Abstract
Objective:
Poorer executive function (EF) has been linked to disinhibited eating in youth, suggesting poor EF predisposes towards obesity, yet the specific nature and extent of interconnections between facets of these domains is unclear. Network analysis provides a promising framework for elucidating these relationships and offers insights into potential maintenance processes.
Method:
Among youth ages 8-17y, a regularized partial correlation network of EF and disinhibited eating facets was estimated to examine centrality and bridge strength for each facet. Computerized neurocognitive tasks assessed EF variables (decision-making, general and food-related inhibitory control, delayed gratification, cognitive flexibility, working memory). Disinhibited eating variables included total carbohydrate + fat intake at a laboratory test meal and self-reported eating in the absence of hunger, emotional eating, and loss-of-control eating severity. A network comparison test across those with and without overweight/obesity was also conducted.
Results:
In the full sample (N=248; Mage = 12.5; 54.8% female; 43.5% non-Hispanic White; BMI %ile = 65.8±27.8), emotional eating in response to depressive symptoms emerged as a central symptom in the network, although carbohydrate + fat intake had the highest bridge strength and was most strongly connected to general inhibitory control (part r=.14). Networks for youths with and without overweight/obesity did not significantly differ in structure or strength.
Discussion:
The link between general inhibitory control and objective palatable food intake may be particularly salient in maintaining maladaptive eating behavior. Interventions targeting EF facets including behavioral disinhibition should be tested to determine if improving EF can reduce propensity for obesity in children.
Keywords: network analysis, executive function, eating behavior, binge eating, loss-of-control eating, child and adolescent, childhood obesity
Introduction
Executive dysfunction, defined as impaired higher-order cognitive processing involved in self-regulation, is a multi-dimensional construct comprising domains such as cognitive flexibility, inhibitory control, working memory, decision-making, and ability to delay gratification (Miyake et al., 2000). Among youth, executive dysfunction has been associated with disinhibited eating behaviors (Kelly et al., 2020; Van Malderen, Goossens, Verbeken, & Kemps, 2018) and may predispose youth to excess weight gain (Goldschmidt, Hipwell, Stepp, McTigue, & Keenan, 2015). Yet, the nature and extent of the interrelationships among specific dimensions of executive dysfunction and disinhibited eating is unclear. The current study aims to explore this gap in the literature by investigating the pathways that connect facets of executive dysfunction and disinhibited eating among youth.
Weaker executive function (EF) has been linked most strongly with disinhibited eating behaviors characterized by a lack of restraint (Maayan, Hoogendoorn, Sweat, & Convit, 2011; Nederkoorn, Dassen, Franken, Resch, & Houben, 2015), such as loss-of-control (LOC)-eating, emotional eating, and overeating in the absence of hunger (Shomaker, Tanofsky-Kraff, & Yanovski, 2011). LOC-eating, the key feature of binge eating disorder (BED) and a precursor to excess weight gain (Tanofsky-Kraff, Yanovski, et al., 2009), has been associated with poor performance on measures of planning and working memory (Goldschmidt et al., 2018) and inhibitory control (Reinblatt et al., 2015) among youth. Children who report greater difficulties with dimensions of EF, such as inhibitory control, working memory, and emotional control, show greater consumption of energy-dense foods both when hungry and in the absence of hunger (Riggs, Spruijt-Metz, Sakuma, Chou, & Pentz, 2010). Further, youth with obesity perform significantly worse than their peers on measures of EF, particularly tasks measuring inhibitory control (Bozkurt et al., 2017; Pearce, Leonhardt, & Vaidya, 2018; Reinert, Po’e, & Barkin, 2013). In addition to poor inhibitory control, obesity has also been linked to poorer performance on measures of ability to delay gratification (Schlam, Wilson, Shoda, Mischel, & Ayduk, 2013) and cognitive flexibility (Groppe & Elsner, 2017). Such results suggest there may be differences in the links between EF and eating behaviors among youth with and without obesity that warrant further investigation.
Grounded in the network theory of psychopathology, which suggests that disorders arise from and are maintained by dynamic relationships among symptoms that may reinforce one another over time (Borsboom, 2017; Cramer, Waldorp, Van Der Maas, & Borsboom, 2010), network analysis can offer insight into how symptoms may be interrelated and reinforcing. This analytic approach allows for identification of central symptoms, or nodes, that have the greatest influence within a network overall, as well as bridge pathways, or links between distinct network communities. As such, central and bridge symptoms may represent promising targets for interventions because they may theoretically disrupt the overall network (central) and/or the connection between co-occurring constructs (bridge). Identifying central and bridge symptoms may offer clinically salient insights into the complex links between dimensions of executive dysfunction and disinhibited eating behaviors.
Building on an emerging literature using network analysis in studies of disinhibited and disordered eating (Brown et al., 2020; DuBois, Rodgers, Franko, Eddy, & Thomas, 2017; Forbush, Siew, & Vitevitch, 2016; Levinson et al., 2018; Levinson et al., 2017; Smith et al., 2018; Vanzhula, Calebs, Fewell, & Levinson, 2019), this investigation examined the links between dimensions of executive dysfunction and facets of disinhibited eating in a sample of healthy youth to identify central facets within an executive dysfunction-disinhibited eating network and bridge pathways between executive dysfunction and disinhibited eating variables. Given that inhibitory control has been most consistently and robustly associated with disinhibited eating (Liang, Matheson, Kaye, & Boutelle, 2014), it was hypothesized that both general and food-related inhibitory control would emerge as key network bridge factors. We conducted an exploratory network comparison test to investigate potential network differences between youth with (OW/OB+) and without (OW/OB−) overweight/obesity.
Methods
Participants and Procedures
Participants were boys and girls, 8-17y, in generally good health. See Supplement for detailed exclusion criteria. All youth and a parent/guardian supplied written assent and consent. Youth completed eating-related questionnaires, semi-structured clinical interviews, and, after an overnight fast, at approximately 10:00 AM, participants consumed a standardized breakfast shake (17% protein, 16% fat, 67% carbohydrate) containing 21% of daily energy needs, as estimated by measured body weight, height, age, and average activity level within the previous week (Craig et al., 2003; Institute of Medicine, 2006). At approximately 12:30 PM, participants completed a laboratory test meal. After the test meal, neurocognitive tasks were administered on a computer. The study procedure was approved by the National Institutes of Health Institutional Review Board.
Measures
Facets of Executive Function were measured using a battery of computerized neurocognitive tasks (see Supplement for additional details). On certain tasks, lower scores typically indicate worse EF (e.g., Iowa Gambling, Delay Discounting, Dimensional Card Sort, Working Memory), while higher scores on other tasks indicate worse EF (e.g., Stop-Signal, Food Go/No-Go). Given this discrepancy, for ease of interpretation in the network, constructs that were originally expected to negatively relate to greater disinhibited eating (i.e. lower scores indicated worse EF) were reverse-scored so that the directionality of the expected relationship with disinhibited eating was hypothesized to be positive for all EF constructs. This reverse-scoring method has been utilized in prior network analytic studies (Evanovich, Marshall, David, & Mumma, 2019). Thus, in final analyses, higher scores indicated greater executive dysfunction for all measures.
Decision-Making for monetary rewards was assessed with the Iowa Gambling Task (IGT) (Bechara, Damasio, Damasio, & Anderson, 1994), which examines decision-making skills including uncertainty, risk, and evaluation of reward and punishment. Performance on IGT is defined as the difference between the number of advantageous and disadvantageous selections across five blocks. Previous studies have used the IGT to assess decision-making in children and adolescents (Garon, Moore, & Waschbusch, 2006; McNally, Shear, Tlustos, Amin, & Beebe, 2012).
General Inhibitory Control was assessed using the Stop Signal Task (SST) (Logan, Cowan, & Davis, 1984; Logan, Schachar, & Tannock, 1997). Participants are asked to make a decision about a series of stimuli. In 25% of the trials, an auditory “stop signal” is presented at variable intervals before the stimulus presentation, which cues participants to inhibit their response. The stop signal presentation timing varies depending on successful or unsuccessful trials. The primary indicator of performance on this task is the stop signal reaction time, calculated by subtracting the mean stop signal delay from the mean reaction time. A longer reaction time (i.e., higher scores) represents poorer inhibitory control.
Food-Related Inhibitory Control was assessed using the Food Go/No-Go (Food GNG) task (Teslovich et al., 2014), which instructed participants to press the space bar when a specific stimulus-type appeared (“Go” trials), but inhibit pressing the space bar when a different stimulus-type appeared (“No-Go” trials). The task contained four randomized, counterbalanced blocks; two of which contained neutral toy stimuli as Go cues and food stimuli as No-Go cues (i.e., Neutral Toy = Go cue/ Food = No-Go cue, hereafter referred to as “Neutral-Go” blocks), while the other two blocks contained food stimuli as Go cues and neutral toy stimuli as No-Go cues (i.e., Food = Go cue/ Neutral Toy = No-Go cue, hereafter referred to as “Food-Go” blocks). The variable of interest is the percentage of total commission errors (inaccurate No-Go trial responses). A higher percentage of commission errors represents worse inhibitory control (Batterink, Yokum, & Stice, 2010; Teslovich et al., 2014). Commission errors were also analyzed separately for Neutral-Go blocks and Food-Go blocks (Teslovich et al., 2014) to examine differentiated measures of food-related inhibitory control.
Ability to Delay Gratification for Food, a component of impulsivity and decision-making, was measured using the Delay Discounting Task (Odum, Baumann, & Rimington, 2006). This task captures one’s ability to modulate a desire for immediate gratification for the sake of longer-term goals. Participants were randomly presented with a series of choices between a portion of palatable food after a specified delay, or a smaller amount of food available immediately. A discounting function is calculated to indicate participant’s willingness to wait for a larger reward.
Cognitive Flexibility, also known as “set-shifting”, or one’s ability to shift responses based on a rule or contingency, was examined using the NIH Toolbox Dimensional Change Card Sort Test (Weintraub et al., 2013). During this 4-minute computerized task, participants match target visual stimuli to one of two choices according to shape or color. Performance was determined by the number of accurate responses. Normed scores were calculated accounting for age, sex, race/ethnicity, and parental education (Casaletto et al., 2015).
Working Memory, the ability to temporarily hold information in mind to guide decision-making, was assessed with the NIH Toolbox List Sorting Working Memory Test (Weintraub et al., 2013). Participants were presented with a series of images for two seconds each, accompanied by an auditory cue, and were instructed to verbally repeat back the visual stimuli from smallest to largest in size. The number of images and variety of sizes successively increased throughout the task, thus further taxing working memory. Performance was determined by the number of accurate responses. Normed scores were created accounting for age, sex, race/ethnicity, and parental education (Casaletto et al., 2015).
Facets of Disinhibited Eating were measured by either self-report or behavioral measures in the laboratory. Higher values on all measures indicate a greater degree of disinhibited eating.
The Eating in the Absence of Hunger Questionnaire for Children (EAH-C) (Tanofsky-Kraff et al., 2008) is a 14-item self-report measure rated on a 5-point Likert scale ranging from 0 = “never” to 4 = “always.” This measure was designed to assess the frequency of precipitants to eating when sated in youth between the ages of 6-19 years old. The measure consists of three subscales: Negative Affect, External Eating, and Fatigue/Boredom. In the current sample, Cronbach’s alphas were: Negative Affect α = .85; External Eating α = .73; Fatigue/Boredom α = .75.
The Emotional Eating Scale for Children (EES-C) (Tanofsky-Kraff, Theim, et al., 2007) is a 26-item self-report measure rated on a 5-point Likert scale from “I have no desire to eat” to “I have a very strong desire to eat.” This measure is designed to assess the urge to cope with negative affect by eating. The EES-C consists of three subscales; Emotional Eating in Response to: Anger/Anxiety/Frustration, Depressive Symptoms, and Feeling Unsettled. In the current sample, Cronbach’s alphas were: Anger/Anxiety/Frustration α = .95; Depressive Symptoms α = .88; Unsettled α = .78.
At the Laboratory Buffet Test Meal, participants were presented with a >10,000 kcal multi-item buffet meal (55% carbohydrate, 12% protein, 33% fat). Before the buffet meal, participants listened to a standardized recording that stated, “Let yourself go and eat as much as you want.” This validated test meal paradigm is designed to promote and capture disinhibited eating (Mirch et al., 2006; Tanofsky-Kraff, McDuffie, et al., 2009). The amount of food consumed was determined by weighing each food item on an electronic balance scale to the nearest gram before and after the meal. Nutrient content data were based on the USDA National Nutrient Database Standard reference database and nutrition labels. The total amount consumed (as kcal) and the amount of energy consumed from carbohydrates + fats (as % of total energy) was calculated for each participant.
Loss-of-Control (LOC)-Eating Severity was assessed immediately following the laboratory buffet test meal using six items adapted from the Eating Disorder Examination (Fairburn & Cooper, 1993) to capture the degree to which LOC eating was experienced during the test meal. A composite score of the six items was used, with greater values indicating a greater sense of LOC eating severity. Cronbach’s alpha was .81 in the current sample.
Data Analytic Plan
Statistical Analyses were conducted using R. Data were screened for outliers and normality. Influential outliers (<1% of data points) were recoded to fall within 1.5 times the interquartile range below or above the 25th or 75th percentile (Behrens, 1997). LOC-eating severity did not achieve normality and was thus log-transformed. An arcsine square-root transformation was conducted for percentage test meal carbohydrate + fat intake. The amount of total energy consumed was statistically adjusted for age, height, and lean mass (kg). Chi-square and t-tests compared demographics and network variables between OW/OB+ and OW/OB− groups.
Missing Data were handled by using multiple imputation with the ‘mice’ (Multiple Imputation via Chained Equations) package, according to the best practices for managing missing data within a network analysis (Van Buuren & Groothuis-Oudshoorn, 2011). The percentage of missing data in the current sample was 17.4%, and the network results were similar both before and after data imputation.
Item Selection.
To reduce multicollinearity, selection of items for inclusion in the network was determined with a combination of theory and an empirical approach (Levinson et al., 2018) using the ‘goldbricker’ function in the package ‘networktools’ (P. Jones, 2018). See Supplement. Several redundant nodes were ultimately removed: Food GNG total commission errors, EAH-C-External Eating, and EES-C-Feeling Unsettled. The final network consisted of 14 nodes (Table 1).
Table 1.
Variables included in final network
| Disinhibited Eating | Executive Dysfunction |
|---|---|
| EES-Ca: Anger/Anxiety/Frustration | Decision-Making |
| EES-C: Depressive Symptoms | SSTd General Inhibitory Control |
| EAH-Cb Negative Affect | GNGe Food-Go Inhibitory Control |
| EAH-C Fatigue/Boredom | GNG Neutral-Go Inhibitory Control |
| Lab Test Meal: Total kcal consumed | Ability to Delay Gratification for Food |
| Lab Test Meal: % Carbs + Fats | Cognitive Flexibility |
| LOCc Eating Severity | Working Memory |
EES-C = Emotional eating scale for children.
EAH-C = Eating in the absence of hunger scale for children.
LOC = loss-of-control.
SST = Stop-Signal Task.
GNG = Food Go/No-Go Task.
For Network Estimation, a regularized partial correlation network of executive dysfunction and disinhibited eating facets was estimated using a graphical least absolute shrinkage and selection operator (GLASSO) estimator with the ‘bootnet’ package (Epskamp et al., 2018). GLASSO-regularized partial correlation networks estimate edges (i.e. the degree of partial correlation between nodes) that are likely to be zero, resulting in a more parsimonious and subsequently, more accurate, network (Epskamp & Fried, 2018). As such, in GLASSO networks, correlations between nodes represent unique partial relationships among constructs, while accounting for all other nodes within the network. Bridge pathways identify which variables in one predetermined “community” (e.g., Disinhibited Eating variables listed in Table 1) are most strongly connected to variables in another community (e.g., Executive Dysfunction variables listed in Table 1), and may help to explain how co-occurrence between two constructs is maintained. The current study focused on bridge-strength and bridge-expected influence centrality indices. Bridge strength is the sum of the absolute value of all edges that exist between node X and all nodes that are not in the same community as node X. Bridge expected influence is the sum of the value of all edges that exist between node X and all other nodes that are not in the same community as node X. Finally, the accuracy of network parameters was analyzed using bootstrapped difference tests, which tests whether network connections and centrality indices for variables statistically differ from one another.
Centrality Indices, namely strength centrality and expected influence, identify which facets are most central within the network, which facets may potentially maintain interactions within and between facets, and which facets may be potential targets for intervention. In networks with unexpected negative edges, expected influence can be interpreted as a more accurate centrality index. See Supplement.
Network Stability was estimated by correlation-stability coefficients (CS-coefficients), namely the strength centrality coefficient, expected influence centrality coefficient, bridge strength centrality, and bridge expected influence (see Supplement). Stability coefficients that range between .20 and .50 are considered acceptable, .50 to .70 are good, and those greater than .70 are considered excellent (Epskamp & Fried, 2018).
A Network Comparison Test was conducted to determine whether the same constructs were connected and whether the strength of the connections differed between two types of samples (youth with overweight or obesity [BMI%ile ≥ 85] vs. BMI%ile < 85) using the ‘NetworkComparisonTest’ package (van Borkulo, Epskamp, & Millner, 2015). The network comparison test assesses whether network structure, or the way in which symptoms interact, is invariant. It uses an omnibus test to determine whether any edges significantly differ between the two networks after multiple corrections. The network comparison test also assesses whether global strength, defined as the overall connectivity, is invariant across the two networks.
Results
Demographics
Two-hundred-forty-eight youth (see Table 2), among whom 35.1% (N=87) had overweight/obesity, participated. Compared to participants with BMI%ile < 85 (OW/OB−), those with OW/OB+ showed significantly higher total energy consumption [t(246) = −6.8, p<.01]; commission errors on GNG Food-Go [t(246) = −2.1, p=.03] and Neutral-Go [t(246) = −2.7, p=.01] inhibitory control; and working memory dysfunction [t(246) = −2.0, p=.05] (Table 2), but did not differ significantly on any of the other network variables, age, sex, or race/ethnicity (ps>.09).
Table 2.
Sample characteristics of network variables for youth with overweight/obesity (OW/OB+) compared to youth without overweight/obesity (OW/OB−).
| Network Variablesa | Total (n = 248) |
OW/OB+ (n = 87) |
OW/OB− (n = 161) |
p |
|---|---|---|---|---|
| Disinhibited Eating | ||||
| EES-Cb Anger/Anxiety/Frustration | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.1 ± 0.2 | .24 |
| EES-C Depressive Symptoms | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.2 | .20 |
| EAH-Cc Negative Affect | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | .91 |
| EAH-C Fatigue/Boredom | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.2 | .18 |
| Test Meal Total Kcal Consumed | 988.1 ± 168.8 | 1078.8 ± 185.3 | 939.1 ± 136.5 | <.01** |
| Test Meal % Carb + Fats | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | .87 |
| Test Meal LOCd Eating Severity | −0.1 ± 0.4 | −0.1 ± 0.4 | −0.2 ± 0.5 | .09 |
| Executive Dysfunction | ||||
| Decision-Making | −1.1 ± 25.8 | −2.4 ± 25.0 | −0.4 ± 26.2 | .55 |
| SSTe General Inhibitory Control | 288.0 ± 74.7 | 298.5 ± 74.1 | 282.3 ±74.7 | .10 |
| GNGf Food-Go Inhibitory Control | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.3 ± 0.2 | .03* |
| GNG Neutral-Go Inhibitory Control | 0.4 ± 0.2 | 0.5 ± 0.2 | 0.4 ± 0.2 | .01* |
| Ability to Delay Gratification for Food | 0.6 ± 0.4 | 0.6 ± 0.4 | 0.6 ± 0.4 | .57 |
| Cognitive Flexibility | 47.9 ± 15.1 | 46.9 ± 14.9 | 48.4 ± 15.2 | .44 |
| Working Memory | 33.2 ± 9.7 | 34.8 ± 9.9 | 32.3 ± 9.5 | .05* |
Values presented are M ± SD.
EES-C = Emotional eating scale for children.
EAH-C = Eating in the absence of hunger scale for children.
LOC = loss-of-control.
SST = Stop-Signal Task.
GNG = Food Go/No-Go Task.
Analyses examining differences between OW/OB+ vs. OW/OB− groups are significant at p < .05
Analyses examining differences between OW/OB+ vs. OW/OB− groups are significant at p < .01
Central Nodes
The final executive dysfunction-disinhibited eating network (Figure 1) included 14 variables (Table 1) and demonstrated excellent stability (i.e., low network error; strength centrality CS-coefficient = 0.75; expected influence stability CS-coefficient = 0.75). Given the network contained positive and negative edges, both strength and expected influence results are reported, as strength and expected influence values may provide unique information when negative edges are present in a network. However, values for strength and expected influence were highly correlated (r = .87, p < .001) in the current dataset.
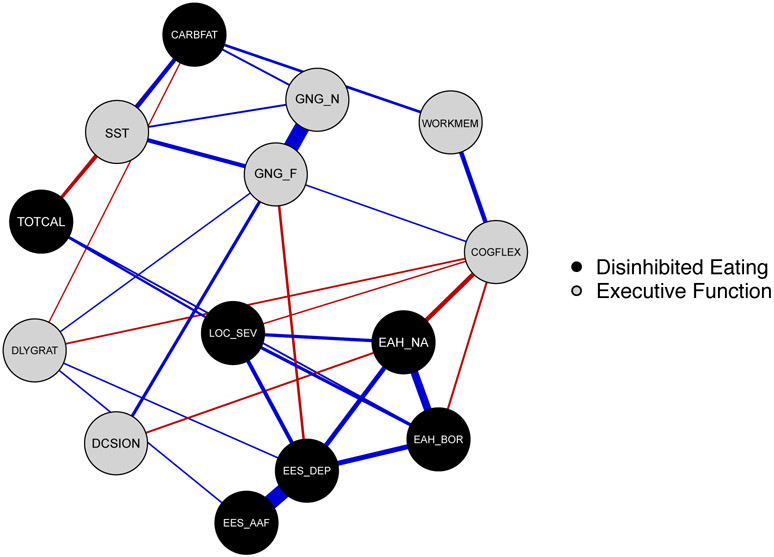
Figure 1.
Executive dysfunction and disinhibited eating behaviors network. Executive Dysfunction behaviors are in gray, and Disinhibited Eating behaviors are in black. Thicker edges (i.e. line connections between nodes) represent stronger relationships. Blue edges indicate positive relationships; red edges indicate negative relationships. Executive Dysfunction labels: DCSION = decision-making, Iowa Gambling Task; SST = inhibitory control, Stop-Signal task reaction time; GNG_F = inhibitory control Food-Go commission errors, Food Go/No-Go task; GNG_N = inhibitory control Neutral-Go commission errors, Food Go/No-Go task; DLYGRAT = ability to delay gratification for food, Delay Discounting task; COGFLEX = cognitive flexibility, NIH Toolbox Dimensional Change Card Sort Test; WORKMEM = working memory, NIH Toolbox List Sorting Working Memory Test. Disinhibited Eating labels: EAH_NA = Eating in the Absence of Hunger Questionnaire for Children-Negative Affect subscale; EAH_BOR = Eating in the Absence of Hunger Questionnaire for Children-Fatigue/Boredom subscale; EES_DEP = Emotional Eating Scale for Children-Depressive Symptoms subscale; EES_AAF = Emotional Eating Scale for Children-Anger/Anxiety/Frustration subscale; TOTCAL = total calories consumed in a laboratory buffet test meal, adjusted for height, age, and lean mass (kg); CARBFAT = percentage of energy consumed from carb carbohydrates + fats in a laboratory buffet test meal; LOC_SEV = loss-of-control eating severity reported at a laboratory buffet test meal.
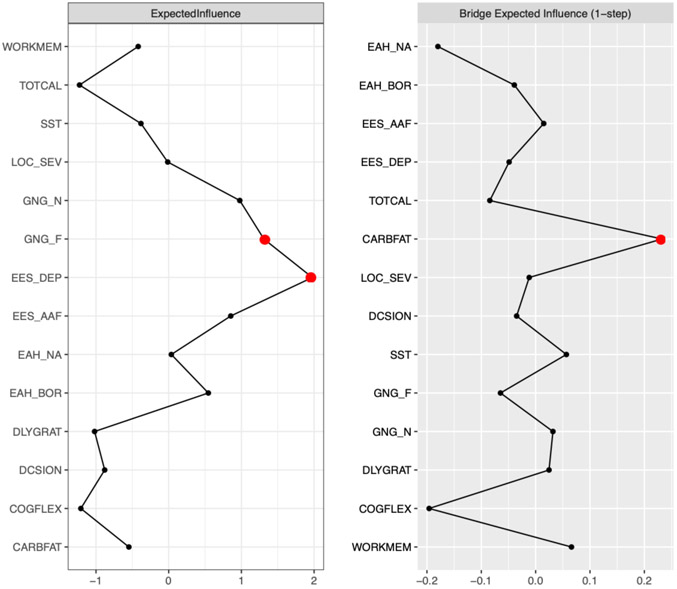
Figure 2 presents strength, expected influence, and bridge centrality graphs. Emotional eating in response to depressive symptoms had the highest strength centrality (strength = 2.12) and had significantly higher strength centrality than all other nodes (Figure 3). The following symptoms had the highest expected influence: Emotional eating in response to depressive symptoms (expected influence = 1.98), and Inhibitory Control-Food GNG Food-Go commission errors (expected influence = 1.31). Emotional eating in response to depressive symptoms had higher expected influence than 92% of other nodes but was not significantly different from Inhibitory Control-Food GNG Food-Go, which had significantly higher expected influence than 77% of other nodes (Figure 3).
Figure 2.
Executive dysfunction-disinhibited eating network strength centrality, expected influence, and bridge expected influence plots. The strongest central and bridge nodes are highlighted with a red dot. Executive Dysfunction labels: DCSION = decision-making, Iowa Gambling Task; SST = inhibitory control, Stop-Signal task reaction time; GNG_F = inhibitory control Food-Go commission errors, Food Go/No-Go task; GNG_N = inhibitory control Neutral-Go commission errors, Food Go/No-Go task; DLYGRAT = ability to delay gratification for food, Delay Discounting task; COGFLEX = cognitive flexibility, NIH Toolbox Dimensional Change Card Sort Test; WORKMEM = working memory, NIH Toolbox List Sorting Working Memory Test. Disinhibited Eating labels: EAH_NA = Eating in the Absence of Hunger Questionnaire for Children-Negative Affect subscale; EAH_BOR = Eating in the Absence of Hunger Questionnaire for Children-Fatigue/Boredom subscale; EES_DEP = Emotional Eating Scale for Children-Depressive Symptoms subscale; EES_AAF = Emotional Eating Scale for Children-Anger/Anxiety/Frustration subscale; TOTCAL = total calories consumed in a laboratory buffet test meal, adjusted for height, age, and lean mass (kg); CARBFAT = percentage of energy consumed from carb carbohydrates + fats in a laboratory buffet test meal; LOC_SEV = loss-of-control eating severity reported at a laboratory buffet test meal.
Figure 3.
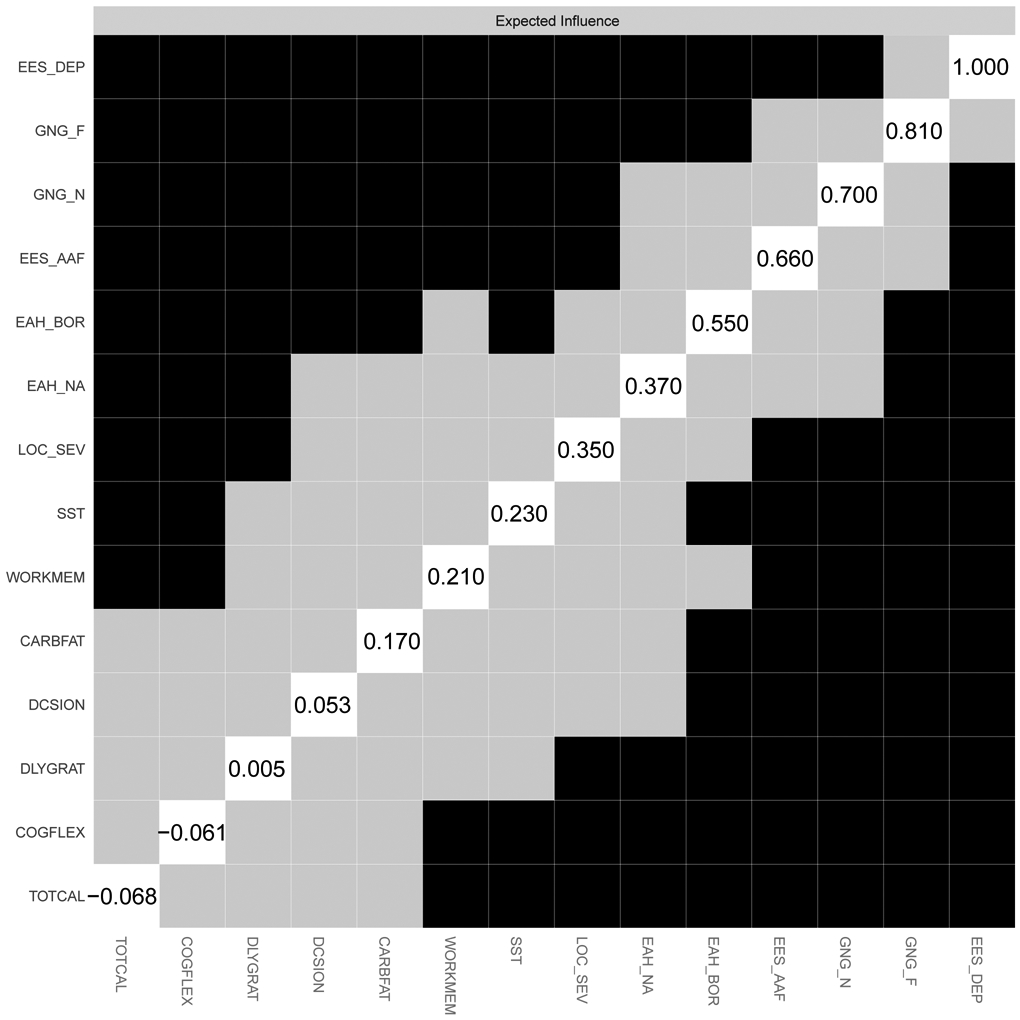
Strength centrality and expected influence difference graphs for the executive dysfunction-disinhibited eating network. Strength centrality difference is presented in panel A; and expected influence difference is presented in panel B. Variables are presented in descending order of strength. Values in the diagonal white squares indicate unstandardized strength values. Black squares indicate a statistically significant difference between nodes at the p < .05 level.
Bridge Pathways
Bridge strength (bridge-strength stability coefficient = .28) and expected influence (bridge-expected influence stability coefficient = .36) showed acceptable stability. The disinhibited eating item, test meal carbohydrate + fat intake, had the highest bridge strength (bridge strength = 0.25) and expected influence (bridge expected influence = 0.23). Because bridge expected influence had better stability than bridge strength, we report and interpret results related to bridge expected influence (Figure 2). Test meal carbohydrate + fat intake had higher bridge expected influence than 77% of other nodes (Figure 4). It was most strongly associated with the following executive dysfunction items: General Inhibitory Control-SST (part r = .14); and working memory (part r = .07).
Figure 4.
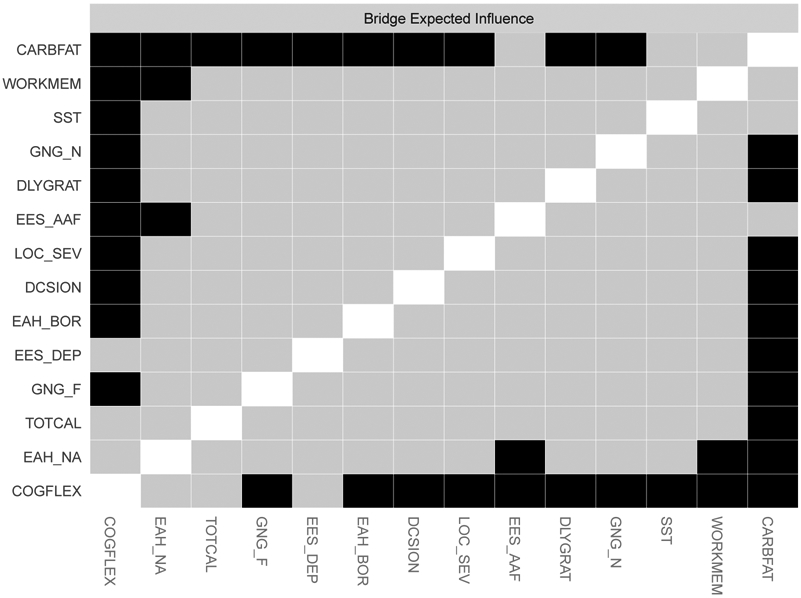
Bridge expected influence difference graph for the executive dysfunction-disinhibited eating network. Symptoms are presented in descending order of expected influence. Black squares indicate a statistically significant difference between nodes at the p < .05 level.
Network Comparison Test
Individuals with OW/OB+ (N=87; 35.1%) were compared to OW/OB− (N=161) using a network comparison test in R. There was no significant difference in network structure invariance (M = 0.26, p = .15) or global strength invariance (S = 0.68; OW/OB+ = 0.91, OW/OB− = 1.59, p = .60) between samples. Thus, the central symptoms and the overall connectivity of the network did not appear to differ significantly between youth with and without overweight/obesity.
Discussion
Network analytic techniques to characterize the complex interrelationships between executive dysfunction and disinhibited eating in a sample of youth across the weight spectrum indicated the most central variable in the overall network was the disinhibited eating variable representing emotional eating in response to feelings of depressive symptoms. In contrast, the disinhibited eating variable representing carbohydrate + fat intake during the laboratory test meal emerged as the variable with the highest bridge strength and expected influence, bridging most strongly with the executive dysfunction variables of general inhibitory control and working memory.
Although emotional eating in response to feelings of depressive symptoms emerged as the most central facet in the overall network, it was not involved in the key bridge pathways. In contrast, the second most central facet (i.e., food-related inhibitory control) also displayed a high expected influence in the overall network and was more conceptually consistent with the key bridge pathway involving general inhibitory control. Central facets should be interpreted with caution, as they may be inflated due to strong connections with similar constructs. For example, as one would expect, emotional eating in response to feelings of depressive symptoms had strong connections with other affect-oriented eating constructs such as emotional eating in response to anger/anxiety/frustration and negative affect motivated eating in the absence of hunger. It should also be noted that the current network analysis is undirected and non-causal. As such, it is possible that emotional eating in response to feelings of depressive symptoms emerged as the most highly central node due to receiving many inputs from other network facets, rather than necessarily causing many outputs. There may be a cycle of maladaptive eating behaviors driven by executive dysfunction that ultimately leads to increased negative affect, which could result in youth engaging in emotional eating in response to feelings of low mood. This cycle aligns with affect theory, which proposes that disinhibited eating may result from maladaptive coping with negative emotions (Heatherton & Baumeister, 1991; Kenardy, Arnow, & Agras, 1996). Engaging in a maladaptive cycle of eating in order to cope with negative emotions may subsequently influence risk for excess weight gain and development of BED (Tanofsky-Kraff, Wilfley, et al., 2007).
Test meal carbohydrate + fat intake emerged as the key bridge facet within the network. Compared to other facets of disinhibited eating that were characterized by self-report, this facet may have had greater relevance in bridging the disinhibited eating and executive dysfunction communities in the network due to its objective nature. Test meal carbohydrate + fat intake bridged most strongly with general inhibitory control, the EF domain most consistently and robustly associated with obesity among youth (Bozkurt et al., 2017; Liang et al., 2014; Pearce et al., 2018; Reinert et al., 2013). Other studies consistently demonstrate that poorer general inhibitory control relates to both LOC-eating and intake of highly-palatable foods among youth (Maayan et al., 2011; Nederkoorn et al., 2015; Reinblatt et al., 2015). Past research has identified a high-risk pediatric obesity phenotype characterized by greater behavioral disinhibition, high sedentary behavior, and high energy intake (Riggs, Huh, Chou, Spruijt-Metz, & Pentz, 2012), suggesting poor general inhibitory control and unhealthy energy patterns may reinforce one another and contribute to development of excess weight gain. The potential clinical implications for youth with disinhibited eating and/or overweight/obesity include that inhibitory control training could theoretically be utilized to disrupt the tendency to consume highly-palatable foods and prevent development of excess weight gain among high-risk youth (Berner, Winter, Matheson, Benson, & Lowe, 2017; Turton, Bruidegom, Cardi, Hirsch, & Treasure, 2016).
The link between objective food intake, particularly of palatable food, and inhibitory control difficulties may be central to maintaining the co-occurrence between executive dysfunction and disinhibited eating. Eating behaviors are generally guided by both automatic and reflective neurocognitive processes (A. Jones, Hardman, Lawrence, & Field, 2018; Strack & Deutsch, 2004). Automatic eating processes involve impulsive behaviors that often occur in response to rewarding properties associated with specific food items, whereas reflective processes involve self-regulatory EF abilities that help individuals override automatic processes using higher-order knowledge or long-term goals. Theoretically, dysfunction in the self-regulatory process may impair one’s ability to override the automatic eating process driven by impulsivity and immediate reward, potentially contributing to greater consumption and subsequent development of excess weight gain. Inhibitory control approaches should be examined as a target for excess weight gain prevention to determine if they disrupt the central links between poor EF and disinhibited eating (Berner et al., 2017; Turton et al., 2016).
Test meal carbohydrate + fat intake also demonstrated a somewhat strong bridge connection with the EF facet of working memory. A recent meta-analysis showed that working memory was more impaired in individuals with both BED and obesity versus individuals only with obesity (Cury et al., 2020). It is possible that working memory may emerge as a more relevant or central bridge connection among an executive dysfunction-disinhibited eating network of individuals with a full-threshold diagnosis of BED. Given that the current sample consisted of healthy, non-treatment-seeking youth, the bridge between working memory and test meal carbohydrate + fat intake suggests that poorer working memory could be a predisposing factor for more severe patterns of disinhibited eating. This relationship should be examined longitudinally to identify whether poorer working memory in childhood relates to later development of BED or other forms of eating pathology.
The network comparison test revealed no significant network differences between OW/OB+ and OW/OB−, indicating that the central symptoms and overall connectivity was similar across groups. The lack of significant differences could be due in part to lack of power given the small number of youth with OW/OB+. Additionally, the generally healthy nature of participants may have limited the range for certain variables, or there may be other unexplored variables that would be important to include in future network investigations. Interestingly, and contrary to hypotheses, food-related inhibitory control was not a significant bridge variable within the network, although it did emerge as a variable with high expected influence in the overall network. It may be that the general inhibitory control task better captured the underlying construct of disinhibition. Additionally, in the current study, youth were presented with an array of standardized food choices at the laboratory test meal, which did not correspond with the food images used in the food-related inhibitory control task. A potentially more accurate reflection of the natural eating environment could allow youth to pre-select their food preferences in both contexts: the laboratory test meal and the computerized food-related inhibitory control task.
Study strengths include a racially diverse sample of boys and girls reflecting the weight spectrum, which suggests generalizability of the results. Further, past research using network analysis to investigate disinhibited and disordered eating have predominantly been limited to use of item-level self-report questionnaire data. In this study, we utilized data collected using multiple methodologies, including both objective eating and self-report measures, as well as computerized neurocognitive tasks. Importantly, including distinct measures of both general and food-related inhibitory control within the same study is advantageous, given general measures are useful for identifying transdiagnostic neurocognitive mechanisms, while food-specific measures can perhaps isolate neurocognitive deficits that are more pronounced for eating-related problems (Berner et al., 2017). However, this study also has limitations. Although it is statistically expected that measures of bridge centrality will be lower than overall network centrality, in the current study bridge strength and expected influence were acceptable, but somewhat low, and should be interpreted with caution. Additionally, although the use of cross-sectional data in this investigation is consistent with most prior network analytic studies of disinhibited and disordered eating, it limits the ability to draw any directional and causal conclusions. Thus, future network analytic studies using prospective data are recommended. Finally, although several of the neurocognitive task scores were adjusted based on age norms, analyses may have been limited in that not all neurocognitive tasks had age-based norms. Given the age range of the current sample, the influence of various neurodevelopmental stages should be considered in future research examining links between disinhibited eating and facets of neurocognitive function.
Findings from the current investigation provide insights into the complex interrelationships between disinhibited eating and executive dysfunction among youth. Understanding which dimensions of executive dysfunction are most strongly linked to disinhibited eating behaviors can offer preliminary guidance for future investigations of targeted interventions. In this study, network analytic techniques helped to identify central symptoms, as well as a bridge pathway between palatable food intake and inhibitory control, which may represent a promising intervention target. Further research is warranted to gain a better understanding of how executive dysfunction and disinhibited eating interact and maintain each other over time.
Supplementary Material
Acknowledgments
DISCLAIMER: The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the National Institutes of Health, USU, or the United States Department of Defense. This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number ZIA-HD00641; J. Yanovski).
Footnotes
CONFLICT OF INTEREST: The authors have no conflicts of interest to declare.
The data that support the findings of this study are available from the corresponding author upon request.
References
- Batterink L, Yokum S, & Stice E (2010). Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. 52(4), 1696–1703. doi: 10.1016/j.neuroimage.2010.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, & Anderson SW (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50, 1–3. [DOI] [PubMed] [Google Scholar]
- Behrens JT (1997). Principles and procedures of exploratory data analysis. Psychological Methods, 2(2), 131–160. [Google Scholar]
- Berner LA, Winter SR, Matheson BE, Benson L, & Lowe MR (2017). Behind binge eating: A review of food-specific adaptations of neurocognitive and neuroimaging tasks. Physiology & Behavior. doi: 10.1016/j.physbeh.2017.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D (2017). A network theory of mental disorders. World Psychiatry, 16(1), 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt H, Özer S, Yılmaz R, Sönmezgöz E, Kazancı Ö, Erbaş O, & Demir O (2017). Assessment of neurocognitive functions in children and adolescents with obesity. Applied Neuropsychology: Child, 6(4), 262–268. [DOI] [PubMed] [Google Scholar]
- Brown TA, Vanzhula IA, Reilly EE, Levinson CA, Berner LA, Krueger A, … Wierenga CE (2020). Body mistrust bridges interoceptive awareness and eating disorder symptoms. Journal of Abnormal Psychology. doi: 10.1037/abn0000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant-Waugh RJ, Cooper PJ, Taylor CL, & Lask BD (1996). The use of the eating disorder examination with children: A pilot study. International Journal of Eating Disorders, 19(4), 391–397. [DOI] [PubMed] [Google Scholar]
- Casaletto KB, Umlauf A, Beaumont J, Gershon R, Slotkin J, Akshoomoff N, & Heaton RK (2015). Demographically corrected normative standards for the English version of the NIH Toolbox Cognition Battery. Journal of the International Neuropsychological Society, 21(5), 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, … Sallis JF (2003). International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise, 35(8), 1381–1395. [DOI] [PubMed] [Google Scholar]
- Cramer AO, Waldorp LJ, Van Der Maas HL, & Borsboom D (2010). Comorbidity: A network perspective. Behavioral and Brain Sciences, 33(2-3), 137. [DOI] [PubMed] [Google Scholar]
- Cury MEG, Berberian A, Scarpato BS, Kerr-Gaffney J, Santos FH, & Claudino AM (2020). Scrutinizing Domains of Executive Function in Binge Eating Disorder: A Systematic Review and Meta-Analysis. Frontiers in Psychiatry, 11. doi: 10.3389/fpsyt.2020.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois RH, Rodgers RF, Franko DL, Eddy KT, & Thomas JJ (2017). A network analysis investigation of the cognitive-behavioral theory of eating disorders. Behaviour Research and Therapy, 97, 213–221. [DOI] [PubMed] [Google Scholar]
- Epskamp S, & Fried EI (2018). A tutorial on regularized partial correlation networks. Psychological Methods, 23(4), 617. [DOI] [PubMed] [Google Scholar]
- Epskamp S, Maris G, Waldorp L, Borsboom D, Irwing P, Hughes D, & Booth T (2018). Network Psychometrics In Handbook of Psychometrics. New York, NY: Wiley-Blackwell. [Google Scholar]
- Evanovich EK, Marshall AJ, David SJ, & Mumma GH (2019). A network conceptualization of the multiple facets of distress tolerance. Anxiety, Stress, & Coping, 32(6), 654–669. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, & Cooper Z (1993). The Eating Disorder Examination (12th edition). In Fairburn CG & Wilson GT (Eds.), Binge eating: Nature, assessment, and treatment. (pp. 317–360). New York, NY, US: Guilford Press. [Google Scholar]
- Forbush K, Siew C, & Vitevitch M (2016). Application of network analysis to identify interactive systems of eating disorder psychopathology. Psychological Medicine, 46(12), 2667. [DOI] [PubMed] [Google Scholar]
- Garon N, Moore C, & Waschbusch DA (2006). Decision making in children with ADHD only, ADHD-anxious/depressed, and control children using a child version of the Iowa Gambling Task. Journal of Attention Disorders, 9(4), 607–619. [DOI] [PubMed] [Google Scholar]
- Goldschmidt AB, Hipwell AE, Stepp SD, McTigue KM, & Keenan K (2015). Weight gain, executive functioning, and eating behaviors among girls. Pediatrics, 136(4), e856–e863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, O'Brien S, Lavender JM, Pearson CM, Le Grange D, & Hunter SJ (2018). Executive functioning in a racially diverse sample of children who are overweight and at risk for eating disorders. Appetite, 124, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe K, & Elsner B (2017). Executive function and weight status in children: A one-year longitudinal perspective. Child Neuropsychology, 23(2), 129–147. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, & Baumeister RF (1991). Binge eating as escape from self-awareness. Psychological Bulletin, 110(1), 86. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2006). Dietary reference intakes: the essential guide to nutrient requirements (Meyers LD, Hellwig JP, & Otten JJ Eds.). Washington, DC: National Academies Press. [Google Scholar]
- Jones A, Hardman CA, Lawrence N, & Field M (2018). Cognitive training as a potential treatment for overweight and obesity: A critical review of the evidence. Appetite, 124, 50–67. [DOI] [PubMed] [Google Scholar]
- Jones P (2018). networktools: Tools for identifying important nodes in networks (Version R package 1.2).
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kelly NR, Jaramillo M, Ramirez S, Altman DR, Rubin SG, Yang SB, … Yanovski JA (2020). Executive functioning and disinhibited eating in children and adolescents. Pediatric Obesity, e12614. doi: 10.1111/ijpo.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenardy J, Arnow B, & Agras WS (1996). The aversiveness of specific emotional states associated with binge-eating in obese subjects. Australian and New Zealand Journal of Psychiatry, 30(6), 839–844. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, 0… Johnson CL (2002). 2000 CDC Growth Charts for the United States: methods and development. Vital and Health Statistics, 11(246), 1–190. [PubMed] [Google Scholar]
- Levinson CA, Brosof LC, Vanzhula I, Christian C, Jones P, Rodebaugh TL, … Weeks JW (2018). Social anxiety and eating disorder comorbidity and underlying vulnerabilities: Using network analysis to conceptualize comorbidity. International Journal of Eating Disorders, 51(7), 693–709. [DOI] [PubMed] [Google Scholar]
- Levinson CA, Zerwas S, Calebs B, Forbush K, Kordy H, Watson H, … Peat C (2017). The core symptoms of bulimia nervosa, anxiety, and depression: A network analysis. Journal of Abnormal Psychology, 126(3), 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Matheson BE, Kaye WH, & Boutelle KN (2014). Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. International Journal of Obesity, 38(4), 494–506. doi: 10.1038/ijo.2013.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, & Davis KA (1984). On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal of Experimental Psychology: Human Perception and Performance, 10(2), 276. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, & Tannock R (1997). Impulsivity and inhibitory control. Psychological Science, 8(1), 60–64. [Google Scholar]
- Maayan L, Hoogendoorn C, Sweat V, & Convit A (2011). Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity, 19(7), 1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally KA, Shear P, Tlustos S, Amin R, & Beebe D (2012). Iowa Gambling Task Performance in Overweight Children and Adolescents At-Risk for Obstructive Sleep Apnea. Journal of the International Neuropsychological Society, 18(3), 481–489. [DOI] [PubMed] [Google Scholar]
- Mirch MC, McDuffie JR, Yanovski SZ, Schollnberger M, Tanofsky-Kraff M, Theim KR, … Yanovski JA (2006). Effects of binge eating on satiation, satiety, and energy intake of overweight children. The American Journal of Clinical Nutrition, 84(4), 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, & Warusawitharana M (2001). Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior, 76(2), 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn C, Dassen FC, Franken L, Resch C, & Houben K (2015). Impulsivity and overeating in children in the absence and presence of hunger. Appetite, 93, 57–61. [DOI] [PubMed] [Google Scholar]
- Odum AL, Baumann AA, & Rimington DD (2006). Discounting of delayed hypothetical money and food: Effects of amount. Behavioural Processes, 73(3), 278–284. [DOI] [PubMed] [Google Scholar]
- Pearce AL, Leonhardt CA, & Vaidya CJ (2018). Executive and reward-related function in pediatric obesity: a meta-analysis. Childhood Obesity, 14(5), 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinblatt SP, Mahone EM, Tanofsky-Kraff M, Lee-Winn AE, Yenokyan G, Leoutsakos J-MS, … Riddle MA (2015). Pediatric loss of control eating syndrome: Association with attention-deficit/hyperactivity disorder and impulsivity. International Journal of Eating Disorders, 48(6), 580–588. doi: 10.1002/eat.22404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert K, Po’e E, & Barkin S (2013). The relationship between executive function and obesity in children and adolescents: a systematic literature review. Journal of Obesity, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs NR, Huh J, Chou C-P, Spruijt-Metz D, & Pentz MA (2012). Executive function and latent classes of childhood obesity risk. Journal of Behavioral Medicine, 35(6), 642–650. [DOI] [PubMed] [Google Scholar]
- Riggs NR, Spruijt-Metz D, Sakuma K-L, Chou C-P, & Pentz MA (2010). Executive cognitive function and food intake in children. Journal of Nutrition Education and Behavior, 42(6), 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam TR, Wilson NL, Shoda Y, Mischel W, & Ayduk O (2013). Preschoolers' delay of gratification predicts their body mass 30 years later. The Journal of pediatrics, 162(1), 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, & Yanovski JA (2011). Disinhibited eating and body weight in youth. In Handbook of Behavior, Food and Nutrition (pp. 2183–2200). New York, NY: Springer. [Google Scholar]
- Smith KE, Crosby RD, Wonderlich SA, Forbush KT, Mason TB, & Moessner M (2018). Network analysis: An innovative framework for understanding eating disorder psychopathology. International Journal of Eating Disorders, 51(3), 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack F, & Deutsch R (2004). Reflective and impulsive determinants of social behavior. Personality and Social Psychology Review, 8(3), 220–247. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, Kozlosky M, Schvey NA, Shomaker LB, … Yanovski JA (2009). Laboratory assessment of the food intake of children and adolescents with loss of control eating. The American Journal of Clinical Nutrition, 89(3), 738–745. doi: 10.3945/ajcn.2008.26886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Ranzenhofer LM, Yanovski SZ, Schvey NA, Faith M, Gustafson J, & Yanovski JA (2008). Psychometric properties of a new questionnaire to assess eating in the absence of hunger in children and adolescents. Appetite, 51(1), 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Theim KR, Yanovski SZ, Bassett AM, Burns NP, Ranzenhofer LM, … Yanovski JA (2007). Validation of the emotional eating scale adapted for use in children and adolescents (EES-C). International Journal of Eating Disorders, 40(3), 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Wilfley DE, Young JF, Mufson L, Yanovski SZ, Glasofer DR, & Salaita CG (2007). Preventing excessive weight gain in adolescents: interpersonal psychotherapy for binge eating. Obesity, 15(6), 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, & Yanovski JA (2009). A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. International Journal of Eating Disorders, 42(1), 26–30. doi: 10.1002/eat.20580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich T, Freidl EK, Kostro K, Weigel J, Davidow JY, Riddle MC, … Mayer L (2014). Probing behavioral responses to food: Development of a food-specific go/no-go task. Psychiatry Research, 219(1), 166–170. doi: 10.1016/j.psychres.2014.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton R, Bruidegom K, Cardi V, Hirsch CR, & Treasure J (2016). Novel methods to help develop healthier eating habits for eating and weight disorders: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 61, 132–155. [DOI] [PubMed] [Google Scholar]
- van Borkulo C, Epskamp S, & Millner A (2015). Network comparison test: permutation-based test of differences in strength of networks. In.
- Van Buuren S, & Groothuis-Oudshoorn K (2011). Mice: multivariate imputation by chained equations in R. Journal of Statistical Software, 45(3), 1–67. [Google Scholar]
- Van Malderen E, Goossens L, Verbeken S, & Kemps E (2018). Unravelling the association between inhibitory control and loss of control over eating among adolescents. Appetite, 125, 401–409. [DOI] [PubMed] [Google Scholar]
- Vanzhula IA, Calebs B, Fewell L, & Levinson CA (2019). Illness pathways between eating disorder and post-traumatic stress disorder symptoms: Understanding comorbidity with network analysis. European Eating Disorders Review, 27(2), 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2011). WASI-II: Wechsler abbreviated scale of intelligence: PsychCorp. [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, … Wallner-Allen K (2013). Cognition assessment using the NIH Toolbox. Neurology, 80(11 Supplement 3), S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.