Abstract
Cerebral hypoperfusion leads to adverse sequalae including dementia. Mid-life higher blood pressure (BP) can lead to low cerebral blood flow (CBF), but older persons may need higher BP to maintain cerebral perfusion. We investigated the associations among late-life BP, CBF, and cognition. Data are from 2498 participants with a mean age of 79.8 (SD 4.7) years of the second exam of the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study. BP was measured, and Phase-Contrast MRI was acquired to estimate total brain CBFPC. Cognitive outcomes included verbal and working memory, processing speed, Mild Cognitive Impairment (MCI), and all-cause dementia. Relationships among late-life BP, CBFPC and cognition were assessed with regression models, controlling for socio-demographics, BP level at midlife (at a mean age of: 49.6 (SD 5.9) years), cardiovascular factors, and total brain volume. In fully adjusted models, each mmHg increase in late-life diastolic BP (DBP) was associated with a −0.082 ml/min/100 ml (95% CI −0.123 to −0.041) lower CBFPC. In contrast, each mmHg increase in late-life systolic BP (SBP) or pulse pressure was associated with a 0.027 ml/min/100ml (95% CI 0.0065 to 0.048) and 0.061 ml/min/100ml (95% CI 0.038 to 0.084) higher late-life CBFPC, respectively. Higher CBFPC was significantly related to higher cognitive scores for psychomotor speed, verbal and working memory and to a lower odd of MCI or dementia, irrespective of late-life BP level. Higher late-life DBP and SBP were differentially associated with CBFPC. Our findings suggest CBF is an important correlate of late-life cognition, independent of BP level.
Keywords: blood pressure, cerebral blood flow, cognitive functioning, dementia, old age
Introduction
Chronic hypoperfusion of the brain is one mechanism underlying the cerebral damage associated with high blood pressure. Chronic hypertension and hemodynamic stress can lead to arterial stiffening, lumen narrowing, changes in neurovascular coupling and cerebral autoregulation—thereby affecting cerebral blood flow and delivery of nutrients and oxygen to the brain.1, 2 With age, these vascular changes tend to lead to an increase in systolic BP (SBP) and a decrease in diastolic BP (DBP)3 and to cerebral vascular damage that may lead to cognitive decline or dementia4, 5,6
In older persons an increased systolic BP and pulse pressure (PP), may be needed to overcome the increased resistance of a cerebral vascular bed that is affected by arteriolosclerosis.7 This concern is raised in the context of adverse outcomes of a lower BP at old age such as falls, cognitive decline, and mortality.8, 9 However, there are few data on the relationship of late-life BP level to CBF in cohorts that better represent the range and distribution of CBF, BP and health status typical in older populations. Further, few studies have compared the associations among BP, CBF and cognitive function in the same population. Current studies indicate a negative, positive or no association between late-life BP and cognitive function,5 whereas CBF may be a more consistent marker.10
We examined the possible paradox between desirable BP levels, CBF, and cognitive function, in the Age Gene/Environment Susceptibility -Reykjavik Study (AGES-RS), a large population-based study with 30-year follow-up between middle and late age. We investigate whether a lower BP is related to a lower CBF at old age. Further we hypothesize that in this older cohort there is an association between a higher CBF and better cognitive function.
Methods
Study population
The data that support the findings of this study are available from the corresponding author upon reasonable request. This study included cohort members from the AGES-RS, which originated from the Reykjavik Study.11 Briefly, the Reykjavik Study (RS) was initiated by the Icelandic Heart Association to study cardiovascular disease in a population-based cohort of persons born between 1907 and 1935 and living in Reykjavik and surroundings in 1967. AGES-RS is based on a random sample of RS cohort survivors who were examined in 2002-2006 and then again 5 years later (2007-2011) to study genetic and environmental factors for disease and disability at old age. AGES–RS was approved by the National Bioethics Committee in Iceland (VSN 00-063) acting as the institutional review board for the Icelandic Heart Association, and by the institutional review board governing the National Institute on Aging. All participants gave written informed consent.
Blood pressure
In both the AGES-RS and the Reykjavik Study, BP was measured twice after 5 min of rest in a sitting position with a standardized cuff (bladder width x length: 15 x 22 cm) according to the World Health Organization and European guidelines. The mean of two consecutive measurements, that were separated by 1-2 min, was used in analyses. Pulse pressure was calculated as [systolic BP minus diastolic BP]. Late-life BP levels were measured at the second visit, concurrently with the CBF MRI measure, when participants had a mean age of 79.8 (SD 4.7) years. The measured mid-life BP levels were taken from the RS exam conducted closest to when the participants were age 50 years (mean age (SD) in years: 49.6 (SD 5.9)). Antihypertensive treatment and other medication were registered by using a questionnaire that participants were asked to fill out.
MRI acquisition and image processing
All consenting participants without contraindications were eligible for brain MRI acquired on a study dedicated 1.5-Tesla Signa Twinspeed Excite system (General Electric Medical Systems, Waukesha, WI), using an 8 channel phased array head cap coil. Described in detail elsewhere12 the image protocol included a three-dimensional spoiled gradient echo (3D-SPGR) T1-weigthed, fast spin echo proton density/T2-weighted, and Fluid-Attenuated Inversion Recovery (FLAIR) sequences for structural imaging. Different tissues volumes, including white matter lesion load (WML) a measure of vascular injury, were segmented automatically using a validated image analysis post-processing pipeline. Total brain volume (TBV) was defined as the sum of volumes of gray matter, white matter and white matter lesions. Intracranial volume (ICV) was estimated as total brain volume plus cerebral spinal fluid volume. A measure of atrophy was defined as the volume of TBV / ICV to give a percent (%TBV).
Total cerebral blood flow (ml/min) (CBF), was estimated with 2D Phase Contrast (PC)-MRI. The PC-MR images were processed using the software package FLOW (Division of Image Processing, Leiden University Medical Center, The Netherlands).13 Total cerebral blood flow was derived from the flow through one standardized oblique axial slice perpendicular to the internal carotid arteries (ICA), and the basal artery (BA) on a PC sagittal angiographic localizer image. Estimated total cerebral blood flow (CBFPC) was defined as the sum of the flow in ICAs and BA and was expressed per 100 ml brain volume in ml/min/100ml. CBFpc was normally distributed.
Confounding or moderating factors
In our analyses we controlled for several factors that may confound the association or moderate the association of BP to CBFPC. Questionnaires were used to assess level of education (dichotomized at primary education) and smoking status (current versus never or former). Measured weight and height were used to calculate Body Mass Index (BMI; kg/m2). Diabetes was defined as a self-reported history of diabetes, use of blood glucose-lowering medication, or fasting serum glucose of ≥7 mmol/L. Hospital records identified events of stroke and heart failure. Coronary calcium load [in mm3], as a measure of atherosclerosis load, was acquired using Computerized tomography, calculated as the sum score of the four coronary arteries and scored in Agatston units.14
Cognitive function
As described previously15, cognitive function was measured by three composite domain scores, verbal memory, working memory and processing speed. The verbal memory compound score included the immediate- and delayed-recall portions of the original California Verbal Learning Test (CVLT).16 The working memory composite includes: Digits Backward,17 the CANTAB spatial working memory test18 and the Stroop Test Part III.19 The processing speed composite includes: the Digit Symbol Substitution Test,17 Figure Comparison20 and the Stroop Test Parts 1&2.19 All tests had a normal distribution and inter-rater reliability was high (Spearman correlations for cognitive tests range from 0.96 - 0.99). Composite domain scores measures were computed by converting raw scores of each cognitive test to standardized z-scores and averaging them across the tests in each composite. Dementia and mild cognitive impairment (MCI) were assessed in a 3-step process, as previously described21 including testing the total population with measures of general cognitive function; detailed assessment of cognitive function of those falling below a cut-point; and among those falling again below a cut-point, a third step that included a neurological examination and a proxy interview. A consensus diagnosis of dementia and sub-types, according to criteria of the Diagnostic and Statistical Manual of Mental Disorders IV22 or of MCI23 was made by a panel including a geriatrician, a neurologist, a neuropsychologist, and a neuroradiologist.
Analytical sample
Of the 2643 participants who had MRI, 138 participants had incomplete data on CBFPC, two had incomplete data on brain volume, and twelve had missing BP measures at mid- or late-life, and 2 extreme outliers for CBF were excluded, leaving 2489 participants available for analysis.
Statistical Analysis
Late-life characteristics of participants are reported as mean (standard deviation), median (inter-quartile range) or number (%) where appropriate. Population characteristics are compared amongst late-life SBP and DBP groups with Chi-square for categorical variables, with ANOVA for normally distributed continuous variables, and with Kruskal-Wallis for non-normally distributed continuous variables. Late-life BP group, was categorized according to BP Joint National Committee (JNC)-8 hypertension guideline7 cut-offs, late-life SBP; <120, 120-<140, 140-<150, 150+, late-life DBP; ≤70, 70-<80, 80-<90, 90+ and pulse pressure (PP); <25th percentile, 25-<75th percentiles and 75th+ percentiles. The association between late-life BP and CBFPC was estimated with linear regression analysis. Non-linearity of associations was tested by entering quadratic terms into an age and sex adjusted model, but terms were not significant. Model 1 included age, sex, education and %TBV; model 2 additionally included coronary calcium load, diabetes mellitus, BMI, heart failure, smoking status, stroke, use of antihypertensive medication (never, both mid- and late-life, only mid- or only late-life), and mid-life BP level of the same late-life measure (i.e., late-life DBP was adjusted for mid-life DBP). The relationships among BP level, CBF, and cognitive function (psychomotor speed, verbal and working memory, and MCI or dementia status) were examined with linear [or logistic] regression adjusting for age, sex, education and %TBV.
In secondary analyses we assessed if the relationship between a higher systolic blood pressure and higher cerebral blood flow may be more pronounced in subgroups of frailer older persons. We assessed whether the following indicators of health status modified the association of late-life BP to CBFPC, including age (median split at 80 years), sex; groups for mid-life SBP and DBP,7 use of antihypertensive treatment, late-life BMI ( ≤18.5, 18.5-24.9, 25-29.9, ≥30 kg/m2), late-life smoking (yes/no), prevalent diabetes mellitus, heart failure, stroke, coronary calcium Agatston score (absent: 0, mild: 1-100, moderate: 101-400, severe >400)24, and upper quartile of abnormal white matter volume (≥29.9 cc). Effect modification by these factors was tested by adding an interaction term in the linear regression model [late-life BP * covariate]. Stratified and interaction analyses were adjusted for age and sex (model 1). P-values for significant interaction-terms will be corrected for multiple testing using the Bonferroni adjustment. We also examined the association of CBF to cognition in strata of BP and we tested if there was significant interaction between late-life BP and CBF on cognitive function. SAS v. 9 was used for all analyses.
Results
Population characteristics
The cohort included 40.9% male; 59.9% used antihypertensive medications in late life (Table 1); descriptive statistics by late-life SBP and DBP categories are found in Tables S1A and S1B. In this cohort, 4.3% of the study population had high DBP (> 90mmHg) and 37.8% had high SBP (>150 mmHg).
Table 1.
Characteristics of the AGES-RS cohort in mid and late life
| Characteristics | Sample n=2489 |
|---|---|
| Late-life age in years (y), mean (SD) | 79.8 (4.7) |
| ≥80 y, n (%) | 1207 (48.49) |
| Male, n (%) | 1017 (40.9) |
| Education, ≤primary school (%) | 504 (20.2) |
| Blood pressure in mmHg, mean (SD) | |
| Midlife SBP | 129.4 (15.2) |
| Midlife DBP | 82.3 (9.1) |
| Late-life SBP | 145.0 (21.05) |
| Late-life DBP | 70.3 (10.7) |
| Use of antihypertensive medication in late-life, n (%) | 1491 (59.9) |
| Late-life characteristics | |
| Body Mass Index, mean (SD) | 26.8 (4.3) |
| Coronary calcium (Agaston units), median (IQR) | 461.07 (92.68, 1302.21) |
| Heart failure, n (%) | 138 (5.5) |
| Type 2 diabetes, n (%) | 317 (12.7) |
| Stroke, n (%) | 208 (8.4) |
| Smoking status, n (%)* | |
| Never | 1308 (52.6%) |
| Former | 977 (39.3%) |
| Current smoker | 158 (6.3%) |
| Cognition* | |
| Normal cognitive function | 2110 (84.8%) |
| Mild Cognitive Impairment | 216 (8.7%) |
| Dementia | 134 (5.4%) |
| Late-life brain MRI measures | |
| Total brain volume in % ICV, mean (SD) | 0.69 (0.042) |
| Abnormal white matter volume, median (IQR) | 15.66 (8.27-29.69) |
| Cerebral blood flow in ml/100ml/min, mean (SD) | 56.7 (10.5) |
smoking status is missing for n=46 and cognitive status is missing for n=29
DBP=diastolic blood pressure, SBP=systolic blood pressure, SD= standard deviation
Late-life BP and CBF
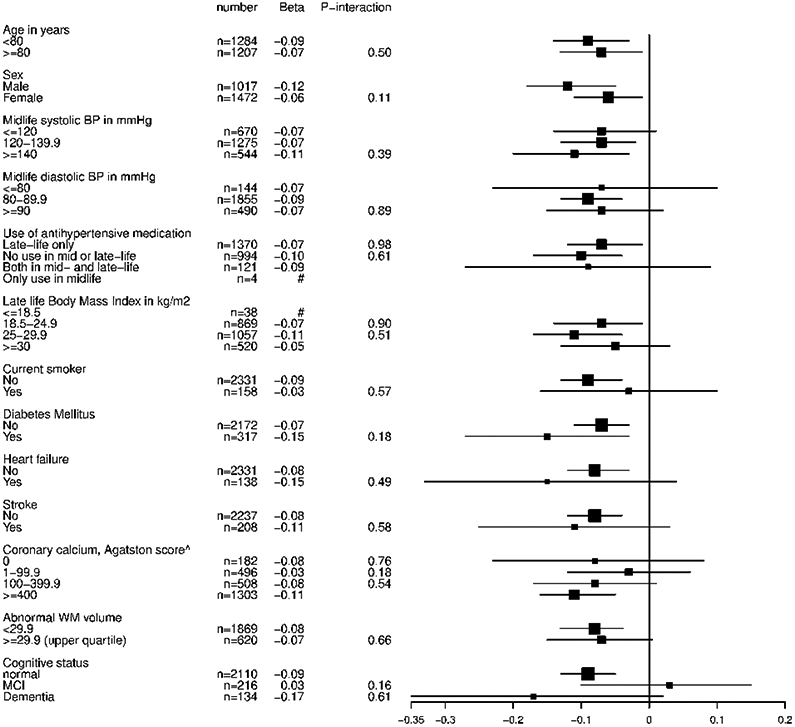
Each mmHg increase in DBP was associated with −0.082 ml/min/100 ml (95% CI −0.123 to −0.041) lower CBFPC (Table 2, Model 2). Each mmHg increase in SBP and in PP was associated with a 0.027 ml/min/100ml (95% CI 0.0065 to 0.048) and 0.061 ml/min/100ml (95%CI 0.038 to 0.084) higher late-life CBFPC, respectively. Mean CBF per BP group is visualized in Figure 1. The positive (for SBP) and negative (for DBP) direction of these associations with CBF were similar across several demographic characteristics, cardiovascular risk factors and sub-clinical vascular disease markers (Figure 2). There was no positive association for SBP and CBFPC, in persons with dementia, but the interaction term for [dementia status * SBP] was not-significant (P-interaction=0.15) (Figure 2).
Table 2.
Relationship between late-life BP and CBF (n=2489): AGES-RS
| 95% CI | ||||
|---|---|---|---|---|
| BP | Beta | Lower | Upper | P |
| DBP | ||||
| Model 1 | −0.081 | −0.121 | −0.042 | <.0001 |
| Model 2 | −0.082 | −0.123 | −0.041 | <.0001 |
| SBP | ||||
| Model 1 | 0.027 | 0.0066 | 0.047 | 0.009 |
| Model 2 | 0.027 | 0.0065 | 0.048 | 0.010 |
| PP | ||||
| Model 1 | 0.060 | 0.037 | 0.082 | <.0001 |
| Model 2 | 0.061 | 0.038 | 0.084 | <.0001 |
BP=blood pressure, CBF=cerebral blood flow, CI= confidence interval, DBP=diastolic blood pressure, SBP=systolic blood pressure, PP=pulse pressure
Model 1: Adjusted for age, sex, education, brain volume
Model 2: Adjusted for sex, education, brain volume, coronary calcium, BMI, DM, heart failure, smoking, stroke, midlife BP, use of antihypertensive medication
Beta indicates change in CBF per 1 mmHg increase in BP, i.e. a positive Beta indicates that a higher BP is related to a higher CBF.
Figure 1. Mean cerebral blood flow (95% CI) per blood pressure group.
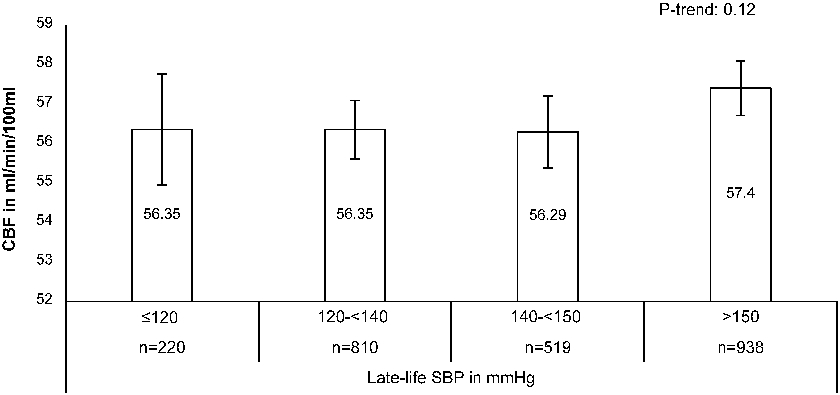
Adjusted for age, sex, brain volume and education. CBF=cerebral blood flow, SBP=systolic blood pressure, PP= pulse pressure, DBP= diastolic blood pressure
Figure 2.
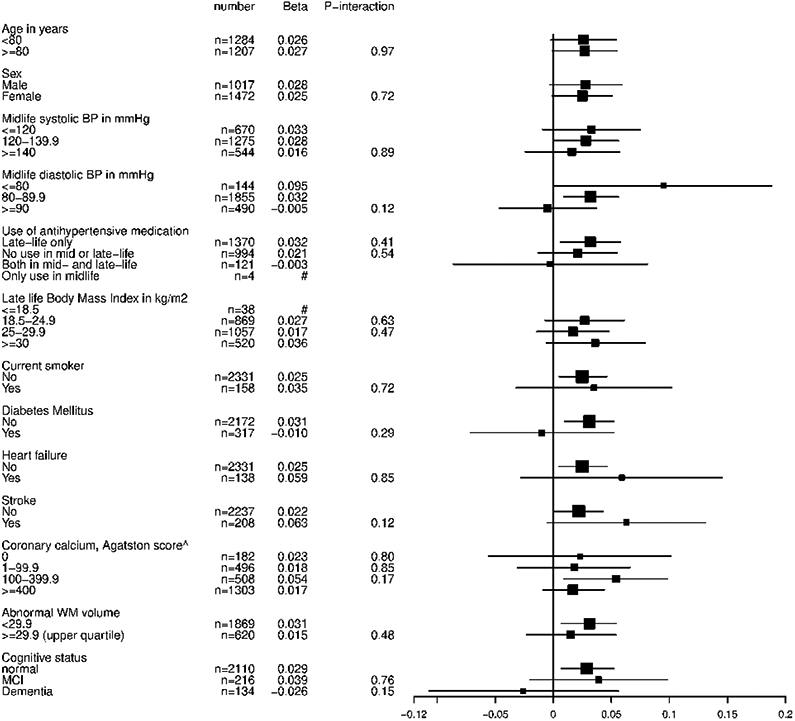
A Stratified analyses for the association between late-life systolic blood pressure and CBF
B Stratified analyses for the association between late-life diastolic blood pressure and CBF
CBF=cerebral blood flow, BP=blood pressure, MCI=mild cognitive impairment, # indicates subgroup too small; data not shown. Analyses are adjusted for age, gender and total brain volume. ^ Grading of coronary calcium, 0: none, mild: 1-99.9, moderate: 100-399.9, severe: ≥400
Late-life BP, CBF and cognition
Both CBFPC and late-life BP (either DBP, SBP or PP) were entered into the models to examine their association with cognition. Higher CBFPC was significantly related to higher cognitive scores for verbal, working memory and psychomotor speed (Table 3A) and to a lower odd of MCI or dementia (Table 3B), while controlling for age, sex, education, BP level and %TBV. In this model with both BP level and CBF, except for higher PP being related to lower working memory scores (Table 3A), levels of DBP, SBP and PP were not associated with any cognitive domain or with MCI or dementia status. Table S2 shows the relationship between CBF and cognitive domains by BP level. Estimates were higher in those with a lower DBP or in the range of SBP between 120 to 140 mmHg, whereas no clear pattern was observed by PP level. There was no significant interaction between late-life BP and CBF on cognitive function (Table S2). There was no significant relationship between midlife DBP, SBP or PP and late-life CBF (Table S3A) or cognitive function (Table S3B). To evaluate the use of a single standardized cuff size, which could potentially lead to faulty BP measurements in those with extremes of BMI, we have rerun BP analyses excluding those in either the lower or the upper quartile of BMI, which provided similar results (data not shown).
Table 3A.
Relationship between BP, CBF and cognition: AGES-RS
| Verbal memory | Working memory | Speed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | ||||||||||
| BP | Beta | Lower | Upper | P | Beta | Lower | Upper | P | Beta | Lower | Upper | P |
| DBP | 0.0028 | −0.00060 | 0.0062 | 0.11 | 0.002 | −0.001 | 0.005 | 0.210 | 0.0015 | −0.0019 | 0.0048 | 0.393 |
| CBF | 0.0060 | 0.0026 | 0.0095 | 0.0006 | 0.004 | 0.001 | 0.007 | 0.001 | 0.0059 | 0.0025 | 0.0093 | 0.001 |
| SBP | −0.00071 | −0.0024 | 0.0010 | 0.42 | −0.0011 | −0.003 | 0.0005 | 0.169 | −0.00071 | −0.0024 | 0.0010 | 0.409 |
| CBF | 0.0059 | 0.0024 | 0.0093 | 0.0009 | 0.0039 | 0.001 | 0.007 | 0.018 | 0.0058 | 0.0024 | 0.0092 | 0.001 |
| PP | −0.0018 | −0.0037 | 0.00012 | 0.066 | −0.002 | −0.004 | −0.00028 | 0.023 | −0.0014 | −0.0033 | 0.0005 | 0.151 |
| CBF | 0.0062 | 0.0027 | 0.0096 | 0.001 | 0.004 | 0.001 | 0.007 | 0.012 | 0.0060 | 0.0026 | 0.0094 | 0.0005 |
Model includes age, sex, education, BP, CBF and brain volume (% ICV)
The Beta’s indicate change in standardized cognitive domain score per 1 mmHg increase in BP, or per 1 ml/min/100ml CBF. The significant positive beta’s for CBF, indicate that a higher CBF is related to a higher cognitive function.
BP=blood pressure, CBF=cerebral blood flow, CI= confidence interval, DBP=diastolic blood pressure, SBP=systolic blood pressure, PP=pulse pressure
Table 3B.
Relationship between BP, CBF and cognitive status: AGES-RS
| Dementia* | MCI# | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| BP | OR | Lower | Upper | p | OR | Lower | Upper | P |
| SBP | 1.00 | 0.99 | 1.01 | 0.68 | 1.00 | 0.99 | 1.007 | 0.92 |
| CBF | 0.97 | 0.95 | 0.99 | 0.003 | 0.98 | 0.97 | 0.999 | 0.03 |
| DBP | 1.00 | 0.98 | 1.02 | 0.80 | 0.99 | 0.98 | 1.006 | 0.23 |
| CBF | 0.97 | 0.95 | 0.99 | 0.003 | 0.98 | 0.97 | 0.998 | 0.03 |
| PP | 1.00 | 0.99 | 1.01 | 0.54 | 1.00 | 1.00 | 1.011 | 0.41 |
| CBF | 0.97 | 0.95 | 0.99 | 0.002 | 0.98 | 0.97 | 0.998 | 0.03 |
Model includes age, sex, education, BP, CBF and brain volume (% ICV)
0= normal cognitive status (n=2011) versus 1= Dementia (n=134) or
0= normal cognitive status (n=2011) versus 1=MCI (n=216)
OR=Odds Ratio.
OR <1 indicates a decreased odd for dementia or MCI with a higher CBF.
BP=blood pressure, CBF=cerebral blood flow, CI= confidence interval, DBP=diastolic blood pressure, SBP=systolic blood pressure, PP=pulse pressure
Discussion
In this large population-based study of 2489 persons followed for 30 years we found that the effect of BP on CBF is different for SBP and DBP. A higher late-life DBP was related to a lower CBFPC, whereas a higher late-life SBP was associated with higher CBFPC. Extensive stratified and interaction analyses suggested these relationships were robust to level of midlife BP, cardiovascular risk factors and use of antihypertensive treatment. Our findings also indicate that CBF is an important correlate of late-life cognition, while accounting for atrophy and for level of BP. There were no systematic differences in CBF modulation of cognition by level of BP (i.e., no significant interaction), but the data suggest that particularly people with lower DBP, or with SBP between 120 and 140 mmHg may have a cognitive benefit of having a higher CBF.
There are few data on the association of BP to CBF in community-based cohorts. A previous study in non-demented older adults25 also showed that higher diastolic BP, but not systolic BP was significantly associated with lower CBF. To better understand the difference in directionality of late-life DBP and SBP with CBF additional studies are needed. We did not observe a relationship between midlife BP and late-life CBF, while in a middle-aged cohort with manifest atherosclerotic disease (SMART-MR Study) both higher SBP and DBP were associated with a decline in CBF.26 Differences in study findings may be attributable to differences in population characteristics such as prevalence of cardiovascular comorbidities.
Whereas the evidence on the association of BP levels to CBF is mixed, there is robust and consistent evidence of an adverse impact of midlife hypertension on late-life cognitive function.4 A higher blood pressure in late-life however has demonstrated no27, a negative,28or a positive relationship with cognitive function.5, 29 Several studies indicate that it is essential to consider history of midlife hypertension in order to understand how late-life BP affects cognitive function. Walker et al. showed that those participants with hypertension in both mid and late-life and participants with midlife hypertension and late-life hypotension had significantly increased risk of subsequent dementia compared with those who remained normotensive.30 Considering midlife BP only, we did not observe a relationship with late-life cognition. However, Muller et al. has previously demonstrated in the AGES-Reykjavik study that those participants with midlife hypertension and late-life lower diastolic BP had the worst memory performance.31 BP is known to decline 3 to 6 years prior to dementia diagnosis.32
Low CBF has been shown to be a consistent marker of dementia,10 although results are mixed in the MCI stage.33 The Rotterdam study showed that dementia risk estimates for low cerebral perfusion were higher in those with higher blood pressure levels at baseline (with significant interaction for mean arterial pressure).34 De Heus et al. also showed that the BP to CBF ratio is higher in dementia and MCI compared to controls.35 In line with these findings, we showed that effect estimates for the relationship between CBF and cognitive function were highest in those with a lower level of DBP or normal range of SBP, although the interaction term for BP and CBF did not reach significance.
Our findings on the complex interplay between blood pressure, CBF and cognitive function may be attributable to several factors, which fall broadly into 3 categories: physiologic changes accumulating with age and disease; co-occurrence of BP changes, low CBF, and increased mortality; or selective survival.
First, with ageing there is an increase in central arterial stiffness, leading to an increase in SBP and decline in DBP, resulting in a higher pulse pressure at old age.36 A higher pulse pressure transmitted into smaller cerebral arterioles and capillary beds can lead to cerebral vascular remodeling, including narrowing of lumen and increased vascular resistance.1, 2 These structural changes may be accompanied by dysfunction of cerebral autoregulation (CA) and neurovascular coupling, which renders the brain vulnerable for hypoperfusion with lower systolic BP. Cerebral hypoperfusion leads to an imbalance of neuronal metabolic supply and demand, thereby precipitating cognitive dysfunction.10 Nevertheless, Heus et al.35 showed that in MCI or dementia CA remains functional (as measured by transcranial Doppler ultrasound in the middle cerebral artery). Possibly, larger cerebral arteries may still be able to compensate to maintain and stabilize CBF with varying BP through CA, while there is localized increased cerebral vascular resistance and impaired CA in smaller arteries.
Second, the positive slope between SBP and CBFPC may be driven by factors at the lower end of the BP distribution, where a lower late-life SBP and lower CBF may be an epiphenomenon of incipient death. Lower late-life CBF has been strongly associated with increased mortality risk,37 and SBP is known to decrease progressively for more than a decade before death, and most steeply in the last 2 life years.38
Finally, selective survival may explain the relationship between a higher SBP and higher CBF as there may have been proportionately more loss from the cohort of those with higher SBP and lower CBF [potential high-risk group for cardiovascular mortality].
Strengths of our study include the population-based setting, the large sample size that is extensively phenotyped for cardiovascular risk and cognitive function, the availability of both midlife and late-life BP data over 30 year follow-up, and comprehensive analyses to explore the association of both level of BP and CBF with cognition. Our extensive stratified analyses provide insight into how markers of biological age influence the complex relationship between BP and CBF.
However, several characteristics of our study need to be considered when interpreting the results. We used Phase-Contrast MRI to estimate total brain CBF, which may be influenced by accuracy of the segmentation of the arteries, assumptions in the acquisition and processing protocols, changes in vascular morphology such as tortuosity and hemoglobin levels.39, 40 Further research is needed to identify factors that contribute to error in CBF measurement. Nevertheless, PC-MRI has proven to be a reliable measure for CBF in the AGES population; the intraclass correlation coefficient (ICC) for whole brain CBF values obtained with PC-MRI against pCASL was 0.80.41 Our estimates of CBF are also consistent with another, on average younger population-based cohort, that also used PC-MRI to measure CBF.10 Second, CBFpc was only measured once so we do not know the start of, how rapid, or to what extent there was a decline from middle to late age in CBF in total or regional brain areas. Also, we cannot determine the directionality of the relationship between a lower CBF and dementia risk, either a lower CBF precedes dementia, or vice versa. Future studies are needed with multiple time points and combined technologies, such as the MRI sequence Arterial Spin Labeling (ASL) and transcranial doppler to give temporal and regional measures of cerebral perfusion and autoregulation.42 The AGES-Reykjavik study included a white community-dwelling sample, with a prevalence of antihypertensive treatment use of 60%, stroke of 8% and diabetes mellitus of 13%, which should be considered when generalizing our results. Future studies should examine whether BP, CBF and cognition show similar associations in multi-racial samples or in samples with a different cardiovascular burden. Finally, a standardized cuff was used to measure BP that was not adjusted for arm circumference, which may have affected BP measurements of those participants with a particularly small or large arm circumference.
Higher late-life diastolic BP and systolic BP were differentially associated with CBFPC. Our findings suggest that late-life CBF is an important correlate of cognition.
Perspectives
In this large population-based study the effect of late-life BP on CBF is different for SBP and DBP. A higher late-life DBP was related to a lower CBF, whereas a higher late-life SBP was associated with higher CBF. Not BP level, but a higher CBF was consistently related to better cognitive outcomes. To better understand BP lowering strategies for optimal cognition, future trials on BP and cognition should include CBF measurements.
Supplementary Material
Novelty and significance.
What is new?
We show that in late-life not BP level, but a lower CBF is consistently related to worse cognitive outcomes.
What is relevant?
To establish late-life BP values and treatment goals for optimizing brain function, it is crucial to consider CBF level.
Summary
This large population-based study in older persons shows that the effect of late-life BP on CBF is different for SBP and DBP, with a higher SBP but a lower DBP relating to a higher CBF. Higher CBF was related to higher cognitive functioning and lower odd of MCI or dementia, irrespective of BP level.
Acknowledgements
The authors would like to thank the participants of the AGES Reykjavik Study and the staff personal at the Icelandic Heart Association.
Sources of funding
This work was supported the National Institutes of Health contract N01-AG-1-2100, the National Institute of Aging Intramural Research Program, the Icelandic Heart Association, and Althingi, the Icelandic Parliament. Additional funding is provided by grants from the Science Fund of Landspitali – The National University Hospital of Iceland and The Helga Jonsdottir and Sigurlidi Kristjansson Memorial Fund. Landspitali-National University Hospital Science Fund and The Helga Jonsdottir and Sigurlidi Kristjansson Memorial Fund.
Footnotes
Disclosures No conflict of interest
References
- 1.de Roos A, van der Grond J, Mitchell G and Westenberg J. Magnetic Resonance Imaging of Cardiovascular Function and the Brain: Is Dementia a Cardiovascular-Driven Disease? Circulation. 2017;135:2178–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorelick PB, Counts SE and Nyenhuis D. Vascular cognitive impairment and dementia. Biochim Biophys Acta. 2016;1862:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng S, Xanthakis V, Sullivan LM and Vasan RS. Blood pressure tracking over the adult life course: patterns and correlates in the Framingham heart study. Hypertension (Dallas, Tex : 1979). 2012;60:1393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, Seshadri S and Zeki Al Hazzouri A. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension (Dallas, Tex : 1979). 2016;68:e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu C, Winblad B and Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. The Lancet Neurology. 2005;4:487–99. [DOI] [PubMed] [Google Scholar]
- 6.Debette S, Schilling S, Duperron MG, Larsson SC and Markus HS. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA neurology. 2019;76:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr., Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr., Narva AS and Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). Jama. 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- 8.Molander L, Lovheim H, Norman T, Nordstrom P and Gustafson Y. Lower systolic blood pressure is associated with greater mortality in people aged 85 and older. Journal of the American Geriatrics Society. 2008;56:1853–9. [DOI] [PubMed] [Google Scholar]
- 9.Shaw BH, Borrel D, Sabbaghan K, Kum C, Yang Y, Robinovitch SN and Claydon VE. Relationships between orthostatic hypotension, frailty, falling and mortality in elderly care home residents. BMC geriatrics. 2019;19:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW and Ikram MA. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation. 2017. [DOI] [PubMed] [Google Scholar]
- 11.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ and Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. American journal of epidemiology. 2007;165:1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigurdsson S, Aspelund T, Forsberg L, Fredriksson J, Kjartansson O, Oskarsdottir B, Jonsson PV, Eiriksdottir G, Harris TB, Zijdenbos A, van Buchem MA, Launer LJ and Gudnason V. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. NeuroImage. 2012;59:3862–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spilt A, Box FM, van der Geest RJ, Reiber JH, Kunz P, Kamper AM, Blauw GJ and van Buchem MA. Reproducibility of total cerebral blood flow measurements using phase contrast magnetic resonance imaging. Journal of magnetic resonance imaging : JMRI. 2002;16:1–5. [DOI] [PubMed] [Google Scholar]
- 14.Gudmundsson EF, Gudnason V, Sigurdsson S, Launer LJ, Harris TB and Aspelund T. Coronary artery calcium distributions in older persons in the AGES-Reykjavik study. European journal of epidemiology. 2012;27:673–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saczynski JS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Jonsson PV, Garcia ME, Kjartansson O, Lopez O, van Buchem MA, Gudnason V and Launer LJ. Cerebral infarcts and cognitive performance: importance of location and number of infarcts. Stroke. 2009;40:677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan E, Fein D, Morris R and Delis D. The wais-r as a neuropsychological instrument. 1991;San Antonio: Psychological Corporation. [Google Scholar]
- 17.Wechsler D Wechsler adult intelligence scale. 1955;New York: Psychological Corporation. [Google Scholar]
- 18.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L and Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–81. [DOI] [PubMed] [Google Scholar]
- 19.Stroop J Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–62. [Google Scholar]
- 20.Salthouse T and Babcock R. Decomposing adult age differences in executive function. Developmental Psychology. 1991;27:763–76. [Google Scholar]
- 21.Qiu C, Cotch MF, Sigurdsson S, Jonsson PV, Jonsdottir MK, Sveinbjrnsdottir S, Eiriksdottir G, Klein R, Harris TB, van Buchem MA, Gudnason V and Launer LJ. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology. 2010;75:2221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. 1994;Washington, DC: American Psychiatric Association. [Google Scholar]
- 23.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M and Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Archives of neurology. 2003;60:1385–9. [DOI] [PubMed] [Google Scholar]
- 24.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ and Weintraub WS. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. Journal of the American College of Cardiology. 2007;49:378–402. [DOI] [PubMed] [Google Scholar]
- 25.Shokouhi M, Qiu D, Samman Tahhan A, Quyyumi AA and Hajjar I. Differential Associations of Diastolic and Systolic Pressures with Cerebral Measures in Older Individuals with Mild Cognitive Impairment. American journal of hypertension. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller M, van der Graaf Y, Visseren FL, Mali WP and Geerlings MI. Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Annals of neurology. 2012;71:825–33. [DOI] [PubMed] [Google Scholar]
- 27.Shang S, Li P, Deng M, Jiang Y, Chen C and Qu Q. The Age-Dependent Relationship between Blood Pressure and Cognitive Impairment: A Cross-Sectional Study in a Rural Area of Xi'an, China. PloS one. 2016;11:e0159485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilander L, Nyman H, Boberg M, Hansson L and Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension (Dallas, Tex : 1979). 1998;31:780–6. [DOI] [PubMed] [Google Scholar]
- 29.Euser SM, van Bemmel T, Schram MT, Gussekloo J, Hofman A, Westendorp RG and Breteler MM. The effect of age on the association between blood pressure and cognitive function later in life. Journal of the American Geriatrics Society. 2009;57:1232–7. [DOI] [PubMed] [Google Scholar]
- 30.Walker KA, Sharrett AR, Wu A, Schneider ALC, Albert M, Lutsey PL, Bandeen-Roche K, Coresh J, Gross AL, Windham BG, Knopman DS, Power MC, Rawlings AM, Mosley TH and Gottesman RF. Association of Midlife to Late-Life Blood Pressure Patterns With Incident Dementia. Jama. 2019;322:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller M, Sigurdsson S, Kjartansson O, Aspelund T, Lopez OL, Jonnson PV, Harris TB, van Buchem M, Gudnason V and Launer LJ. Joint effect of mid- and late-life blood pressure on the brain: the AGES-Reykjavik study. Neurology. 2014;82:2187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu C, von Strauss E, Winblad B and Fratiglioni L. Decline in blood pressure over time and risk of dementia: a longitudinal study from the Kungsholmen project. Stroke. 2004;35:1810–5. [DOI] [PubMed] [Google Scholar]
- 33.Sierra-Marcos A Regional Cerebral Blood Flow in Mild Cognitive Impairment and Alzheimer's Disease Measured with Arterial Spin Labeling Magnetic Resonance Imaging. Int J Alzheimers Dis. 2017;2017:5479597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW and Ikram MA. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation. 2017;136:719–728. [DOI] [PubMed] [Google Scholar]
- 35.de Heus RAA, de Jong DLK, Sanders ML, van Spijker GJ, Oudegeest-Sander MH, Hopman MT, Lawlor BA, Olde Rikkert MGM and Claassen J. Dynamic Regulation of Cerebral Blood Flow in Patients With Alzheimer Disease. Hypertension (Dallas, Tex : 1979). 2018;72:139–150. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell GF, Lacourcière Y, Ouellet JP, Izzo JL Jr., Neutel J, Kerwin LJ, Block AJ and Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation. 2003;108:1592–8. [DOI] [PubMed] [Google Scholar]
- 37.Sabayan B, van der Grond J, Westendorp RG, Jukema JW, Ford I, Buckley BM, Sattar N, van Osch MJ, van Buchem MA and de Craen AJ. Total cerebral blood flow and mortality in old age: a 12-year follow-up study. Neurology. 2013;81:1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgado J, Bowman K, Ble A, Masoli J, Han Y, Henley W, Welsh S, Kuchel GA, Ferrucci L and Melzer D. Blood Pressure Trajectories in the 20 Years Before Death. JAMA internal medicine. 2018;178:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang J, Kokeny P, Ying W, Magnano C, Zivadinov R and Mark Haacke E. Quantifying errors in flow measurement using phase contrast magnetic resonance imaging: comparison of several boundary detection methods. Magnetic resonance imaging. 2015;33:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ismaili ARA, Vestergaard MB, Hansen AE, Larsson HBW, Johannesen HH, Law I and Henriksen OM. Components of day-to-day variability of cerebral perfusion measurements - Analysis of phase contrast mapping magnetic resonance imaging measurements in healthy volunteers. PloS one. 2018;13:e0197807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sigurdsson S, Forsberg L, Aspelund T, van der Geest RJ, van Buchem MA, Launer LJ, Gudnason V and van Osch MJ. Feasibility of Using Pseudo-Continuous Arterial Spin Labeling Perfusion in a Geriatric Population at 1.5 Tesla. PloS one. 2015;10:e0144743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozturk ED and Tan CO. Human cerebrovascular function in health and disease: insights from integrative approaches. Journal of physiological anthropology. 2018;37:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


