Abstract
Although executive dysfunction is the characteristic cognitive marker of behavioral variant frontotemporal dementia (bvFTD), episodic memory deficits are relatively common, and may be present even during the prodromal disease phase. In a cohort of mutation carriers with mild behavioral and/or cognitive symptoms consistent with prodromal bvFTD, we aimed to investigate patterns of performance on an abbreviated list learning task, with a particular focus on recognition memory. We further aimed to characterize the cognitive prodromes associated with the three major genetic causes of frontotemporal dementia, as emerging evidence suggests there may be subtle differences in cognitive profiles among carriers of different genetic mutations. Participants included 57 carriers of a pathogenic mutation in microtubule-associated protein tau (MAPT, N=23), or progranulin (GRN, N=15), or a or a hexanucleotide repeat expansion in chromosome 9 open reading frame 72 (C9orf72, N=19), with mild cognitive and/or behavioral symptoms consistent with prodromal bvFTD. Familial non-carriers were included as controls (N=143). All participants completed a comprehensive neuropsychological examination, including an abbreviated list learning test assessing episodic memory recall and recognition. MAPT mutation carriers performed worse than non-carriers in terms of list recall, and had difficulty discriminating targets from distractors on the recognition memory task, primarily due to the endorsement of distractors as targets. MAPT mutation carriers also showed nonverbal episodic memory and semantic memory dysfunction (object naming). GRN mutation carriers were variable in performance and overall the most dysexecutive. Slowed psychomotor speed was evident in C9orf72 repeat expansion carriers. Identifying the earliest cognitive indicators of bvFTD is of critical clinical and research importance. List learning may be a sensitive cognitive marker for incipient dementia in MAPT and potentially a subset of GRN carriers. Our results highlight that distinct cognitive profiles may be evident in carriers of the three disease-causing genes during the prodromal disease stage.
Keywords: behavioral variant frontotemporal dementia, episodic memory, neuropsychology, prodromal disease, genetic frontotemporal dementia
1. INTRODUCTION
Frontotemporal lobar degeneration (FLTD) is a pathological process that results in progressive atrophy of the frontal and temporal lobes, and presents clinically most often as frontotemporal dementia (FTD). Around one third of all FTD cases have a strong family history (Goldman score ≤3), and 10-15% follow a known autosomal dominant inheritance pattern (Greaves & Rohrer, 2019). The main genetic causes of FTD are mutations in microtubule-associated protein tau (MAPT) or progranulin (GRN), or a hexanucleotide repeat expansion in chromosome 9 open reading frame 72 (C9orf72), all of which are highly penetrant (Onyike & Diehl-Schmid, 2013). Studying pathogenic mutation carriers is considered the ‘gold standard’ for characterizing the prodrome of FTD because pathology can be predicted. Understanding the disease prodrome is critical in the context of clinical trials, which are set to begin imminently with genetic mutation carriers among the first enrolled; thus, measures that can accurately pinpoint prodromal changes are increasingly sought after. Furthermore, early detection of clinical manifestations of disease, especially in familial cases, is increasingly recognized as important for optimal patient care, as it can guide early counselling and management strategies.
The vast majority of genetic FTD cases present with the clinical phenotype of behavioral variant FTD (bvFTD), though occasionally with a primary language or motor phenotype. BvFTD is a progressive disorder primarily affecting behavior, personality, and social cognition. The hallmark behavioral changes in bvFTD include apathy and socially inappropriate behavior (Neary et al., 1998; Rascovsky et al., 2011). According to the most recent bvFTD diagnostic criteria (Rascovsky et al., 2011), the neuropsychological profile includes executive dysfunction (i.e., deficits in higher-order cognitive skills such as planning, generation, reasoning, cognitive switching, etc.) in the context of relatively preserved episodic memory and visuospatial skills. In some cases, episodic memory dysfunction may tilt clinicians away from a diagnosis of FTD and towards dementia due to Alzheimer’s disease. Despite this, episodic memory dysfunction may be present in a significant subset, even up to half, of bvFTD cases at some stage during the disease course (Bertoux et al., 2014, 2018; Fernández-Matarrubia et al., 2017; Hornberger et al., 2010; Hornberger & Piguet, 2012; Johnen & Bertoux, 2019; Poos et al., 2018). In fact, emerging evidence suggests it may be one of the first domains affected in bvFTD (Ramanan et al., 2017; Schubert et al., 2016), including in carriers of FTLD-associated genetic mutations (Cheran et al., 2019; Jiskoot et al., 2016, 2018; Lee et al., 2017; Olney et al., 2020). However, in determining the frequency and nature of early episodic memory1 weaknesses in genetic FTD, differences in cognitive profiles among genetic mutations need to be considered (Poos et al., 2020; Rohrer et al., 2015).
A small number of studies have presented detailed cognitive profiles of MAPT, GRN, and C9orf72 carriers, some with dementia and some in the preclinical or prodromal phase. Note that most studies of genetic mutation carriers do not stratify by clinical phenotype due to limited numbers and overlap between phenotypes. Nevertheless, given the frontal lobe involvement in FTLD, it is not surprising that executive dysfunction and generative (e.g. verbal fluency) impairments have been found in mutation carriers across all three genes at the preclinical and dementia stages of disease (Snowden et al., 2015; Staffaroni, Bajorek, et al., 2020). The cognitive weaknesses most characteristic of MAPT mutation carriers are in object naming / semantic memory and social cognition, and episodic memory difficulties have also been consistently reported (Cheran et al., 2019; Jiskoot et al., 2016, 2018; Olney et al., 2020; Pickering-Brown et al., 2008; Poos et al., 2020; Rohrer et al., 2010, 2015; Spina et al., 2008). GRN mutation carriers may also display episodic memory deficits (Jiskoot et al., 2016; Rohrer et al., 2008; van Swieten & Heutink, 2008), and have been shown to be apraxic (Le Ber et al., 2008; Pickering-Brown et al., 2008; Snowden et al., 2015), have working memory problems (Hallam et al., 2014; Rohrer et al., 2015), and might be the most severely dysexecutive of the three (Pickering-Brown et al., 2008; Poos et al., 2020). C9orf72 repeat expansion carriers seem to have a less distinctive, more ‘diffuse’, and potentially milder pattern of deficits, spanning the domains of episodic memory, executive function, processing speed and language (Lee et al., 2017; Mahoney, Beck, et al., 2012; Mahoney, Downey, et al., 2012; Poos et al., 2020). Variation in cognitive profiles among mutation groups can partly be explained by phenotypic variability (e.g., logopenic and nonfluent variants of primary progressive aphasia are more common in GRN carriers), but cognitive differences among mutations are still apparent when the samples are restricted to bvFTD phenotypes (Poos et al., 2020).
Despite the cognitive variability among genetic groups, episodic memory difficulties, in terms of delayed recall, have been documented during the presymptomatic and early symptomatic disease stage in carriers of all three genetic mutations (Cheran et al., 2019; Jiskoot et al., 2016; Lee et al., 2017). These findings are largely based on free recall of word lists, suggesting that list learning task performance may be a useful marker of early cognitive decline in genetic carriers. However, the extent to which such impairment reflects memory (i.e. amnestic) deficits per se in genetic bvFTD is unknown. Free recall also relies heavily on sustained, effortful retrieval of information. Hence, free recall is highly susceptible to apathy, variable effort, or executive dysfunction, as well as semantic memory or language impairments, all of which can be present in bvFTD.
Recognition memory, a relatively less examined aspect of episodic memory in prodromal bvFTD, requires discrimination between learned targets and distractor items. Recognition measures are more structured, examiner-guided memory paradigms, which allow problems with effortful retrieval to be overcome by cueing (i.e. presenting the target stimulus as a recognition option) (Johnen & Bertoux, 2019), and may therefore provide more direct insight into memory integrity. Preliminary evidence suggests that early symptomatic MAPT mutation carriers are less successful at discriminating between targets and distractors than controls, and there is thus speculation that there may be true amnesia in bvFTD caused by MAPT mutations (Cheran et al., 2019; van den Berg et al., 2020). However, recognition memory paradigms have unique susceptibilities to cognitive deficits outside of the memory domain. Close examination of error types (e.g., ‘misses’ [missing the target] vs. ‘false positives’ [endorsing a distractor as a target]), can be informative, as false positives have been linked to executive problems (Flanagan et al., 2016) and semantic memory problems (van den Berg et al., 2020). No study to date has explored errors in recognition memory performance in prodromal bvFTD and potential differences among mutation carrier groups. Investigating the relative contributions of semantic and executive problems, alongside true amnestic deficits, to weakened performance on list learning tasks can help shape clinical recommendations or management strategies (e.g., presenting information in a different way to aid semantic problems, assistance with planning for executive dysfunction, writing down information to help with amnesia).
A clear understanding of the earliest cognitive symptoms of genetic bvFTD, including any differences among genetic mutations, is critical and timely with clinical trials on the horizon. Reduced performance relative to controls on list learning tasks has been reported in carriers of all three disease-causing genes; thus, we aimed to cross-sectionally investigate whether an abbreviated list learning task is a useful candidate tool to signal incipient bvFTD in pathogenic MAPT mutation, GRN mutation and/or C9orf72 repeat expansion carriers. We expected that all three carrier groups would display reduced recall compared to non-carriers, with MAPT mutation carriers showing the greatest reduction (Poos et al., 2020). Furthermore, we examined recognition memory performance in the three prodromal mutation carrier groups to determine whether any retrieval difficulties can be overcome with a stimulus cue, which would suggest an absence of true amnesia. Again, based on previous literature (Cheran et al., 2019; Rohrer et al., 2015), we hypothesized that MAPT mutation carriers would show the weakest performance of the three carrier groups. Finally, we assessed a range of additional cognitive functions in the three carrier groups for the purpose of 1) identifying cognitive dysfunctions that may contribute to performance on episodic memory tasks, and 2) characterizing the broader cognitive prodromes of bvFTD due to MAPT and GRN mutations and the C9orf72 repeat expansion.
2. MATERIALS AND METHODS
In the following sections we report how we determined our sample size, all inclusion/exclusion criteria, and all measures in the study. Inclusion/exclusion criteria were established prior to data analysis.
2.1. Participants and Clinical Evaluation
Participants were enrolled in Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL; U54 NS092089) and/or Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS; U01 AG045390) studies; both are now incorporated into the ARTFL LEFFTDS Longitudinal Frontotemporal Lobar Degeneration (ALLFTD; U19 AG063911) consortium. Details regarding the recruitment, clinical and neuropsychological assessment, clinical/genetic/imaging characterization, and other procedures are published elsewhere (Boeve et al., 2020; Heuer et al., 2020; Kornak et al., 2019; Miyagawa, Brushaber, Syrjanen, Kremers, Fields, et al., 2020; Miyagawa, Brushaber, Syrjanen, Kremers, Wszolek, et al., 2020; Olney et al., 2020; Ramos et al., 2020; Rosen et al., 2020; Staffaroni, Cobigo, et al., 2020). DNA was collected for genotyping of FTLD-associated genes (Ramos et al., 2020). Based on the data frozen in January 2020, a total of 211 participants, including prodromal mutation carriers and familial non-carriers, were identified for inclusion. Of the current sample, 68 were identified as carriers of a pathogenic variant of the MAPT, GRN, or C9orf72 genes with mild cognitive and/or behavioral symptoms consistent with being in the prodromal phase of disease (see below), and 143 were familial non-carriers (i.e., have a known autosomal dominant FTLD-causing genetic mutation in their family, but do not carry the mutation). Because the focus of the current study is on prodromal bvFTD, and motor or language phenotypes can complicate interpretation of neuropsychological scores, participants deemed by the evaluating clinician as displaying a primary, secondary or tertiary clinical phenotype of amyotrophic lateral sclerosis (ALS), primary progressive aphasia (PPA), corticobasal syndrome (CBS), or progressive supranuclear palsy (PSP) were excluded from all analyses (n = 10). One participant who carried both a C9orf72 repeat expansion and a GRN mutation was also excluded, resulting in a final carrier group of N = 57 (MAPT = 23; GRN = 15; C9orf72 = 19) (see Table 1).
Table 1.
Demographics of the MAPT, GRN and C9orf72 groups and non-carriers
| Non-carriers n = 143 |
MAPT n = 23 |
GRN n = 15 |
C9orf72 n = 19 |
|
|---|---|---|---|---|
| Age Mean (SD) Range |
49.5 (11.7) 30-80 |
48.4 (9.9) 31-67 |
61.9 (9.9)*† 49-80 |
56.6 (9.2)* 36-74 |
| Education Mean (SD) Range |
15.8 (2.5) 12-20 |
15.7 (2.9) 12-22 |
15.4 (2.8) 12-20 |
14.7 (2.7) 12-20 |
| Sex (M:F) | 57:86 | 14:9 | 7:8 | 6:13 |
| CDR®+NACC FTLD Sum of Boxes Mean (SD) Range |
0 (0) 0-0 |
1.59 (1.01) 0.5-3.0 |
1.42 (0.80) 0.5-3.0 |
1.73 (1.08) 0.5-3.0 |
Note. Age and education are in years.
= significant difference vs. non-carriers pTukey < .05
= significant difference vs. MAPT pTukey < .05. Non-carriers were not included in the CDR®+NACC FTLD Sum of Boxes ANOVA.
Neurologists completed clinical evaluations and neurological examinations with all participants at one of 18 study sites across the United States. Symptom severity was determined via the National Alzheimer’s Coordinating Center (NACC) Clinical Dementia Rating (CDR®) + FTLD module (Knopman et al., 2008), which is abbreviated to CDR®+NACC FTLD, as per Miyagawa et al. (2020). The CDR®+NACC FTLD requires the evaluating clinician to assign a rating of 0-3 indicating symptom severity (0=none, 0.5=questionable, 1=mild, 2=moderate, 3=severe) in the six CDR® domains of Memory, Orientation, Judgment & Problem Solving, Community Affairs, Home & Hobbies, Personal Care (Hughes et al., 1982), plus two supplemental domains of Behavior and Language. An algorithm combines all domain ratings into a global score (0-3) (Miyagawa, Brushaber, Syrjanen, Kremers, Wszolek, et al., 2020). In line with our aim to investigate prodromal cognitive changes, a global CDR®+NACC FTLD score of 0.5 was an inclusion criterion for the mutation carrier group; that is, all mutation carriers were judged by the evaluating clinician to have cognitive and/or behavioral changes consistent with the prodromal phase of disease. We discuss this cohort as ‘prodromal bvFTD’ because 1) the vast majority of carriers of a pathogenic variant of the MAPT, GRN, or C9orf72 genes go on to develop a bvFTD phenotype (Snowden et al., 2015), and 2) we excluded clinician-assigned motor and language phenotypes (ALS, PPA, CBS, PSP). Indeed, where longitudinal data were available (n=41; 70% of the sample), 80% (n=33) progressed to bvFTD or a displayed a stable mild behavioral impairment. The remaining 20% (n=8; 1 MAPT, 3 GRN, 4 C9orf72) were judged to display a predominantly cognitive presentation, or behavioral changes that appeared ‘less stable’ over time (i.e. bounced between CDR®+NACC FTLD of 0.5 and 0 across multiple visits). All non-carriers were rated as a global CDR®+NACC FTLD of 0, considered ‘clinically normal’, and selected to be ≥ 30 years old. Over 97% of the current cohort self-identified as white/Caucasian.
The ARTFL/LEFFTDS/ALLFTD studies received local ethics approval through individual study sites; all participants or their surrogates provided informed written consent.
2.2. Neuropsychological Assessment
All participants completed a neuropsychological assessment in a quiet room, administered by certified study personnel. Participants were monitored for signs of distress or fatigue, which would prompt a discontinuation of testing.
The primary cognitive outcome was an abbreviated version of the California Verbal Learning Test Second Edition (Delis et al., 2000) (CVLT-SF), which assesses learning, recall and recognition memory in the form of a verbal list learning task. The CVLT-SF comprises four learning trials of nine words, which belong to three semantic categories (fruits, clothing, tools). After a ten-minute delay there is a free recall trial and a cued recall trial (category cue). Finally, there is a recognition component in which the examiner reads a list of the nine target words (e.g. blueberry, shoe), nine semantically-related foils (e.g. pear, skirt), and nine unrelated foils (e.g. cloud, knee)2, and the participant indicates whether each word was on the original target list. To assess learning and recall memory we derived the following metrics: total immediate recall (total number of correct words recalled across all learning trials); total intrusions (total number of non-target words across learning trials); delayed free recall (number of correct words recalled after a ten-minute delay); percentage of final learning recalled after delay (percent retention =[delayed recall / final learning trial total correct] * 100); cued recall (number of correct words recalled after a category cue). From the recognition component, we calculated total correct hits (number of targets correctly identified), false positives (number of foils incorrectly identified as targets, either semantically-related or unrelated), the ability to discriminate between targets and foils (discriminability index=1-[{target misses + false positives}/27]). A measure of response bias was also included, which is the tendency to favor ‘yes’ or ‘no’ responses (=all ‘yes’ / all ‘no’), and is theoretically distinct from discriminability in that a participant could have poor discriminability but no response bias (Kramer et al., 2005).
Additional tests from the NACC Uniform Data Set (UDS) v.3.0 battery were also administered in order to obtain more detailed cognitive information. The Craft Story 21 immediate and delayed (20 min) recall provided a measure of narrative episodic memory, and we calculated the percentage of information retained after the delay ([delay / immediate] *100). The Benson Complex Figure copy and delayed (10-15min) recall assessed visuospatial skills and nonverbal memory, respectively, and again the percentage of information retained was calculated ([recall / copy] *100). The Multilingual Naming Test (MINT) assessed confrontation naming. Verbal fluency tasks provided measures of verbal initiation and generation with either a semantic/category (animals) or phonemic/letter (F, L) cue. Trail Making A gauged psychomotor speed, Trail Making B assessed executive function (set-shifting), and Trails B/A ratio score was computed to separate the executive set-shifting component of Trails B from psychomotor speed (Arbuthnott & Frank, 2000). Number Span forward and backward provided measures of auditory attention and working memory, respectively. Finally, global cognition was assessed with the Montreal Cognitive Assessment (MoCA), which is a screening tool designed to briefly assess orientation, memory, visuospatial skills, executive function, attention, working memory, and language (Nasreddine et al., 2005).
2.3. Statistical Analyses
All statistical analyses were performed using JASP version 0.11.1.0 or IBM SPSS version 26 software. Means and standard deviations were computed for all demographic and cognitive variables. To investigate demographic differences between groups, we ran one-way between-groups (MAPT vs. GRN vs. C9orf72 vs. non-carriers) ANOVAs with education and age as the outcome variables, and the Chi-Square test of independence to determine whether there was a relationship between group and sex. We conducted a one-way between-groups (MAPT vs. GRN vs. C9orf72) ANOVA with CDR®+NACC FTLD Sum of Boxes (sum of individual domain scores) as the outcome variable, to determine whether there were differences among mutation carrier groups on this more fine-grained estimate of disease severity. To examine primary memory outcomes and broader cognitive performance in the genetic mutation carrier and non-carrier groups, we conducted a series of one-way between-groups (MAPT vs. GRN vs. C9orf72 vs. non-carriers) ANCOVAs, with memory and cognitive scores as the outcome variables, and age, sex, and education as covariates. Omnibus tests that were significant or trending towards significance (p = .051-.060) were followed up with post-hoc/-tests, and Tukey-corrected p-values are reported for these post-hoc analyses. Group difference estimates and Tukey-corrected 95% confidence intervals (CIs) are also reported. For each of the ANOVA and ANCOVA models and corresponding post-hoc t-tests, a family-wise significance level of α=0.05 was used. For all other analyses, the significance level of α=0.05 was used.
3. RESULTS
3.1. Demographics
The groups differed in age (F(3,196)=7.61, p<0.001). Consistent with the literature (Rohrer et al., 2015), the GRN group was significantly older than the MAPT group (Mean Difference=13.43, 95% CI=3.84, 23.02, pTukey=.002) and non-carriers (Mean Difference=12.35, 95% CI=4.51, 20.19, pTukey<.001). The C9orf72 group was estimated to be >7 years older than non-carriers (Mean Difference=7.11, 95% CI=0.06, 14.17, pTukey=.047), and the MAPT group (Mean Difference=8.20, 95% CI=−0.76, 17.15, pTukey=.086), though only the C9orf72 vs. non-carrier comparison was statistically significant at the family-wise α=0.05 level. There were no significant group differences in years of education3, F(3,196)=0.95, p=.420, disease severity as estimated by CDR®+NACC FTLD Sum of Boxes3, F(2,54)=0.44, p=.644, nor sex distribution, χ2(3)=6.61, p=.203 (all post-hoc comparisons p>.05).
3.2. Neuropsychological Assessment
Means and standard deviations for all cognitive variables are provided in Table 2. Omnibus test results, as well as group difference estimates and 95% CIs for the post hocs discussed in text, are available in Table 3 (see Appendix A for descriptive CVLT-SF figures; for the full set of post hoc comparisons see Supplemental Tables 1 and 2). On the CVLT-SF, in terms of learning and recall, we found statistically significant group differences on the following metrics: total immediate recall, delayed free recall, percent retention, and cued recall. The MAPT group had significantly lower total immediate recall than non-carriers. On delayed free recall, the MAPT group retrieved significantly fewer words than non-carriers and the C9orf72 group. Estimates indicated that the MAPT group also recalled fewer words than the GRN group, but this was not statistically significant. The MAPT group retained a lower percentage of information than non-carriers, C9orf72, and GRN. The MAPT group also recalled fewer words with a category cue compared to non-carriers, and although estimates indicated that the MAPT group performed below C9orf72 and GRN on cued recall, group differences were not statistically significant. The number of intrusions was not statistically different among groups, and all estimated mean group differences were <1, indicating limited clinical impact.
Table 2.
Neuropsychological test scores for the MAPT, GRN, and C9orf72 groups and non-carriers: raw means (standard deviations) shown.
| Non-carriers n = 143 |
MAPT n = 23 |
GRN n = 15 |
C9orf72 n = 19 |
|
|---|---|---|---|---|
| Memory Tests | ||||
| CVLT-SF | ||||
| Immediate recall (/36) | 29.17 (3.72) | 26.14 (7.27)* | 27.40 (6.05) | 28.06 (4.96) |
| Delayed Free Recall (/9) | 7.29 (1.68) | 5.64 (3.06)* | 6.73 (2.79) | 7.24 (1.75)† |
| % Retention | 88.91 (16.79) | 66.90 (36.03)* | 84.79 (29.34)† | 86.52 (26.37)† |
| Cued Recall (/9) | 7.59 (1.42) | 6.36 (2.95)* | 7.20 (2.24) | 7.47 (1.84) |
| Intrusions | 0.44 (0.84) | 0.96 (1.36) | 0.60 (1.60) | 0.28 (0.83) |
| Recognition Discriminability (0-1) | 0.96 (0.06) | 0.90 (0.15)* | 0.90 (0.19) | 0.94 (0.09) |
| Recognition Hits (/9) | 8.61 (0.72) | 8.27 (1.42) | 8.60 (0.83) | 8.35 (0.79) |
| Recognition False Positives | 0.64 (1.15) | 2.00 (2.94)* | 2.33 (4.37) | 1.12 (2.31) |
| Semantically-related FPs | 0.53 (1.00) | 1.36 (1.94) | 1.53 (2.80) | 0.94 (1.98) |
| Unrelated FPs | 0.11 (0.36) | 0.59 (1.46)* | 0.80 (1.86)* | 0.18 (0.39) |
| Response Bias | 0.53 (0.13) | 0.65 (0.33) | 0.84 (0.74)* | 0.58 (0.33) |
| Benson Figure | ||||
| % Retention | 81.55 (16.19) | 72.00 (24.42) | 79.17 (13.07) | 75.34 (18.04) |
| Craft Story | ||||
| % Retention (verbatim) | 91.07 (15.44) | 81.79 (32.09) | 85.13 (15.57) | 88.67 (16.69) |
| Other Cognitive Tests | ||||
| Multilingual Naming Test | ||||
| Raw /32 | 30.24 (1.63) | 28.26 (3.49)* | 29.73 (1.53) | 29.90 (1.70) |
| Trail Making | ||||
| A (sec) | 23.92 (8.45) | 25.09 (7.20) | 29.53 (6.17) | 33.90 (14.56)* |
| B (sec) | 58.23 (26.58) | 67.00 (32.07) | 109.67 (84.79)*† | 82.84 (30.35) |
| Trails B Errors | 0.28 (0.55) | 0.22 (0.42) | 1.00 (1.46)*† | 0.53 (0.70) |
| B/A ratio | 2.48 (0.83) | 2.64 (0.92) | 3.51 (2.17)* | 2.62 (0.86) |
| Verbal Fluency | ||||
| Category (Animals) | 23.36 (5.68) | 21.22 (5.10) | 20.93 (6.81) | 21.16 (5.11) |
| Letter (F+L) | 28.68 (7.93) | 27.83 (9.25) | 24.73 (10.19) | 25.37 (7.19) |
| Number Span | ||||
| Forward | 9.02 (2.39) | 9.17 (2.17) | 7.53 (2.30) | 8.26 (2.31) |
| Backward | 7.83 (2.39) | 8.00 (2.70) | 6.40 (2.90) | 7.74 (2.31) |
| Benson Figure Copy (/17) | 15.84 (1.15) | 16.00 (0.95) | 14.87 (1.77) | 15.47 (1.31) |
| MoCA Total Score (/30) | 27.18 (2.13) | 25.65 (3.58) | 24.27 (4.22)* | 25.84 (3.13) |
Note.
= significant difference vs. non-carriers pTukey < .05
= significant difference vs. MAPT pTukey < .05. Analyses are adjusted for age. FPs = False Positives. CVLT-SF = California Verbal Learning Test Short Form. MoCA = Montreal Cognitive Assessment.
Table 3.
Results of the ANCOVAs and post hoc comparisons for main analyses of neuropsychological data.
| Omnibus test | Post hoc comparisons | |||
|---|---|---|---|---|
| F-Statistic (df), p-value | Estimated mean difference | 95% CI Lower, Upper |
pTukey | |
| Memory Tests | ||||
| CVLT-SF | ||||
| Immediate recall | F(3,187) = 2.71, p = .046 | MAPT < non-carriers: −2.89 | −5.51, −0.26 | .025 |
| Delayed Free Recall | F(3,186) = 4.51, p = .004 | MAPT < non-carriers: −1.60 | −2.75, −0.44 | .003 |
| MAPT < C9orf72: −1.84 | −3.51, −0.16 | .025 | ||
| MAPT < GRN: −1.59 | −3.28, 0.15 | .086 | ||
| % Retention | F(3,187) = 6.32, p < .001 | MAPT < non-carriers: −21.43 | −34.32, −8.55 | <.001 |
| MAPT < C9orf72: −21.01 | −39.22, −2.80 | .017 | ||
| MAPT < GRN: −21.74 | −41.07, −2.41 | .021 | ||
| Cued Recall | F(3,185) = 3.11, p = .028 | MAPT < non-carriers: −1.16 | −2.18, −0.14 | .019 |
| MAPT < C9orf72: −1.32 | −2.79, 0.16 | .098 | ||
| MAPT < GRN: −1.26 | −2.79, 0.28 | .148 | ||
| Intrusions | F(3,187) = 2.36, p = .073 | |||
| Discriminability | F(3,184) = 3.71, p = .013 | MAPT < non-carriers: −0.06 | −0.12, −0.01 | .017 |
| GRN < non-carriers: −0.05 | −0.11, 0.02 | .273 | ||
| Recognition Hits | F(3,185) = 1.49, p = .220 | |||
| Recognition FPs | F(3,184) = 4.36, p = .005 | MAPT < non-carriers: 1.30 | 0.13, 2.47 | .023 |
| GRN < non-carriers: 1.38 | −0.06, 2.82 | .066 | ||
| Semantic FPs | F(3,184) = 2.87, p = .038 | MAPT < non-carriers: 0.81 | −0.05, 1.67 | .073 |
| GRN < non-carriers: 0.76 | −0.30, 1.82 | .251 | ||
| Unrelated FPs | F(3,184) = 4.67, p = .004 | MAPT < non-carriers: 0.49 | 0.02, 0.96 | .037 |
| GRN < non-carriers: 0.62 | 0.04, 1.20 | .030 | ||
| Response Bias | F(3,184) = 5.37, p = .001 | GRN < non-carriers: 0.29 | 0.09, 0.49 | .001 |
| Benson Figure % retention | F(3,19l) = 2.55, p = .057 | MAPT < non-carriers: −10.09 | −20.03, −0.14 | .045 |
| MAPT< GRN: −12.35 | −27.25, 2.56 | .142 | ||
| Craft Story % retention | F(3,192) = 1.96, p = .121 | |||
| Other Cognitive Tests | ||||
| Multilingual Naming Test | F(3,193) = 8.34, p < .001 | MAPT < non-carriers: −2.08 | −3.16, −0.99 | <.001 |
| MAPT < C9orf72: −1.90 | −3.42, −0.38 | .008 | ||
| Trails A (sec) | F(3,193) = 4.94, p = .002 | C9orf72 < non-carriers: 8.12 | 2.60, 13.64 | .001 |
| C9orf72 < MAPT: 6.58 | −0.43, 13.59 | .075 | ||
| C9orf72 < GRN: 5.62 | −2.07, 13.31 | .224 | ||
| Trails B (sec) | F(3,193) = 6.75, p < .001 | GRN < non-carriers: 40.30 | 15.68, 64.93 | <.001 |
| GRN < MAPT: 30:58 | 0.78,60.38 | .42 | ||
| Trails B Errors | F(3,193) = 3.73, p = .012 | GRN < non-carriers: 0.59 | 0.11, 1.06 | .010 |
| GRN < MAPT: 0.66 | 0.09, 1.24 | .017 | ||
| B/A ratio | F(3,193) = 3.58, p = .015 | GRN < non-carriers: 0.89 | 0.17, 1.62 | .009 |
| GRN < C9orf72: 0.90 | 0.01, 1.79 | .046 | ||
| Category Fluency | F(3,192) = 1.40, p = .243 | |||
| Letter Fluency | F(3,192) = 0.94, p = .422 | |||
| Number Span Forward | F(3,191) = 1.37, p = .254 | |||
| Number Span Backward | F(3,191) = 1.05, p = .372 | |||
| Benson Figure Copy | F(3, 192) = 2.37 p = .072 | |||
| MoCA Total | F(3,193) = 6.06, p < .001 | GRN < non-carriers: −2.66 | −4.54, −0.78 | .002 |
| MAPT < non-carriers: −1.35 | −2.86, 0.15 | .095 | ||
| C9orf72 < non-carriers: −1.10 | −2.76, 0.56 | .318 | ||
Note. Bold denotes statistically significantly difference between groups at α = 0.05. CI = Tukey adjusted confidence interval. Analyses are adjusted for age. < denotes poorer performance, regardless of whether better performance is related to higher raw scores (e.g. retention) or lower raw scores (e.g. errors). Only post hoc comparisons discussed in text are presented here, for full set of post hoc comparisons see Supplemental Tables 1 and 2. CVLT-SF = California Verbal Learning Test Short Form. MoCA = Montreal Cognitive Assessment.
With regard to recognition, we found significant group-level differences in recognition discriminability and number of false positive errors, including both semantic and unrelated false positives. The MAPT group was significantly weaker than non-carriers at discriminating between targets and distractor items. Although estimates indicated that GRN also had a discriminability weakness compared to non-carriers, wide confidence intervals indicated high variability in the GRN group, and the difference was not statistically significant. Poor discriminability appeared to be driven by over endorsement of distractor items, rather than missing the targets, as the ‘correct hits’ omnibus test was not significant and all mean group differences were <1 (indicating limited clinical impact). However, the MAPT group made significantly more false positive errors than non-carriers. Estimates indicated that the GRN group also had an increase in false positives compared to non-carriers, though this did not reach significance. A breakdown of error types revealed that both MAPT and GRN made more unrelated false positives than non-carriers. Estimates suggested that the MAPT also made more semantically-related false positives than non-carriers and the GRN group, though these differences were not statistically significant. Response bias showed significant difference at the group level, with GRN having a stronger bias towards ‘yes’ responses than non-carriers.
We found divergent results on the additional tasks assessing episodic memory: the percentage of information recalled after a delay on the Benson Figure and Craft Story. On the Benson Figure, the MAPT group recalled significantly less figure information than non-carriers, and there was a numerically similar MAPT vs. GRN difference though confidence intervals were wide. However, there were no significant omnibus differences on the Craft Story, and large confidence intervals preclude interpretation. In terms of language, group differences were found on the MINT (naming), with the MAPT group performing below non-carriers and the C9orf72 group, suggesting some degree of naming difficulty in the MAPT group. The MINT has a ceiling effect so a group difference of two words may be clinically informative. Trails A also showed group differences, but estimates indicated that it was the C9orf72 group that was >5 seconds slower than non-carriers, MAPT, and GRN. This indicates a potential problem with processing speed in C9orf72 repeat expansion carriers. In terms of executive functioning, measured via Trails B, there were significant group-level differences in time (seconds) and errors, as well as the Trails B/A ratio score. This time, the GRN group was the lowest performing group: GRN mutation carriers were an estimated 30-40 seconds slower than non-carriers and MAPT mutation carriers, and also made more errors than non-carriers and MAPT mutation carriers. GRN carriers also had a significantly higher Trails B/A ratio (worse performance) than non-carriers and the C9orf72 group. In terms of visuospatial skills, there were no significant group differences on the Benson Figure Copy, but the GRN group scored almost 1 point less than non-carriers, and >1 point less than MAPT, which may have clinical significance. There were no significant group differences on letter or category fluency, with wide confidence intervals and small estimated group differences (<3.4 on letter fluency, <2.2 on category fluency). Likewise, there were no significant group differences on Number Span forward or backward, and again large confidence intervals for the estimates. Finally, group-level differences were found on the MoCA total score, with the GRN group scoring significantly lower than non-carriers, suggesting that global cognition was reduced. The estimated MAPT vs. non-carrier and C9orf72 vs. non-carrier differences were <1.5 points and not significant. Taken together, these results hint at gene-specific cognitive profiles. To aid clinicians in determining whether group means on the NACC UDS v.3.0 neuropsychological tests fell into the range of clinical impairment, we have provided figures in the Supplementary Material depicting the data in terms of USA-specific, age-, sex-, and education-adjusted z-scores (Kornak et al., 2019) (see Supplementary Figure 1).
3.3. Exploratory Analyses
The analyses above suggest that MAPT and GRN mutation carriers both have some degree of difficulty relative to non-carriers on CVLT-SF recognition discriminability, endorsing more false positive responses. False positive errors may occur due to memory problems, executive deficits, or language / semantic dysfunction (e.g., Flanagan et al., 2016; van den Berg et al., 2020). To explore these potential contributing factors in the MAPT and GRN groups, we ran a series of exploratory analyses using MINT total score as a measure of naming / semantic memory, Benson Figure percent recall as a measure of nonverbal episodic memory (because Figure memory is less likely to be confounded by semantic impairment), and Trails B/A ratio as a measure of executive function.
Firstly, we re-ran the between-groups (MAPT vs. GRN vs. C9orf72 vs. non-carriers) ANCOVA analysis above, with CVLT-SF recognition discriminability as the outcome variable, and either MINT score, Figure Recall, or Trails B/A ratio added as a covariate (alongside demographics), to see whether the group differences in recognition discriminability held when these other cognitive functions were taken into account. When MINT score was added as a covariate, the omnibus group difference in recognition memory discriminability was no longer significant (F(3,183) = 1.08, p = .357), though MINT was a significant covariate (p <.001). The MAPT group was no longer significantly below non-carriers (Mean Difference = −0.02, 95% CI = −0.08, 0.03, pTukey = .673). When Figure recall was added as a covariate, the omnibus group difference in recognition memory remained significant (F(3,181) = 2.99, p = .033), and Figure Recall was a significant covariate (p <.001). The MAPT vs. non-carrier group was no longer significantly below non-carriers (Mean Difference = −0.05, 95% CI = −0.10, 0.00, pTukey = .084), yet the estimated GRN vs. non-carrier difference in recognition discriminability did not change, suggesting a potential small role for nonverbal episodic memory in the MAPT but not the GRN group. When Trails B/A ratio was added as a covariate, the omnibus group difference in recognition memory discriminability remained significant (F(3,181) = 3.01, p = .031), and Trails B/A ratio was a significant covariate (p <.001). However, in this case, the MAPT vs. non-carrier group difference remained significant and essentially unchanged (Mean Difference = −0.06, 95% CI = −0.11, −0.01, pTukey = . 022), but the GRN Marginal Mean increased to 0.93 (unadjusted = 0.90; see Table 2). This tentatively suggests that executive function was a contributing factor in the GRN but not MAPT group.
Secondly, Spearman’s partial correlations (controlling for age, sex, education) between the CVLT-SF recognition discriminability index and MINT score, Figure Recall, and Trails B/A ratio within the MAPT and GRN carrier groups. In the MAPT group, there were small to medium sized correlations between recognition discriminability and MINT score (rspearman = .23) and Figure recall (rSpearman = .36), numerically larger than the correlation with Trails B/A ratio (rspearman = −.12). By contrast, in the GRN group, numerically the largest correlation was between discriminability and Trails B/A ratio (rSpearman = −.55), and a moderately sized correlation between discriminability and MINT (rSpearman = .40). There was no indication of any relationship between discriminability and Figure Recall in the GRN group (rSpearman = .08).
None of the correlations reached statistical significance in these small samples, and the values should be interpreted with high caution given the group sizes. However, taken together, these analyses tentatively suggest that semantic / language and episodic memory dysfunction may be contributing to weakened CVLT-SF recognition discriminability in the MAPT group; we found no evidence that executive dysfunction was a factor. In contrast, executive dysfunction appeared to be most strongly related to weakened CVLT-SF recognition performance in the GRN group, with a smaller potential role of semantic dysfunction.
Finally, although the focus of our exploratory analyses was on recognition memory, we note that the recall aspects of the CVLT-SF, such as immediate and cued recall, as well as percentage of words retained after a delay, may be influenced by semantic and/or executive dysfunction. When the ANCOVA analyses with CVLT-SF recall metrics as the outcome variables were re-run with naming (MINT) added as a covariate, only the percentage of words retained after a delay remained significant (F(3,186) = 2.74, p = .045), with the MAPT group performing below non-carriers (Mean Difference = −13.91, 95% CI = −26.98, −0.85, pTukey = .032). Consistent with our exploratory CVLT-SF recognition findings, this hints at a role of semantic dysfunction but this deficit does not appear to fully explain the findings in MAPT mutation carriers. When Trails B/A ratio was entered as a covariate, all omnibus group differences remained significant (all p <.05).
4. DISCUSSION
Studying the earliest or prodromal phase of bvFTD has proven challenging for the field. Sporadic cases of bvFTD are commonly misdiagnosed (Lanata & Miller, 2016; Woolley et al., 2011), and tend only to come to the attention of FTD specialists when they are past the prodromal phase. This underscores the value of genetic mutation carriers, whom we can track through symptom onset and early disease (Tavares et al., 2020). The current study investigated whether episodic memory weaknesses, on list learning in particular, might signal early cognitive difficulties in carriers of a pathogenic variant of the MAPT, GRN, or C9orf72 genes who will likely go on to develop bvFTD, and whether recall difficulties in any of the three carrier groups could be overcome by a recognition cue; that is, whether memory difficulties exist primarily at the level of retrieval or storage. We further aimed to characterize the cognitive profiles of the three carrier groups by examining performance on other neuropsychological tests.
Although previous studies have reported reduced performance on list learning tasks in preclinical carriers of all three disease-causing genes, we found MAPT mutation carriers to perform the weakest on the CVLT-SF. This was true for recall and recognition-based metrics derived from the task. Previous findings regarding recognition memory in MAPT carriers are mixed: in one longitudinal study of cognitive function in the years leading to symptom onset, MAPT carriers did not show significant decline on Rey Auditory Verbal Learning Test recognition (Jiskoot et al., 2018), but another cross-sectional study reported significantly lower recognition memory performance in preclinical and early symptomatic MAPT carriers compared to non-carrier controls (Cheran et al., 2019). We replicated the Cheran et al. (2019) finding of low recognition memory discriminability; however, the authors did not report target misses vs. false positives. In the current study, we observed an over endorsement of distractors as targets (false positive errors) rather than target misses. False positive errors may occur for several possible reasons, including forgetting the material, executive deficits (e.g., ‘disinhibited’ or perseverative responding), problems with language (e.g., semantics or concept representations), or any combination of these. Increased false positives have been documented in both Alzheimer’s disease and bvFTD (Ricci et al., 2012; van den Berg et al., 2020), and there is evidence that both memory and executive processes are associated with high false positive rates (Flanagan et al., 2016). Further, weaker language skills are associated with lower recognition discriminability in bvFTD (van den Berg et al., 2020), which is particularly relevant for verbally-mediated memory tasks such as the CVLT. It is difficult to fully disentangle the relative contributions of memory, executive, and language skills in the context of a single list learning test, but examining performance on other cognitive tasks may provide some insight.
In our study, MAPT mutation carriers were the only genetic group to perform worse than non-carriers on naming. Increasing evidence suggests that semantic memory deficits, which can contribute to impaired naming, are often present in MAPT mutation carriers (Grossman, 2010; Pickering-Brown et al., 2008; Snowden et al., 2015), and some degree of naming dysfunction has been documented in MAPT mutation carriers at the presymptomatic or prodromal disease stage (Cheran et al., 2019; Jiskoot et al., 2018; Rohrer et al., 2015). In addition, in the current study, MAPT mutation carriers performed significantly lower than non-carriers on the nonverbal episodic memory task (Benson Figure recall % retained), and group difference estimates supported the clinical impact of this (>10% less figure retained after a delay in the MAPT group vs. non-carriers and GRN). Nonverbal memory is less likely to be confounded by semantic impairment. By contrast, we did not find evidence of executive dysfunction in the MAPT group. Taken together, it seems that the discriminability weakness in MAPT carriers, specifically the high number of false positive errors, reflects some degree of semantic disruption as well as genuine memory difficulty. The exploratory analyses tentatively support this suggestion, but we cannot truly disentangle the contributions of other cognitive skills in this limited sample4.
Overall, our MAPT results are consistent with a temporal lobe-predominant disorder, supported by neuroimaging findings of early atrophy in the anterior and medial temporal lobes (Domínguez-Vivero et al., 2020; Greaves & Rohrer, 2019; Olney et al., 2020; Rohrer et al., 2010). This may explain some of the heterogeneity in the literature regarding episodic memory in bvFTD: a MAPT mutation might be a risk factor for early memory decline due to anteromedial temporal lobe neurodegeneration.
In contrast to MAPT, we did not find that GRN mutation carriers had statistically significant difficulty relative to controls with recall or recognition discriminability on the CVLT-SF. However, inspection of the means and group difference estimates (Table 2, Appendix A Figures) shows that, on average, GRN mutation carriers performed similarly to MAPT mutation carriers in terms of discriminability, suggesting some degree of weakness. GRN mutation carriers also made significantly more unrelated false positive errors than non-carriers. The fact that the discriminability difference between GRN mutation carriers and controls was not statistically significant appears to be due to wider variability in performance. In fact, of the three disease-causing genes, GRN is the most diverse in clinical presentation, and great variability in cognitive profiles has been documented (Le Ber et al., 2008). This is consistent with atrophy patterns in GRN, which may be widespread and asymmetrical, with some divergence in cognitive profiles documented in left vs. right dominant atrophy (Le Ber et al., 2008); this stands in contrast to the circumscribed symmetrical atrophy patterns characteristic of MAPT (Rohrer et al., 2010; Rohrer & Warren, 2011). It may be that a subset of GRN mutation carriers present with a truly amnestic cognitive profile (Brouwers et al., 2007; Kelley et al., 2010). However, we did not find evidence for this upon close inspection of each individual in our current cohort. Rather, low recognition discriminability in GRN mutation carriers appeared to be driven by a high number of false positive errors, reflective of a dysexecutive syndrome. Consistent with previous research (Pickering-Brown et al., 2008; Poos et al., 2020), our GRN group was the most dysexecutive of the three carrier groups, performing poorly on an executive test of set-shifting (Trails B).
Notably, the GRN group had significantly higher Trails B/A ratios than non-carriers, suggesting that the executive difficulty could not be accounted for by slowed psychomotor speed. A ratio score of >3 is thought to reflect a clinical impairment of set-shifting, validated against other cognitive switching tasks (Arbuthnott & Frank, 2000), and the GRN group Mean was 3.5 (C9orf72 Mean = 2.6; MAPT Mean = 2.6). Further, the GRN group showed a significantly more liberal response bias on the recognition portion of the CVLT-SF than non-carriers, which lends support to a dysexecutive profile (van den Berg et al., 2020).
Interestingly, while theoretically executive dysfunction is linked to false positive errors, set-shifting (the aspect of executive functioning measured by Trails B) has not been a focus of previous research; rather, verbal disinhibition is the facet of executive function most closely linked to false positive list learning errors (Flanagan et al., 2016). However, a set-shifting problem may lead to difficulty switching between ‘yes’ and ‘no’ responses as the task demands. Unfortunately, we did not have any standard neuropsychological measures of verbal response inhibition (e.g. Stroop, Hayling test) in the current study. It is possible that such a measure would have been more strongly related to false positive errors in the GRN group, and we may have found a relationship in the MAPT group as well. This remains an open question for future research, and underscores the importance of including multiple tests of executive function in the assessment of prodromal bvFTD, for clinical and theoretical reasons.
In comparison to MAPT and GRN mutations, cognitive deficits associated with C9orf72 repeat expansions have been described as ‘milder’ and more ‘diffuse’ (Poos et al., 2020). Our current findings do not challenge that position; numerically the C9orf72 group scored the highest of the three carrier groups across all CVLT-SF metrics, the Craft Story, and MINT, suggesting that verbal and memory skills remained relatively preserved; small estimated group differences (vs. non-carriers) support this. In fact, the only test on which C9orf72 group performed below non-carriers and other genetic groups was Trails A. Slowed processing speed in the absence of other deficits is consistent with widespread mild neurodegeneration, and indeed neuroimaging studies of C9orf72 repeat expansion carriers have reported degeneration encompassing frontal and temporal lobes, as well as subcortical areas and the cerebellum (Mahoney, Downey, et al., 2012)
Somewhat unexpectedly, we did not find significant differences on verbal fluency tasks among any of the three carrier groups and non-carriers, although wide confidence intervals highlight variability. It is well established that patients with bvFTD exhibit verbal initiation and generation dysfunction on both phonemic/letter and semantic/category fluency tasks (Libon et al., 2009; Rascovsky et al., 2007; Staffaroni, Bajorek, et al., 2020), even at the prodromal stage (Cheran et al., 2019; Jiskoot et al., 2018). It may be that longitudinal declines on fluency tasks are more informative than single assessments, but this is speculative. Another domain where no significant differences between mutation carriers and non-carriers were found was digit span, though again variability and wide confidence intervals incite caution in interpretation. Rohrer et al. (2015) reported that Digit Span backward showed the earliest decline for GRN mutation carriers. In the current study, numerically the GRN group performed the lowest so it is possible we were underpowered to detect a statistically significant difference.
A major question for future research remains: how do we translate findings from genetic cohorts to sporadic bvFTD? One recent paper suggested that familial and sporadic bvFTD are clinically similar, and therefore clinical tools developed in the context of familial bvFTD may be applicable to sporadic cases (Heuer et al., 2020). However, whether this is true for the prodrome presentation remains an open question, and our current results show little consistency between the prodromal cognitive profiles of the three main autosomal dominant genetic mutation carrying genes. Identifying the cognitive prodrome of sporadic bvFTD is an ongoing effort in the field. It is possible that with disease progression, the different mutations converge into a more homogenous cognitive profile, but, with clinical trials imminent, the earliest cognitive markers of decline are of high importance. These findings should be taken into account when developing outcome measures for trials, as subtle differences among cognitive profiles in different mutations have implications for clinical trial endpoints. Furthermore, the current findings are applicable to early cognitive management strategies in cases where genetic status is known, and longitudinal analyses will be valuable in investigating this.
As with any study of rare genetic mutation carriers, the conclusions are limited by small sample sizes. Given the statistical trends in the data, it is likely that we were underpowered to detect differences between mutation carrier groups on most of the cognitive measures, hence our focus on estimates and confidence intervals. However, our sample sizes are comparable to influential studies in the field (Rohrer et al., 2015), and our results are strengthened by matching the genetic groups for disease severity. Furthermore, we maximized phenotypic homogeneity in the genetic groups by excluding motor and language phenotypes, rendering our results highly applicable to the cognitive prodrome of bvFTD. However, we acknowledge the significant overlap between clinical FTLD phenotypes (Murley et al., 2020), and we cannot rule out the possibility that a portion of our prodromal carriers will go on to develop motor and language phenotypes (i.e. not bvFTD). Our conclusions are also limited by the fact that our study design was cross-sectional, lending itself to simple analyses that were not corrected for multiple comparisons (to minimize the risk of Type II error in this small sample size). It is promising that through national and international consortia, future studies will have access to larger numbers of longitudinally-followed FTLD cases, with clinical and cognitive data, on which more complex statistical modelling can be performed.
Characterizing the earliest cognitive markers of disease in genetic mutation carriers is of high clinical and research importance. Identifying specific cognitive weaknesses early in the disease course can inform clinical management strategies. Carriers of FTLD-associated genetic mutations are rare, but provide an invaluable opportunity to examine and define the prodromal disease stage. Taken together, our findings suggest that list learning tasks, particularly recall and recognition, may be sensitive cognitive markers for incipient bvFTD, most reliably for MAPT mutation carriers but potentially also for a subset of GRN mutation carriers. However, we emphasize that results should be interpreted in the context of the broader cognitive profile (including other episodic memory tasks) and genetic results if known, and we highlight that the utility of list learning tasks in distinguishing early FTD from Alzheimer’s disease is questionable (Flanagan et al., 2016). Our findings also add to the growing body of literature demonstrating distinct cognitive profiles in carriers of a pathogenic variant of the MAPT, GRN, or C9orf72 genes. Overall, we found poor naming and episodic memory to be characteristic of MAPT mutation carriers, predominant executive dysfunction in GRN mutation carriers, mildly slowed processing speed in C9orf72 repeat expansion carriers.
Supplementary Material
Highlights.
Prodromal cognitive profiles differ among genetic causes of frontotemporal dementia
Weak verbal recognition memory may signal early FTD in MAPT and GRN mutations
Episodic memory and semantic weaknesses are most characteristic of MAPT mutations
Executive dysfunction is most characteristic of GRN mutations
Mildly slowed processing speed is evident in C9orf72 repeat expansions
ACKNOWLEDGEMENTS AND FUNDING
Data collection and dissemination of the data presented in this manuscript were supported by the ALLFTD Consortium (U19: AG063911, funded by the National Institute on Aging and the National Institute of Neurological Disorders and Stroke) and the former ARTFL & LEFFTDS Consortia (ARTFL: U54 NS092089, funded by the National Institute of Neurological Disorders and Stroke and National Center for Advancing Translational Sciences; LEFFTDS: U01 AG045390, funded by the National Institute on Aging and the National Institute of Neurological Disorders and Stroke). The manuscript has been reviewed by the ALLFTD Executive Committee for scientific content. The authors acknowledge the invaluable contributions of the study participants and families as well as the assistance of the support staffs at each of the participating sites. No part of the study procedures was pre-registered prior to the research being conducted.
Abbreviations:
- ALS
Amyotrophic lateral sclerosis
- bvFTD
Behavioral variant frontotemporal dementia
- C9orf72
Chromosome 9 open reading frame 72
- CBS
Corticobasal syndrome
- CDR
Clinical Dementia Rating
- CI
Confidence interval
- CVLT-SF
California Verbal Learning Test – Short Form
- FTD
Frontotemporal dementia
- FTLD
Frontotemporal lobar degeneration
- GRN
Progranulin
- MAPT
Microtubule-associated protein tau
- MINT
Multilingual Naming Test
- NACC
National Alzheimer’s Coordinating Center
- PPA
Primary progressive aphasia
- PSP
Progressive supranuclear palsy
- UDS
Uniform Data Set
APPENDIX A
Figure A.
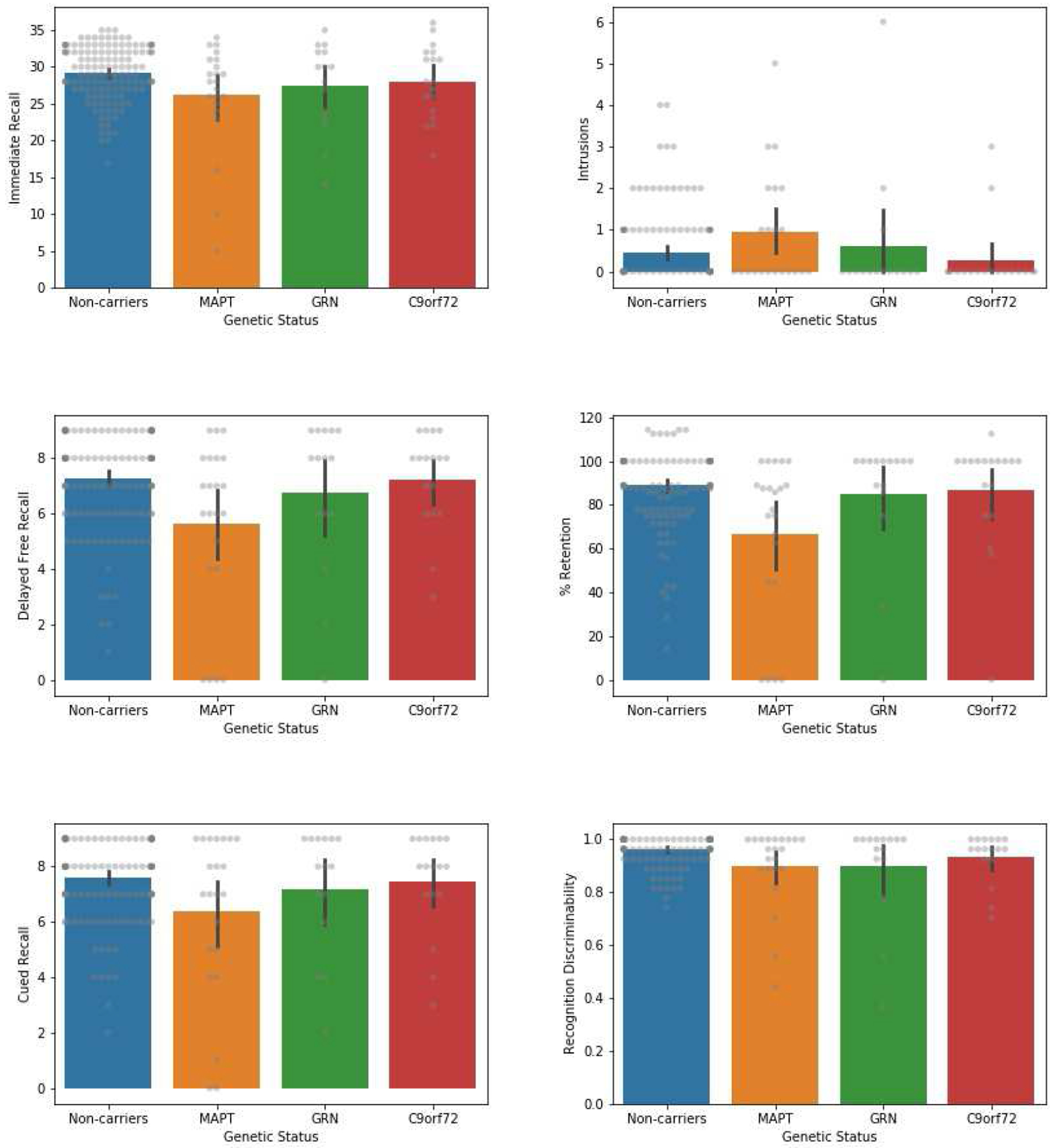
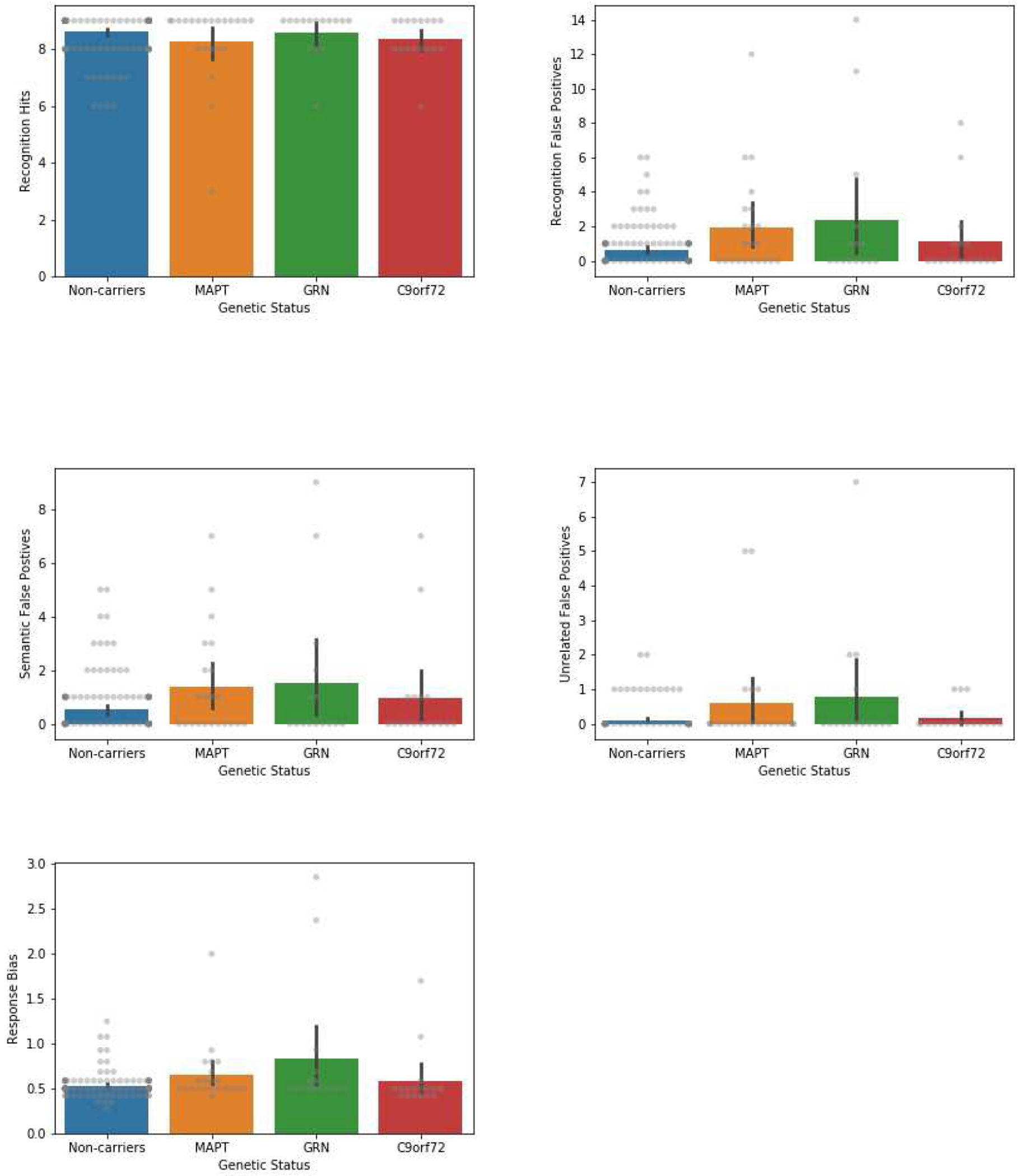
Bar plots showing mean performance on CVLT-SF metrics, compared across genetic carrier (MAPT, GRN, 9orf72) and non-carrier groups. Descriptive only (uncorrected). Error bars represent 95% confidence intervals. Raw data depicted in gray.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY STATEMENT
To maintain confidentiality of sensitive clinical and genetic information, these data cannot be archived in a public repository. All data are available upon request from the ALLFTD Executive Committee at https://www.allftd.org/.
COMPETING INTERESTS
M.S. Barker, Ph.D.: Nothing to disclose
M. Manoochehri, B.A.: Nothing to disclose
S.J. Rizer, M.A.: Nothing to disclose
B.S. Appleby, M.D.: Has received research funding from CDC, NIH, Ionis, & Alector
D. Brushaber, B.S.: Nothing to disclose
S.I. Dev, Ph.D.: Nothing to disclose
K.L. Devick, Ph.D.: Nothing to disclose
B.C. Dickerson, M.D.: Research support from NIH, Alzheimer’s Drug Discovery Foundation, consulting for Acadia, Arkuda, Axovant, Lilly, Biogen, Merck, Novartis, Wave LifeSciences. Editorial duties with payment for Elsevier (Neuroimage: Clinical and Cortex). Royalties from Oxford University Press and Cambridge University Press.
J.A. Fields, Ph.D., L.P.: Receives research funding from the NIH.
T.M. Foroud, Ph.D.: Nothing to disclose
L.K. Forsberg, Ph.D.: Nothing to disclose
D.R. Galasko, M.D.: Paid consultant for Biogen, vTv Pharmaceuticals, Cognition Theraptutics, Fujirebio and Amprion and received payment as a journal Editor from Springer.
N. Ghoshal, M.D., Ph.D.: Has participated or is currently participating in clinical trials of anti-dementia drugs sponsored by the following companies: Bristol Myers Squibb, Lilly/Avid Radiopharmaceuticals, Janssen, Novartis, Pfizer, Wyeth, SNIFF (The Study of Nasal Insulin to Fight Forgetfulness) study, and A4 (The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease) trial. She receives research support from Tau Consortium and Association for Frontotemporal Dementia and is funded by the NIH.
N.R. Graff-Radford, MBBCh: Takes part in multicenter studies funded by Biogen, AbbVie, and Lilly.
M. Grossman, M.D., Ed.D.: Nothing to disclose
H.W. Heuer, Ph.D.: Nothing to disclose
G-Y. Hsiung, M.D.: Has received research support as a clinical trials site investigator from Anavax, Biogen, Eli Lilly and Roche; and has received research funding from the CIHR, Alzheimer Society of Canada, and NIH.
J. Kornak, Ph.D.: Has provided expert witness testimony for 1) Teva Pharmaceuticals in Forest Laboratories Inc. et al. v. Teva Pharmaceuticals USA, Inc., Case Nos. 1:14-cv-00121 and 1:14-cv-00686 (D. Del. filed Jan. 31, 2014 and May 30, 2014) regarding the drug Memantine; 2) for Apotex/HEC/Ezra in Novartis AG et al. v. Apotex Inc., No. 1: 15-cv-975 (D. Del. filed Oct. 26, 2015, regarding the drug Fingolimod; 3) on behalf of Puma Biotechnology in Hsingching Hsu et al, vs. Puma Biotechnology, INC., et al. 2018 regarding the drug Neratinib and 4) on behalf of Hikma Pharmaceuticals in Amarin Pharma, Inc vs. Hikma Pharmaceuticals in 2019. He receives research support from the NIH.
I. Litvan, M.D.: Research is supported by the National Institutes of Health grants: 5P50AG005131-33, 2R01AG038791-06A, U01NS090259, U01NS100610, U01NS80818, R25NS098999, P20GM109025; U19 AG063911-1; 1R21NS114764-01A1; Parkinson Study Group, Michael J Fox Foundation, Parkinson Foundation, Lewy Body Association, Parkinson Foundation, Roche, Abbvie, Biogen, EIP-Pharma and Biohaven Pharmaceuticals. She was member of a Lundbeck Advisory Board and Corticobasal Degeneration Solutions. She receives her salary from the University of California San Diego and as Chief Editor of Frontiers in Neurology.
I.R. Mackenzie, M.D.: Scientific advisory board member for Prevail Therapeutics.
M.F. Mendez, M.D.: Nothing to disclose
B. Pascual, Ph.D. Nothing to disclose
K.P. Rankin, Ph.D.: Funding from the National Institutes of Health, Quest Diagnostics, the Rainwater Charitable Foundation, and the Marcus Foundation.
K. Rascovsky, Ph.D.: Nothing to disclose
A.M. Staffaroni, Ph.D.: Receives research funding from the NIA-NIH and Larry L. Hillblom Foundation.
M.C. Tartaglia, M.D.: Nothing to disclose
S. Weintraub, Ph.D.: Nothing to disclose
B. Wong, Ph.D.: Nothing to disclose
B.F. Boeve, M.D.: Has served as an investigator for clinical trials sponsored by GE Healthcare and Axovant. He receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program and the Little Family Foundation.
A.L. Boxer, M.D., Ph.D.: Receives research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration and the Bluefield Project to Cure Frontotemporal Dementia. He has served as a consultant for AGTC, Alector, Arkuda, Arvinas, Bioage, Ionis, Lundbeck, Passage BIO, Samumed, Ono, Sangamo, Stealth,Transposon, UCBand Wave, and received research support from Avid, Eisai, Biogen and Roche.
H. Rosen, M.D.: has received research support from Biogen Pharmaceuticals, has consulting agreements with Wave Neuroscience and Ionis Pharmaceuticals, and receives research support from NIH.
J. Goldman, M.S., M.Phil.: Receives research support from NIH, HDSA, New York State Department of Health (RFA #1510130358).
E.D. Huey, M.D.: Nothing to disclose
S. Cosentino, Ph.D.: Nothing to disclose
For the purpose of this paper, we define ‘episodic memory’ as the learning of new information, as tested via list learning tasks, story memory, or complex figure recall. We note that there is no evidence of an autonoetic dimension at play in these assessments.
Note that these stimuli are for example purposes only and are not the actual stimulus items.
Given the non-significant F-statistics, Bayesian ANOVAs (priors based on Cauchy distribution) were conducted to determine evidence for the null hypothesis. Education: BF10 = 0.143, meaning the data are ~7 times more likely under the null hypothesis (BF01 = 1/0.143 = 6.99). CDR®+NACC FTLD Sum of Boxes: BF10 = 0.194, meaning the data are ~5 times more likely under the null hypotheses (BF01 = 1/0.194 = 5.15). Both these results are considered strong evidence for the null hypothesis.
We also note that semantic dysfunction likely contributed to the MAPT group performing lower than the GRN and C9orf72 groups on other CVLT-SF metrics, such as immediate, cued, and delayed recall, though semantic deficits do not entirely explain the relative CVLT-SF weakness in the MAPT group (see Exploratory Analyses).
REFERENCES
- Arbuthnott K, & Frank J (2000). Trail Making Test, Part B as a Measure of Executive Control: Validation Using a Set-Switching Paradigm. Journal of Clinical and Experimental Neuropsychology, 22(4), 518–528. 10.1076/1380-3395(200008)22:4;1-0;FT518 [DOI] [PubMed] [Google Scholar]
- Bertoux M, de Souza LC, Corlier F, Lamari F, Bottlaender M, Dubois B, & Sarazin M (2014). Two distinct amnesic profiles in behavioral variant frontotemporal dementia. Biological Psychiatry, 75(7), 582–588. [DOI] [PubMed] [Google Scholar]
- Bertoux M, Flanagan EC, Hobbs M, Ruiz-Tagle A, Delgado C, Miranda M, Ibáñez A, Slachevsky A, & Hornberger M (2018). Structural Anatomical Investigation of Long-Term Memory Deficit in Behavioral Frontotemporal Dementia. Journal of Alzheimer’s Disease, 62(4), 1887–1900. 10.3233/JAD-170771 [DOI] [PubMed] [Google Scholar]
- Boeve B, Bove J, Brannelly P, Brushaber D, Coppola G, Dever R, Dheel C, Dickerson B, Dickinson S, Faber K, Fields J, Fong J, Foroud T, Forsberg L, Gavrilova R, Gearhart D, Ghoshal N, Goldman J, Graff-Radford J, … LEFFTDS Consortium. (2020). The longitudinal evaluation of familial frontotemporal dementia subjects protocol: Framework and methodology. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 16(1), 22–36. 10.1016/j.jalz.2019.06.4947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers N, Nuytemans K, Zee J van der, Gijselinck I, Engelborghs S, Theuns J, Kumar-Singh S, Pickut BA, Pals P, Dermaut B, Bogaerts V, Pooter TD, Serneels S, Broeck MV den, Cuijt I, Mattheijssens M, Peeters K, Sciot R, Martin J-J, … Sleegers K (2007). Alzheimer and Parkinson Diagnoses in Progranulin Null Mutation Carriers in an Extended Founder Family. Archives of Neurology, 64(10), 1436–1446. 10.1001/archneur.64.10.1436 [DOI] [PubMed] [Google Scholar]
- Cheran G, Wu L, Lee S, Manoochehri M, Cines S, Fallon E, Lynch T, Heidebrink J, Paulson H, Goldman J, Huey E, & Cosentino S (2019). Cognitive Indicators of Preclinical Behavioral Variant Frontotemporal Dementia in MAPT Carriers. Journal of the International Neuropsychological Society, 25(2), 184–194. 10.1017/S1355617718001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH, & Ober BA (2000). Manual for the California verbal learning test (CVLT-II). The Psychological Corporation. [Google Scholar]
- Domínguez-Vivero C, Wu L, Lee S, Manoochehri M, Cines S, Brickman AM, Rizvi B, Chesebro A, Gazes Y, Fallon E, Lynch T, Heidebrink JL, Paulson H, Goldman JS, Huey E, & Cosentino S (2020). Structural Brain Changes in Pre-Clinical FTD MAPT Mutation Carriers. Journal of Alzheimer’s Disease: JAD. 10.3233/JAD-190820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Matarrubia M, Matías-Guiu JA, Cabrera-Martin MN, Moreno-Ramos T, Valles-Salgado M, Carreras JL, & Matías-Guiu J (2017). Episodic Memory Dysfunction in Behavioral Variant Frontotemporal Dementia: A Clinical And FDG-PET Study. Journal of Alzheimer’s Disease, 57(4), 1251–1264. 10.3233/JAD-160874 [DOI] [PubMed] [Google Scholar]
- Flanagan EC, Wong S, Dutt A, Tu S, Bertoux M, Irish M, Piguet O, Rao S, Hodges JR, Ghosh A, & Hornberger M (2016). False Recognition in Behavioral Variant Frontotemporal Dementia and Alzheimer’s Disease—Disinhibition or Amnesia? Frontiers in Aging Neuroscience, 8. 10.3389/fnagi.2016.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves CV, & Rohrer JD (2019). An update on genetic frontotemporal dementia. Journal of Neurology, 266(8), 2075–2086. 10.1007/s00415-019-09363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M (2010). Primary progressive aphasia: Clinicopathological correlations. Nature Reviews Neurology, 6(2), 88–97. 10.1038/nrneurol.2009.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam BJ, Jacova C, Hsiung G-YR, Wittenberg D, Sengdy P, Bouchard-Kerr P, Slack P, Rademakers R, Baker M, Chow TW, Levine B, Feldman HH, & Mackenzie IR (2014). Early neuropsychological characteristics of progranulin mutation carriers. Journal of the International Neuropsychological Society, 20(7), 694–703. [DOI] [PubMed] [Google Scholar]
- Heuer HW, Wang P, Rascovsky K, Wolf A, Appleby B, Bove J, Bordelon Y, Brannelly P, Brushaber D. e., Caso C, Coppola G, Dickerson B, Dickinson S, Domoto-Reilly K, Faber K, Ferrall J, Fields J, Fishman A, Fong J, … on behalf of the ARTFL and LEFFTDS consortia. (2020). Comparison of sporadic and familial behavioral variant frontotemporal dementia (FTD) in a North American cohort. Alzheimer’s & Dementia, 16(1), 60–70. 10.1002/alz.12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, & Piguet O (2012). Episodic memory in frontotemporal dementia: A critical review. Brain, 135(3), 678–692. 10.1093/brain/aws011 [DOI] [PubMed] [Google Scholar]
- Hornberger M, Piguet O, Graham AJ, Nestor PJ, & Hodges JR (2010). How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology, 74(6), 472–479. 10.1212/WNL.0b013e3181cef85d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, & Martin RL (1982). A new clinical scale for the staging of dementia. The British Journal of Psychiatry: The Journal of Mental Science, 140, 566–572. 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- Jiskoot LC, Dopper EGP, Heijer T den, Timman R, van Minkelen R, van Swieten JC, & Papma JM (2016). Presymptomatic cognitive decline in familial frontotemporal dementia: A longitudinal study. Neurology, 87(4), 384–391. 10.1212/WNL.0000000000002895 [DOI] [PubMed] [Google Scholar]
- Jiskoot LC, Panman JL, van Asseldonk L, Franzen S, Meeter LHH, Donker Kaat L, van der Ende EL, Dopper EGP, Timman R, van Minkelen R, van Swieten JC, van den Berg E, & Papma JM (2018). Longitudinal cognitive biomarkers predicting symptom onset in presymptomatic frontotemporal dementia. Journal of Neurology, 265(6), 1381–1392. 10.1007/s00415-018-8850-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnen A, & Bertoux M (2019). Psychological and Cognitive Markers of Behavioral Variant Frontotemporal Dementia–A Clinical Neuropsychologist’s View on Diagnostic Criteria and Beyond. Frontiers in Neurology, 10, 594. 10.3389/fneur.2019.00594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BJ, Haidar W, Boeve BF, Baker M, Shiung M, Knopman DS, Rademakers R, Hutton M, Adamson J, Kuntz KM, Dickson DW, Parisi JE, Smith GE, & Petersen RC (2010). Alzheimer Disease—like Phenotype Associated With the c.154delA Mutation in Progranulin. Archives of Neurology, 67(2), 171–177. 10.1001/archneurol.2010.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, Miller BL, & Mercaldo N (2008). Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain, 131(11), 2957–2968. 10.1093/brain/awn234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornak J, Fields J, Kremers W, Farmer S, Heuer HW, Forsberg L, Brushaber D, Rindels A, Dodge H, Weintraub S, Besser L, Appleby B, Bordelon Y, Bove J, Brannelly P, Caso C, Coppola G, Dever R, Dheel C, … Rosen H (2019). Nonlinear Z-score modeling for improved detection of cognitive abnormality. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 11, 797–808. 10.1016/j.dadm.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Rosen HJ, Du A-T, Schuff N, Hollnagel C, Weiner MW, Miller BL, & Delis DC (2005). Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology, 19(6), 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanata SC, & Miller BL (2016). The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J Neurol Neurosurg Psychiatry, 87(5), 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, Rovelet-Lecrux A, Hahn-Barma V, van der Zee J, Clot F, Bakchine S, Puel M, Ghanim M, Lacomblez L, Mikol J, Deramecourt V, Lejeune P, de la Sayette V, Belliard S, Vercelletto M, … Brice A (2008). Phenotype variability in progranulin mutation carriers: A clinical, neuropsychological, imaging and genetic study. Brain, 131(3), 732–746. 10.1093/brain/awn012 [DOI] [PubMed] [Google Scholar]
- Lee SE, Sias AC, Mandelli ML, Brown JA, Brown AB, Khazenzon AM, Vidovszky AA, Zanto TP, Karydas AM, Pribadi M, Dokuru D, Coppola G, Geschwind DH, Rademakers R, Gorno-Tempini ML, Rosen HJ, Miller BL, & Seeley WW (2017). Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. NeuroImage: Clinical, 14, 286–297. 10.1016/j.nicl.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, McMillan C, Gunawardena D, Powers C, Massimo L, Khan A, Morgan B, Farag C, Richmond L, Weinstein J, Moore P, Coslett HB, Chatterjee A, Aguirre G, & Grossman M (2009). Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology, 73(7), 535–542. 10.1212/WNL.0b013e3181b2a4f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, Yeatman T, Warrington EK, Schott JM, Fox NC, Rossor MN, Hardy J, Collinge J, Revesz T, Mead S, & Warren JD (2012). Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: Clinical, neuroanatomical and neuropathological features. Brain, 135(3), 736–750. 10.1093/brain/awr361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Downey LE, Ridgway GR, Beck J, Clegg S, Blair M, Finnegan S, Leung KK, Yeatman T, Golden H, Mead S, Rohrer JD, Fox NC, & Warren JD (2012). Longitudinal neuroimaging and neuropsychological profiles of frontotemporal dementia with C9ORF72 expansions. Alzheimer’s Research & Therapy, 4(5), 41. 10.1186/alzrt144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa T, Brushaber D, Syrjanen J, Kremers W, Fields J, Forsberg LK, Heuer HW, Knopman D, Kornak J, Boxer A, Rosen H, Boeve B, & ARTFL/LEFFTDS Consortium. (2020). Use of the CDR® plus NACC FTLD in mild FTLD: Data from the ARTFL/LEFFTDS consortium. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 16(1), 79–90. 10.1016/j.jalz.2019.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa T, Brushaber D, Syrjanen J, Kremers W, Fields J, Forsberg LK, Heuer HW, Knopman D, Kornak J, Boxer A, Rosen HJ, Boeve BF, Appleby B, Bordelon Y, Bove J, Brannelly P, Caso C, Coppola G, Dever R, … Wszolek Z (2020). Utility of the global CDR® plus NACC FTLD rating and development of scoring rules: Data from the ARTFL/LEFFTDS Consortium. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 16(1), 106–117. 10.1002/alz.12033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murley AG, Coyle-Gilchrist I, Rouse MA, Jones PS, Li W, Wiggins J, Lansdall C, Rodr®guez PV, Wilcox A, Tsvetanov KA, Patterson K, Lambon Ralph MA, & Rowe JB (2020). Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes. Brain, 143(5), 1555–1571. 10.1093/brain/awaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, & Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings JL, & Benson DF (1998). Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology, 51(6), 1546–1554. 10.1212/wnl.51.6.1546 [DOI] [PubMed] [Google Scholar]
- Olney NT, Ong E, Goh S-YM, Bajorek L, Dever R, Staffaroni AM, Cobigo Y, Bock M, Chiang K, Ljubenkov P, Kornak J, Heuer HW, Wang P, Rascovsky K, Wolf A, Appleby B, Bove J, Bordelon Y, Brannelly P, … ARTFL and LEFFTDS consortia. (2020). Clinical and volumetric changes with increasing functional impairment in familial frontotemporal lobar degeneration. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 16(1), 49–59. 10.1016/j.jalz.2019.08.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyike CU, & Diehl-Schmid J (2013). The epidemiology of frontotemporal dementia. International Review of Psychiatry, 25(2), 130–137. 10.3109/09540261.2013.776523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering-Brown SM, Rollinson S, Du Plessis D, Morrison KE, Varma A, Richardson AMT, Neary D, Snowden JS, & Mann DMA (2008). Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: Comparison with patients with MAPT and no known mutations. Brain, 131(3), 721–731. 10.1093/brain/awm331 [DOI] [PubMed] [Google Scholar]
- Poos JM, Jiskoot LC, Leijdesdorff SMJ, Seelaar H, Panman JL, van der Ende EL, Mol MO, Meeter LHH, Pijnenburg YAL, Donker Kaat L, de Jong FJ, van Swieten JC, Papma JM, & van den Berg E (2020). Cognitive profiles discriminate between genetic variants of behavioral frontotemporal dementia. Journal of Neurology. 10.1007/s00415-020-09738-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poos JM, Jiskoot LC, Papma JM, van Swieten JC, & van den Berg E. (2018). Meta-analytic Review of Memory Impairment in Behavioral Variant Frontotemporal Dementia. Journal of the International Neuropsychological Society, 24(6), 593–605. 10.1017/S1355617718000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S, Bertoux M, Flanagan E, Irish M, Piguet O, Hodges JR, & Hornberger M (2017). Longitudinal Executive Function and Episodic Memory Profiles in Behavioral–Variant Frontotemporal Dementia and Alzheimer’s Disease. Journal of the International Neuropsychological Society, 23(1), 34–43. 10.1017/S1355617716000837 [DOI] [PubMed] [Google Scholar]
- Ramos EM, Dokuru DR, Van Berlo V, Wojta K, Wang Q, Huang AY, Deverasetty S, Qin Y, van Blitterswijk M, Jackson J, Appleby B, Bordelon Y, Brannelly P, Brushaber DE, Dickerson B, Dickinson S, Domoto-Reilly K, Faber K, Fields J, … Coppola G (2020). Genetic screening of a large series of North American sporadic and familial frontotemporal dementia cases. Alzheimer’s & Dementia, 16(1), 118–130. 10.1002/alz.12011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EGP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini M-L, Rosen H,… Miller BL (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Thal LJ, & Galasko D (2007). Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology, 21(1), 20–30. 10.1037/0894-4105.21.1.20 [DOI] [PubMed] [Google Scholar]
- Ricci M, Graef S, Blundo C, & Miller LA (2012). Using the Rey Auditory Verbal Learning Test (RAVLT) to Differentiate Alzheimer’s Dementia and Behavioural Variant Fronto-Temporal Dementia. The Clinical Neuropsychologist, 26(6), 926–941. 10.1080/13854046.2012.704073 [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, van Minkelen R, Rombouts SA, Cardoso MJ, Clegg S, Espak M, Mead S, Thomas DL, De Vita E, Masellis M, Black SE, Freedman M, Keren R, MacIntosh BJ, … Rossor MN (2015). Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia initiative (GENFI) study: A cross-sectional analysis. The Lancet Neurology, 14(3), 253–262. 10.1016/S1474-4422(14)70324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Modat M, Ourselin S, Mead S, Fox NC, Rossor MN, & Warren JD (2010). Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. NeuroImage, 53(3), 1070–1076. 10.1016/j.neuroimage.2009.12.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, & Warren JD (2011). Phenotypic signatures of genetic frontotemporal dementia: Current Opinion in Neurology, 24(6), 542–549. 10.1097/WCO.0b013e32834cd442 [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Barnes J, Mead S, Beck J, Pepple T, Boyes R, Omar R, Collinge J, Stevens JM, Warrington EK, Rossor MN, & Fox NC (2008). Mapping the progression of progranulin-associated frontotemporal lobar degeneration. Nature Clinical Practice. Neurology, 4(8), 455–460. 10.1038/ncpneuro0869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Boeve BF, & Boxer AL (2020). Tracking disease progression in familial and sporadic frontotemporal lobar degeneration: Recent findings from ARTFL and LEFFTDS. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 16(1), 71–78. 10.1002/alz.12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert S, Leyton CE, Hodges JR, & Piguet O (2016). Longitudinal Memory Profiles in Behavioral-Variant Frontotemporal Dementia and Alzheimer’s Disease. Journal of Alzheimer’s Disease: JAD, 51(3), 775–782. 10.3233/JAD-150802 [DOI] [PubMed] [Google Scholar]
- Snowden JS, Adams J, Harris J, Thompson JC, Rollinson S, Richardson A, Jones M, Neary D, Mann DM, & Pickering-Brown S (2015). Distinct clinical and pathological phenotypes in frontotemporal dementia associated with MAPT, PGRN and C9orf72 mutations. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 16(7–8), 497–505. 10.3109/21678421.2015.1074700 [DOI] [PubMed] [Google Scholar]
- Spina S, Farlow MR, Unverzagt FW, Kareken DA, Murrell JR, Fraser G, Epperson F, Crowther RA, Spillantini MG, Goedert M, & Ghetti B (2008). The tauopathy associated with mutation +3 in intron 10 of Tau: Characterization of the MSTD family. Brain, 131(1), 72–89. 10.1093/brain/awm280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni AM, Bajorek L, Casaletto KB, Cobigo Y, Goh S-YM, Wolf A, Heuer HW, Elahi FM, Ljubenkov PA, Dever R, Kornak J, Appleby B, Bove J, Bordelon Y, Brannelly P, Brushaber D, Caso C, Coppola G, Dheel C, … on behalf of the ARTFL/LEFFTDS consortium. (2020). Assessment of executive function declines in presymptomatic and mildly symptomatic familial frontotemporal dementia: NIH-EXAMINER as a potential clinical trial endpoint. Alzheimer’s & Dementia, 16(1), 11–21. 10.1016/j.jalz.2019.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni AM, Cobigo Y, Goh S-YM, Kornak J, Bajorek L, Chiang K, Appleby B, Bove J, Bordelon Y, Brannelly P, Brushaber D, Caso C, Coppola G, Dever R, Dheel C, Dickerson BC, Dickinson S, Dominguez S, Domoto-Reilly K, … ARTFL/LEFFTDS consortium. (2020). Individualized atrophy scores predict dementia onset in familial frontotemporal lobar degeneration. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 16(1), 37–48. 10.1016/j.jalz.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares TP, Mitchell DGV, Coleman KK, Coleman BL, Shoesmith CL, Butler CR, Santana I, Danek A, Gerhard A, de Mendonca A, Borroni B, Tartaglia MC, Graff C, Galimberti D, Tagliavini F, Moreno F, Frisoni G, Rowe JB, Levin J, … Finger E (2020). Early symptoms in symptomatic and preclinical genetic frontotemporal lobar degeneration. Journal of Neurology, Neurosurgery & Psychiatry, jnnp-2020-322987. 10.1136/jnnp-2020-322987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E, Poos JM, Jiskoot LC, Heijnen LM, Franzen S, Steketee RME, Meijboom R, de Jong FJ, Seelaar H, van Swieten JC, & Papma JM (2020). Differences in Discriminability and Response Bias on Rey Auditory Verbal Learning Test Delayed Recognition in Behavioral Variant Frontotemporal Dementia and Alzheimer’s Disease. Journal of the International Neuropsychological Society: JINS, 1–9. 10.1017/S1355617720000375 [DOI] [PubMed] [Google Scholar]
- van Swieten JC, & Heutink P (2008). Mutations in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. The Lancet Neurology, 7(10), 965–974. 10.1016/S1474-4422(08)70194-7 [DOI] [PubMed] [Google Scholar]
- Woolley JD, Khan BK, Murthy NK, Miller BL, & Rankin KP (2011). The diagnostic challenge of psychiatric symptoms in neurodegenerative disease; rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. The Journal of Clinical Psychiatry, 72(2), 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


