Knee and hip replacements are the 3rd and 4th most common inpatient surgeries performed in the United States, respectively [1,53]. While intended to relieve pain and improve function, up to 44% of knee replacement patients and 27% of hip replacement patients reports persistent postoperative joint pain [69]. Both preoperative risk factors and postoperative pain management influence surgical outcomes. Preoperative risk factors include pain severity/chronicity and psychological distress (e.g., anxiety) [2,25,31,36,37,39,68]. Barriers to effective postoperative pain management include perceived helplessness, insufficient pain coping education, and lack of non-pharmacological interventions [47,68]. Thus, identifying scalable, time-limited interventions with preoperative and postoperative impacts is critical for maximizing surgical outcomes. Surgical patients could benefit from non-pharmacological, preoperative interventions that a) teach psychological techniques for immediately decreasing pain and distress, and that b) can be self-administered as needed.
Mind-body interventions (MBIs), such as mindfulness meditation, hypnotic suggestion, and cognitive-behavioral therapy (CBT), are promising, non-pharmacological pain management strategies [2,22,28,68]. Mindfulness meditation (MM) involves non-reactively observing thoughts, feelings and body sensations as they arise [7,11,34,65]. Hypnosis (HS) modulates the placebo response via suggestions to alter thoughts, feelings, and body sensations [12,55]. CBT is a form of psychotherapy that challenges dysfunctional thoughts as a means of modifying emotional and behavioral responses, and is commonly considered the gold standard behavioral treatment for pain [6,13]. Despite sharing operational processes, these three MBIs represent distinct clinical approaches. Most notably, CBT relies on logical means to consciously dispute dysfunctional thoughts, emotions, and behaviors, while MM and HS leverage attentional techniques to nonreactively observe the mind at work or increase responsiveness to suggestions for change, respectively. MS and HS also differ in that the former is a self-regulated attentional process whereas the latter is dependent on suggestions provided by a therapist. Thus, while evidence suggests that delivering mindfulness [10,40], hypnosis [4,14,16,33,48–50,52,63], and CBT [59,64] preoperatively can improve postoperative outcomes, given their putative mechanistic differences, it may be that these three MBIs differentially impact postoperative outcomes. To date, there are no large-scale, comparative studies of the pre- and postoperative impacts of these three MBIs. Furthermore, to our knowledge, no study has compared very brief forms (i.e., 15-minute interventions) of these MBIs. Brief MBIs may be more feasible to implement in clinical settings and thus present a promising avenue for improving surgical outcomes. Determining which type of very brief preoperative MBI is most likely to improve pre- and postoperative symptomology has significant implications for surgical patients and medical professionals charged with optimizing limited resources.
This randomized controlled trial (RCT) compared preoperative MM, HS, and cognitive-behavioral education (CBE) interventions all delivered in a very brief (i.e., 15-minute), single session format for patients undergoing total joint arthroplasty (TJA) of the knee or hip. In a prior RCT (N=244), we demonstrated that brief MM and HS decreased acute pain, anxiety, and the desire to take analgesic medication to a significantly greater extent than a CBE control condition among inpatients with a variety of clinical pain conditions [21]. In light of these prior results, we hypothesized that TJA patients receiving either a brief MM or HS intervention would report less preoperative pain intensity, pain unpleasantness, pain medication desire, and anxiety, as well as better physical functioning six weeks following surgery, compared with patients receiving CBE.
Methods
Trial Design and Randomization
This was a single-site, three-arm, parallel-group RCT (trial registry: NCT03665727). The local IRB approved all procedures. Participants provided informed consent after reviewing an informed consent cover letter with study personnel. Patients and the public were not involved in the design or conduct of this study.
Participants were patients at an academic medical center scheduled for knee or hip TJA. At a preoperative visit, patients were encouraged to attend a 2-hour preoperative education program, Joint Academy, during which patients learned from a team of medical professionals (i.e., nurses, physical therapists, psychologists) how to ensure the best possible outcome for their TJA. First, a nurse discussed health optimization, anesthesia choice, typical length of hospital stay, and wound care. Second, a physical therapist discussed prehabilitation exercises, the physical therapy timeline, and the process of functional recovery. Finally, the psychologist delivered the MBI. Joint Academy was held once a week and conducted in groups of 9±3 patients. Weekly group cohorts were randomized via a computer-generated randomization schedule with simple random allocation (1:1:1) to one of three MBIs: MM, HS, and CBE. Randomization sequence was generated by a researcher uninvolved with intervention delivery or data collection before the trial began, and any differences in random allocation were purely due to chance. Patients who attended Joint Academy from January 2018 to January 2019 were eligible to participate. Patients experiencing surgical complications and those scheduled for multiple, consecutive surgeries were excluded from the study, due to the possibility that an additional surgeries or procedures would confound their 6-week physical function assessment.
Interventions
Study interventions were matched in terms of format (group), duration (15 minutes), and frequency (once). Each intervention was delivered according to a manualized protocol by a licensed psychologist with eight years of experience using CBT in clinical work and seven years of experience using mindfulness-based interventions and hypnotic suggestion. The same individual delivered all interventions to prevent therapist effects, whereas adherence to a manualized protocol helped to protect against potential bias or treatment diffusion. All three interventions began with a 5-minute presentation about biopsychosocial aspects of pain followed by a 10-minute MBI script. MM consisted of instruction in focused attention on breath and body sensations and metacognitive monitoring and acceptance of discursive thoughts, negative emotions, and pain. This script closely followed a standardized mindfulness induction script (see supplementary materials) validated in prior research on mindfulness in medical settings [21]. HS consisted of a hypnosis session in which patients were invited to focus on sensations of floating before imagining a richly detailed, pleasant scene of their choosing. Next, suggestions for transforming pain into sensations of warmth, coolness, or tingling were provided. This script closely followed a standardized hypnotic induction script (see supplementary materials) validated in prior research on hypnosis during acute medical procedures [42]. CBE consisted of psychoeducation about the link between thoughts, emotions, and behavior and provided instruction in the use logic to dispute maladaptive thoughts about pain that might otherwise exacerbate pain and distress. This script (see supplementary materials) accorded with standard cognitive restructuring techniques employed in CBT interventions for pain [32,66]. We selected a CBE arm for this study to provide a rigorous, active control for a range of non-specific therapeutic factors that might otherwise confound assessment of mindfulness and hypnotic suggestion’s specific mechanisms of action, including attention by a caring professional, and the expectation of therapeutic benefit.
All participants received standard medical care for TJA. Surgical and anesthetic protocols were highly consistent as all TJAs were performed by one of four surgeons working at the same orthopedic center. For all primary TJAs of the knee or hip an intra-operative periarticular joint infiltration was used, consisting of a standardized cocktail of clonidine, morphine, epinephrine, toradol, ropivicaine, and saline. Some patients also received a pre- or post-operative adductor canal single shot nerve block (See Table 1). Post-operatively, patients received a standardized analgesic regimen that included ASA 81 mg BID for deep venous thrombosis prophylaxis, Tylenol, an NSAID (e.g., Naproxen, Celebrex, Mobic), and Tramadol. For patients still experiencing pain, a breakthrough orally administered narcotic (Oxycodone) was used. There was no difference in the amount of postoperative opioids administered between groups. Intravenous narcotics were avoided and PCA opioids are never used at this orthopedic center.
Table 1.
Baseline Characteristics of Participant Sample by Treatment Condition
| Total | Mindfulness Meditation | Hypnotic Suggestion | Cognitive Behavioral Education | Test Statistic | Statistical Significance | |
|---|---|---|---|---|---|---|
| N | 285 | 106 | 89 | 90 | ||
| Cohorts | 47 | 16 | 15 | 16 | ||
| Sociodemographic Characteristics | ||||||
| Age, mean (SD) | 65.44 (10.56) | 65.52 (10.75) | 63.20 (10.67) | 67.56 (9.88) | F=3.88 | p=.02 |
| Women, No. (%) | 181 (63.5) | 63 (59.4) | 60 (67.4) | 58 (64.4) | χ2=1.38 | p=.50 |
| Race, No. (%) | χ2=13.80 | p=.31 | ||||
| American Indian or Alaska Native | 2 (0.7) | - | 2 (2.2) | - | ||
| Asian | 1 (0.4) | - | - | 1 (1.1) | ||
| Black or African American | 2 (0.7) | 2 (1.9) | - | - | ||
| Native Hawaiian or Other Pacific Islander | 3 (1.1) | - | 1 (1.1) | 1 (1.1) | ||
| White or Caucasian | 262 (91.9) | 98 (92.5) | 80 (88.9) | 84 (93.3) | ||
| Other | 9 (3.2) | 3 (2.8) | 4 (4.5) | 2 (2.2) | ||
| Choose not to disclose | 6 (2.1) | 3 (2.8) | 1 (1.1) | 2 (2.2) | ||
| Hispanic Ethnicity, No. (%) | 12 (4.2) | 4 (3.8) | 4 (4.5) | 4 (4.4) | χ2=1.77 | p=.78 |
| Scheduled Surgery, No. (%) | χ2=8.42 | p=.02 | ||||
| Hip Arthroplasty | 104 (36.5) | 46 (43.4) | 36 (40.4) | 22 (24.4) | ||
| Knee Arthroplasty | 181 (63.5) | 60 (56.6) | 53 (59.6) | 68 (75.6) | ||
| BMI, mean (SD) | 30.35 (5.98) | 29.52 (5.82) | 31.31 (6.10) | 30.41 (5.97) | F=2.12 | p=.12 |
| Median Income, mean (SD) | $75,862 ($20,421) | $78,566 ($21,920) | $72,143 (17,595) | $76,354 ($20,855) | F=2.46 | p=.09 |
| Readmissions, mean (SD) | 0.56 (0.98) | 0.55 (0.93) | 0.52 (0.97) | 0.62 (1.05) | F=0.28 | p=.76 |
| Physical Therapy Sessions, mean (SD) | 6.41 (7.74) | 6.12 (7.48) | 7.25 (8.20) | 5.91 (7.59) | F=0.78 | p=.46 |
| Charlson Comorbidity Index, mean (SD) | 1.79 (2.2) | 1.78 (2.25) | 1.97 (2.35) | 1.64 (1.90) | F=0.50 | p=.61 |
| Tobacco Use, No. (%) | 81 (28.4) | 28 (26.4) | 26 (29.2) | 27 (30.0) | χ2=0.35 | p=.84 |
| Alcohol Use, No. (%) | 132 (46.3) | 47 (44.3) | 46 (51.7) | 39 (43.4) | χ2=1.52 | p=.47 |
| Drug Use, No. (%) | 14 (4.9) | 4 (3.8) | 3 (3.4) | 7 (7.8) | χ2=2.33 | p=.31 |
| Surgeon, No. (%) | χ2=9.59 | p=.14 | ||||
| Surgeon A | 7 (2.5) | 5 (4.7) | - | 2 (2.2) | ||
| Surgeon B | 66 (23.2) | 28 (26.4) | 14 (15.7) | 24 (26.7) | ||
| Surgeon C | 116 (40.7) | 41 (38.7) | 42 (47.2) | 33 (36.7) | ||
| Surgeon D | 96 (33.7) | 32 (30.2) | 33 (37.1) | 31 (34.4) | ||
| Nonsteroidal Anti-Inflammatory Drug Use, No. (%) | 189 (66.3) | 75 (70.8) | 56 (62.9) | 58 (64.4) | χ2=1.54 | p=.46 |
| Prescription Opioid Use, No. (%) | 76 (26.7) | 26 (24.5) | 27 (30.3) | 23 (25.6) | χ2=0.92 | p=.63 |
| Psychotropic Medication Use, No. (%) | 68 (23.9) | 28 (26.4) | 17 (19.1) | 23 (25.6) | χ2=1.63 | p=.44 |
| Spinal Anesthesia, No. (%) | 218 (76.5) | 82 (77.4) | 65 (73.0) | 71 (78.9) | χ2=0.92 | p=.63 |
| Nerve Block, No. (%) | 11 (3.9%) | 2 (1.9%) | 4 (4.5%) | 5 (5.6%) | χ2=1.91 | p=.39 |
| Baseline Measures of Preoperative Outcomes | ||||||
| Pain Intensity a | 4.59 (4.30 to 4.89) | 4.42 (3.93 to 4.91) | 4.71 (4.17 to 5.24) | 4.65 (4.12 to 5.18) | F=0.34 | p=.71 |
| Pain Unpleasantness a | 4.40 (4.08 to 4.72) | 4.14 (3.61 to 4.66) | 4.56 (3.99 to 5.13) | 4.51 (3.93 to 5.08) | F=0.70 | p=.50 |
| Pain Medication Desire a, c | 0.91 (0.78 to 1.03) | 0.96 (0.75 to 1.16) | 0.97 (0.75 to 1.19) | 0.80 (0.57 to 1.02) | F=0.75 | p=.47 |
| Anxiety a | 4.62 (4.29 to 4.95) | 4.95 (4.41 to 5.50) | 4.49 (3.91 to 5.08) | 4.40 (3.82 to 4.99) | F=1.07 | p=.34 |
| Baseline Measure of Postoperative Outcome and Timing of Assessments | ||||||
| Physical Functioning b | 37.77 (37.04 to 38.83) | 37.87 (37.04 to 38.69) | 38.19 (35.26 to 41.12) | 37.24 (36.32 to 38.16) | F=0.59 | p=.56 |
| Days from Preoperative Assessment to Surgery | 20.28 (17.81 to 22.75) | 20.55 (16.37 to 24.73) | 17.23 (11.51 to 22.94) | 21.75 (18.01 to 25.49) | F=0.98 | p=.38 |
| Days from Surgery to Postoperative Assessment | 52.22 (48.67 to 55.77) | 54.58 (48.10 to 61.05) | 51.63 (45.44 to 57.83) | 50.03 (44.20 to 55.85) | F=0.57 | p=.56 |
Baseline measures of preoperative outcome scores’ range is 0 to 10. Higher scores indicate more of the respective construct.
Baseline measure of postoperative outcome score’s range is 23.21 to 55.20. Higher scores indicate better physical functioning.
Square root transformed values reported due to negatively skewed raw values.
Measures
Sociodemographic and diagnostic information as well as preoperative physical functioning levels were obtained from patient medical records. Preoperative, self-report measures of acute clinical symptoms were administered immediately before and after the MBI, which comprised a 15-minute interval. The postoperative physical function assessment was completed during a routine, postoperative follow-up visit 6-weeks after surgery.
Preoperative Clinical Symptom Assessment.
Pain intensity [15], pain unpleasantness [15], pain medication desire [24], and anxiety [41,70,71] were measured with individual items rated on a numeric rating scale (0–10), a widely used and validated approach to measuring clinical pain and related symptomology [21,27].
Postoperative Physical Function Assessment.
Self-reported physical function was assessed with the Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function (-PF) computer adaptive test. The PROMIS-PF has demonstrated reliability, validity, and sensitivity to clinical change, with higher scores indicating greater functional ability [17,18,30,35].
PROMIS-PF scores were obtained through medical record review. Medical staff gathering PROMIS-PF data during routine pre- and postoperative visits were blind to experimental condition. Patients’ most proximal preoperative PROMIS-PF score was included if that assessment occurred in the 6 weeks preceding surgery (x̄ days=16, S.D.=15). Postoperatively, patients’ PROMIS-PF score most proximal to their 6-week follow-up was included (x̄ days post-surgery=48, S.D.=19). Days from preoperative PROMIS-PF assessment to surgery (F=0.15, p=.86) and days from surgery to postoperative PROMIS-PF assessment (F=0.55, p=.58) did not differ between MBI conditions.
Power Calculation
For linear mixed modeling, a priori power analysis was conducted using Optimal Design [62]. An estimated total sample size of 250 participants in 47 cohorts was determined necessary to detect an overall between-group (MM vs. HS vs. CBE) effect on baseline-adjusted outcomes (f=0.25, or of medium size) with 80% power, two-sided, p < 0.05.
Statistical Analysis
Following our prespecified analysis plan, between-group differences were assessed by fitting linear mixed models for each preoperative (pain intensity, pain unpleasantness, pain medication desire, anxiety) and postoperative (physical functioning) outcome. Mixed models were conducted from an intention-to-treat (ITT) framework using full information maximum likelihood estimation, and included random intercepts for individuals nested within each weekly cohort to account for clustering. For the preoperative mixed models, outcome variables were regressed on intervention group (MM vs. HS vs. CBE) after covarying baseline values and scheduled surgery. For the postoperative mixed model, physical functioning was regressed on intervention group (MM vs. HS vs. CBE) after covarying baseline physical functioning, scheduled surgery, and days from surgery to postoperative assessment to account for variance in postoperative recovery time. In accordance with the classical ANCOVA approach endorsed by Frison and Pocock [19] for analyzing clinical trial outcomes, covarying baseline values performs statistical matching on the prerandomization scores and ensures that comparisons of postrandomization values by treatment group are independent of baseline differences. Sensitivity analysis was also performed for the physical functioning outcome adjusting for all sociodemographic characteristics listed in Table 1, including those baseline characteristics found to differ between groups (i.e., age, scheduled surgery), as well as analgesic use. To determine whether surgery type (knee or hip) moderated experimental outcomes, we tested the interaction between treatment condition (dummy-coded for each study intervention relative to usual care) and a dichotomous variable representing surgery type. Finally, we examined within-group effects in the pre- and postoperative outcomes. All tests and CIs were 2-sided and statistical significance was defined as a P value less than .05. All linear models were conducted using SPSS version 25.
Results
Of the 481 participants scheduled to attend Joint Academy, 316 attended, with 271 participants (95%) completing the entire preoperative survey. Of the 316 randomized participants, 31 were excluded from the study due to surgery cancellation (n=10) or ineligibility (n=9), or their medical record number was unavailable (n=12). Postoperative PROMIS-PF assessment scores were available for 74% of participants (see supplementary materials for CONSORT Flowchart). Per our a priori power analysis, recruitment was stopped after the 47th cohort. No intervention related harms or unintended effects occurred during this study.
Treatment conditions were similar in sociodemographic characteristics and acute clinical symptomology (Table 1). Mean pain intensity, pain unpleasantness, and anxiety scores indicated moderate levels of acute clinical symptomology during Joint Academy. As pain medication desire may simply serve as a proxy for pain intensity and pain unpleasantness in opioid naïve patients, we examined bivariate correlations between pain medication desire, pain intensity, and pain unpleasantness. Pain medication desire was significantly associated with both pain intensity and pain unpleasantness in both opioid naïve (intensity r = .57, p < .001; unpleasantness r = .65, p < .001) and opioid using (intensity r = .60, p < .001; unpleasantness r = .61, p < .001) patients. Missing data analyses on self-reported physical functioning scores at pre- and postoperative assessment points revealed the observed missing data pattern was consistent with data being missing completely at random (Little’s MCAR test: χ2(2)=1.17, p=.56).
Preoperative Outcomes
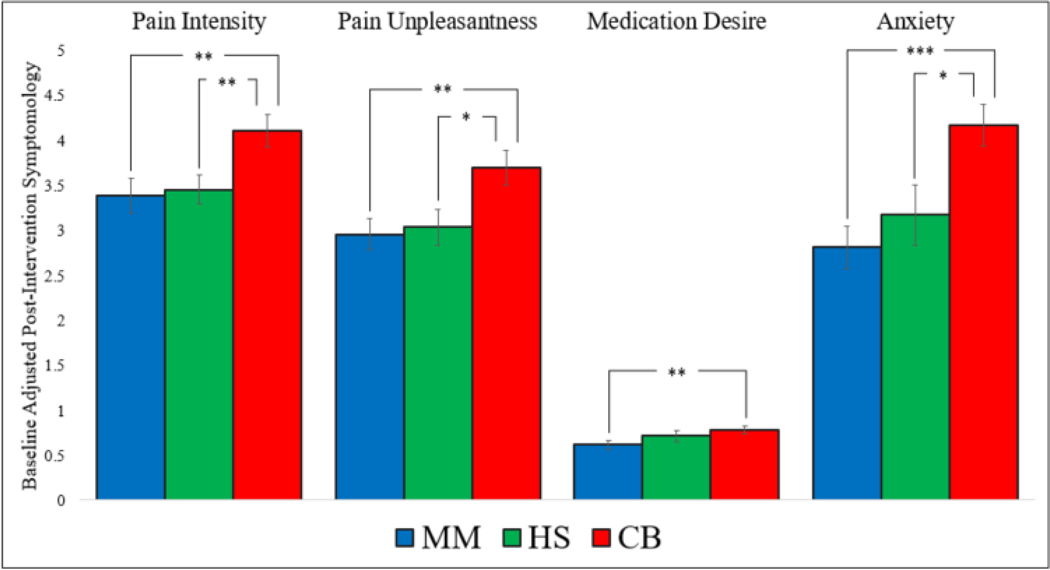
Immediately after the MBI, a significant effect of MBI condition was observed for pain intensity, pain unpleasantness, pain medication desire, and anxiety (Table 2). Planned pairwise comparisons indicated that patients randomized to MM or HS reported significantly less pain intensity, pain unpleasantness, and anxiety than those randomized to CBE. Additionally, patients randomized to MM reported significantly less pain medication desire than those randomized to CBE. No difference was observed between MM and HS on any of the four preoperative outcomes (Figure 1). Significant within-group effects were observed for each preoperative outcome (Table 3).
Table 2.
Outcomes by Treatment Group and Mean (95% CI) Differences Between Treatment Groups (Adjusted Analyses)a,b
| Pain Intensity a | Pain Unpleasantness a | Pain Medication Desire a, d | Anxiety a | Physical Function b | ||
|---|---|---|---|---|---|---|
| Estimated Marginal Means | Mindfulness Meditation | 3.38 (3.00 to 3.76) | 2.96 (2.61 to 3.30) | 0.62 (0.52 to 0.71) | 2.81 (2.35 to 3.28) | 43.59 (42.02 to 45.16) |
| Hypnotic Suggestion | 3.45 (3.14 to 3.77) | 3.03 (2.64 to 3.43) | 0.72 (0.59 to 0.84) | 3.17 (2.52 to 3.82) | 41.08 (39.29 to 42.87) | |
| Cognitive Behavioral Education | 4.11 (3.75 to 4.46) | 3.70 (3.32 to 4.08) | 0.78 (0.70 to 0.86) | 4.17 (3.72 to 4.63) | 39.87 (38.04 to 41.71) | |
| F | 5.27 | 4.98 | 3.63 | 8.82 | 4.79 | |
| Omnibus P Value | .006 | .008 | .028 | <.001 | .010 | |
| Cohen’s d | .40 | .39 | .32 | .54 | .41 | |
| Pairwise Comparisons | Mindfulness vs. Cognitive behavioral education | −.72 c (−1.22 to −0.22) | −.74 c (−1.24 to −0.25) | −0.17 c (−0.29 to −0.04) | −1.35 c (−2.14 to −0.57) | 3.71 c (1.29 to 6.14) |
| Hypnosis vs. Cognitive behavioral education | −.65 c (−1.15 to −0.19) | −.66 c (−1.21 to −0.12) | −0.07 (−0.21 to 0.08) | −1.00 c (−1.82 to −0.19) | 1.21 (−1.13 to 3.54) | |
| Mindfulness vs. Hypnosis | −.07 (−0.56 to 0.42) | −.08 (−0.60 to 0.45) | −0.10 (−0.26 to 0.06) | −0.35 (−1.14 to 0.44) | 2.51 c (0.13 to 4.88) | |
Estimates are from linear mixed models adjusting for baseline score and scheduled surgery.
Estimates are from linear mixed models adjusting for baseline score, scheduled surgery, and time from surgery.
P value is less than .05 for pairwise comparison.
Square root transformed values reported due to negatively skewed raw values.
Note. Pain intensity, pain unpleasantness, pain medication desire, and anxiety were not measured in the usual care condition as these patients did not attend Joint Academy
Figure 1.
Bar graphs representing baseline adjusted pain intensity, pain unpleasantness, pain medication desire, and anxiety by condition. MM=Mindfulness Meditation. HS = Hypnotic Suggestion. CBE = Cognitive Behavioral Pain Psychoeducation.
Table 3.
Within-Group Effects for Preoperative and Postoperative Outcomes
| Mindfulness Meditation | Hypnotic Suggestion | Cognitive Behavioral Education | ||||
|---|---|---|---|---|---|---|
| Outcome | t | Cohen’s d | t | Cohen’s d | t | Cohen’s d |
| Pain Intensity | 5.91*** | .59 | 6.35*** | .70 | 3.63*** | .39 |
| Pain Unpleasantness | 7.11*** | .70 | 7.57*** | .83 | 4.52*** | .49 |
| Pain Medication Desire | 4.78*** | .48 | 3.72*** | .41 | 2.12* | .23 |
| Anxiety | 7.47*** | .74 | 5.56*** | .61 | 2.39* | .25 |
| Physical Function | 5.69*** | .94 | 1.83 | .38 | 1.43 | .22 |
6-week Postoperative Outcome
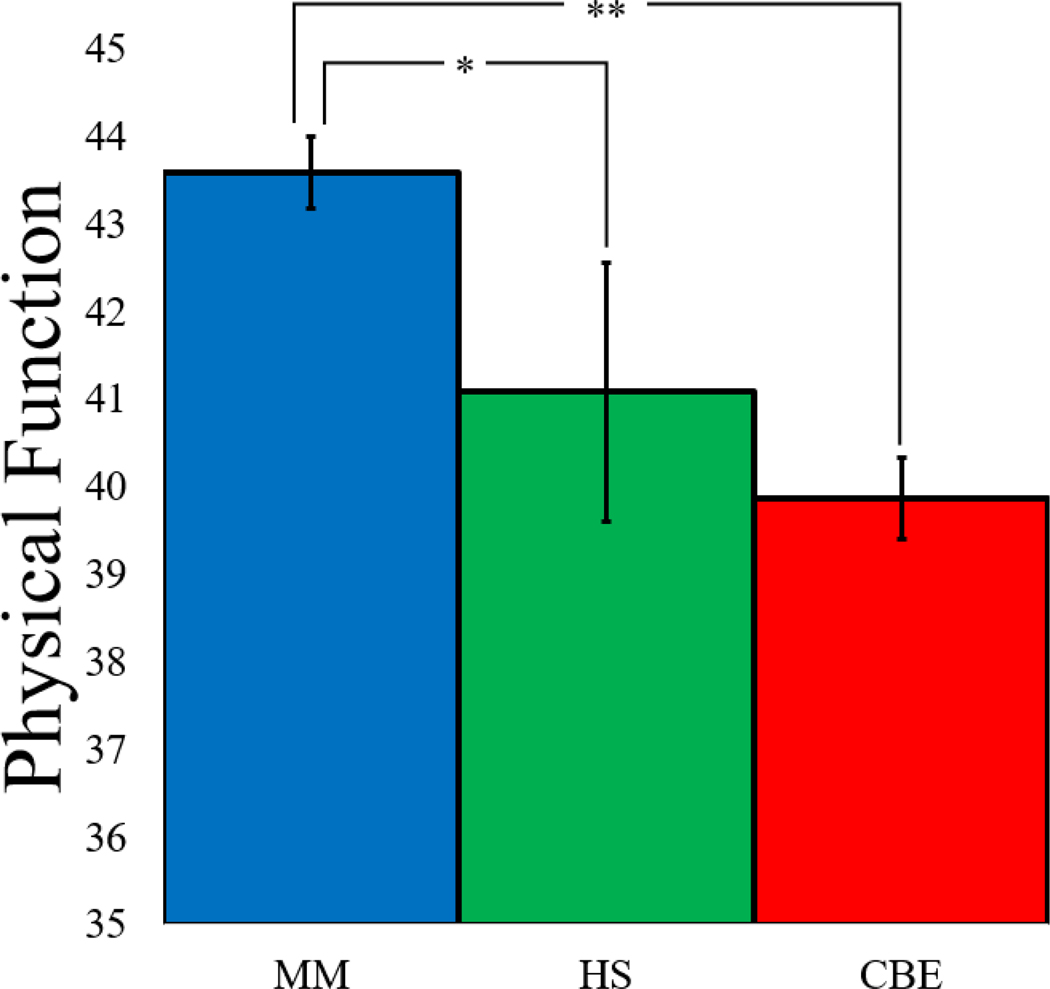
After surgery, a significant effect of MBI condition was observed for postoperative physical function (Table 2). Planned pairwise comparisons indicated that participants randomized to MM reported significantly greater physical function than those randomized to HS or CBE and usual care patients (Figure 2). A sensitivity analysis, adjusting for all sociodemographic characteristics listed in Table 1, did not alter these results (F=6.65, p=.002), and a significant within-group effect was observed for the MM condition only (Table 3). Moderation analysis revealed non-significant interactions between intervention condition and surgery type, indicating that surgery type did not differentially impact the three interventions. Opioid (F=1.40, p=.249) and NSAIDs (F=0.63, p=.535) prescriptions did not differ by MBI condition [Opioid: MM=28 (26%), HS=16 (18%), CBE=28 (31%); NSAIDs: MM=69 (65%), HS=62 (70%), CBE=67 (74%)] at the 6-week postoperative visit.
Figure 2.
Bar graph depicting baseline adjusted physical function by condition. MM=Mindfulness Meditation. HS = Hypnotic Suggestion. CBE = Cognitive Behavioral Pain Psychoeducation.
Discussion
In addition to pharmacological pain management, surgical patients need effective, nonpharmacological pain management strategies [20,25,39,47]. Unfortunately, little evidence exists on the comparative efficacy of brief, preoperative, MBIs designed to teach surgical patients how to manage the pain and distress that often accompany surgery. In one of the largest clinical trials of MBIs ever conducted, we examined the effects of MBIs on preoperative pain, pain medication desire, and anxiety as well as postoperative physical function following primary TJA. Results revealed a single, 15-minute MM or HS intervention immediately reduced preoperative pain intensity from joint disease (−24% and −27%, respectively), as well as pain unpleasantness (−29% and −34%, respectively), and anxiety (−43% and −29%, respectively) in patients preparing for TJA - improving important preoperative risk factors known to influence postoperative outcomes [25,39]. MM also decreased pain medication desire by 35%.
At 6-week postoperative follow-up, patients receiving MM reported significantly better physical function when compared with usual care patients as well as those receiving preoperative HS or CBE. Beyond statistical significance, patients receiving preoperative MM reported postoperative physical function scores indicative of clinically significant improvement. Typically, TJA patients do not realize clinically significant improvements in physical function 6-weeks after surgery, with 6-week physical function scores tending to be very similar to preoperative scores [35]. However, in the present study, the level of physical function reported by patients in the MM arm rose by nearly 5.5 points on the PROMIS-PF (i.e., to 43.36), to a level of physical function more commonly seen 3-months after surgery [35]. Thus, it appears that a single session of preoperative mindfulness training accelerated TJA patients’ recovery from surgery, allowing them to more quickly engage in more strenuous physical activities, such as manual labor, housework, yardwork, and hiking.
Differences in efficacy observed between MM and HS may be a result of the distinct psychological mechanisms of these two MBIs. Mindfulness aims to promote acceptance, reduce pain catastrophizing, and foster reappraisal of pain as innocuous sensory information rather than as an emotionally-laden indicator of bodily harm [23]. In contrast, hypnosis aims to reduce pain by dissociating awareness from the body and using imagination to superimpose pleasurable sensations onto the painful body part [57]. Despite their apparent differences, neuroimaging demonstrates that mindfulness and hypnosis both alleviate pain by modulating corticothalamic activity integral to processing ascending nociceptive input [9,58,74,75]. Although both techniques may attenuate nociceptive peripheral afference from joint disease or the surgical site, the therapeutic mechanisms of mindfulness may be especially well suited for improving physical function, which is highly influenced by known targets of mindfulness including cognitive schemas, attentional biases, and distress intolerance [5,38,43,44,51]. Future studies could use psychophysiological measures to discriminate mind-body interventions; for instance, heart rate variability (HRV) has been shown to distinguish the analgesic mechanisms of mindfulness versus slow breathing [3].
Study findings are consistent with evidence that mindfulness and hypnosis relieve clinical pain and reduce emotional distress [10,22,26,29,40,60,72,73]. Additionally, these results are congruent with our previous RCT, which found that 15 minutes of MM or HS decreased inpatients’ pain intensity, pain unpleasantness, pain medication desire, and anxiety compared with a psychoeducation control [21]. Taken together, these two trials suggest that very brief mindfulness and hypnosis interventions have the capacity to relieve pre- and postoperative clinical symptomology. Combining preoperative MM -- and potentially HS -- with postoperative, inpatient support may result in even better postoperative outcomes. This combined approach may be particularly valuable for decreasing chronic postsurgical pain, which is estimated to occur in 8%−20% of TJA patients [35], and chronic opioid use, which has been observed in 41% of knee TJA patients [54]. Future studies are needed to directly investigate whether adding postoperative booster sessions to preoperative MM training has added benefit. Of further interest for future study may be the synergistic effects of mindful surgeons [45,46] operating on mindful patients, and whether having both parties trained in mindfulness has added benefit.
Despite study strengths, including the use of an active, rigorous CBE control condition, limitations should be noted. First, physical function was assessed by self-report, which may be subject to bias. Future studies could use actigraphy to objectively quantify physical function. Second, participants were patients who voluntarily attended a preoperative education program before TJA, and thus may have been more motivated to engage in behavioral strategies than other patients. As such, these results may not generalize to all TJA patients. Third, although this study did not find surgery type to moderate experimental outcomes, knee and hip TJA patients may require distinct intervention strategies. Behavioral treatment development research could tailor pre-/postoperative training packages for a variety of surgical procedures to meet patients’ individual needs while maximizing treatment adherence and transportability into medical settings. Fourth, the majority of participants were white and living in the mountain west. As such, the generalizability of findings to other populations is unknown. Fifth, we were unable to track intervention usage after preoperative training. While participants were not explicitly instructed to continue practicing the MBIs, MM may have been perceived as more easily self-administered than HS, increasing the likelihood that participants would continue using mindfulness skills to manage pain and distress. Future studies should assess patient use of MBIs pre- and postoperatively to determine whether intervention dosage impacts postoperative outcomes. Finally, in the present trial, though there were no observed differences in opioid prescription rates, we were unable to assess intervention effects on actual opioid consumption. Future studies should track postoperative pain and medication use to cessation as both may impact physical function, and extend the postoperative follow-up period to examine longer-term outcomes.
Conclusions
TJA is one of the most common surgical procedures performed [1,53], with total knee and hip arthroplasty rates expected to increase 100% to 400% by 2040 [8,56,61,67]. This study demonstrates a single session of a simple, scripted MM intervention had measurable effects on physical function six weeks following surgery, implicating its potential for improving surgical outcomes for the millions of patients receiving TJA each year.
Supplementary Material
Acknowledgments
Funding/support:
E.L.G. was supported by grant number R01DA042033 from the National Institutes of Health during the preparation of this manuscript. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Eric Garland, PhD, LCSW is the Director of the Center on Mindfulness and Integrative Health Intervention Development. The Center provides Mindfulness-Oriented Recovery Enhancement (MORE), mindfulness-based therapy, and cognitive behavioral therapy in the context of research trials for no cost to research participants; however, Dr. Garland has received honoraria and payment for delivering seminars, lectures, and teaching engagements (related to training clinicians in mindfulness) sponsored by institutions of higher education, government agencies, academic teaching hospitals, and medical centers. Dr. Garland receives royalties from the sale of books related to MORE. Dr. Garland also is a consultant to BehaVR, LLC.
Footnotes
Conflict of interest disclosure:
The other authors declare that they have no competing interests.
Data Sharing Statement:
De-identified study data will be made available to qualified parties upon request following manuscript publication.
CONSORT Flow Chart.
Flow of participants through trial comparing mindfulness medication with hypnotic suggestion and cognitive-behavioral pain psychoeducation among patients undergoing total joint arthroplasty of the knee or hip.
Note. MRN = Medical Record Number
References
- [1].Abbott TEF, Fowler AJ, Dobbs TD, Harrison EM, Gillies MA, Pearse RM. Frequency of surgical treatment and related hospital procedures in the UK: a national ecological study using hospital episode statistics. BJA Br J Anaesth 2017;119:249–257. [DOI] [PubMed] [Google Scholar]
- [2].Abid Azam M, Weinrib AZ, Montbriand J, Burns LC, McMillan K, Clarke H, Katz J. Acceptance and Commitment Therapy to manage pain and opioid use after major surgery: Preliminary outcomes from the Toronto General Hospital Transitional Pain Service. Can J Pain 2017;1:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Adler-Neal AL, Waugh CE, Garland EL, Shaltout HA, Diz DI, Zeidan F. The Role of Heart Rate Variability in Mindfulness-Based Pain Relief. J Pain 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Akgul A, Guner B, Çırak M, Çelik D, Hergünsel O, Bedirhan S. The Beneficial Effect of Hypnosis in Elective Cardiac Surgery: A Preliminary Study. Thorac Cardiovasc Surg 2016;64:581–588. [DOI] [PubMed] [Google Scholar]
- [5].Banka TR, Ruel A, Fields K, YaDeau J, Westrich G. Preoperative Predictors of Postoperative Opioid Usage, Pain Scores, and Referral to a Pain Management Service in Total Knee Arthroplasty. HSS J 2015;11:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beck AT. Cognitive therapy: Nature and relation to behavior therapy. Behav Ther 1970;1:184–200. [Google Scholar]
- [7].Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, Velting D, Devins G. Mindfulness: A Proposed Operational Definition. Clin Psychol Sci Pract 2004;11:230–241. [Google Scholar]
- [8].Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–1330. [DOI] [PubMed] [Google Scholar]
- [9].Del Casale A, Ferracuti S, Rapinesi C, De Rossi P, Angeletti G, Sani G, Kotzalidis GD, Girardi P. Hypnosis and pain perception: An Activation Likelihood Estimation (ALE) meta-analysis of functional neuroimaging studies. J Physiol-Paris 2015;109:165–172. [DOI] [PubMed] [Google Scholar]
- [10].Dindo L, Zimmerman MB, Hadlandsmyth K, StMarie B, Embree J, Marchman J, Tripp-Reimer T, Rakel B. Acceptance and Commitment Therapy for Prevention of Chronic Post-surgical Pain and Opioid Use in At-Risk Veterans: A Pilot Randomized Controlled Study. J Pain 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dreyfus G. Is mindfulness present-centred and non-judgmental? A discussion of the cognitive dimensions of mindfulness. Contemp Buddhism 2011;12:41–54. [Google Scholar]
- [12].Elkins GR, Barabasz AF, Council JR, Spiegel D. Advancing research and practice: The revised APA Division 30 definition of hypnosis. Int J Clin Exp Hypn 2015;63:1–9. [DOI] [PubMed] [Google Scholar]
- [13].Ellis A Reason and emotion in psychotherapy. 1962. [Google Scholar]
- [14].Enqvist B, Fischer K. Preoperative hypnotic techniques reduce consumption of analgesics after surgical removal of third mandibular molars: a brief communication. Int J Clin Exp Hypn 1997;45:102–108. [DOI] [PubMed] [Google Scholar]
- [15].Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–158. [DOI] [PubMed] [Google Scholar]
- [16].Faymonville ME, Mambourg PH, Joris J, Vrijens B, Fissette J, Albert A, Lamy M. Psychological approaches during conscious sedation. Hypnosis versus stress reducing strategies: a prospective randomized study. Pain 1997;73:361–367. [DOI] [PubMed] [Google Scholar]
- [17].Fries JF, Cella D, Rose M, Krishnan E, Bruce B. Progress in assessing physical function in arthritis: PROMIS short forms and computerized adaptive testing. J Rheumatol 2009;36:2061–2066. [DOI] [PubMed] [Google Scholar]
- [18].Fries JF, Krishnan E, Rose M, Lingala B, Bruce B. Improved responsiveness and reduced sample size requirements of PROMIS physical function scales with item response theory. Arthritis Res Ther 2011;13:R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med 1992;11:1685–1704. [DOI] [PubMed] [Google Scholar]
- [20].Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res 2017;10:2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Garland EL, Baker AK, Larsen P, Riquino MR, Priddy SE, Thomas E, Hanley AW, Galbraith P, Wanner N, Nakamura Y. Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. J Gen Intern Med 2017;32:1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Garland EL, Brintz C, Hanley A, Roseen E, Atchley R, Gaylord S, Faurot K, Yaffe J, Fiander M, Keefe F. Mind-Body Therapies for Opioid-Treated Pain: A Systematic Review and Meta-Analysis. JAMA Intern Med in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Garland EL, Gaylord SA, Palsson O, Faurot K, Mann JD, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med 2012;35:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol 2014;82:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. The Lancet 2019;393:1537–1546. [DOI] [PubMed] [Google Scholar]
- [26].Hammond DC. Hypnosis in the treatment of anxiety-and stress-related disorders. Expert Rev Neurother 2010;10:263–273. [DOI] [PubMed] [Google Scholar]
- [27].Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual analog scale for pain (vas pain), numeric rating scale for pain (nrs pain), mcgill pain questionnaire (mpq), short-form mcgill pain questionnaire (sf-mpq), chronic pain grade scale (cpgs), short form-36 bodily pain scale (sf-36 bps), and measure of intermittent and constant osteoarthritis pain (icoap). Arthritis Care Res 2011;63. [DOI] [PubMed] [Google Scholar]
- [28].HEAL Initiative Research Plan. Natl Inst Health NIH 2018. Available: https://www.nih.gov/research-training/medical-research-initiatives/heal-initiative/heal-initiative-research-plan. Accessed 15 Jul 2019.
- [29].Hilton L, Hempel S, Ewing BA, Apaydin E, Xenakis L, Newberry S, Colaiaco B, Maher AR, Shanman RM, Sorbero ME, others. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med 2017;51:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS physical function item bank in orthopaedic patients. J Orthop Res 2011;29:947–953. [DOI] [PubMed] [Google Scholar]
- [31].Ip HYV, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumptiona qualitative systematic review. Anesthesiol J Am Soc Anesthesiol 2009;111:657–677. [DOI] [PubMed] [Google Scholar]
- [32].Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: A randomized trial. PAIN 2010;150:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Joudi M, Fathi M, Izanloo A, Montazeri O, Jangjoo A. An Evaluation of the Effect of Hypnosis on Postoperative Analgesia following Laparoscopic Cholecystectomy. Int J Clin Exp Hypn 2016;64:365–372. [DOI] [PubMed] [Google Scholar]
- [34].Kabat-Zinn J Full Catastrophe Living. NY: Delacorte Press, 1990. p. [Google Scholar]
- [35].Kagan R, Anderson MB, Christensen JC, Peters CL, Gililland JM, Pelt CE. The recovery curve for the patient-reported outcomes measurement information system patient-reported physical function and pain interference computerized adaptive tests after primary total knee arthroplasty. J Arthroplasty 2018;33:2471–2474. [DOI] [PubMed] [Google Scholar]
- [36].Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother 2009;9:723–744. [DOI] [PubMed] [Google Scholar]
- [37].Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: From risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain 2019;3:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kaunisto MA, Jokela R, Tallgren M, Kambur O, Tikkanen E, Tasmuth T, Sipilä R, Palotie A, Estlander A-M, Leidenius M, Ripatti S, Kalso EA. Pain in 1,000 Women Treated for Breast CancerA Prospective Study of Pain Sensitivity and Postoperative Pain. Anesthesiol J Am Soc Anesthesiol 2013;119:1410–1421. [DOI] [PubMed] [Google Scholar]
- [39].Kim DH, Pearson-Chauhan KM, McCarthy RJ, Buvanendran A. Predictive factors for developing chronic pain after total knee arthroplasty. J Arthroplasty 2018;33:3372–3378. [DOI] [PubMed] [Google Scholar]
- [40].Kiran U, Ladha S, Makhija N, Kapoor PM, Choudhury M, Das S, Gharde P, Malik V, Airan B. The role of Rajyoga meditation for modulation of anxiety and serum cortisol in patients undergoing coronary artery bypass surgery: A prospective randomized control study. Ann Card Anaesth 2017;20:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lang EV, Berbaum KS, Faintuch S, Hatsiopoulou O, Halsey N, Li X, Berbaum ML, Laser E, Baum J. Adjunctive self-hypnotic relaxation for outpatient medical procedures: a prospective randomized trial with women undergoing large core breast biopsy. Pain 2006;126:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lang EV, Joyce JS, Spiegel D, Hamilton D, Lee KK. Self-hypnotic relaxation during interventional radiological procedures: effects on pain perception and intravenous drug use. Int J Clin Exp Hypn 1996;44:106–119. [DOI] [PubMed] [Google Scholar]
- [43].Lautenbacher S, Huber C, Kunz M, Parthum A, Weber PG, Griessinger N, Sittl R. Hypervigilance as predictor of postoperative acute pain: its predictive potency compared with experimental pain sensitivity, cortisol reactivity, and affective state. Clin J Pain 2009;25:92–100. [DOI] [PubMed] [Google Scholar]
- [44].Lautenbacher S, Huber C, Schöfer D, Kunz M, Parthum A, Weber PG, Roman C, Griessinger N, Sittl R. Attentional and emotional mechanisms related to pain as predictors of chronic postoperative pain: A comparison with other psychological and physiological predictors. PAIN® 2010;151:722–731. [DOI] [PubMed] [Google Scholar]
- [45].Lebares CC, Guvva EV, Olaru M, Sugrue LP, Staffaroni AM, Delucchi KL, Kramer JH, Ascher NL, Harris HW. Efficacy of Mindfulness-Based Cognitive Training in Surgery: Additional Analysis of the Mindful Surgeon Pilot Randomized Clinical Trial. JAMA Netw Open 2019;2:e194108–e194108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lebares CC, Hershberger AO, Guvva EV, Desai A, Mitchell J, Shen W, Reilly LM, Delucchi KL, O’Sullivan PS, Ascher NL, Harris HW. Feasibility of Formal Mindfulness-Based Stress-Resilience Training Among Surgery Interns: A Randomized Clinical Trial. JAMA Surg 2018;153:e182734–e182734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lin RJ, Reid MC, Liu LL, Chused AE, Evans AT. The barriers to high-quality inpatient pain management: a qualitative study. Am J Hosp Palliat Med 2015;32:594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mackey EF. An Extension Study Using Hypnotic Suggestion as an Adjunct to Intravenous Sedation. Am J Clin Hypn 2018;60:378–385. [DOI] [PubMed] [Google Scholar]
- [49].Mackey EF. Effects of hypnosis as an adjunct to intravenous sedation for third molar extraction: a randomized, blind, controlled study. Int J Clin Exp Hypn 2010;58:21–38. [DOI] [PubMed] [Google Scholar]
- [50].Marc I, Rainville P, Masse B, Verreault R, Vaillancourt L, Vallée E, Dodin S. Hypnotic analgesia intervention during first-trimester pregnancy termination: an open randomized trial. Am J Obstet Gynecol 2008;199:469.e1–9. [DOI] [PubMed] [Google Scholar]
- [51].Masselin-Dubois A, Attal N, Fletcher D, Jayr C, Albi A, Fermanian J, Bouhassira D, Baudic S. Are Psychological Predictors of Chronic Postsurgical Pain Dependent on the Surgical Model? A Comparison of Total Knee Arthroplasty and Breast Surgery for Cancer. J Pain 2013;14:854–864. [DOI] [PubMed] [Google Scholar]
- [52].Montgomery GH, Bovbjerg DH, Schnur JB, David D, Goldfarb A, Weltz CR, Schechter C, Graff-Zivin J, Tatrow K, Price DD, Silverstein JH. A Randomized Clinical Trial of a Brief Hypnosis Intervention to Control Side Effects in Breast Surgery Patients. J Natl Cancer Inst 2007;99:1304–1312. [DOI] [PubMed] [Google Scholar]
- [53].Most Common Hospital Inpatient Operations - HCUP Fast Stats. n.d. Available: https://www.hcup-us.ahrq.gov/faststats/NationalProceduresServlet. Accessed 23 Dec 2018.
- [54].Namba RS, Inacio MC, Pratt NL, Graves SE, Roughead EE, Paxton EW. Persistent opioid use following total knee arthroplasty: a signal for close surveillance. J Arthroplasty 2018;33:331–336. [DOI] [PubMed] [Google Scholar]
- [55].Oakley DA, Halligan PW. Hypnotic suggestion: opportunities for cognitive neuroscience. Nat Rev Neurosci 2013;14:565–576. [DOI] [PubMed] [Google Scholar]
- [56].Patel A, Pavlou G, Mújica-Mota RE, Toms AD. The epidemiology of revision total knee and hip arthroplasty in England and Wales: a comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone Jt J 2015;97:1076–1081. [DOI] [PubMed] [Google Scholar]
- [57].Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychol Bull 2003;129:495. [DOI] [PubMed] [Google Scholar]
- [58].Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997;277:968–71. [DOI] [PubMed] [Google Scholar]
- [59].Rolving N, Nielsen CV, Christensen FB, Holm R, Bünger CE, Oestergaard LG. Preoperative cognitive-behavioural intervention improves in-hospital mobilisation and analgesic use for lumbar spinal fusion patients. BMC Musculoskelet Disord 2016;17:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Seminowicz DA, Burrowes SA, Kearson A, Zhang J, Krimmel SR, Samawi L, Furman AJ, Keaser ML, Gould NF, Magyari T. Enhanced mindfulness-based stress reduction in episodic migraine: a randomized clinical trial with magnetic resonance imaging outcomes. Pain 2020;161:1837–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Singh JA, Yu S, Chen L, Cleveland JD. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J Rheumatol 2019:jrheum–170990. [DOI] [PubMed] [Google Scholar]
- [62].Spybrook J, Raudenbush SW, Liu X, Congdon R, Martínez A. Optimal design for longitudinal and multilevel research: Documentation for the “Optimal Design” software. Ann Arbor Univ Mich Sch Educ Hierarchical Models Proj 2006. [Google Scholar]
- [63].Syrjala KL, Cummings C, Donaldson GW. Hypnosis or cognitive behavioral training for the reduction of pain and nausea during cancer treatment: a controlled clinical trial. Pain 1992;48:137–146. [DOI] [PubMed] [Google Scholar]
- [64].Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE. Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain 1995;63:189–198. [DOI] [PubMed] [Google Scholar]
- [65].Tang Y-Y, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci 2015;16:213–225. [DOI] [PubMed] [Google Scholar]
- [66].Thorn BE, Kuhajda MC. Group cognitive therapy for chronic pain. J Clin Psychol 2006;62:1355–1366. [DOI] [PubMed] [Google Scholar]
- [67].Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, Woods RJ, Lieberman DE. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci 2017;114:9332–9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Weinrib AZ, Azam MA, Birnie KA, Burns LC, Clarke H, Katz J. The psychology of chronic post-surgical pain: new frontiers in risk factor identification, prevention and management. Br J Pain 2017;11:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. PAIN® 2011;152:566–572. [DOI] [PubMed] [Google Scholar]
- [70].Young Q-R, Ignaszewski A, Fofonoff D, Kaan A. Brief screen to identify 5 of the most common forms of psychosocial distress in cardiac patients: validation of the screening tool for psychological distress. J Cardiovasc Nurs 2007;22:525–534. [DOI] [PubMed] [Google Scholar]
- [71].Young Q-R, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-item measures for depression and anxiety: Validation of the Screening Tool for Psychological Distress in an inpatient cardiology setting. Eur J Cardiovasc Nurs 2015;14:544–551. [DOI] [PubMed] [Google Scholar]
- [72].Zeidan F, Adler-Neal AL, Wells RE, Stagnaro E, May LM, Eisenach JC, McHaffie JG, Coghill RC. Mindfulness-Meditation-Based Pain Relief Is Not Mediated by Endogenous Opioids. J Neurosci 2016;36:3391–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zeidan F, Gordon NS, Merchant J, Goolkasian P. The effects of brief mindfulness meditation training on experimentally induced pain. J Pain 2009;11:199–209. [DOI] [PubMed] [Google Scholar]
- [74].Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 2011;31:5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zeidan F, Vago DR. Mindfulness meditation–based pain relief: a mechanistic account. Ann N Y Acad Sci 2016;1373:114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.