Introduction:
Chronic pain and mental health disorders have converged as a critical comorbidity [21]. Symptoms of prolonged pain overlap with those of anxiety and depression, such as sympathetic arousal, heightened stress sensitivity, insomnia, appetitive changes, and fatigue [62]. Reduced quality of life reinforces cognitive dysfunction; patients who feel greater helplessness, catastrophizing, and anger are at greater risk for suicidal ideation and attempts [48,58]. The complexity of inflammatory pain in particular includes dysregulated and impaired reward evaluation, emotional decision-making, and sustainment of physical and mental activity [2,7,63]. This multifactorial experience may increase risk of opioid misuse and use disorder in patients, reinforcing the need to understand how pain alters motivational and emotional states [19]. Thermal hypersensitivity induced by Complete Freund’s Adjuvant (CFA) has been a useful model yielding such key translational information.
Injection of CFA into the rodent hind-paw induces an inflammatory condition characterized by mechanical hypersensitivity, thermal hyperalgesia, and swelling, which model aspects of human pain conditions featuring inflammation [25]. CFA has been repeatedly shown to reduce reward-seeking in progressive ratio tasks for acquiring sucrose pellets and low doses of heroin [19,35,52]. Such a model of inflammatory-pain-induced negative affect may extend to other domains of behavior classically described as “anxiety-like” or “depressive-like.” While motivational deficits reliably appear at least in the first 48 hours, these other behavioral changes may emerge at a longer interval from injection [35,42,52].
For example, chronic unpredictable mild stress as a reliable model of stress-induced negative affect (and of aspects of human major depressive disorder) requires four weeks of varied stressors to evoke differences in exploratory behavior and stress coping strategy, as measured by the Forced Swim Test (FST), Elevated Plus Maze (EPM), and Open Field Test (OFT) [1,5,13,23,37,40,45,59]. These assays, with their predictive validity for anxiolytics and antidepressants, have for decades been used in translational research on the development of aspects of anxiety- and depressive-like behaviors; however, the effects of CFA-induced inflammatory pain on these measures are unclear [16,18,32,33,47,55,60,61,65,67]. The contradictory results among previous studies may be due to minute but not trivial differences in methodology: the lighting and timing of experiments, the particular phenotyping assays chosen, the order in which these assessments were conducted, etc. The diverse factors that can influence these outcomes during inflammatory pain remain to be determined.
In the current study, we hypothesized that if inflammatory pain persists for the same duration as models of chronic stress, it may be itself a comparable stressor with similar outcomes. We performed a systematic study, considering the variables of injection-to-assessment interval, testing combination and order, individual differences across mice. Finally, we conducted a meta-analysis on similar studies to explore the context of our work. Regardless of the order, combination, and time point at which the EPM, OFT, and FST were conducted, we found no significant reduction in exploratory behavior or adoption of passive stress coping strategies. Furthermore, our meta-analysis found no significant effect of CFA on immobility in the FST, and only a modest effect of CFA on exploratory behavior among male C57BL/6 mice.
Methods:
Animals:
A total of 182 (Fig. S1) male wild-type C57BL/6J mice (Jackson Laboratories) were housed in groups of 4 to 5, in standard cages with corn-cob bedding and nesting material, on a 12/12-hour dark/light cycle (lights on at 7:00 AM), and received food ad libitum throughout the experiment. Mice were 8 weeks of age at CFA injection. All procedures were approved by the Washington University Institutional Animal Care and Use Committee (IACUC) in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All efforts were made to adhere to the Animal Research: Reporting of In Vivo Experiments guidelines (Table S1).
CFA Administration:
Mice naïve to behavioral testing were injected in alternating saline/CFA order. Mice were anesthetized with 2% isoflurane and sedation was confirmed by the absence of a reflex during a toe pinch. CFA (50 μL, Thermo Fisher), or sterile saline as a control, was injected into the plantar surface of the right hind-paw; this volume and unilateral strategy have previously been shown to induce thermal hyperalgesia and mechanical hypersensitivity persisting for weeks [14,34–36,54,66]. Comparison of individual mice injected with saline or CFA was used to determine the behavioral alteration induced by inflammatory pain.
Experimental Design:
Handling and habituation.
Mice were habituated for 7 days to the vivarium prior to any manipulation, injected with CFA, and handled twice a week for the 4- or 6-week interval to onset of behavior testing. Mice were habituated to the experimental room for 2 days x 1 hour before the first behavior test. All mice were confirmed to exhibit no ostensible stress response to handling or the environment (shaking, vocalizations, jumping) immediately prior to behavioral testing. Additionally, mice were tested in alternative saline – CFA order to avoid the confound of time of day or circadian rhythm effects across the duration of a testing day/session.
Note. CFA administration results in visually obvious paw edema while handling the animal, preventing a fully blinded experimental design. However, all data collection was video-recorded in black and white, and analysis was conducted with Any-Maze video tracking software and confirmed with triplicate, blinded, manual scoring after the conclusion of the Hargreaves assay. Additionally, no other adverse health consequences were observed.
Apparatus:
Forced Swim Test:
A 2L beaker is filled with 25°C water to the 1.8L mark. Mice are gently placed into the water for a 6-minute period of forced swim in normal room lighting. The experimenter remains out of sight. Upon completion, mice are gently taken out of the water, dried with towels, and placed on a heating pad in a clean, bedding-free cage to dry and recover. All trials are video-recorded in side-view. Tests are manually scored by a blinded observer 3 times after the conclusion of all experiments. The total time spent immobile, time spent immobile per minute of test, and latency to immobility in seconds were measured.
Elevated Zero Maze:
The EZM is a circular, elevated track divided into quarters, with 50 cm diameter, 20 cm walls on the closed arms, and a 1cm rim on the open arms. Mice are gently placed onto the center of one open arm and allowed to freely explore for 10 minutes in normal room lighting. The experimenter leaves the room after placing animals on the apparatus. All trials are video-recorded in top-view, analyzed with Any-Maze tracking software, and results confirmed with blinded manual scoring. The time spent in and entries into the open arms as totals and per minute of the test, average duration of entry into the open arms, and time spent in the open arms across time of day tested were measured.
Open Field Test:
The OFT is a square, matte light gray box with dimensions 50 cm x 50 cm x 50 cm. Mice are gently placed in the center of the arena and allowed to freely explore for 15 minutes in dim lighting (approximately 25 lux). The experimenter leaves the room after placing the animals in the arena. All trials are video-recorded in top-view, analyzed with Any-Maze tracking software, and tracking fidelity confirmed by a blinded manual observer. The time spent in and entries into the center as totals and per minute of test, average duration of entry into the center, time spent in the center across time of day tested, total entries and time spent in the corners, distance traveled as total and per minute of the test, number of rears, number of fecal boli, and time spent grooming were measured.
Hargreaves Test for Plantar Thermal Hyperalgesia:
Animals are placed in 10 cm x 10 cm x 10 cm enclosures with matte light gray walls, Plexiglass front windows, perforated lids, and no bottom, on a glass surface. Animals are habituated for 3 × 1 hour sessions the day prior to testing, and again for 1 hour on day of testing. A radiant heat source is applied to the plantar surface of the hind-paw through the glass, and the latency of paw withdrawal in seconds is recorded. Five measurements with at least a 5-minute interval between trials are obtained for each hind-paw of CFA- and saline-injected mice [8]. The intensity of the light is adjusted so that baseline latencies are approximately 12 s in contralateral/naïve paws. A cutoff time of 20 s is imposed to prevent tissue damage. Twenty-four hours after the conclusion of the Hargreaves assay, mice were euthanized by rapid cervical dislocation.
Electronic von Frey test for Mechanical Allodynia:
Animals are placed in 10 cm x 10 cm x 10 cm enclosures with matte light gray walls, Plexiglass front windows, perforated lids, and no bottom, on an elevated rack with chicken-wire flooring. Animals are habituated for 3 × 1 hour sessions the day prior to testing, and again for 1 hour on day of testing. The electronic von Frey wand (BioSeb) is applied to the plantar surface of the hind-paw through a gap in the chicken wire, and the threshold of force, in grams, at which the paw is withdrawn is recorded. Three measurements with at least a 5-minute interval between trials are obtained for each hind-paw of CFA- and saline-injected mice.
Caliper measurement for paw swelling.
After the conclusion of a Hargreaves or von Frey test session, mice were gently scruffed and right hind-paw swelling was measured by a digital caliper (Uline).
Statistics:
Post-hoc group assignment.
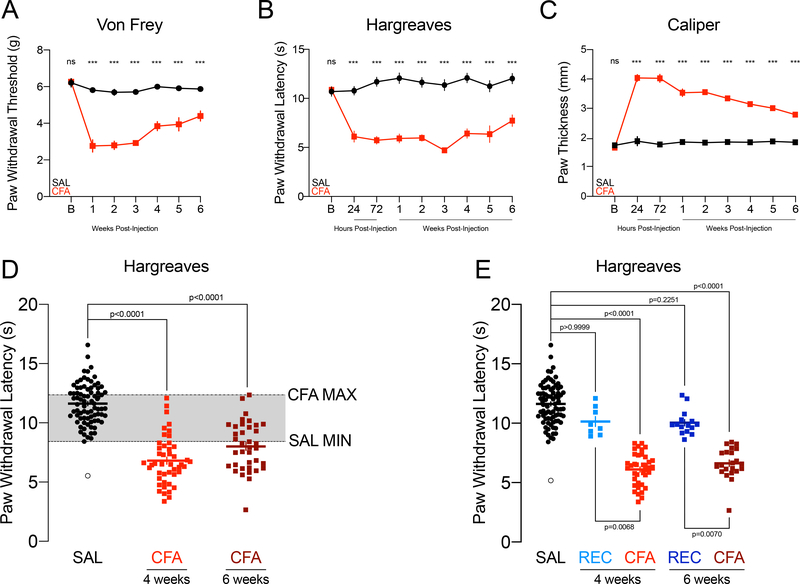
We first confirmed that CFA-induced thermal hyperalgesia persists for up to six weeks. Male C57BL/6J mice were tested in the von Frey and Hargreaves tests and paw thickness was measured with a caliper at baseline. Mice were then injected with saline or CFA into the right hind-paw and tested 24 and 72 hours later in the Hargreaves test, and weekly in the electronic von Frey and Hargreaves tests thereafter for six weeks with a caliper measurement of paw swelling conducted after each session. Mechanical allodynia persisted for six weeks in CFA-injected mice albeit with slow and incomplete recovery (two-way repeated measures ANOVA: Paw F(1,16)=388.9, p<0.0001; Week F(4.116,65.850)=20.84, p<0.0001; Paw x Week F(6,96)=12.27, p<0.0001; Sidak’s multiple comparisons between SAL and CFA per week: baseline p>0.9999, weeks 1 to 4 p<0.0001, week 5 p=0.0029, week 6 p=0.0034, Fig. 1A). Thermal hyperalgesia persisted for six weeks in CFA-injected mice with shallow and incomplete recovery (two-way repeated measures ANOVA: Paw F(1,16)=303.6, p<0.0001; Time F(4.693,75.09)=5.441, p=0.0003; Paw x Time F(8,128)=8.340, p<0.0001; Sidak’s multiple comparisons between SAL and CFA per time point: baseline p>0.9999, 24 to 72 hours and weeks 1 to 4 p<0.0001, week 5 p=0.0028, week 6 p=0.0005, Fig. 1B). Paw swelling persisted with recovery for six weeks in CFA-injected mice (two-way repeated measures ANOVA: Paw F(1,16)=850.2, p<0.0001; Week F(8,128)=44.75, p=0<0.0001; Paw x Week F(8,128)=39.80, p<0.0001; Sidak’s multiple comparisons between SAL and CFA per time point: baseline p=0.9979, 24 hours to week 6 p<0.0001, Fig. 1C). A separate group of mice used for the behavior experiments were tested only once in the Hargreaves, 48 hours after the FST, EZM, or OFT. A subset of these CFA-injected mice exhibited paw withdrawal latency values overlapping with those of the saline-injected mice, suggesting partial or full resolution of the thermal hypersensitivity putatively induced by CFA injection four or six weeks prior (one-way ANOVA F(2,60)=111.6, p<0.0001, n=80 SAL, n=46 CFA 4-weeks, n=37 CFA 6-weeks; Dunnett’s SAL vs. CFA 4-weeks p<0.0001, SAL vs. CFA 6-weeks p<0.0001; Fig. 1D). One saline-injected mouse exhibited a paw withdrawal latency more than 2 standard deviations below the saline average, suggesting a lesion or confounding damage induced by the injection itself; its data are represented in white-filled circles in the relevant figures. The mice whose Hargreaves measurements fell within the saline span (8.42 to 16.57 seconds) were categorized into the REC(overed) group and represented separately from CFA mice in the figures; however, these mice were not excluded from data analysis (Kruskal-Wallis test: KWstat=120.3, p<0.0001, n=80 SAL, n=8 REC 4-weeks, n=38 CFA 4-weeks, n=15 REC 6-weeks, n=22 CFA 6-weeks; Dunn’s SAL vs. CFA 4-weeks p<0.0001, SAL vs. CFA 6-weeks p<0.0001, REC 4-weeks vs. CFA 4-weeks p=0.0068, REC 6-weeks vs. CFA 6-weeks p=0.0070, SAL vs. REC 4-weeks p>0.9999, SAL vs. REC 6-weeks p=0.2251; Fig. 1E). Separating CFA-injected mice into those ostensibly continuing to exhibit thermal hyperalgesia and those whose sensitivity is ostensibly returning to saline/expected levels attempts to infer whether the experience of persistent inflammatory pain itself is responsible for any behavioral effects observed, rather than simply CFA injection. As the Hargreaves test was the final behavioral assessment for each experiment, such allocation to REC was limited to the purposes of post-hoc data analysis and representation, and mice treated no differently during the experiment itself. Furthermore, as the possibility of this event was beyond control, REC n varies across behavioral experiment/cohort. REC groups with at least four mice were tested for normality and compared to SAL and CFA in one-way ANOVAs or Kruskal-Wallis tests followed by the appropriate Sidak’s or Dunn’s post-hoc tests. In all cases, SAL and CFA+REC are also compared in unpaired t-tests or Mann-Whitney tests.
FIGURE 1.
CFA injection induces mechanical allodynia, thermal hyperalgesia, and paw swelling for most mice for at least six weeks.
A: Paw withdrawal thresholds in the von Frey assay for mechanical allodynia of mice injected with saline or CFA and tested weekly for six weeks (n=9,9).
B: Paw withdrawal latencies in the Hargreaves assay for thermal hyperalgesia of mice injected with saline or CFA and tested at 24 hours, 72 hours, and weekly for six weeks (n=9,9).
C: Paw thickness and swelling as measured by caliper of mice injected with saline or CFA and tested at 24 hours, 72 hours, and weekly for six weeks (n=9,9).
D: Paw withdrawal latencies in the Hargreaves assay for thermal hyperalgesia of mice injected saline or CFA, and tested four or six weeks later in behavioral tests (n=80 SAL, n=46 CFA 4-weeks, n=37 CFA 6-weeks). Gray shading indicates overlap between SAL and CFA values.
E: Paw withdrawal latencies in the Hargreaves assay for thermal hyperalgesia of mice injected saline or CFA, and tested four or six weeks later in behavioral tests, with REC mice represented distinct from CFA (n=80 SAL, n=8 REC 4-weeks, n=38 CFA 4-weeks, n=15 REC 6-weeks, n=22 CFA 6-weeks).
Statistical analysis of behavioral data.
Sample sizes were decided based on previous studies that reported n=10–12 as enough power to assess significance among experimental groups. All data were tested for normal distribution in the Shapiro-Wilk test, provided group n≥4 as per our statistical analysis software requirement. Analysis across three groups was conducted in an ordinary one-way ANOVA with Sidak’s or Dunnett’s multiple comparisons (or the Brown-Forsythe ANOVA with Dunnett’s T3 multiple comparisons where standard deviations were significantly different, or the nonparametric alternative Kruskall-Wallis with Dunn’s multiple comparisons if not normally distributed per statistical analysis software recommendation). Analysis between two groups was conducted with two-tailed unpaired t-tests (with Welch’s correction where standard deviations were significantly different, or the nonparametric alternative Mann-Whitney if not normally distributed per statistical analysis software recommendation). For comparisons across both duration of a test or time point tested and group, two-way ANOVA for repeated measures with post-hoc Sidak’s multiple comparisons were used. For correlations, Pearson or the non-parametric alternative Spearman’s rank correlation coefficient were used. All data are presented as mean ± SEM, with individual values visible on each figure. Significance threshold was set at p<0.05. Statistical analyses were conducted with GraphPad PRISM 8.
Meta-Analysis:
In April 2020, we systematic searched Ovid Medline, EMBASE, Web of Science, and Scopus databases with a medical-librarian-designed and -guided search to identify publications reporting in vivo animal modelling of Complete Freund’s Adjuvant-induced changes in the FST, EPM, Elevated Zero Maze (EZM), and OFT. The search strategies were implemented in Ovid Medline 1946-, Embase 1947-, Scopus 1823-, and Web of Science 1900-, and were established using a combination of standardized terms and key words including, but not limited to: (Freund’s adjuvant) AND (anxiety OR depression OR emotion OR negative affect) AND (mice OR rats). Two independent reviewers (DJB and NM) conducted abstract screening and full-text review. Agreement between reviewers was over 90% and any disagreements resolved by consulting a third (JAM). Studies included for this meta-analysis were adult, male, C57BL/6 (N, J, or unknown sub-strain included) mice injected into the plantar surface of one hind-paw with CFA, and subsequently tested in the FST, EPM, EZM, or OFT. Data were abstracted to a data manager platform (Covidence) with a focus on time spent immobile in the FST, time spent in the open arms of the EPM or EZM, and time spent in the center of the OFT. Outcome data represented graphically were abstracted using a digital ruler (WebPlot Digitizer). In studies that evaluated multiple time points per animal, only the first instance of testing was included. The Pitzer et al., 2019 study evaluated multiple discrete cohorts that differed by grouped vs. isolated housing, right vs. left paw injected, and J vs. N sub-strain. Rather than collapsing these discrete cohorts with a fixed-effects model, we include each experiment separately. Standardized mean differences were calculated to Hedge’s g and weighted with the inverse variance method in a random-effects model. Results are presented in SMD units along with 95% confidence intervals, and a 95% prediction interval for the summary effect. The I2 statistic, i.e. the proportion of total variance among studies that is due to true differences in effect size rather than chance alone, was used to quantify heterogeneity in the data set. The Meta-Essentials spreadsheet package was used to perform these statistical analyses [57].
Limitations:
This study follows our previously published work investigating CFA-induced negative affect, as defined by motivational deficits for acquiring sucrose pellets, in male C57BL/6J mice and male Sprague-Dawley rats [19,35]. The current work is similarly limited to one species, one strain, one sex, and one model of inflammatory pain to permit the granularity of our analyses. Investigating the effect of inflammatory or neuropathic pain on rats, other strains or sub-strains of mice such as CD1 or C57BL/6N, and female rodents is just as critical to better understanding the intersection of pain and emotion, as the results here may not extrapolate to these other populations. Furthermore, the present study focuses only on three assays of exploratory behavior and stress coping strategy, as our group’s previously published work addresses motivational, hedonistic, and appetitive behaviors elsewhere. Lastly, the meta-analysis of similar literature was conducted primarily to inform and guide the interpretation and discussion of our results. This meta-analysis is limited without a registered protocol, quality assessments of risks of bias, meta-regression or stratified meta-analysis, and analyses of publication bias.
Supplemental data:
The supplemental file is also available open-source on Figshare (https://figshare.com/s/6bab90f9fb282e3f875f) and the Open Science Framework (https://osf.io/2wpjn/?view_only=5974c94d968d4e55bf6fce986992298e).
Results:
Inflammatory pain persisting for four or six weeks does not induce passive stress coping strategy or reduce exploratory behavior.
As both depression and anxiety are highly prevalent among patients suffering from chronic pain, pre-clinical pain research has been devoted to leveraging the tools of psychiatric drug screening: the forced swim test, the elevated plus maze, and the open field, among others. We hypothesized that modeling pain-induced negative affect may require a time course of inflammatory pain similar to that of chronic unpredictable/mild stress, at least four weeks of which increases immobility in the FST [1,5,13,23,38,40,45,59].
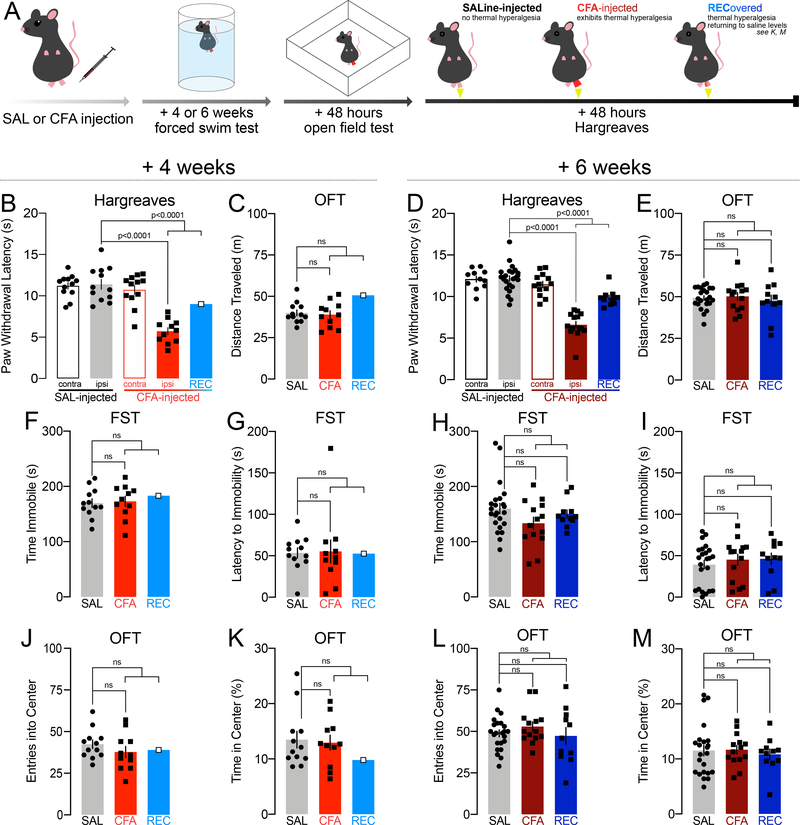
Separate cohorts of male C57BL/6J mice were injected with saline or CFA into the plantar surface of the right hind-paw (Fig. 2A) and tested four or six weeks later in the FST. All were followed 48 hours later by the OFT. Experiments concluded another 48 hours later with the Hargreaves plantar test for thermal hypersensitivity (Fig. 2A).
FIGURE 2.
Inflammatory pain persisting for four or six weeks does not induce passive stress coping strategy during the forced swim test, does not reduce exploratory behavior in the open field test, and does not change locomotion. White-filled data points in the REC group indicate where REC n<4 and was not compared to SAL and CFA in ordinary one-way ANOVAs or Kruskall-Wallis tests. Lines compare SAL vs. CFA, and SAL vs. REC, line ending in a bracket compares SAL vs. CFA+REC together.
A: Schematic of experimental design.
B: Paw withdrawal thresholds in the Hargreaves assay for thermal hyperalgesia by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=11), compared with mice injected with CFA which recovered (n=1), and with mice injected with saline (n=12).
C: Distance traveled in the open field by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=11), compared with mice injected with CFA which recovered (n=1), and with mice injected with saline (n=12).
D: Paw withdrawal thresholds in the Hargreaves assay for thermal hyperalgesia by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=14), compared with mice injected with CFA which recovered (n=11), and with mice injected with saline (n=23).
E: Distance traveled in the open field by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=14), compared with mice injected with CFA which recovered (n=11), and with mice injected with saline (n=23).
F: Time spent immobile in the FST by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=11), compared with mice injected with CFA which recovered (n=1), and with mice injected with saline (n=12).
G: Latency to immobility in the FST by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=11), compared with mice injected with CFA which recovered (n=1), and with mice injected with saline (n=12).
H: Time spent immobile in the FST by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=14), compared with mice injected with CFA which recovered (n=11), and with mice injected with saline (n=23).
I: Latency to immobility in the FST by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=14), compared with mice injected with CFA which recovered (n=11), and with mice injected with saline (n=23).
J: Entries into the center of the OFT by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=11), compared with mice injected with CFA which recovered (n=1), and with mice injected with saline (n=12).
K: Time spent in the center of the OFT by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=11), compared with mice injected with CFA which recovered (n=1), and with mice injected with saline (n=12).
L: Entries into the center of the OFT by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=14), compared with mice injected with CFA which recovered (n=11), and with mice injected with saline (n=23).
M: Time spent in the center of the OFT by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=14), compared with mice injected with CFA which recovered (n=11), and with mice injected with saline (n=23).
Four weeks after CFA injection, thermal hyperalgesia persisted for all but one mouse (one-way ANOVA: F(3,43)=27.12, p<0.0001; Sidak’s SAL IPSI vs. CFA IPSI p<0.0001, n=12 SAL, n=11 CFA; n=1 REC; Sidak’s SAL IPSI vs. CFA IPSI + REC p<0.0001) (Fig. 2B). To analyze a direct comparison between control, saline-injected mice and CFA-injected mice exhibiting thermal hyperalgesia, CFA-injected mice whose paw withdrawal latencies were within the saline span were separated into the “recovered” (REC) group for data representation. The REC group in this cohort comprised insufficient samples for ordinary one-way ANOVAs or Kruskal-Wallis tests; thus, unpaired t-tests and Mann-Whitney tests were used to compare saline and CFA-injected mice confirmed to express thermal hypersensitivity (SAL vs. CFA), as well as saline and CFA-injected mice regardless of thermal hypersensitivity status (SAL vs. CFA+REC).
No differences in locomotor activity were observed between saline- and CFA-injected mice 4 weeks after injection (SAL vs. CFA unpaired t-test, t21=0.3474, p=0.7317, n=11–12; SAL vs. CFA+REC unpaired t-test, t22=0.01417, p=0.9888, n=12,12) (Fig. 2C).
In a separate cohort, tested 6 weeks after CFA injection, 44% of the mice exhibited paw withdrawal latencies within the saline span, suggesting partial or full reversal of thermal hyperalgesia (Kruskal-Wallis: KWstat=48.45, p<0.0001, Dunn’s SAL ipsi vs. CFA ipsi p<0.0001, CFA ipsi vs. REC p=0.3672, SAL ipsi vs. REC p=0.0058, n= 23 SAL, n=14 CFA, n=11 REC; one-way ANOVA: F(3,92)=36.73, p<0.0001, Sidak’s SAL ipsi vs. CFA ipsi + REC p<0.0001, n=23 SAL, n=25 CFA+REC) (Fig. 2D). Statistical comparisons across all groups (SAL vs. CFA vs. REC) as well as between SAL and all CFA-injected mice regardless of thermal hyperalgesia status (SAL vs. CFA+REC) are provided.
There were also no differences in locomotor activity 6 weeks after injection (one-way ANOVA: F(2,45)=0.2991, p=0.7429, Dunnett’s SAL vs. CFA p=0.9314, SAL vs. REC p=0.8203, n=11–23; SAL vs. CFA+REC unpaired t-test with Welch’s correction: t41.26=0.09274, p=0.9266, n=23–25) (Fig. 2E).
CFA did not increase immobility (SAL vs. CFA unpaired t-test: t21=0.2448, p=0.8090, n=11–12; SAL vs. CFA+REC unpaired t-test: t22=0.3314, p=0.7435, n=12,12; Fig. 2F) and did not decrease latency to immobility (SAL vs. CFA Mann-Whitney: U=56, p=0.5658, n=11–12; SAL vs. CFA+REC Mann-Whitney: U=62, p=0.5899, n=12,12; Fig. 2G) in the FST 4 weeks after injection. Time spent immobile (Kruskal-Wallis test: KWstat=2.647, p=0.2662, Dunn’s SAL vs. CFA p=0.3113, SAL vs. REC p>0.9999, n=11–23; SAL vs. CFA+REC Mann-Whitney: U=220, p=0.1682, n=23–25; Fig. 2H) and the latency to immobility (Kruskal-Wallis: KWstat=1.113, p=0.5732, Dunn’s SAL vs. CFA p>0.9999, SAL vs. REC p>0.9999, n=11–23; SAL vs CFA+REC Mann-Whitney: U=236.5, p=0.2979, n=23–25; Fig. 2I) were also unchanged 6 weeks after CFA injection.
Saline- and CFA-injected mice also did not differ in the number of entries into (SAL vs. CFA unpaired t-test: t21=1.114, p=0.2778, n=11–12; SAL vs. CFA+REC unpaired t-test: t22=1.139, p=0.2669, n=12,12; Fig. 2J), or time spent in the center (SAL vs. CFA Mann-Whitney: U=64.50, p=0.9398, n=11–12; SAL vs. CFA+REC Mann-Whitney: U=69.50, p=0.8985, n=12,12; Fig. 2K) of an open field 4 weeks after the induction of inflammatory pain. Six weeks after injection, CFA also did not decrease entries into (one-way ANOVA: F(2,45)=0.7814, p=0.4639, Dunnett’s SAL vs. CFA p=0.5231, SAL vs. REC p=0.9204, n=11–23; SAL vs. CFA+REC unpaired t-test t46=0.4605, p=0.6474, n=23–25; Fig. 2L), or time spent in (Kruskal-Wallis: KWstat=0.4067, p=0.8160, Dunn’s SAL vs. CFA p>0.9999, SAL vs. REC p>0.9999, n=11–23; SAL vs. CFA+REC Mann-Whitney: U=276, p=0.8180, n=23–25; Fig. 2M) the center of the OFT regardless of thermal hyperalgesia status.
Time spent immobile across duration of the FST; distance traveled, entries into, and time spent in the center across the duration of the OFT; time spent in the center across time of day tested; average duration of entry into the center, entries and time spent in the corners, fecal boli, rearing, and time spent grooming in the OFT for both 4 and 6 weeks post-injection are represented in Fig. S2. Group behavior did not significantly vary on these measures.
Inflammatory pain persisting for four or six weeks does not reduce exploratory behavior or induce locomotor deficits.
We next investigated the potential impact of CFA-induced inflammatory pain on exploratory behavior in a novel, anxiogenic environment using the EZM. We used different cohorts of mice from those tested in the FST, to dissociate the effects of one stressful test on another and investigate a distinct domain of affect.
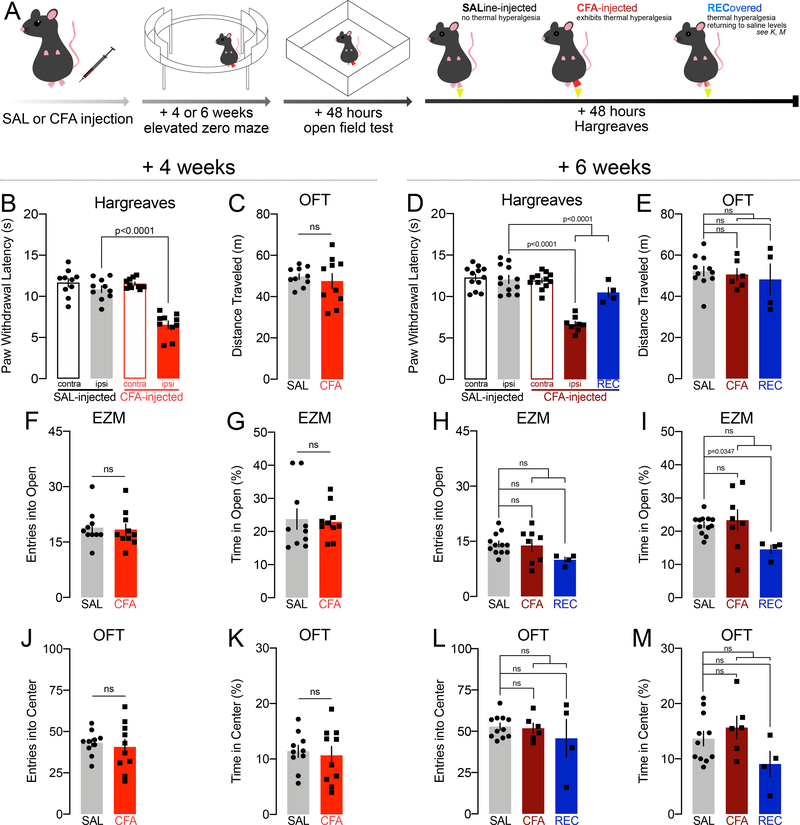
Separate cohorts of C57BL/6J mice were tested 4 or 6 weeks after CFA or saline injection in the hind-paw in the EZM (this variation was chosen because it lacks the ambiguous center zone of the elevated plus maze) (Fig. 3A). All tests were followed 48 hours later by the OFT to assess effects of inflammatory pain on locomotion. Experiments concluded another 48 hours later with the Hargreaves plantar test for thermal hypersensitivity (Fig. 3A).
FIGURE 3.
Inflammatory pain persisting for four or six weeks does not reduce exploratory behavior during the elevated zero maze or the open field test, nor results in locomotor deficits. White-filled data points in the REC group indicate where REC n<4 and was not compared to SAL and CFA in ordinary one-way ANOVAs or Kruskall-Wallis tests. Lines compare SAL vs. CFA, and SAL vs. REC, line ending in a bracket compares SAL vs. CFA+REC together.
A: Schematic of experimental design.
B: Paw withdrawal thresholds in the Hargreaves assay for thermal hyperalgesia by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=10), compared with mice injected with saline (n=10).
C: Distance traveled in the open field by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=10), compared with mice injected with saline (n=10).
D: Paw withdrawal thresholds in the Hargreaves assay for thermal hyperalgesia by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=8), compared with mice injected with CFA which recovered (n=4), and with mice injected with saline (n=12).
E: Distance traveled in the open field by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=6), compared with mice injected with CFA which recovered (n=4), and with mice injected with saline (n=11).
F: Entries into the open arms of the EZM by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=10), compared with mice injected with saline (n=10).
G: Time spent in the open arms of the EZM by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=10), compared with mice injected with saline (n=10).
H: Entries into the open arms of the EZM by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=8), compared with mice injected with CFA which recovered (n=4), and with mice injected with saline (n=12).
I: Time spent in the open arms of the EZM by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=8), compared with mice injected with CFA which recovered (n=4), and with mice injected with saline (n=12).
J: Entries into the center of the OFT by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=10), compared with mice injected with saline (n=10).
K: Time spent in the center of the OFT by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=10), compared with mice injected with saline (n=10).
L: Entries into the center of the OFT by mice injected with CFA and sustaining thermal hyperalgesia for six weeks (n=6), compared with mice injected with CFA which recovered (n=4), and with mice injected with saline (n=11).
M: Time spent in the center of the OFT by mice injected with CFA and sustaining thermal hyperalgesia for six (n=6), compared with mice injected with CFA which recovered (n=4), and with mice injected with saline (n=11).
In this cohort, all mice exhibited thermal hypersensitivity 4 weeks after injection (one-way ANOVA: F(3,36)=35.29, p<0.0001, Sidak’s SAL ipsi vs. CFA ipsi p<0.0001, n=10 SAL, n=10 CFA) (Fig. 3B). Similarly to our previous observations, CFA did not impact locomotor activity 4 weeks after injection (unpaired t-test with Welch’s correction t11.82=0.5606, p=0.5855, n=10,10) (Fig. 3C).
Six weeks after the induction of inflammatory pain, however, 33% of mice exhibited paw withdrawal latencies within the saline span, suggesting partial or full reversal of thermal hyperalgesia (one-way ANOVA: F(4,43)=28.56, p<0.0001; Sidak’s SAL ipsi vs. CFA ipsi p<0.0001, CFA ipsi vs. REC p=0.0001, SAL ipsi vs. REC p=0.1896, SAL IPSI vs. CFA+REC p<0.0001, n=12 SAL, n=8 CFA, n=4 REC) (Fig. 3D). Statistical comparisons across all groups (SAL vs. CFA vs. REC) as well as between SAL and all CFA-injected mice regardless of thermal hyperalgesia status (SAL vs. CFA+REC) are provided.
Open field test data is unavailable for three animals (2 CFA, 1 SAL) due to a technical failure in which video and data were not recorded during the open field test. Similarly to our previous observations, CFA did not impact locomotor activity 6 weeks after injection in this cohort (Brown-Forsythe ANOVA: F(2,5.273)=0.1912, p=0.8315, Dunnett’s T3 Sal vs. CFA p=0.8920, SAL vs. REC p=0.8575, n=4–11; SAL vs. CFA+REC: unpaired t-test: t19=0.6411, p=0.5291, n=10–11 Fig. 3E).
Four weeks after injection, CFA did not change exploratory behavior. In the EZM, in which mice can make active decisions about entering and retreating from an exposed space across a visible borderline, CFA did not decrease entries into (unpaired t-test: t18=0.2356, p=0.8164, n=10,10; Fig. 3F) or time spent (Mann-Whitney: U=41, p=0.5149, n=10,10; Fig. 3G) in the open arms.
Six weeks after injection, CFA also did not reduce the number of entries (one-way ANOVA: F(2,21)=2.429, p=0.1125, Dunnett’s SAL vs. CFA p=0.9621, p=0.0807, n=12 SAL, n=8 CFA, n=4 REC; SAL vs. CFA+REC unpaired t-test: t22=1.134, p=0.2690, n=12,12; Fig. 3H) into the open arms of the EZM. “Recovered” mice spent significantly less time in the open arms of the EZM than SAL-injected mice, although there was no significant reduction in open arm time between saline mice and all mice injected with CFA regardless of thermal hypersensitivity status (Kruskall-Wallis: KWstat=6.812, p=0.0332; Dunn’s SAL vs. CFA p>0.9999, SAL vs. REC p=0.0347, n=4–12; SAL vs. CFA+REC unpaired t-test with Welch’s correction: t13.56=0.6125, p=0.5503, n=12,12; Fig. 3I).
Saline- and CFA-injected mice also did not differ in the number of entries into (unpaired t-test: t18=0.4620, p=0.6496, n=10,10; Fig. 3J), or time spent in (unpaired t-test: t18=0.3753, p=0.7119, n=10,10; Fig. 3K), the center of the OFT arena 4 weeks after the induction of inflammatory pain. Entries into (Brown-Forsythe ANOVA: F(2,3.962)=0.3157, p=0.7461, Dunnett’s T3 SAL vs. CFA p=0.9492, SAL vs. REC p=0.8000, n=4–11; SAL vs. CFA+REC unpaired t-test with Welch’s correction: t12.65=0.6882, p=0.5037, n=10–11; Fig. 3L) and time spent in (one-way ANOVA: F(2,18)=2.509, p=0.1094, Dunnett’s SAL vs. CFA p=0.6266, SAL vs. REC p=0.1902, n=4–11; SAL vs. CFA+REC unpaired t-test: t19=0.2868, p=0.7774, n=10–11; Fig. 3M) the center of the OFT were also unchanged in CFA-injected mice 6 weeks later.
Entries into and time spent in the open arms across the duration of the test, the average duration of an entry into the open arms, and time spent in the open arms across time of day tested in the EZM for both 4 and 6 weeks post-injection are represented in Fig. S3. Distance traveled, entries into, and time spent in the center across the duration of the OFT; time spent in the center across time of day tested; average duration of entry into the center, entries and time spent in the corners, number of fecal boli, number of rears, and time spent grooming in the OFT for both 4 and 6 weeks post-injection are represented in Fig. S4. Group behavior did not significantly vary on these measures either.
Effects of inflammatory pain persisting for four weeks on exploratory behavior and stress coping strategy when testing order is reversed.
The testing experience of the FST and EZM may be inherently stressful and therefore confounding to the OFT assessment [4,15]. Therefore, reversing the order of behavioral tests administered would determine whether the effect of CFA on stress coping strategy and exploratory behavior depends on the experimental design itself.
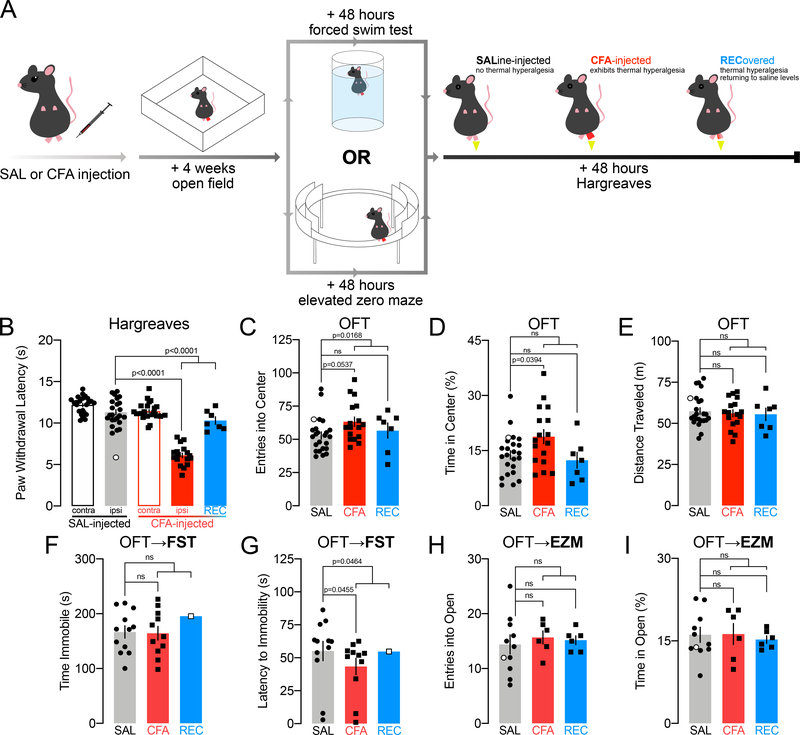
Male C57BL/6J mice were injected with saline or CFA into the plantar surface of the right hind-paw and tested four weeks later, first in the OFT (Fig. 4A). Discrete cohorts were tested 48 hours later in the FST or EPM, and all concluded with a final Hargreaves plantar test (Fig. 4A). One saline-injected animal exhibited paw withdrawal latencies more than 2 standard deviations lower than the saline average (5.68 s) suggesting a lesion or confounding damage induced by the injection itself; while this animal has not been excluded from analysis, its data are represented in white-filled data points in the figures.
FIGURE 4.
Inflammatory pain persisting for four weeks may alter exploratory behavior and passive coping strategy, when testing order is reversed. White-filled data points in the REC group indicate where REC n<4 and was not compared to SAL and CFA in ordinary one-way ANOVAs or Kruskall-Wallis tests. White-filled data points in the SAL group indicate mouse with a putative paw lesion. Lines compare SAL vs. CFA, and SAL vs. REC, line ending in a bracket compares SAL vs. CFA+REC together.
A: Schematic of experimental design.
B: Paw withdrawal thresholds in the Hargreaves assay for thermal hyperalgesia by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=17), compared with mice injected with CFA which recovered (n=7), and with mice injected with saline (n=23).
C: Entries into the center of the OFT by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=17), compared with mice injected with CFA which recovered (n=7), and with mice injected with saline (n=23).
D: Time spent in the center of the OFT by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=17), compared with mice injected with CFA which recovered (n=7), and with mice injected with saline (n=23).
E: Distance traveled in the open field by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=17), compared with mice injected with CFA which recovered (n=7), and with mice injected with saline (n=23).
F: Time spent immobile in the FST by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=11), compared with mice injected with CFA which recovered (n=1), and with mice injected with saline (n=12).
G: Latency to immobility in the FST by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=11), compared with mice injected with CFA which recovered (n=1), and with mice injected with saline (n=12).
H: Entries into the open arms of the EZM by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=6), compared with mice injected with CFA which recovered (n=6), and with mice injected with saline (n=10).
I: Time spent in the open arms of the EZM by mice injected with CFA and sustaining thermal hyperalgesia for four weeks (n=6), compared with mice injected with CFA which recovered (n=6), and with mice injected with saline (n=10).
Although thermal hypersensitivity persisted in most mice four weeks after injection of CFA, in this cohort, nearly a third exhibited paw withdrawal latencies within the saline span (one-way ANOVA: F(4,89)=73.58, p<0.0001, SAL IPSI vs. CFA IPSI p<0.0001; CFA IPSI vs. REC p<0.0001, SAL IPSI vs. REC p=0.2533, SAL IPSI vs. CFA IPSI + REC p<0.0001, n=23 SAL, n=17 CFA, n=7 REC; including saline with PWL of 5.68 s: one-way ANOVA: F(4,91)=60.48, p<0.0001, Sidak’s SAL IPSI vs. CFA IPSI p<0.0001, SAL IPSI vs. CFA IPSI + REC p<0.0001) (Fig. 4B).
Statistical comparisons across all groups (SAL vs. CFA vs. REC) as well as between SAL and all CFA-injected mice regardless of thermal hyperalgesia status (SAL vs. CFA+REC) are provided.
Surprisingly, CFA injection increased entries into the center of the arena when mice were tested in the OFT first (Kruskal-Wallis: KWstat=5.879 p=0.0529, Dunn’s SAL vs. CFA p=0.0537, SAL vs. REC p=0.6426, n=23 SAL, n=17 CFA, n=7 REC; SAL vs. CFA+REC Mann-Whitney: U=164.5, p=0.0168, n=23–24; Fig. 4C). CFA injection increased time spent in the center only for mice with elevated thermal hypersensitivity (one-way ANOVA: F(2,44)=3.745, p=0.0315, Dunnett’s SAL vs. CFA p=0.0394, SAL vs. REC p=0.8574, n=7–23; SAL vs. CFA+REC unpaired t-test: t45=1.595, p=0.1178, n=23–24; Fig. 4D) without increasing locomotor activity (Kruskall-Wallis: KWstat=0.1372, p=0.9337, Dunn’s SAL vs. CFA p>0.9999, SAL vs. REC p>0.9999, n=7–23; SAL vs. CFA+REC Mann-Whitney: U=275, p=0.9916, n=23–24; Fig. 4E). The inclusion of the saline-injected mouse with an aberrant average paw withdrawal latency did not change the outcome of these analyses (entries SAL vs. CFA+REC Mann-Whitney U=184, p=0.0314, n=24,24; time SAL vs. CFA unpaired t-test t39=2.302, p=0.0268, n=17–24; distance SAL vs. CFA+REC Mann-Whitney U=282, p=0.9106, n=24,24).
Mice tested in the FST afterwards exhibited no differences in time spent immobile (SAL vs. CFA unpaired t-test: t21=0.1450, p=0.8861, n=12 SAL, n=11 CFA; SAL vs. CFA+REC unpaired t-test: t22=0.01135, p=0.9910, n=12, 12; Fig. 4F) but CFA-injected mice exhibited a shorter latency to immobility (SAL vs. CFA Mann-Whitney: U=33.50, p=0.0455, n=11–12; SAL vs. CFA+REC Mann-Whitney: U=37.50, p=0.0464, n=12,12; Fig. 4G).
A separate group was tested in the EZM; data are unavailable for one mouse (SAL) which fell off the EZM and did not complete the trial. No CFA-induced changes were observed in entries into (one-way ANOVA: F(2,19)=0.1825, p=0.8346, Dunnett’s SAL vs. CFA p=0.7949, SAL vs. REC p=0.9180, n=10 SAL, n=6 CFA; SAL vs. CFA+REC unpaired t-test with Welch’s correction: t11.84=0.5449, p=0.5959, n=10–12; Fig. 4H), or time spent in (one-way ANOVA: F(2,19)=0.1182, p=0.8892, Dunnett’s SAL vs. CFA p=0.9974, SAL vs. REC p=0.8854, n=6–10; SAL vs. CFA+REC unpaired t-test: t20=0.2234, p=0.8255, n=10–12; Fig. 4I) the open arms. The inclusion of the saline-injected mouse with an aberrant average paw withdrawal latency did not change the outcome of these analyses (entries SAL vs. CFA+REC unpaired t-test with Welch’s correction t13.72=0.7150, p=0.4866, n=11–12; time SAL vs. CFA+REC unpaired t-test t21=0.08362, p=0.9342, n=11–12).
Distance traveled, entries into center, and time spent in the center of the OFT; time spent in the center of the OFT across time of day tested; average duration of an entry to the center, entries into corners, time in corners, fecal boli, number of rears, and time spent grooming in the OFT; entries and time spent in the open arms of the EZM across the duration of the test; time spent in the open arms of the EZM across time of day tested; and time spent immobile in the FST across the duration of the test are represented in Fig. S5. Group behavior did not significantly vary on these measures either.
Measures of exploratory behavior do not reliably correlate between two tests of behavior in a novel, anxiogenic environment.
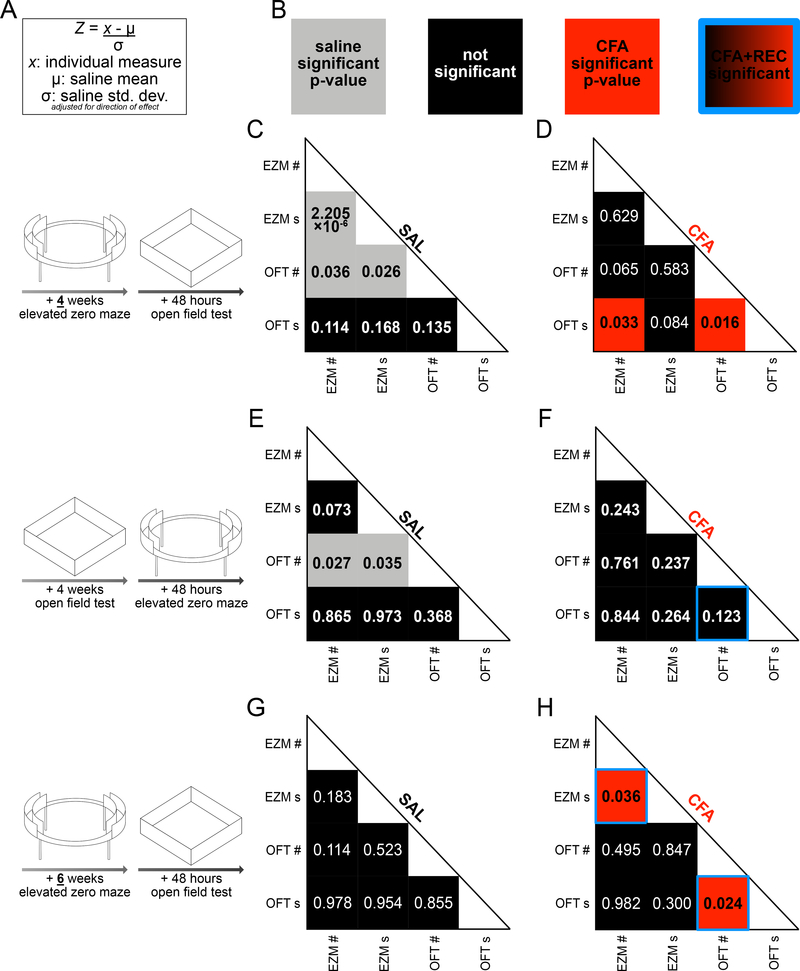
While CFA only had an effect on exploratory behavior in the OFT when mice were naïve to other behavioral phenotyping at the time of the OFT assessment, this effect did not correspond to differences in exploratory behavior in the EZM in the same mice. This suggests these behavioral measurements may not consistently correlate with each other across individuals. To determine how an individual mouse’s performance in one behavioral test may correspond to its performance in another, we analyzed the previously described behavioral data in correlation matrices [24]. To normalize EZM and OFT data on the same scale, entries into the center and open arms, and time spent in the center and open arms, were converted into z-scores. Each individual data point in each group was subtracted from the saline average and divided by the saline standard deviation (Fig. 5A). In the correlation matrices, gray indicates a significant p-value for saline-injected mice, red indicates a significant p-value for CFA-injected mice, black indicates a non-significant correlation, and a blue border around a square of any color indicates a significant p-value when CFA and REC are combined as all mice injected with CFA regardless of thermal hyperalgesia status (Fig. 5B). In mice injected with saline and tested 4 weeks later in the EZM, followed 48 hours later by the OFT, entries into the open arms correlated with time spent in the open arms of the EZM (Spearman r=0.991, p<0.0001, n=10, Fig. 5C). Entries into the center of the OFT correlated with entries into the open arms of the EZM (Spearman r=0.676, p=0.036, n=10, Fig. 5C) and time spent in the open arms of the EZM (Spearman r=0.707, p=0.026, n=10, Fig. 5C). In mice injected with CFA in the same cohort, time spent in the center of the OFT correlated with entries into the center of the OFT (Pearson r=0.733, p=0.016, n=10, Fig. 5D) and with entries into the open arms of the EZM (Pearson r=0.674, p=0.033, n=10, Fig. 5D). In mice injected with saline and tested 4 weeks later in the OFT, followed 48 hours later by the EZM (the reverse experimental design), entries into the center of the OFT negatively correlated with entries into the open arms of the EZM (Spearman r=−0.709, p=0.027, n=10, Fig. 5E) and time spent in the open arms of the EZM (Spearman r=−0.685, p=0.035, n=10, Fig. 5E). In mice injected with CFA in the same cohort, no significant correlations among behavioral measurements were observed (n=6) except when CFA and REC were combined as a group of all mice injected with CFA regardless of thermal hyperalgesia status, in which case entries into and time spent in the center of the OFT were correlated (Pearson r=0.733, p=0.007, n=12, Fig. 5F). Most measures also did not correlate between the FST and OFT regardless of the order of testing (Fig. S6).
FIGURE 5.
Measures of exploratory behavior do not consistently or reliably correlate between two tests of behavior in a novel, anxiogenic environment.
A: Equation used to calculate z-score for individual measurements of entries into open arms of EZM, time spent in open arms of EZM, entries into center of OFT, and time in center of OFT.
B: Key for correlations. Each box contains the p-value of the strength of correlation between the measurements aligned with its location. Gray boxes contain significant p-values for saline groups. Black boxes contain insignificant p-values. Red boxes contain significant p-values for CFA groups.
C: In saline-injected mice tested four weeks later first in the EZM and later in the OFT, time spent in the open arms of the EZM correlates only to entries into the open arms of the EZM and entries into the center of the OFT. Time spent in the center of the OFT does not correlate to any other measure (n=10).
D: In CFA-injected mice tested four weeks later first in the EZM and later in the OFT, time in the center of the OFT correlates to entries into the center of the OFT and entries into the open arms of the EZM. Time in the open arms of the EZM does not correlate to time in the center of the OFT or entries into the open arms of the EZM (n=10).
E: In saline-injected mice tested four weeks later first in the OFT and later in the EZM, entries into the center of the OFT correlates to entries into the open arms of the EZM and time spent in the open arms of the EZM. Time in the center of the OFT does not correlate to any other measure (n=10).
F: In CFA-injected mice tested four weeks later first in the OFT and later in the EZM, no behavioral measures correlate (n=6).
G: In saline-injected mice tested six weeks later first in the EZM and later in the OFT, no behavioral measures are associated except when CFA+REC mice are grouped back together, in which case time and entries into the center of the OFT are significantly correlated (n=11).
H: In CFA-injected mice tested first six weeks later in the EZM and later in the OFT, entries into and time spent in the open arms are significantly correlated, and entries into and time spent in the center of the OFT are significantly correlated (n=6). These significant associations persist when CFA+REC mice are grouped back together (n=10).
Paw withdrawal threshold in the Hargreaves assay does not correlate to average Z-score of behavior in the forced swim test, elevated zero maze, and open field test.
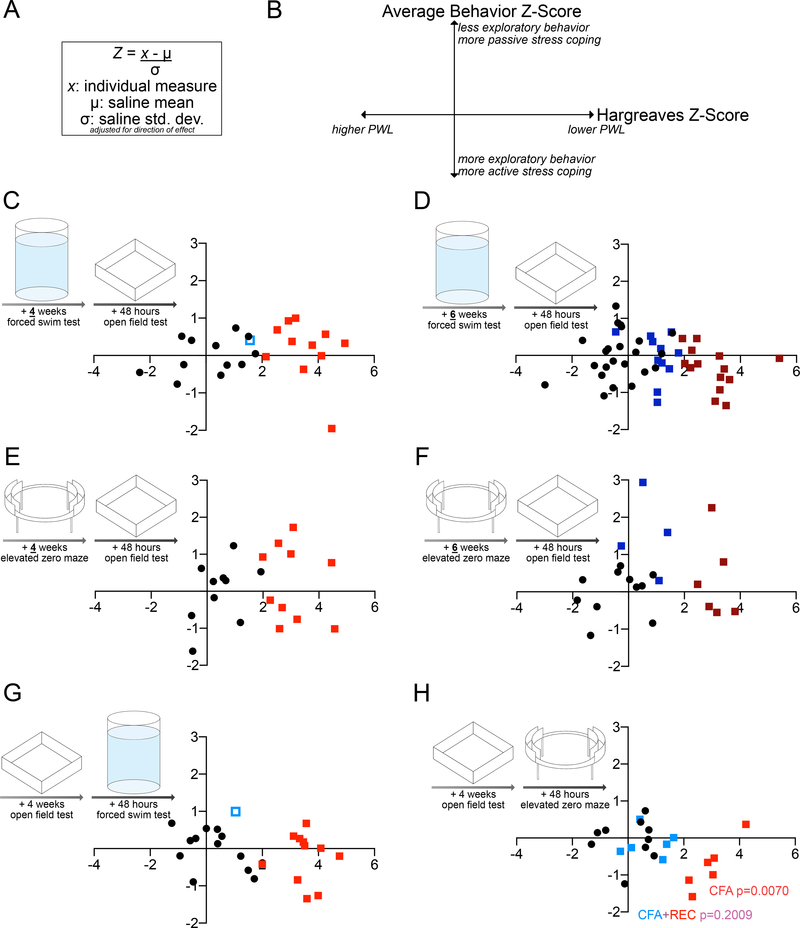
Although this study evaluated two distinct domains of negative affect across three behavioral assays, the data analysis still relied on binary categorizations and are represented as single endpoints. Recent evidence from pre-clinical models of stress susceptibility have suggested rectifying the reliance on single endpoints by converting behavioral data into z-scores, which quantify the relationship of individual data points to a control mean, and averaging those z-scores for a composite assessment of affective state [20,24,44].
To generate a composite behavioral score, time spent immobile in the FST, latency to immobility in the FST, entries into the open arms of the EZM, time spent in the open arms of the EZM, entries into the center of the OFT, and time spent in the center of the OFT, were converted into z-scores for each individual mouse across all our experimental designs as previously described (Fig. 6A). Each individual data point in each group was subtracted from the saline average, and this was divided by the saline standard deviation (Fig. 6A). Hargreaves paw withdrawal latencies were similarly converted to z-scores, allowing for correlations between thermal hypersensitivity thresholds and the average behavioral score. All z-scores were adjusted for direction of effect such that higher behavior z-scores on the y-axis corresponded to less exploratory behavior and more passive stress coping, and higher Hargreaves Z-scores indicated more thermal hypersensitivity (Fig. 6B).
FIGURE 6.
Paw withdrawal threshold in the Hargreaves assay does not correlate to average Z-score of behavior in the forced swim test, elevated zero maze, and open field test.
A: Equation used to calculate z-score for individual measurements of latency to immobility in FST, time spent immobile in FST, OR entries into open arms of EZM, time spent in open arms of EZM; AND entries into center of OFT, time in center of OFT.
B: Key for correlations. The average z-score for FST/OFT or EZM/OFT behavior lies on the Y-axis; higher values correspond to fewer entries and less time spent into the open arms OR less time immobile and longer latency to immobility, and fewer entries and less time spent in the center.
C: Individual paw withdrawal threshold in the Hargreaves assay plotted versus average Z-score of time spent immobile in the FST, latency to immobility in the FST, entries into center of the OFT, and time spent in the center of the OFT, four weeks after CFA or saline injection (n=11–12).
D: Individual paw withdrawal threshold in the Hargreaves assay plotted versus average Z-score of time spent immobile in the FST, latency to immobility in the FST, entries into center of the OFT, and time spent in the center of the OFT, six weeks after CFA or saline injection (n=11–23).
E: Individual paw withdrawal threshold in the Hargreaves assay plotted versus average Z-score of entries into the open arms of the EZM, time spent in the open arms of the EZM, and time spent in the center of the OFT, four weeks after CFA or saline injection (n=10,10).
F: Individual paw withdrawal threshold in the Hargreaves assay plotted versus average Z-score of entries into the open arms of the EZM, time spent in the open arms of the EZM, and time spent in the center of the OFT, six weeks after CFA or saline injection (n=4–11).
G: Individual paw withdrawal threshold in the Hargreaves assay plotted versus average Z-score of time spent immobile in the FST, latency to immobility in the FST, entries into center of the OFT, and time spent in the center of the OFT, four weeks after CFA or saline injection (order of behavior testing reversed from A) (n=11–12).
H: Individual paw withdrawal threshold in the Hargreaves assay plotted versus average Z-score of entries into the open arms of the EZM, time spent in the open arms of the EZM, and time spent in the center of the OFT, four weeks after CFA or saline injection (order of behavior testing reversed from C) (n=6–10).
Composite behavior scores mostly did not correlate to Hargreaves paw withdrawal latency, for saline- or CFA-injected mice. Regardless of injection, mice tested four or six weeks after injection in the FST followed by the OFT did not have a correlation between behavioral outcomes and paw withdrawal latency (SAL Pearson r=−0.3706, p=0.2594, n=12; CFA Spearman r=−0.3182, p=0.3415, n=11; CFA+REC Spearman r=−0.3427, p=0.2762, n=12 Fig. 6C; SAL Pearson r=0.2373, p=0.2757, n=23; CFA Pearson r=0.−0.3259, p=0.2555, n=14; REC Pearson r=−0.2545, p=0.4502, n=11; CFA+REC Pearson r=−0.3746, p=0.0651, n=25 Fig. 5D). Regardless of injection, behavior scores and paw withdrawal latencies also did not correlate in mice tested four or six weeks after injection in the EZM followed by the OFT (SAL Pearson r=0.4550, p=0.1864, n=10; CFA Pearson r=−0.1731, p=0.6325, n=10 Fig. 6E; SAL Pearson r=0.2244, p=0.5071, n=11; CFA Pearson r=−0.2024, p=0.7006, n=6; REC Pearson r=−0.1729, p=0.8271, n=4; CFA+REC r=−0.5400, p=0.1071, n=10 Fig. 6F). There was no association between behavior and paw withdrawal in mice tested four weeks after injection in the OFT first, followed by the FST (SAL Pearson r=−0.4512, p=0.1410, n=12; CFA Pearson r=−0.05600, p=0.8701, n=11; CFA+REC Pearson r=−0.3920, p=0.2075, n=12 Fig. 6G). There was a significant association between lower composite behavioral scores and higher thermal hyperalgesia in mice injected with CFA and tested first in the OFT, followed by the EZM, but this correlation did not persist when all mice injected with CFA regardless of thermal hyperalgesia status were grouped, i.e., CFA+REC (SAL Pearson r=0.06132, p=0.8664, n=10; CFA r=0.9310, p=0.0070, n=6, REC r=−0.1193, p=0.9821, n=6; CFA+REC Pearson r=−0.2009, p=0.5312, n=12 Fig. 6H). Individual z-scores for the FST time immobile and latency to immobility, the OFT entries into and time spent in the center, the average of those four z-scores per individual, and the Hargreaves z-scores from the cohort of animals tested in the FST and OFT 6 weeks after injection are provided for example in Fig. S7. Ultimately, these results emphasize that there is no relationship between CFA-induced persistent inflammatory pain and exploratory behavior or stress coping behavior.
Previously published effects of CFA on stress coping strategy and exploratory behavior are heterogeneous.
To gauge the translational limitations or benefits of the FST, EPM, and OFT in basic pain research and provide a summary and context of previously published findings, three discrete meta-analyses were conducted on studies reporting the time spent immobile in the FST, time spent in the open arms of the EPM, or in the center of the OFT, after varying intervals post-injection in male C57BL/6 mice injected with saline or CFA. Five databases were searched in April 2020 with inclusion criteria targeting male C57BL/6 mice injected once and unilaterally in either hind-paw (Table S2). Out of 1395 total search results, 15 studies were included in 3 separate meta-analyses (Figure S8).
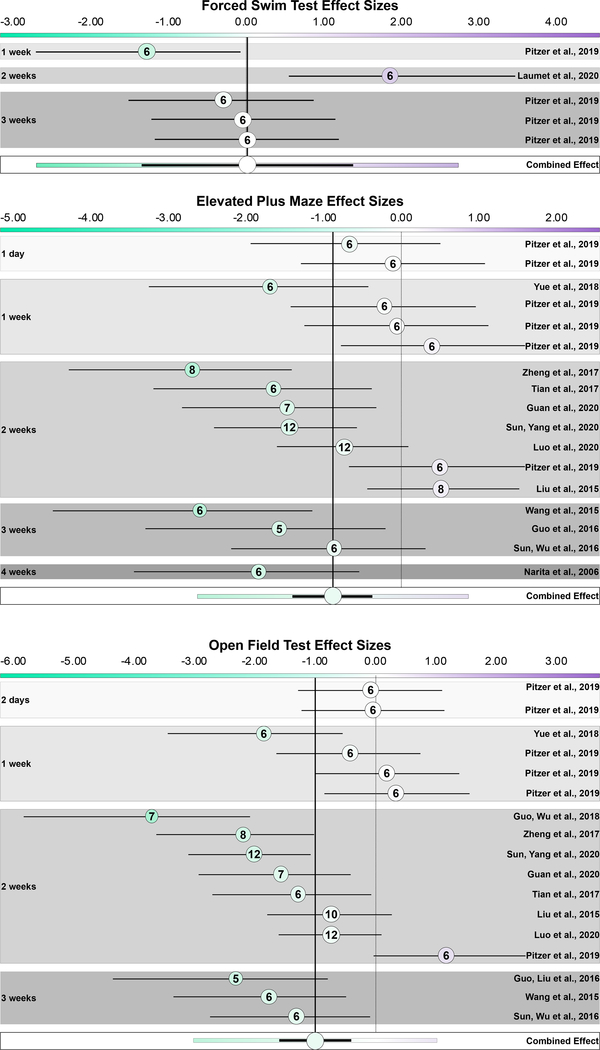
Five discrete cohorts from two studies, conducted at one-, two-, and three- weeks post-injection, evaluated immobility in the FST (Fig. 7A). Substantial heterogeneity is observed although three out of five cohorts have non-significant effects (I2 = 69.19%). The combined effect is not significant as well, although the meta-analysis predicts that 95% of future studies may have effect sizes ranging from −2.70 to 2.74 (Hedges g = 0.02 [95% CI −1.34 to 1.38], p = 0.966, n = 60) (Fig. 7A). This range quantifies the current uncertainty of the degree to which CFA alters coping strategy to an inescapable stressor: studies using other strains of mice, rats, or the tail suspension test alternative do not consistently demonstrate an effect of CFA either [28,34,61,67].
FIGURE 7.
There is high heterogeneity of CFA effect size on stress coping strategy and exploratory behavior, among previously published studies. Each panel is a distinct meta-analysis for each behavioral test: forced swim test, elevated plus maze, and open field test. Hedges g effect sizes align on a divergent color scale unique to each panel; green saturation corresponds to negative values, purple saturation corresponds to positive values, and they converge to white at 0. The dashed line marks 0; the solid line marks the combined effect size, and both are provided for comparison to where individual studies lie on the scale. Studies are ordered by interval between CFA injection and assessment. The size of the circle corresponds to the precision of the study and consequently its weight in the combined effect size in a random-effects model. The color of the circle aligns on the effect size scale. The number inside the circle indicates the lower of saline or CFA n. The black lines indicate 95% confidence intervals. In the combined effect, the colored lines extending past the black 95% confidence interval are the 95% prediction interval.
A: Effect sizes of individual studies measuring immobility in the forced swim test, ordered by interval between injection and assessment date. Among previously published studies, there is no significant effect of CFA on FST immobility and 95% of future studies will have effect sizes that fall on either side of 0. Heterogeneity is high.
B: Effect sizes of individual studies measuring time spent in the open arms of the elevated plus maze, ordered by interval between injection and assessment date. Among previously published studies, there is a significant effect of CFA on EZM time in open arms, but 95% of future studies will have effect sizes that fall on either side of 0. Heterogeneity is high.
C: Effect sizes of individual studies measuring time spent in the center of an open field, ordered by interval between injection and assessment date. Among previously published studies, there is a significant effect of CFA on OFT time in center, but 95% of future studies will have effect sizes that fall on either side of 0. Heterogeneity is high.
The combined effect of 17 discrete cohorts from 12 studies assessing time spent in the open arms of the EPM indicates a modest but significant combined effect of CFA on such exploratory behavior (Hedges g = −0.88 [95% CI −1.40 to −0.37], p < 0.001, n = 238) (Fig. 7B) [27,47]. Similarly to FST experiments, there is substantial heterogeneity between studies, with 95% of future studies predicted to have effect sizes ranging from −2.63 to 0.86 (I2 = 67.06%) (Fig. 7B) [16,18,32,33,36,47,55,56,60,64–66]. When restricting the meta-analysis to cohorts tested at the same single time point, a significant combined effect is still observed at three weeks after the induction of inflammatory pain (Hedges g = −0.95 [95% CI −2.02 to 0.12], p = 0.031, n = 120 comparisons, p = 0.031, I2 = 77.88%). Of these 7 studies, 4 individually have non-significant effect sizes. The prediction interval suggests that 95% of future studies will have effect sizes between −3.54 and 1.65.
Finally, 17 discrete cohorts from 12 studies evaluated exploratory behavior in the OFT (Fig. 7C) [16–18,32,33,47,55,56,60,64–66]. The summary estimate indicates a small but significant decrease in time spent in the center of an open field arena after CFA injection and that 95% of future studies will have effect sizes ranging from −3.02 to 1.02 (Hedges g = −1.00 [95% CI −1.60 to −0.40], p < 0.001, n = 239, I2 = 72.30%) (Fig. 7C). Restricting the meta-analysis to cohorts tested at the same single time point indicated a significant combined effect only at three weeks post-injection; however, half the included studies had non-significant individual effect sizes (Hedges g = −1.30 [95% CI −2.43 to −0.18], p = 0.006, n = 133, I2 = 78.79%).
The age, side injected, sub-strain, housing condition of each cohort and lighting condition of each study for the FST, EPM, and OFT meta-analyses are detailed in Tables S3, S4, and S5 respectively.
The results from these three meta-analyses suggest that the FST, EPM, and OFT are inconsistent in their representation of inflammatory-pain-altered behaviors. Many of these studies had under-powered group sizes (n = 6, most commonly) that may have biased observed effects, and some heterogeneity may also be due to whether subjects were naïve to testing or had experienced other, potentially stressful behavioral assessments prior to the FST, EPM, or OFT. We took into consideration these parameters ourselves, to systematically evaluate the degree to which long-term inflammatory pain can affect these metrics.
Discussion:
The current study considers how the particular choice, combination, and order of behavioral tests may determine behavioral outcomes; how multiple measurements across different tests relate to one another; how behavior may change across the duration of an assessment; and how the duration of inflammatory pain may determine its phenotype. Using six discrete experimental designs, our study describes few differences observed between saline- and CFA-injected animals, regardless of thermal hyperalgesia status. Our results also provide the first systematic quantification of the effect of CFA injection on the three most commonly used behavioral tests in rodent models of pain: the FST, EPM/EZM, and OFT.
CFA-induced persistent pain has long been an effective tool for studying inflammation and nociception; however, our understanding of the degree to which this condition impacts activity and behavior remains inconclusive [12,41,49]. While some studies have observed disturbances in sleep, gait, and social interaction, others have been unable to replicate these results or identify similar changes in locomotor activity, sleep, or food intake [31,43,46,53,61]. Novel analgesics may fail so often in clinical trials because of the difficulty in holistically representing the reduced quality of life and emotional experience of pain in the rodent model organism [9].
To better understand our results and the informative limits of such phenotyping, we conducted a targeted meta-analysis on similar studies, i.e. any in which male C57BL/6 mice were once unilaterally injected with saline or CFA, and later compared in the FST, EPM or EZM, or OFT. These meta-analyses identified no significant effect of CFA on FST immobility and indicated a modest but significant effect of CFA on time spent in the center of an OFT and in the open arms of elevated mazes. The prediction intervals of the summary effects for both the EPM and OFT meta-analyses crossed the line of null hypothesis (effect size = 0), however, suggesting that 95% of future studies in a similar population will have true effect sizes that may be positive or negative, or show no effect at all. While the confidence intervals indicate the probable range in which the true effect lies, the prediction interval is a distribution of most possible effects, and thus the range of most future results. Therefore, although the meta-analyses indicated a small but significant effect of CFA on exploratory behavior, these statistics also suggest continued inconclusiveness of future data.
All three meta-analyses also indicated high heterogeneity of effect size (>65%), suggesting the influence of variables such as sub-strain (N vs. J), injected paw (right vs. left), or methodological details specific to each test (lighting and duration). Although the inclusion criteria restricted to studies using male C57BL/6 mice, there is perhaps even greater heterogeneity among studies using other mouse strains, rats, or females.
The variance may also be due in part to individual differences among mice, even in such an inbred strain [37]. Even if the average of groups is equivalent, outliers may be informative if the same individual has a pattern of similar behavior across multiple assessments. Composite behavioral scoring for multiple assessments improves on conventional reliance on a single endpoint and may cross-validate such observations [20,44]. To identify whether certain mice were more susceptible to the effects of CFA, commonly reported behavioral measurements from each behavioral test (i.e. EPM and OFT, or FST and OFT) were converted into z-scores and averaged for each individual. Although there was expected variation in composite z-score, and degree of its correlation to thermal hypersensitivity, there was no evidence that a subpopulation was affected in ways that most others were not. Other studies have similarly demonstrated that the affective component of pain, as modeled by performance in a place escape/avoidance paradigm, does not correlate with performance in an EZM in CFA-injected animals [51].
The subset of CFA-injected mice without thermal hyperalgesia (“recovered,” REC) were not significantly different from saline- or CFA-injected mice with lower paw withdrawal latencies, on nearly any behavioral measure across all six experimental designs. In one exception, REC mice spent less time in the open arms of the EZM than saline-injected, but not CFA-injected mice exhibiting thermal hyperalgesia, six weeks after injection. Separating mice injected with CFA into those exhibiting the typical depressed paw withdrawal latency, and those whose inflammation had somewhat or fully recovered, permits a direct comparison between mice ostensibly experiencing inflammatory pain itself, and those without. Almost no meaningful differences were observed when saline-injected mice were compared to all mice injected with CFA, regardless of thermal hyperalgesia status.
Although the rodent as a model organism is otherwise well-suited for exploring allostatic phenomena and identifying druggable targets, there has been recent and justified questioning of the face and construct validity of the FST and EPM for any aspect of human behavior. The classical rhetoric of “anxiety-like” and “depressive-like” behaviors has been rejected, as has the reliance on only one these assays for conclusions about the etiology or treatment of major depressive disorder or generalized anxiety disorder. The predictive validity of the FST for antidepressants and of the EPM and OFT for anxiolytics is now more of a limitation. More conservatively, the EPM and OFT provide novel, anxiogenic environments for exploratory behavior, and the FST provides an inescapable stressor to measure active versus passive coping [10].
This reinterpretation fits into the National Institute of Mental Health’s Research Domain Criteria, a research classification system with the strategic goal of improving the translatability of neuroscience research and boosting its relevance to clinical decisions [11]. Considering what these assessments represent of fundamental and primary behavioral functions, rather than of inherent psychopathology, may yield more meaningful answers. If these assays were to represent inherent psychopathology, it would stand to reason that measurements in one could associate with or predict performance in another. Our correlational analyses of the two most commonly reported outcomes for each test, however, found they mostly did not correlate to each another, such that low exploratory behavior in the EPM did not coincide with similar performance in the OFT, etc.
This was true regardless of the order or combination of tests administered; however, mice tested in the OFT first when naïve to other measures such as the EZM or FST exhibited a slight increase in exploratory behavior in the open field. Experiencing the OFT 48 hours prior to the FST resulted in a decrease in latency to immobility in the FST among mice injected with CFA as well; however, exploratory behavior was most reduced by CFA three weeks after injection according to the meta-analysis. Exploring this discrepancy further is merited and may identify a critical period in which effects of CFA on exploratory behavior are clearest.
Although mice are ill-suited for modeling subjective states such as pain and mood, motivated behavior has emerged as one alternative tool for uncovering the dynamic biology of pain. Pain relief is rewarding, and the progression to chronic pain is largely encoded by the same mesocorticolimbic circuitry governing reward and aversion [6,39]. The translational implications are critical; patients may lose motivation to comply with treatment protocols or may misuse opioid medications [26,29]. Our laboratory has demonstrated how CFA-induced inflammatory pain decreases heroin self-administration at lower doses but increases it at higher doses; this coincides with desensitization of mu opioid receptors in the ventral tegmental area [19]. We have further identified how inflammatory pain recruits and hyperactivates the kappa opioid receptor system in the nucleus accumbens to induce a similar pain-induced motivational deficit in a progressive ratio task for sucrose pellets [35].
Interestingly, the disparity between effects on motivated behavior but not on stress coping strategy has been observed before, as maternal deprivation stress can similarly reduce the breakpoint for sucrose self-administration but does not impact FST immobility [30]. These alterations in operant behavior and reward consumption may still associate, instead, with alterations in exploratory behavior and reactivity to a novel, anxiogenic environment. Thus far, this has only been observed in the sciatic nerve cuff model of neuropathic pain, as a reduction in the latency to feed during a novelty-suppressed feeding task [3,50].
Pre-clinical models of pain may therefore instead be tools with which to study how pain-induced alterations in endogenous opioid systems result in such other maladaptive behaviors. Because of the overlapping circuitry of pain and motivation, leveraging reward- or aversion-based behavior may yield greater insights for novel pain therapeutics. Recent advances in animal tracking precision, such as Deep Lab Cut, allow for the identification of subtle but potentially meaningful patterns in behavior in such tasks [22]. It is imperative that future studies expand behavioral phenotyping into multiple dimensions of analysis and domains of modeling. Thinking beyond binary outcomes, controlling for the intrinsic anxiogenic nature of such testing, and quantifying the associations among multiple measurements is the future of translatability in rodent models. Moreover, agnostically appraising the previously published literature with meta-analyses can inform the scope and methodology of future studies, such that ultimately, we maximize the information gleaned from limited resources.
Supplementary Material
Acknowledgements & Funding:
We would like to thank Dr. Robert Gereau Ph.D., Dr. Ream Al-Hasani Ph.D., Dr. Meaghan Creed Ph.D., and Dr. Simon Haroutounian Ph.D., M.Sc. for their valuable insights and feedback. The authors declare no conflict of interest or competing financial interests. This work was supported by National Institute on Drug Abuse grants DA041781, DA042499, DA045463 to JAM.
References
- [1].Anderson EM, Gomez D, Caccamise A, McPhail D, Hearing M. Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiol Stress 2019;10:100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain 2004;108:129–136. [DOI] [PubMed] [Google Scholar]
- [3].Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I. The Anterior Cingulate Cortex Is a Critical Hub for Pain-Induced Depression. Biological Psychiatry 2015;77:236–245. [DOI] [PubMed] [Google Scholar]
- [4].Bernal-Morales B, Guillén-Ruiz G, Cueto-Escobedo J, Rodríguez-Landa JF, Contreras CM. Sensitivity to diazepam after a single session of forced swim stress in weaning Wistar rats. Acta Pharm 2018;68:381–388. [DOI] [PubMed] [Google Scholar]
- [5].Biala G, Pekala K, Boguszewska-Czubara A, Michalak A, Kruk-Slomka M, Budzynska B. Behavioral and Biochemical Interaction Between Nicotine and Chronic Unpredictable Mild Stress in Mice. Mol Neurobiol 2017;54:904–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cahill CM, Taylor AMW, Cook C, Ong E, Morón JA, Evans CJ. Does the kappa opioid receptor system contribute to pain aversion? Front Pharmacol 2014;5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet 2004;363:978–988. [DOI] [PubMed] [Google Scholar]
- [8].Cheah M, Fawcett JW, Andrews MR. Assessment of Thermal Pain Sensation in Rats and Mice Using the Hargreaves Test. Bio Protoc 2017;7. doi: 10.21769/BioProtoc.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cobos EJ, Portillo-Salido E. “Bedside-to-Bench” Behavioral Outcomes in Animal Models of Pain: Beyond the Evaluation of Reflexes. Curr Neuropharmacol 2013;11:560–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci 2017;8:955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 2013;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Doron R, Lotan D, Versano Z, Benatav L, Franko M, Armoza S, Kately N, Rehavi M. Escitalopram or novel herbal mixture treatments during or following exposure to stress reduce anxiety-like behavior through corticosterone and BDNF modifications. PLoS ONE 2014;9:e91455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Feehan AK, Zadina JE. Morphine immunomodulation prolongs inflammatory and postoperative pain while the novel analgesic ZH853 accelerates recovery and protects against latent sensitization. Journal of Neuroinflammation 2019;16:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gonzalez LE, File SE. A five minute experience in the elevated plus-maze alters the state of the benzodiazepine receptor in the dorsal raphe nucleus. J Neurosci 1997;17:1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guan S-Y, Zhang K, Wang X-S, Yang L, Feng B, Tian D-D, Gao M-R, Liu S-B, Liu A, Zhao M-G. Anxiolytic effects of polydatin through the blockade of neuroinflammation in a chronic pain mouse model. Mol Pain 2020;16:1744806919900717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guo B, Wang J, Yao H, Ren K, Chen J, Yang J, Cai G, Liu H, Fan Y, Wang W, Wu S. Chronic Inflammatory Pain Impairs mGluR5-Mediated Depolarization-Induced Suppression of Excitation in the Anterior Cingulate Cortex. Cereb Cortex 2018;28:2118–2130. [DOI] [PubMed] [Google Scholar]
- [18].Guo H-L, Xiao Y, Tian Z, Li X-B, Wang D-S, Wang X-S, Zhang Z-W, Zhao M-G, Liu S-B. Anxiolytic effects of sesamin in mice with chronic inflammatory pain. Nutr Neurosci 2016;19:231–236. [DOI] [PubMed] [Google Scholar]
- [19].Hipólito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, Whittington R, Comer SD, Carlton SM, Walker BM, Bruchas MR, Morón JA. Inflammatory Pain Promotes Increased Opioid Self-Administration: Role of Dysregulated Ventral Tegmental Area μ Opioid Receptors. J Neurosci 2015;35:12217–12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Ménard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci 2015;35:16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hooten WM. Chronic Pain and Mental Health Disorders: Shared Neural Mechanisms, Epidemiology, and Treatment. Mayo Clin Proc 2016;91:955–970. [DOI] [PubMed] [Google Scholar]
- [22].Hsu AI, Yttri EA. B-SOiD: An Open Source Unsupervised Algorithm for Discovery of Spontaneous Behaviors. bioRxiv 2019:770271. [Google Scholar]
- [23].Jindal A, Mahesh R, Bhatt S. Etazolate rescues behavioral deficits in chronic unpredictable mild stress model: modulation of hypothalamic-pituitary-adrenal axis activity and brain-derived neurotrophic factor level. Neurochem Int 2013;63:465–475. [DOI] [PubMed] [Google Scholar]
- [24].Johnson A, Rainville JR, Rivero-Ballon GN, Dhimitri K, Hodes GE. Testing the Limits of Sex Differences Using Variable Stress. Neuroscience 2020. [DOI] [PubMed] [Google Scholar]
- [25].Knight B, Katz DR, Isenberg DA, Ibrahim MA, Le Page S, Hutchings P, Schwartz RS, Cooke A. Induction of adjuvant arthritis in mice. Clin Exp Immunol 1992;90:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Koob GF. Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psychiatry 2020;87:44–53. [DOI] [PubMed] [Google Scholar]
- [27].Laumet G, Edralin JD, Dantzer R, Heijnen CJ, Kavelaars A. CD3+ T cells are critical for the resolution of comorbid inflammatory pain and depression-like behavior. Neurobiol Pain 2020;7:100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Le AM, Lee M, Su C, Zou A, Wang J. AMPAkines have novel analgesic properties in rat models of persistent neuropathic and inflammatory pain. Anesthesiology 2014;121:1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Letzen JE, Seminowicz DA, Campbell CM, Finan PH. Exploring the potential role of mesocorticolimbic circuitry in motivation for and adherence to chronic pain self-management interventions. Neurosci Biobehav Rev 2019;98:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Leventopoulos M, Russig H, Feldon J, Pryce CR, Opacka-Juffry J. Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology 2009;56:692–701. [DOI] [PubMed] [Google Scholar]
- [31].Leys LJ, Chu KL, Xu J, Pai M, Yang HS, Robb HM, Jarvis MF, Radek RJ, McGaraughty S. Disturbances in slow-wave sleep are induced by models of bilateral inflammation, neuropathic, and postoperative pain, but not osteoarthritic pain in rats. Pain 2013;154:1092–1102. [DOI] [PubMed] [Google Scholar]
- [32].Liu Y, Yang L, Yu J, Zhang Y-Q. Persistent, comorbid pain and anxiety can be uncoupled in a mouse model. Physiol Behav 2015;151:55–63. [DOI] [PubMed] [Google Scholar]
- [33].Luo L, Sun T, Yang L, Liu A, Liu Q-Q, Tian Q-Q, Wang Y, Zhao M-G, Yang Q. Scopoletin ameliorates anxiety-like behaviors in complete Freund’s adjuvant-induced mouse model. Mol Brain 2020;13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Maciel IS, Silva RBM, Morrone FB, Calixto JB, Campos MM. Synergistic effects of celecoxib and bupropion in a model of chronic inflammation-related depression in mice. PLoS ONE 2013;8:e77227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Massaly N, Copits BA, Wilson-Poe AR, Hipólito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, Klaas A, Walker BM, Neve R, Cahill CM, Shoghi KI, Gereau RW, McCall JG, Al-Hasani R, Bruchas MR, Morón JA. Pain-Induced Negative Affect Is Mediated via Recruitment of The Nucleus Accumbens Kappa Opioid System. Neuron 2019;102:564–573.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 2006;31:739–750. [DOI] [PubMed] [Google Scholar]
- [37].Nasca C, Menard C, Hodes G, Bigio B, Pena C, Lorsch Z, Zelli D, Ferris A, Kana V, Purushothaman I, Dobbin J, Nassim M, DeAngelis P, Merad M, Rasgon N, Meaney M, Nestler EJ, McEwen BS, Russo SJ. Multidimensional Predictors of Susceptibility and Resilience to Social Defeat Stress. Biol Psychiatry 2019;86:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, Battaglia G, Mathé AA, Pittaluga A, Lionetto L, Simmaco M, Nicoletti F. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA 2013;110:4804–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci 2014;17:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].di Nuzzo L, Orlando R, Tognoli C, Di Pietro P, Bertini G, Miele J, Bucci D, Motolese M, Scaccianoce S, Caruso A, Mauro G, De Lucia C, Battaglia G, Bruno V, Fabene PF, Nicoletti F. Antidepressant activity of fingolimod in mice. Pharmacol Res Perspect 2015;3:e00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 2009;14:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Okun A, McKinzie DL, Witkin JM, Remeniuk B, Husein O, Gleason SD, Oyarzo J, Navratilova E, McElroy B, Cowen S, Kennedy JD, Porreca F. Hedonic and motivational responses to food reward are unchanged in rats with neuropathic pain. Pain 2016;157:2731–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Parent AJ, Beaudet N, Beaudry H, Bergeron J, Bérubé P, Drolet G, Sarret P, Gendron L. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav Brain Res 2012;229:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Peña CJ, Smith M, Ramakrishnan A, Cates HM, Bagot RC, Kronman HG, Patel B, Chang AB, Purushothaman I, Dudley J, Morishita H, Shen L, Nestler EJ. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat Commun 2019;10:5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Peng Y-L, Liu Y-N, Liu L, Wang X, Jiang C-L, Wang Y-X. Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J Neuroinflammation 2012;9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pitzer C, Kuner R, Tappe-Theodor A. Voluntary and evoked behavioral correlates in inflammatory pain conditions under different social housing conditions. Pain Rep 2016;1:e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pitzer C, La Porta C, Treede R-D, Tappe-Theodor A. Inflammatory and neuropathic pain conditions do not primarily evoke anxiety-like behaviours in C57BL/6 mice. Eur J Pain 2019;23:285–306. [DOI] [PubMed] [Google Scholar]
- [48].Racine M Chronic pain and suicide risk: A comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry 2018;87:269–280. [DOI] [PubMed] [Google Scholar]
- [49].Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006;27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ramaker MJ, Dulawa SC. Identifying fast-onset antidepressants using rodent models. Molecular Psychiatry 2017;22:656–665. [DOI] [PubMed] [Google Scholar]
- [51].Refsgaard LK, Hoffmann-Petersen J, Sahlholt M, Pickering DS, Andreasen JT. Modelling affective pain in mice: Effects of inflammatory hypersensitivity on place escape/avoidance behaviour, anxiety and hedonic state. Journal of Neuroscience Methods 2016;262:85–92. [DOI] [PubMed] [Google Scholar]
- [52].Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC. Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 2014;345:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sheahan TD, Siuda ER, Bruchas MR, Shepherd AJ, Mohapatra DP, Gereau RW, Golden JP. Inflammation and nerve injury minimally affect mouse voluntary behaviors proposed as indicators of pain. Neurobiol Pain 2017;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shi M, Wang J-Y, Luo F. Depression Shows Divergent Effects on Evoked and Spontaneous Pain Behaviors in Rats. The Journal of Pain 2010;11:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sun T, Luo L, Tian Q-Q, Wang W-J, Liu Q-Q, Yang L, Zhang K, Zhang W, Zhao M-G, Yang Q. Anxiolytic Effects of 8-O-Acetyl Shanzhiside Methylester on Acute and Chronic Anxiety via Inflammatory Response Inhibition and Excitatory/Inhibitory Transmission Imbalance. Neurotox Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sun T, Wang J, Li X, Li Y-J, Feng D, Shi W-L, Zhao M-G, Wang J-B, Wu Y-M. Gastrodin relieved complete Freund’s adjuvant-induced spontaneous pain by inhibiting inflammatory response. Int Immunopharmacol 2016;41:66–73. [DOI] [PubMed] [Google Scholar]
- [57].Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res Synth Methods 2017;8:537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tang NKY, Beckwith P, Ashworth P. Mental Defeat Is Associated With Suicide Intent in Patients With Chronic Pain. Clin J Pain 2016;32:411–419. [DOI] [PubMed] [Google Scholar]
- [59].Thakare VN, Patil RR, Oswal RJ, Dhakane VD, Aswar MK, Patel BM. Therapeutic potential of silymarin in chronic unpredictable mild stress induced depressive-like behavior in mice. J Psychopharmacol (Oxford) 2018;32:223–235. [DOI] [PubMed] [Google Scholar]
- [60].Tian J, Tian Z, Qin S-L, Zhao P-Y, Jiang X, Tian Z. Anxiolytic-like effects of α-asarone in a mouse model of chronic pain. Metab Brain Dis 2017;32:2119–2129. [DOI] [PubMed] [Google Scholar]
- [61].Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 2011;152:990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vachon-Presseau E, Roy M, Martel M-O, Caron E, Marin M-F, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ, Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain 2013;136:815–827. [DOI] [PubMed] [Google Scholar]
- [63].Verdejo-García A, López-Torrecillas F, Calandre EP, Delgado-Rodríguez A, Bechara A. Executive function and decision-making in women with fibromyalgia. Arch Clin Neuropsychol 2009;24:113–122. [DOI] [PubMed] [Google Scholar]
- [64].Wang D-S, Tian Z, Guo Y-Y, Guo H-L, Kang W-B, Li S, Den Y-T, Li X-B, Feng B, Feng D, Zhao J-N, Liu G, Zhao M-G. Anxiolytic-like effects of translocator protein (TSPO) ligand ZBD-2 in an animal model of chronic pain. Mol Pain 2015;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yue J, Wang X-S, Guo Y-Y, Zheng K-Y, Liu H-Y, Hu L-N, Zhao M-G, Liu S-B. Anxiolytic effect of CPEB1 knockdown on the amygdala of a mouse model of inflammatory pain. Brain Res Bull 2018;137:156–165. [DOI] [PubMed] [Google Scholar]
- [66].Zheng J, Jiang Y-Y, Xu L-C, Ma L-Y, Liu F-Y, Cui S, Cai J, Liao F-F, Wan Y, Yi M. Adult Hippocampal Neurogenesis along the Dorsoventral Axis Contributes Differentially to Environmental Enrichment Combined with Voluntary Exercise in Alleviating Chronic Inflammatory Pain in Mice. J Neurosci 2017;37:4145–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhong F, Liu L, Wei J-L, Hu Z-L, Li L, Wang S, Xu J-M, Zhou X-F, Li C-Q, Yang Z-Y, Dai R-P. Brain-Derived Neurotrophic Factor Precursor in the Hippocampus Regulates Both Depressive and Anxiety-Like Behaviors in Rats. Front Psychiatry 2018;9:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.