Abstract
Background and Purpose:
Perfusion imaging can risk stratify patients with symptomatic intracranial stenosis. We aim to determine the association between perfusion delay and length of hospital stay (LOS) in symptomatic middle cerebral artery (MCA) stenosis patients.
Methods:
This is a retrospective study of consecutive patients admitted to a comprehensive stroke center over 5 years with ischemic stroke or TIA within 7 days of symptom onset due to MCA stenosis (50–99%) and underwent perfusion imaging. Patients were divided into three groups: mismatch volume ≥ 15 cc based on T max > 6 sec delay, T max 4–6 sec delay, and < 4 sec delay. The outcome was LOS, both as a continuous variable and categorical (≥ 7 days (prolonged LOS) vs. <7 days). We used adjusted regression analyses to determine the association between perfusion categories and LOS.
Results:
178 of 194 patients met the inclusion criteria. After adjusting for age and NIHSS, T max > 6 sec mismatch was associated with prolonged LOS (OR 2.94 95% CI 1.06 – 8.18; p = 0.039), but T max 4–6 sec was not (OR 1.45 95% CI 0.46 – 4.58, p = 0.528). We found similar associations when LOS was a continuous variable for T max > 6 sec (β coefficient=2.01, 95% CI 0.05–3.97, p=0.044) and T max 4–6 sec (β coefficient=1.24, 95% CI −0.85–3.34, p=0.244).
Conclusion:
In patients with symptomatic MCA stenosis, T max > 6 sec perfusion delay is associated with prolonged LOS. Prospective studies are needed to validate our findings.
Keywords: Intracranial Atherosclerosis, Stroke, Perfusion, Outcome
Introduction
Length of hospital stay (LOS) in patients with ischemic stroke is a surrogate marker of increased morbidity and is influenced by several factors including stroke severity, medical comorbidities and complications,1,2 as well as in-hospital stroke recurrence.3 In addition, LOS increases the risk of hospital complications4 as well as health care cost.5 Therefore, it is of paramount importance to reduce LOS.
Intracranial atherosclerotic disease is the most common cause of stroke worldwide.6 It accounts for nearly 10% of ischemic strokes in the United States7 and up to 50% of ischemic strokes in China8 and carries a high risk of recurrence.9–12 Recent data suggests that perfusion imaging may also help predict recurrent ischemic stroke risk in patients with ischemic stroke due to large artery stenosis13 but the optimal prognostic threshold of the penumbra as a predictor of recurrent stroke remains to be established.
We aim to determine the association between delay on baseline T max (< 4 sec, 4–6 sec, and > 6 sec) perfusion weighted imaging in the territory of the middle cerebral artery (MCA) and LOS in patients with recently symptomatic MCA stenosis.
Methods
Patient population
This is a retrospective study of consecutive patients admitted to a comprehensive stroke center over a 5-year period with: (1) acute ischemic stroke or TIA within 7 days of symptom onset, (2) related to middle cerebral artery stenosis (50–99%), and (3) underwent perfusion imaging (CT or MR perfusion) processed with RAPID software on admission. To limit the effect of infarct size on LOS in our patient population, we excluded patients with core infarct ≥ 30 cc.
Predictors
Perfusion imaging was reviewed and processed using the RAPID software. Core infarct on MR perfusion was defined as apparent diffusion coefficient ≤ 620 and on CT perfusion as CBF < 30%.14, 15 Core infarct and T max based mismatch volumes were determined similar to prior studies16, 17, patients were then divided into three groups: perfusion delay volume ≥ 15 cc based on T max > 6 sec delay, perfusion delay volume ≥ 15 cc based on T max 4–6 sec delay, and no perfusion delay or volume ≥ 15 cc based on T max < 4 sec. Patients meeting criteria for both T max > 6 sec and T max 4–6 sec were considered as T max > 6 sec.
Outcome
The primary outcome in the study was hospital length of stay in days. We also divided the outcome into two groups: prolonged length of stay (≥ 7 days) and non-prolonged length of stay (< 7 days), similar to a prior study.2
Covariates
Covariates collected in this study were: age, sex, admission NIHSS score, and core infarct.
Statistical analysis
We used univariable and adjusted linear and binary logistic regression analyses to determine the association between perfusion mismatch and LOS both as a continuous variable and categorical (≥ 7 days (prolonged LOS) vs. <7 days). We also built receiver operating curves to determine the optimal volume threshold associated with prolonged LOS in each of T max categories (T max > 6 sec and T max 4–6 sec).
Results
Main results
Out of 194 patients, 178 met the inclusion criteria. Reasons for exclusion were 15 patients had core infarct volume ≥ 30 cc and the LOS was not recorded on 1 patient. The mean age was 70.1 ± 15.4 years, 52.8% (94/178) were women, the median (IQR) NIHSS was 4 (1–9); 83.2% underwent MR perfusion; 69 patients (38.8%) had a mismatch volume ≥ 15 cc based on T max > 6 sec and 55 patients 30.7% had a mismatch volume ≥ 15 cc based on T max 4–6 sec. The median (IQR) LOS was 4 days (2–8) and 57 patients (32.0%) had prolonged length of stay.
Univariates analysis
Baseline demographics, NIHSS score, and core infarct differences between the three groups are shown in the Table. In univariate analyses, when compared to group 3, patients in group 1 were more likely to have prolonged length of stay [42.0% (29/69) vs. 22.2% (12/54), p = 0.022]. In addition, the median (IQR) LOS in days was longer in group 3 vs. group 1 [5 (2–11) vs. 3 (2–6), p = 0.005]. Furthermore, when compared to group 3, patients in group 2 had a similar rate of prolonged LOS [29.1% (16/55) vs. 22.2% (12/54), p = 0.512]. Furthermore, the median (IQR) LOS in days was non-significantly higher in group 2 vs. group 1 (4 (2–7) vs. 3 (2–6), p = 0.06).
Table.
Baseline and imaging characteristics and length of stay based perfusion delay definition
| T max < 4 sec or no delay (n = 54) |
T max 4–6 sec (n = 55) |
T max > 6 sec (n = 69) |
|
|---|---|---|---|
| Age in years | 72.0 ± 14.7 | 73.6 ± 15.5 | 69.5 ± 16.1 |
| Sex (% men) | 35.2% (19) | 41.8% (23) | 60.9% (42) |
| NIHSS score (median IQR) | 2 (1 – 7) | 5 (1 – 10) | 6 (2 – 11) |
| Core infarct in cc | 1.7 ± 4.9 | 2.3 ± 6.0 | 3.9 ± 7.6 |
| Length of stay in days (median IQR) | 3 (2 – 6) | 4 (2 – 7) | 5 (2 – 11) |
| Prolonged Length of stay (≥ 7 days) | 22.2% (12) | 29.1% (6) | 42.0% (29) |
Data represents mean ± standard deviation unless otherwise indicated; NIHSS: National Institute of Health Stroke Scale; IQR: Interquartile range; Max: Maximum; n: Number of subjects
Multivariable models
After adjusting for age and NIHSS, the T max > 6 sec mismatch definition was associated with prolonged LOS (OR 2.94 95% CI 1.06 – 8.18; p = 0.039) but T max 4–6 sec definition was not (OR 1.45 95% CI 0.46 – 4.58, p = 0.528). When core infarct volume was added to regression model, the results were unchanged for T max > 6 sec (OR 2.48 95% CI 1.08 – 5.65, p = 0.031) and T max 4–6 sec (OR 1.36 95% CI 0.54 – 3.44, p = 0.516).
We found similar associations when LOS was considered as a continuous variable for T max > 6 sec (β coefficient=2.01, 95% CI 0.05–3.97, p=0.044) and T max 4–6 sec (β coefficient=1.24, 95% CI −0.85–3.34, p=0.244).
Receiver operator curves
In receiver operating curves, the T max > 6 sec mismatch definition performed slightly better (AUC 0.62, 95% CI 0.53 – 0.71, p =0.010) than the T max 4–6 sec definition (AUC 0.61, 95% CI 0.53 – 0.70, p = 0.016) in predicting prolonged LOS and the optimal perfusion delay volume for T max > 6 sec was 10 cc (sensitivity 0.61 and specificity 0.63) whereas for T max 4–6 sec it was 39 cc (sensitivity 0.61 specificity 0.56). (Figure)
Figure.
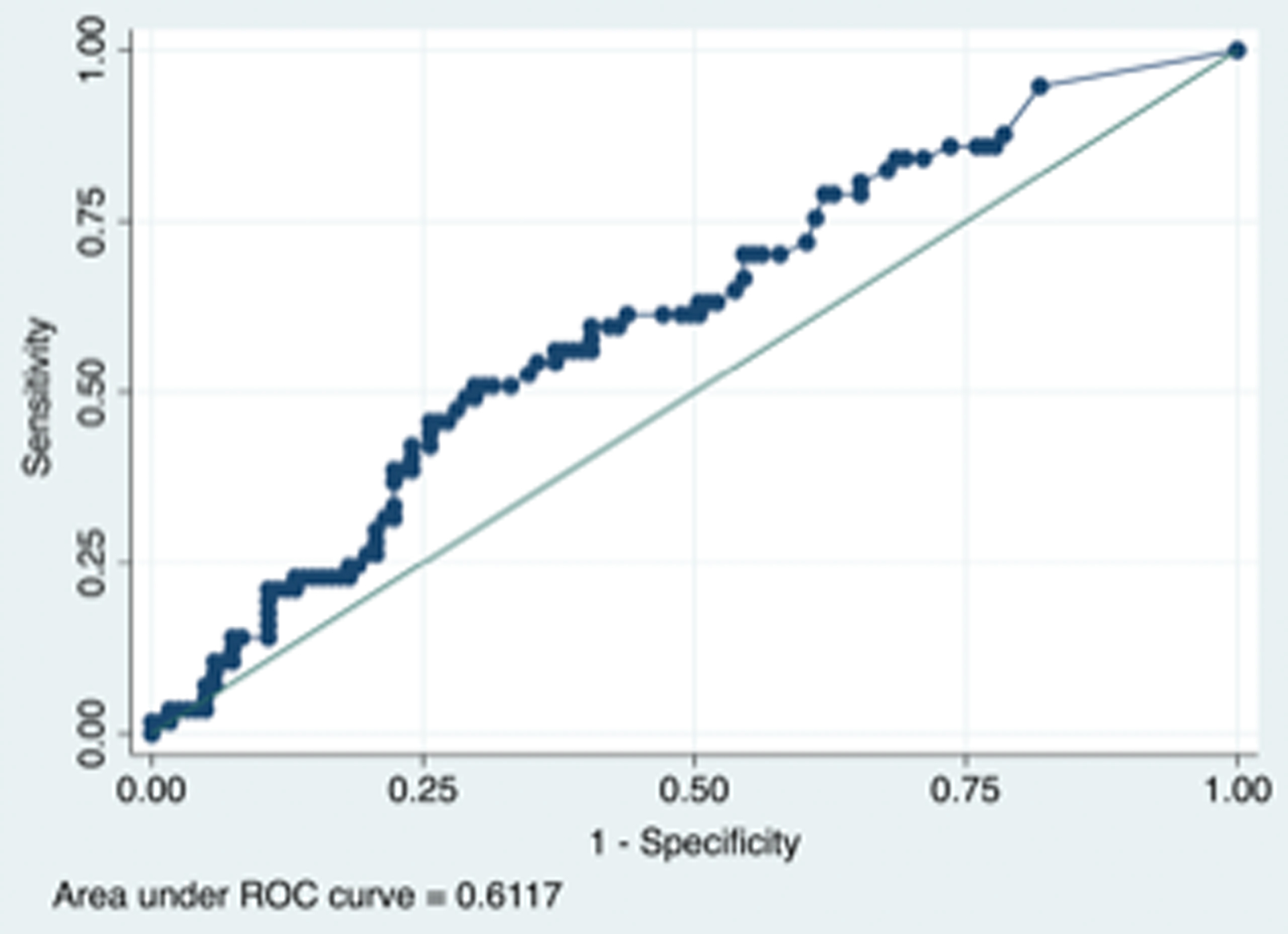
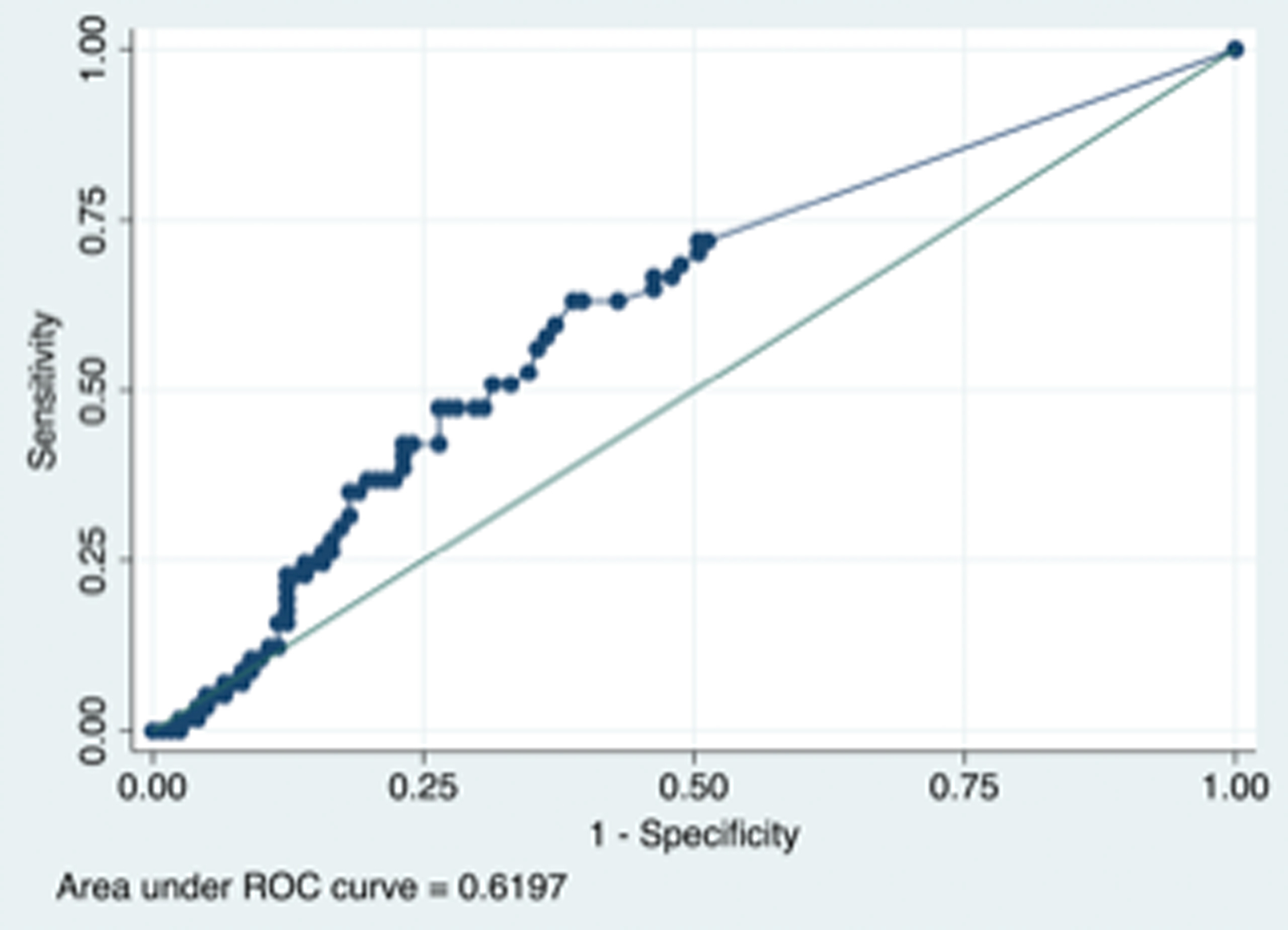
Figure 1 A legend. Receiver operative curves (ROC) showing the predictive ability of infarct volume and prolonged length of stay (≥ 7 days) based on a T maximum delay > 6 sec definition of perfusion delay (left side) with Area Under Curve (AUC) 0.62 (95% Confidence Interval 0.53 – 0.71, p = 0.010)
Figure 1 B legend. Receiver operative curves (ROC) showing the predictive ability of infarct volume and prolonged length of stay (≥ 7 days) legend based on T max delay 4–6 definition of perfusion delay (right side) with AUC 0.61 (95% Confidence Interval 0.53 – 0.70, p = 0.016).
Discussion
Our study showed that perfusion delay in patients with symptomatic middle cerebral artery stenosis is associated with hospital length of stay. This association was more pronounced in patients meeting our pre-defined criteria for mismatch based on T max > 6 sec as opposed to T max 4–6 seconds. It is noteworthy, however, that there appears to be a graded relationship between T max threshold and increased length stay, particularly that 10 cc or more of perfusion delay volume with T max > 6 sec delay appears to be an optimal threshold that predicts prolonged LOS whereas nearly 40 cc or more of perfusion delay volume with T max 4–6 sec delay appears to be an optimal threshold that predicts prolonged LOS.
There are several reasons to explain the association between perfusion delay and increased LOS in hospitalized patients with ischemic stroke in the setting of middle cerebral artery stenosis. It is possible that patients with worse perfusion delay had more severe strokes, but the association remained significant even after adjusting for NIHSS score and core infarct volume. It is also possible that patients with worse perfusion delay were more likely to have in-hospital deterioration, which may have prolonged their hospital LOS. On the other hand, it may have been that providers were more likely to keep the patients with more severe perfusion deficits in the hospital longer for close monitoring.
Perfusion based imaging has been used to aid with acute stroke treatment decisions in patients with emergent proximal large vessel intracranial occlusion,18–20 and studies have shown the benefit of mechanical thrombectomy in patients with large vessel occlusions selected using perfusion based imaging selection from 6 to 24 hours from symptom onset.21,22 It is likely that perfusion imaging can help risk stratify patients with intracranial stenosis, as well.
In general, intracranial atherosclerosis can cause ischemic stroke either by distal embolization, perforator disease, or by impaired blood flow/perfusion across a highly stenosed artery.6, 23 Rupture of the atherosclerotic plaque leads to thrombus formation. Smaller thrombi can therefore break loose and embolize distally causing distal infarcts related. In addition, the unstable plaque can extend to occlude perforating branches leading to deep perforator infarcts. Furthermore, plaque rupture can cause luminal narrowing impeding blood flow and thus causing infarcts related to reduced distal perfusion.6,23,24
Although medical treatment with antithrombotic and anti-lipidemic agents can help stabilize atherosclerotic plaques and intracranial arterial thrombogenesis and reduce the risk of embolization, it is unlikely to improve blood flow or perfusion in the affected territory and thus may not be effective in preventing recurrent stroke in patients with impaired distal blood flow or perfusion. In addition, hemodynamic augmentation has no consistently shown benefit.25 Therefore, patients with ICAS causing impaired distal blood flow or perfusion may benefit from revascularization beyond aggressive medical management alone.24
Our study confirms findings from another study26 suggesting that patients with 15 cc or more of T max > 6 sec mismatch may be a higher risk group but large prospective studies are needed to validate these findings in an attempt to study the safety and efficacy of reperfusion in patients with symptomatic intracranial stenosis and impaired distal perfusion.
Our study has several major limitations. These include its single center, observational nature, and selection bias on who received perfusion imaging. In addition, we did not have data on in-hospital medical complications such as infection or recurrent stroke as well as ischemic lesion location and topography, which are major shortcomings, limiting our ability to draw strong conclusions. Finally, data on comorbidities and treatments was not available in our study and therefore imbalanced comorbidities and treatments between the perfusion delay groups could have contributed to prolonged length of stay in patients with perfusion delay. Our study is hypothesis generating and associations found in our study do not suggest causality. Prospective studies with data on treatments and comorbidities are needed to uncover specific reasons for increased length of stay in patients with intracranial stenosis and perfusion delay.
On the other hand, our study has several strengths including its large sample size of intracranial atherosclerosis patients with perfusion imaging obtained and standardization of imaging software used for imaging analysis.
Therefore, our study suggests that in patients with recently symptomatic MCA stenosis, the T max > 6 sec definition for mismatch, but not T max 4–6 sec, is associated with prolonged LOS. Given our study limitations, prospective studies are needed to validate our findings and define the optimal mismatch threshold in patients with symptomatic MCA stenosis.
Acknowledgement and Disclosure:
Dr. Radoslav recioeved funding from Medtronic, Perflow Medical, Rapid Medical, Spartan Micro, Boehringer Ingelheim, and Society of vascular Interventional Neurology, and he is share-houlder and co-founder of ICAD endovascular. Dr. Romano recieved grant support from NIH/NINDS to the University of Miami, Miller School of Medicine for role as multiple PI of MyRIAD study (R01 NS084288). All other authors have no relevant financial disclosures.
References
- 1.Chang KC, Tseng MC, Weng HH, Lin YH, Liou CW, Tan TY. Prediction of length of stay of first-ever ischemic stroke. Stroke 2002;33:2670–4 [DOI] [PubMed] [Google Scholar]
- 2.Koton S, Bornstein NM, Tsabari R, Tanne D. Derivation and validation of the prolonged length of stay score in acute stroke patients. Neurology 2010;74:1511–6 [DOI] [PubMed] [Google Scholar]
- 3.Yu F, Liu X, Yang Q, Fu Y, Fan D. In-hospital recurrence in a chinese large cohort with acute ischemic stroke. Sci Rep 2019;9:14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosman M, Rachminov O, Segal O, Segal G. Prolonged patients’ in-hospital waiting period after discharge eligibility is associated with increased risk of infection, morbidity and mortality: A retrospective cohort analysis. BMC Health Serv Res 2015;15:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YC, Hu CJ, Lee TH, et al. The impact factors on the cost and length of stay among acute ischemic stroke. J Stroke Cerebrovasc Dis 2013;22:e152–8 [DOI] [PubMed] [Google Scholar]
- 6.Banerjee C, Chimowitz MI. Stroke caused by atherosclerosis of the major intracranial arteries. Circ Res 2017;120:502–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: The northern manhattan stroke study. Neurology 1995;45:659–63 [DOI] [PubMed] [Google Scholar]
- 8.Wong LK. Global burden of intracranial atherosclerosis. Int J of Stroke 2006;1:158–9 [DOI] [PubMed] [Google Scholar]
- 9.Yaghi S, Rostanski SK, Boehme AK, et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA Neurol 2016;73:572–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sangha RS, Naidech AM, Corado C, Ansari SA, Prabhakaran S. Challenges in the medical management of symptomatic intracranial stenosis in an urban setting. Stroke 2017;48:2158–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (sammpris): The final results of a randomised trial. Lancet 2014;383:333–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacchetti DC, Cutting SM, McTaggart RA, et al. Perfusion imaging and recurrent cerebrovascular events in intracranial atherosclerotic disease or carotid occlusion. Int J Stroke 2018;13:592–9 [DOI] [PubMed] [Google Scholar]
- 14.Campbell BC, Christensen S, Levi CR, et al. Cerebral blood flow is the optimal ct perfusion parameter for assessing infarct core. Stroke 2011;42:3435–40 [DOI] [PubMed] [Google Scholar]
- 15.Mokin M, Levy EI, Saver JL, et al. Predictive value of rapid assessed perfusion thresholds on final infarct volume in swift prime (solitaire with the intention for thrombectomy as primary endovascular treatment). Stroke 2017;48:932–8 [DOI] [PubMed] [Google Scholar]
- 16.Yaghi S, Grory BM, Prabhakaran S, et al. Infarct pattern, perfusion mismatch thresholds, and recurrent cerebrovascular events in symptomatic intracranial stenosis. J Neuroimaging 2019;29:640–4 [DOI] [PubMed] [Google Scholar]
- 17.de Havenon A, Khatri P, Prabhakaran S, et al. Hypoperfusion distal to anterior circulation intracranial atherosclerosis is associated with recurrent stroke. J Neuroimaging 2020;30:468–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemmens R, Mlynash M, Straka M, et al. Comparison of the response to endovascular reperfusion in relation to site of arterial occlusion. Neurology 2013;81:614–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jovin TG, Saver JL, Ribo M, et al. Diffusion-weighted imaging or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with trevo (dawn) trial methods. Int J Stroke 2017;12:641–52 [DOI] [PubMed] [Google Scholar]
- 20.Lansberg MG, Straka M, Kemp S, et al. Mri profile and response to endovascular reperfusion after stroke (defuse 2): A prospective cohort study. Lancet Neurol 2012;11:860–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McTaggart RA, Yaghi S, Sacchetti DC, et al. Mechanical embolectomy for acute ischemic stroke beyond six hours from symptom onset using mri based perfusion imaging. J Neurol Sci 2017;375:395–400 [DOI] [PubMed] [Google Scholar]
- 22.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21 [DOI] [PubMed] [Google Scholar]
- 23.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol 2013;12:1106–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaghi S, Prabhakaran S, Khatri P, Liebeskind DS. Intracranial atherosclerotic disease. Stroke 2019;50:1286–93 [DOI] [PubMed] [Google Scholar]
- 25.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2019;50:e344–e418 [DOI] [PubMed] [Google Scholar]
- 26.Yaghi S, Khatri P, Prabhakaran S, et al. What threshold defines penumbral brain tissue in patients with symptomatic anterior circulation intracranial stenosis: An exploratory analysis. J Neuroimaging 2019;29:203–5 [DOI] [PubMed] [Google Scholar]


