qPCR analysis.
Another important source of input to the NAc is via dopaminergic projections from the VTA. GLP-1Rs are found in this area (Merchenthaler et al., 1999) and intra-VTA injections of Ex-4 have been shown to reduce drug-primed reinstatement of cocaine-seeking behavior (Hernandez et al., 2018). In addition, systemic injections of Ex-4 were found to attenuate amphetamine-, cocaine-, and nicotine-induced accumbal dopamine release (Egecioglu et al., 2013b), showing that activation of GLP-1R can modulate drug-associated dopaminergic release in the NAc. Moreover, the protective effects of systemic Ex-4 on cocaine-seeking are blocked by infusion of a GLP-1R antagonist directly into the VTA (Egecioglu et al., 2013a; Hernandez et al., 2018). That said, we did not find changes in the expression of D2 receptors in the NAcS associated with Ex-4 treatment.
Finally, we focused on the expression of receptors associated with the homeostasis-related hormones leptin and orexin. Leptin is an adipose-derived peptide hormone that can attenuate drug-related behaviors (Shen, Jiang, Liu, Wang, & Ma, 2016). However, in this experiment, Ex-4 did not have an effect on LepR1 mRNA expression in the NAcS. On the other hand, we did see a marked increase in the expression of OX1 in the NAcS. This receptor is exclusively activated by Orexin A, which is produced mainly by neurons within the lateral hypothalamus, and has an important role in arousal and feeding motivated behavior (de Lecea et al., 1998; James, Mahler, Moorman, & Aston-Jones, 2017; Sakurai et al., 1998). Orexins increase food intake, but its modulatory role also has been extended to non-feeding reward related processes such as addiction (de Lecea et al., 1998; Harris, Wimmer, & Aston-Jones, 2005; James et al., 2017; Sweet, Levine, Billington, & Kotz, 1999). Indeed, it has been observed that opioids can increase the number of orexin-producing neurons (Thannickal et al., 2018). In this context, our results may suggest that heroin self-administration increases the number of orexin neurons and its transmission. Treatment with Ex-4, then, may have reversed this effect, reducing levels of orexin in the NAcS, and leading to a compensatory increase in the expression of the OX1. Although further testing is required, this hypothesis is consistent with previous research showing that GLP-1Rs in the lateral hypothalamus are critical for the control of ingestive behaviors, body weight, and food reinforcement in rats (Lopez-Ferreras et al., 2018). In addition, blocking the effects of OX1 with systemic administration of the OX1 antagonist, SB-334867, dose-dependently blocked reinstatement of seeking for cocaine (Smith, Tahsili-Fahadan, & Aston-Jones, 2010), alcohol (Jupp, Krstew, Dezsi, & Lawrence, 2011; Lawrence, Cowen, Yang, Chen, & Oldfield, 2006), nicotine (Plaza-Zabala, Martín-García, de Lecea, Maldonado, & Berrendero, 2010) and remifentanil (Porter-Stransky, Bentzley, & Aston-Jones, 2017). As such, the molecular results raise the specter that drugs of abuse hijack not only reward substrates, but also substrates engaged by a potent state of physiological ‘need’ – in this case the ‘need’ for drug. Future studies, then, must be conducted to test the merits of this hypothesis and to parse the roles of the GLP-1R and the OX1 in the Ex-4-induced eventual acceptance of the natural reward cue and in the reduction of both cue- and drug-induced reinstatement of heroin-seeking behavior.
CONCLUSION
Our results demonstrate that treatment with the GLP-1R agonist, Ex-4, attenuates cue-induced heroin seeking and drug-induced reinstatement of heroin seeking in rats. This observation is consistent with other published data showing that treatment with GLP-1R agonists can reduce cue and/or drug-induced seeking for other drugs of abuse. Currently, there are only two opioid medications (methadone and buprenorphine), and one non-opioid medication (naloxone), approved for the treatment of OUD and both classes present problems for the patients. While medicated assisted treatment (MAT) using opioids is effective, and buprenorphine has been found effective in the extended access model (Coffey, 2018; Sorge, Rajabi, & Stewart, 2005), MAT has the associated stigma of replacing one opioid with another and treatment with the opioid blocker naloxone often shows poor compliance from patients. For this reason, while MATs are very effective in both animal models and in humans, the findings in this study are important as they reveal a novel non-opioid for the potential treatment of OUD. Fortunately, the extensive research done with GLP-1R agonists in the context of type 2 diabetes and obesity (Lovshin & Drucker, 2009; Shukla et al., 2015) provides several formulations with differing half-lives (e.g., Exenatide, Liraglutide, Semaglutide), allowing researchers to further their understanding of the role of GLP-1 in addiction, and to develop new treatment regimens that may prevent relapse. Indeed, such studies are underway to test whether treatment with a GLP-1R agonist can reduce craving, withdrawal, and relapse in humans in treatment for an OUD (Bunce, 2020).
Supplementary Material
ACKNOWLEDGMENTS
Authors thank the National Institute on Drug Abuse for generously providing heroin. Support for this research was provided by grants from NIH DA009815 and DA050325 (to PSG) and the Pennsylvania Department of Health, Tobacco Settlement Funds SAP# 410007972 (to PSG) and by the Elliot S. Vesell Professorship in Pharmacology (to KEV). Exendin-4 is not approved for the treatment of substance use disorder, and its use in this study is investigational. We thank Sarah Ballard for her technical assistance. We also want to thank Dr. Andras Hajnal for his assistance on dissecting the brains.The authors report no financial interest or conflicts of interests.
Source of Funding:
Support for this research was provided by grants from NIH DA009815 and DA050325 (to PSG) and the Pennsylvania Department of Health, Tobacco Settlement Funds SAP# 410007972 (to PSG) and by the Elliot S. Vesell Professorship in Pharmacology (to KEV).
Footnotes
Conflicts of Interest
The authors report no financial interest or conflicts of interests.
REFERENCES
- Alhadeff AL, Rupprecht LE, & Hayes MR (2012). GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology, 153(2), 647–658. doi: 10.1210/en.2011-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, & Aldridge JW (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current opinion in pharmacology, 9(1), 65–73. doi: 10.1016/j.coph.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, & Koepsell TD (2007). Release from prison--a high risk of death for former inmates. The New England journal of medicine, 356(2), 157–165. doi: 10.1056/NEJMsa064115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornebusch AB, Fink-Jensen A, Wortwein G, Seeley RJ, & Thomsen M. (2019). Glucagon-Like Peptide-1 Receptor Agonist Treatment Does Not Reduce Abuse-Related Effects of Opioid Drugs. eNeuro, 6(2). doi: 10.1523/ENEURO.0443-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce S. (2020). Use of a GLP-1 R Agonist to Treat Opioid Use Disorder. In: Milton S Hershey Medical Center. [Google Scholar]
- Cason AM, & Grigson PS (2013). Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physiol Behav, 112-113, 96–103. doi: 10.1016/j.physbeh.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease control and Prevention. (2017, 8/13/17). Retrieved from https://www.cdc.gov/drugoverdose/epidemic/index.html
- Coffey A. (2018). Sleep and Circadian Disruption as as Aspect of Opioid Addiction in Rats and Humans. (Doctor of Philosophy). Pennsylvania State University, Retrieved from https://etda.libraries.psu.edu/catalog/14978aac5186 [Google Scholar]
- Colechio EM, Imperio CG, & Grigson PS (2014). Once is too much: conditioned aversion develops immediately and predicts future cocaine self-administration behavior in rats. Behavioral neuroscience, 128(2), 207–216. doi: 10.1037/a0036264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, . . . Sutcliffe JG (1998). The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A, 95(1), 322–327. doi: 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, & Koob GF (2013). Escalation of drug self-administration as a hallmark of persistent addiction liability. Behavioural pharmacology, 24(5–6), 356–362. doi: 10.1097/FBP.0b013e3283644d15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, & Jerlhag E. (2013a). The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PloS one, 8(10), e77284. doi: 10.1371/journal.pone.0077284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, & Jerlhag E. (2013b). The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PloS one, 8(7), e69010. doi: 10.1371/journal.pone.0069010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J, Kleinman WA, Singh L, Singh G, & Raufman JP (1992). Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. The Journal of biological chemistry, 267(11), 7402–7405. [PubMed] [Google Scholar]
- Engel JA, & Jerlhag E. (2014). Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS drugs, 28(10), 875–886. doi: 10.1007/s40263-014-0178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, & Volkow ND (2007). Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug and alcohol dependence, 87(2–3), 233–240. doi: 10.1016/j.drugalcdep.2006.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS (1997). Conditioned taste aversions and drugs of abuse: A reinterpretation. Behavioral neuroscience, 111(1), 129–136. doi:Doi 10.1037/0735-7044.111.1.129 [DOI] [PubMed] [Google Scholar]
- Grigson PS, & Hajnal A. (2007). Once is too much: Conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behavioral neuroscience, 121(6), 1234–1242. doi: 10.1037/0735-7044.121.6.1234 [DOI] [PubMed] [Google Scholar]
- Grigson PS, & Twining RC (2002). Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behavioral neuroscience, 116(2), 321–333. [PubMed] [Google Scholar]
- Harris GC, Wimmer M, & Aston-Jones G. (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature, 437(7058), 556–559. doi: 10.1038/nature04071 [DOI] [PubMed] [Google Scholar]
- Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, & Schmidt HD (2018). Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 43(10), 2000–2008. doi: 10.1038/s41386-018-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, O’Donovan B, Ortinski PI, & Schmidt HD (2019). Activation of glucagon-like peptide-1 receptors in the nucleus accumbens attenuates cocaine seeking in rats. Addiction Biology, 24(2), 170–181. doi: 10.1111/adb.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, & Nestler EJ (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annual review of neuroscience, 29, 565–598. doi: 10.1146/annurev.neuro.29.051605.113009 [DOI] [PubMed] [Google Scholar]
- Imperio CG, & Grigson PS (2015). Greater avoidance of a heroin-paired taste cue is associated with greater escalation of heroin self-administration in rats. Behavioral neuroscience, 129(4), 380–388. doi: 10.1037/bne0000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, & Aston-Jones G. (2017). A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr Top Behav Neurosci, 33, 247–281. doi: 10.1007/7854_2016_57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Casswell S, & Zhang JF (1995). The economic costs of alcohol-related absenteeism and reduced productivity among the working population of New Zealand. Addiction, 90(11), 1455–1461. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, & Lawrence AJ (2011). Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin₁ receptors. Br J Pharmacol, 162(4), 880–889. doi: 10.1111/j.1476-5381.2010.01088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ (2011). Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci, 12(11), 638–651. doi: 10.1038/nrn3105 [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, & Oldfield B. (2006). The orexin system regulates alcohol-seeking in rats. Br J Pharmacol, 148(6), 752–759. doi: 10.1038/sj.bjp.0706789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, & Ahmed SH (2007). Intense sweetness surpasses cocaine reward. PloS one, 2(8), e698. doi: 10.1371/journal.pone.0000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI, & Koob GF (1999). Drugs of abuse and the brain. Proceedings of the Association of American Physicians, 111(2), 99–108. [DOI] [PubMed] [Google Scholar]
- Lopez-Ferreras L, Richard JE, Noble EE, Eerola K, Anderberg RH, Olandersson K, . . . Skibicka KP (2018). Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Molecular psychiatry, 23(5), 1157–1168. doi: 10.1038/mp.2017.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovshin JA, & Drucker DJ (2009). Incretin-based therapies for type 2 diabetes mellitus. Nature reviews. Endocrinology, 5(5), 262–269. doi: 10.1038/nrendo.2009.48 [DOI] [PubMed] [Google Scholar]
- McDonald RV, Parker LA, & Siegel S. (1997). Conditioned sucrose aversions produced by naloxone-precipitated withdrawal from acutely administered morphine. Pharmacol Biochem Behav, 58(4), 1003–1008. doi: 10.1016/s0091-3057(97)00313-4 [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, & Shughrue P. (1999). Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of comparative neurology, 403(2), 261–280. [DOI] [PubMed] [Google Scholar]
- Nachman M, & Ashe JH (1973). Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiology & behavior, 10(1), 73–78. doi: 10.1016/0031-9384(73)90089-9 [DOI] [PubMed] [Google Scholar]
- Nair P, Black MM, Schuler M, Keane V, Snow L, Rigney BA, & Magder L. (1997). Risk factors for disruption in primary caregiving among infants of substance abusing women. Child Abuse & Neglect, 21(11), 1039–1051. doi:Doi 10.1016/S0145-2134(97)00064-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori HR, Spanagel R, & Hansson AC (2012). Neurocircuitry for modeling drug effects. Addict Biol, 17(5), 827–864. doi: 10.1111/j.1369-1600.2012.00485.x [DOI] [PubMed] [Google Scholar]
- Novak U, Wilks A, Buell G, & McEwen S. (1987). Identical mRNA for preproglucagon in pancreas and gut. European journal of biochemistry, 164(3), 553–558. [DOI] [PubMed] [Google Scholar]
- Nozaki M. (1976). Assessment of morphine-type physical dependence liability: a screening method using the rat. Psychopharmacology (Berl), 47(3), 225–235. doi: 10.1007/BF00427606 [DOI] [PubMed] [Google Scholar]
- Nunez C, Földes A, Laorden ML, Milanes MV, & Kovács KJ (2007). Activation of stress-related hypothalamic neuropeptide gene expression during morphine withdrawal. J Neurochem, 101(4), 1060–1071. doi: 10.1111/j.1471-4159.2006.04421.x [DOI] [PubMed] [Google Scholar]
- Nyland JE, & Grigson PS (2013). A drug-paired taste cue elicits withdrawal and predicts cocaine self-administration. Behavioural brain research, 240, 87–90. doi: 10.1016/j.bbr.2012.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YS, Sammartino F, Young NA, Corrigan J, Krishna V, & Rezai AR (2019). Anatomical review of the ventral capsule/ventral striatum and the nucleus accumbens to guide target selection for deep brain stimulation for obsessive-compulsive disorder. World neurosurgery. doi: 10.1016/j.wneu.2019.01.254 [DOI] [PubMed] [Google Scholar]
- Parkes D, Jodka C, Smith P, Nayak S, Rinehart L, Gingerich R, . . . Young A. (2001). Pharmacokinetic actions of exendin-4 in the rat: Comparison with glucagon-like peptide-1. Drug Development Research, 53(4), 260–267. doi: 10.1002/ddr.1195 [DOI] [Google Scholar]
- Plaza-Zabala A, Martín-García E, de Lecea L, Maldonado R, & Berrendero F. (2010). Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci, 30(6), 2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter-Stransky KA, Bentzley BS, & Aston-Jones G. (2017). Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol, 22(2), 303–317. doi: 10.1111/adb.12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, & Scholl L. (2016). Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. Mmwr-Morbidity and Mortality Weekly Report, 65(50–51), 1445–1452. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, . . . Yanagisawa M. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell, 92(5), 1 page following 696. doi: 10.1016/s0092-8674(02)09256-5 [DOI] [PubMed] [Google Scholar]
- Seip KM, & Morrell JI (2007). Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology, 194(3), 309–319. doi: 10.1007/s00213-007-0841-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, & Stewart J. (1995). Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl), 119(3), 334–341. doi: 10.1007/BF02246300 [DOI] [PubMed] [Google Scholar]
- Sharma AN, Pise A, Sharma JN, & Shukla P. (2015). Glucagon-like peptide-1 (GLP-1) receptor agonist prevents development of tolerance to anti-anxiety effect of ethanol and withdrawal-induced anxiety in rats. Metabolic brain disease, 30(3), 719–730. doi: 10.1007/s11011-014-9627-z [DOI] [PubMed] [Google Scholar]
- Shen M, Jiang C, Liu P, Wang F, & Ma L. (2016). Mesolimbic leptin signaling negatively regulates cocaine-conditioned reward. Translational psychiatry, 6(12), e972. doi: 10.1038/tp.2016.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AP, Buniak WI, & Aronne LJ (2015). Treatment of obesity in 2015. Journal of cardiopulmonary rehabilitation and prevention, 35(2), 81–92. doi: 10.1097/HCR.0000000000000112 [DOI] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, & Aston-Jones G. (2010). Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology, 58(1), 179–184. doi: 10.1016/j.neuropharm.2009.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, . . . Fink-Jensen A. (2015). The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiology & behavior, 149, 262–268. doi: 10.1016/j.physbeh.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Rajabi H, & Stewart J. (2005). Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology, 30(9), 1681–1692. doi: 10.1038/sj.npp.1300712 [DOI] [PubMed] [Google Scholar]
- Sweet DC, Levine AS, Billington CJ, & Kotz CM (1999). Feeding response to central orexins. Brain Res, 821(2), 535–538. doi: 10.1016/s0006-8993(99)01136-1 [DOI] [PubMed] [Google Scholar]
- Thannickal TC, John J, Shan L, Swaab DF, Wu MF, Ramanathan L, . . . Siegel JM (2018). Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Science translational medicine, 10(447). doi: 10.1126/scitranslmed.aao4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Dencker D, Wortwein G, Weikop P, Egecioglu E, Jerlhag E, . . . Molander A. (2017). The glucagon-like peptide 1 receptor agonist Exendin-4 decreases relapse-like drinking in socially housed mice. Pharmacology Biochemistry and Behavior, 160, 14–20. doi: 10.1016/j.pbb.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Twining RC, Bolan M, & Grigson PS (2009). Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behavioral neuroscience, 123(4), 913–925. doi: 10.1037/a0016498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Freet CS, Wheeler RA, Reich CG, Tompers DA, Wolpert SE, & Grigson PS (2016). The role of dose and restriction state on morphine-, cocaine-, and LiCl-induced suppression of saccharin intake: A comprehensive analysis. Physiol Behav, 161, 104–115. doi: 10.1016/j.physbeh.2016.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Russell TI, Zhang M, & Shaham Y. (2019). Operant Social Reward Decreases Incubation of Heroin Craving in Male and Female Rats. Biological psychiatry, 86(11), 848–856. doi: 10.1016/j.biopsych.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, & Carelli RM (2008). Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron, 57(5), 774–785. doi: 10.1016/j.neuron.2008.01.024 [DOI] [PubMed] [Google Scholar]
- Wise RA, Yokel RA, & DeWit H. (1976). Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science, 191(4233), 1273–1275. doi: 10.1126/science.1257748 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kahng MW, Elkind JA, Weir VR, Hernandez NS, Stein LM, & Schmidt HD (2020). Activation of GLP-1 receptors attenuates oxycodone taking and seeking without compromising the antinociceptive effects of oxycodone in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 45(3), 451–461. doi: 10.1038/s41386-019-0531-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.