Abstract
The prevalence of Attention Deficit/Hyperactivity Disorder (ADHD) has been increasing. Research suggests that exposure to endocrine disrupting chemicals such as phthalates may play a role, but studies of in utero phthalate exposure and ADHD-related symptoms beyond early childhood are limited. We investigated associations between measures of in utero phthalate exposure and ADHD symptoms, such as inattention and impulsivity, in childhood (age 6–11 years, n=221) and in adolescence (age 9–18 years, n=200), as well as cross-sectional relationships between phthalate exposure and ADHD symptoms in adolescence (n= 491) among participants in the Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) cohort. Women provided urine samples up to three times during pregnancy and adolescents provided a urine sample at 9–18 years of age for phthalate metabolite measurement. We administered the Conners’ Continuous Performance Test (CPT) when children were age 6–11 years and again at 9–18 years of age. We used multivariable linear regression to examine associations between the geometric mean of phthalate metabolite levels across pregnancy and CPT scores in childhood or adolescence separately, adjusting for age, years schooling (at 9–18 only), maternal education, and specific gravity. Although average in utero phthalate concentrations were not associated with CPT scores in childhood, interquartile range (IQR) increases of in utero MBzP, MCPP, and MBP were associated with 4.2%, 4.7%, and 4.5% (p<0.05) higher Omissions scores in adolescence, respectively, indicating higher inattention. In utero MiBP levels were also associated with higher Inter-Stimulus Interval (ISI) and Variability scores (5.4% and 5.5% per IQR, p<0.05) in adolescence. In addition, urinary DEHP metabolite levels during adolescence were cross-sectionally associated with poorer scores on several CPT indices indicating greater inattention. These findings suggest that in utero phthalate exposure may have adverse effects on attention, but these effects may not appear until adolescence, a period of extensive neurodevelopment. Future research investigating the long-term effects of in utero phthalate exposure on attention and ADHD in adolescence, as well as identification of potential mechanisms involved, is needed.
Keywords: phthalates, attention, ADHD, gestational exposure, adolescence
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is an increasingly common neurobehavioral disorder diagnosed in childhood characterized by increased inattention, impulsivity, and/or hyperactivity that interferes with functioning and development (NIMH 2019). In 2016, an estimated 9.4% of children in the US aged 2–17 years had an ADHD diagnosis according to parental reports (CDC 2016). Estimates of worldwide ADHD prevalence range from 3–8% among children and adolescents (Polanczyk et al. 2007; Thomas et al. 2015) and from 1–4% among adults (Fayyad et al. 2017). Indeed, approximately 60% of children diagnosed with ADHD continue to have symptoms in adulthood (Turgay et al. 2012), likely resulting in work, school, and social impairments (NIMH 2019). The World Health Organization estimated that on average, workers with ADHD lost 22 days per year of work performance compared to workers without ADHD (de Graaf et al. 2008), and children, adolescents, and adults with ADHD around the world report poorer quality of life (Brod et al. 2012; Caci et al. 2014). In the US, the estimated total annual cost of ADHD is $143 to $266 billion (Doshi et al. 2012). Despite the increasing prevalence and enormous cost of ADHD, risk factors are not well defined. Genetic and environmental factors, particularly exposures occurring during early neurodevelopment, are both thought to play a role (NIMH 2019).
Previous studies suggest that in utero exposure to phthalates, a group of chemicals utilized as plasticizers and solvents in a wide range of consumer and industrial applications (CDC 2019) may increase ADHD symptoms, such as inattention, impulsivity, or hyperactivity, in early to middle childhood (Engel et al. 2010; Engel et al. 2018; Kobrosly et al. 2014; Ku et al. 2020). However, not all studies reported statistically significant findings (Gascon et al. 2015), and there were inconsistencies regarding sex-specific effects. In addition, many studies have relied solely on parent-reported behavior, measured prenatal phthalate levels only once or twice despite highly variable levels during pregnancy, and have not followed children through to adolescence, a period of extensive brain development.
Phthalate exposure during childhood has been associated with higher risk of ADHD (Chopra et al. 2014; Hu et al. 2017; Park et al. 2015) and higher inattention, hyperactivity, and impulsivity (Hu et al. 2017; Kim et al. 2018; Park et al. 2014; Park et al. 2015) measured through both parental report and performance based assessments. However, these studies are limited by their cross-sectional study design, and some did not adjust for confounders, such as age. Many of these studies also excluded children taking medication for ADHD and children with leaning disabilities, a common co-ocurring diagnosis, and there were inconsistencies in sex-specific effects.
To address some of these knowledge gaps, the aims of this study were to examine relationships between measures of phthalate exposure across gestation and ADHD symptoms in childhood and adolescence, as well as cross-sectional relationships between adolescent measures of phthalate exposure and ADHD symptoms, using a prospective, repeated measures study design.
2. Methods
2.1. Study design
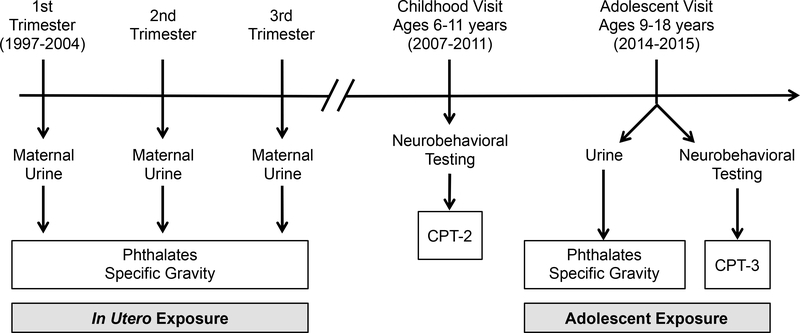
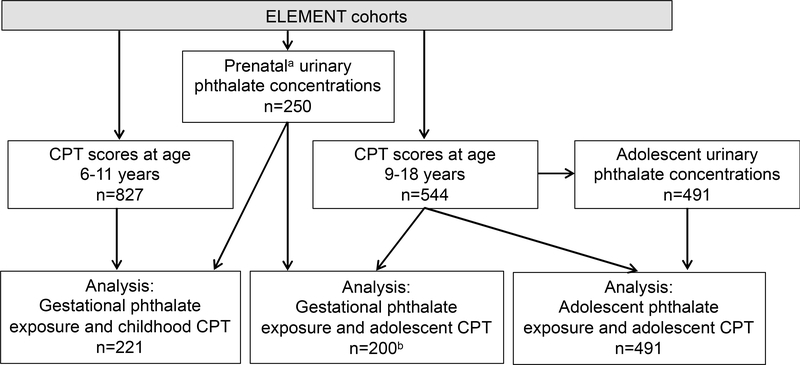
Participants were a subset of women originally recruited into the Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) project, a longitudinal birth cohort in Mexico City (Perng et al. 2019). This analysis includes mothers who were recruited from maternity hospitals during their first trimester of pregnancy between 1997 and 2004, participated in up to three prenatal study visits (mean gestational age: T1=13.4 weeks, T2=25.1 weeks, T3=34.3 weeks), and followed through delivery (Figure 1). At each prenatal visit women provided a urine sample and completed interview-based questionnaires as previously described (Perng et al. 2019). In a subsequent pilot study, urinary phthalate metabolite concentrations were measured in prenatal samples collected from 250 ELEMENT mothers. When the index children born to ELEMENT mothers were 6–11 years of age, a subset were re-recruited to participate in a follow-up study (childhood visit), including a battery of neurobehavioral testing (n=827). ELEMENT children were again re-recruited at 9–18 years of age (adolescent visit), at which point the adolescent provided a urine sample and completed additional neurobehavioral testing and interview-based questionnaires (n=544). The present analysis includes 221 participants with prenatal phthalate and childhood outcome measures and 200 participants with prenatal phthalate and adolescent outcome measures, with 195 participants having outcome measures at both time points (Figure 2). The cross-sectional analysis includes 491 participants with both phthalate and outcome measures in adolescence. Information on socioeconomic status (SES) was collected at both the childhood and adolescent visits using the Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública (AMAI) version 13×6, a validated scale consisting of thirteen questions on housing quality, services, material goods, and head of household education, which classifies households into six SES categories (AMAI 2000). Research protocols were approved by the Institutional Review Board at University of Michigan, and the Research, Biosafety and Ethics in Research at the Mexico National Institute of Public Health. Prior to enrollment, informed consent from mothers and informed assent from offspring were obtained.
Figure 1.
Data collection timeline
Figure 2.
Analysis sample sizes
Maternal prenatal urinary phthalate concentrations are a proxy measure of gestational phthalate exposure to their child. b n=45 participants aged 9–11, n=155 participants aged 12–18.
2.2. Phthalate metabolite measurements
Phthalate metabolites were measured in maternal urine samples collected during each trimester of pregnancy and in adolescent urine collected at 9–18 years of age. Urine samples were frozen and stored at −80°C until analysis at NSF International (Ann Arbor, MI). We measured monoethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), monoisobutyl phthalate (MiBP), monobenzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP) using liquid chromatography–tandem mass spectrometry (LC–MS/MS) as previously described (Lewis et al. 2013). In adolescent samples, we measured 3 additional metabolites of the plasticizers di-isononyl phthalate (DiNP) and di-isodecyl phthalate (DiDP) (mono-carboxyisooctyl phthalate (MCOP), mono-carboxyisononyl phthalate (MCNP), and mono-isononyl phthalate (MNP)). Phthalate metabolite concentrations below the limit of detection (LOD) were imputed with the LOD/√2. We calculated a di-2-ethylhexyl phthalate (DEHP) metabolite summary measure (ΣDEHP) for each sample by dividing individual MEHP, MEHHP, MEOHP, and MECPP concentrations by their molar mass and summing them. Specific gravity (SG) was measured using a handheld digital refractometer (Atago Co., Ltd., Tokyo, Japan) at the time of sample analysis.
2.3. Neurobehavioral assessments
At age 6–11 years, participants completed the Conners’ Continuous Performance Test, Second Edition (CPT-II), a computer-based assessment of attention and impulsivity that provides information on specific ADHD behaviors (Conners 2004). Diagnosis of ADHD requires clinical evaluation and self- and caregiver-based reports of behavior, but objective assessments of ADHD symptoms such as the CPT are critical tools in the diagnostic process (Emser et al. 2018; Hall et al. 2016; Slobodin 2020). At 9–18 years of age, participants were re-recruited and completed an updated version of the Conners’ CPT (CPT-3) (Conners 2014). The CPT-II and CPT-3 both consist of the examinee pressing the spacebar whenever letters other than “X” are shown on the computer screen. Letters are displayed for 250 milliseconds at intervals of 1, 2, or 4 seconds, with a total of 360 trials over 14 minutes. Scores for a number of indices are produced: 1) Omissions (missed targets), a measure of inattention; 2) Commissions (incorrect response to non-targets), a measure of impulsivity; 3) Hit Reaction Time (HRT), which is higher with inattention and lower with impulsivity; and 4) HRT standard deviation (HRT-SD), a measure of reaction time consistency. HRTs for Block Change and ISI Change measure the change in speed across blocks of trials and across different inter-stimulus intervals (ISIs), respectively, with lower scores indicating sustained or increased speed at later blocks or longer ISIs. The Detectability score is a measure of the examinee’s ability to differentiate targets from non-targets, and the Variability score is a measure of variability of reaction time consistency across blocks of trials. Lastly, Response Style is a measure of whether the examinee favors a conservative (accuracy over speed = higher scores), liberal (speed over accuracy = lower scores) or balanced response. Raw scores can be converted to age- and sex-adjusted t-scores with a mean of 50 and standard deviation of 10.
2.4. Statistical analyses
Distributions of urinary exposure and CPT outcome measures were assessed prior to analysis. Phthalate metabolite concentrations were natural log-transformed for use in regression analyses, as were CPT t-scores for Response Style, Commissions, and Omissions at 6–11 years, and Omissions, HRT, HRT-SD, and Variability at 9–18 years. T-scores for all other CPT indices were normally distributed and were not transformed prior to analyses. Spearman correlations were performed to evaluate relationships between CPT-II scores at 6–11 years and CPT-3 scores at 9–18 years among participants in both follow-up visits (n=195). Geometric mean (GM) phthalate metabolite concentrations across pregnancy were calculated for each individual using all available measurements from prenatal visits 1, 2, and 3 for each metabolite to reflect overall exposure during in utero development. Adolescent phthalate metabolite concentrations were compared to prenatal GM values using Spearman correlations (n=200).
Individual prenatal GM concentrations were entered into separate linear regression models to assess relationships between in utero phthalate exposure and either CPT-II scores at 6–11 years or CPT-3 scores at 9–18 years. To evaluate cross-sectional relationships between exposures and outcomes, adolescent phthalate concentrations were entered into regression models as predictors of concurrent CPT-3 scores. Results are presented as the percent difference in score (95% confidence interval) per interquartile range (IQR) increase in phthalate metabolite using the following equations:
Phthalate concentrations and outcomes log-transformed:
Phthalate concentrations log-transformed, outcomes not log-transformed:
All models were adjusted a priori for child age at assessment, sex, and maternal education, as well as urinary specific gravity to account for urinary dilution. Models predicting CPT-3 scores at 9–18 years of age were additionally adjusted for the number of years the child had attended school. To assess linearity of associations, prenatal and peripubertal phthalate concentrations were categorized into tertiles and entered into regression models in place of the continuous exposure biomarkers. In separate sensitivity analyses, we additionally adjusted for household socioeconomic status at the time of CPT administration (Fortenberry et al. 2014; Watkins et al. 2016a), ADHD medication (n=5), gestational age at birth, or birth weight, and evaluated sex*exposure interactions.
3. Results
3.1. Participant Characteristics
Mothers of ELEMENT children included in this analysis were on average 26.9 (SD: ±5.7) years of age and had 10.9 (±2.9) years of education upon enrollment (Supplemental Table S1) and gave birth to their index child at 38.7 (± 1.6) weeks gestation. Participant characteristics were highly similar to those of the original ELEMENT cohorts (Perng et al. 2019).
At the childhood visit, the mean age among 106 male and 115 female participants was 8.1 (±1.2) years, while the mean participant age at the adolescent visit was 14.6 (±2.1) years, with 262 males and 282 females, and 8.4 (±2.0) years of schooling (Supplemental Table S1). At the childhood visit, mean age adjusted t-scores for all CPT indices were slightly above the validated norm of 50 but within the normal range (SD±10), and scores did not significantly differ by sex (Table 1). At the adolescent visit, mean scores for most indices were within 1–3 points of the validated norm of 50, with some significant differences between males and females. Girls had lower (better) scores for Detectability and Commissions compared to boys, but higher (poorer) HRT and Block Change scores. Among participants who performed the CPT at both visits (n=195), correlations between scores at the two time-points were weak to moderate for most indices, with the exception of Response Style and Block Change, which were not significantly correlated (Supplemental Table S2).
Table 1.
Distribution of neurobehavioral outcome measures
| CPT (6–12 years of age) | All Participants n= 221 | Boys n= 106 | Girls n=115 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | |
| Response Style | 49.3 | 50.8 | 7.2 | 49.8 | 50.8 | 6.5 | 49.0 | 50.8 | 7.8 |
| Detectability | 52.4 | 51.7 | 8.8 | 52.5 | 52.8 | 7.1 | 52.3 | 50.8 | 10.1 |
| Omissions | 51.3 | 55.4 | 12.9 | 52.1 | 55.8 | 14.0 | 51.2 | 55.0 | 11.8 |
| Commissions | 52.4 | 51.2 | 8.8 | 52.4 | 51.6 | 7.7 | 53.6 | 50.8 | 9.8 |
| Hit Reaction Time | 51.4 | 52.6 | 10.4 | 51.3 | 52.7 | 10.3 | 52.0 | 52.6 | 10.5 |
| HRT SE | 57.6 | 57.1 | 10.8 | 56.2 | 56.7 | 10.4 | 58.1 | 57.5 | 11.2 |
| HRT block change | 50.0 | 51.3 | 10.1 | 50.6 | 51.6 | 10.2 | 49.8 | 51.1 | 10.0 |
| HRT ISI change | 53.7 | 54.3 | 12.2 | 52.5 | 53.6 | 12.3 | 54.4 | 55.0 | 12.1 |
| Variability | 57.6 | 56.5 | 10.0 | 55.5 | 56.0 | 9.8 | 58.6 | 56.9 | 10.2 |
| CPT (9–18 years of age) | n=544 | n=262 | n=282 | ||||||
| Response Style | 52 | 52.3 | 9.3 | 52 | 51.8 | 9.0 | 53 | 52.9 | 9.6 |
| Detectability* | 49 | 49.2 | 9.8 | 50 | 50.0 | 9.6 | 48 | 48.4 | 9.9 |
| Omissions | 47 | 50.6 | 10.5 | 47 | 51.1 | 10.9 | 47 | 50.1 | 10.1 |
| Commissions* | 47 | 48.0 | 8.7 | 49 | 49.2 | 8.3 | 46 | 47.0 | 9.0 |
| Hit Reaction Time* | 51 | 52.3 | 8.6 | 49 | 50.6 | 7.8 | 53 | 53.9 | 8.9 |
| HRT SD | 48 | 50.5 | 10.8 | 48 | 50.6 | 11.5 | 48 | 50.5 | 10.1 |
| HRT block change*a | 51 | 51.4 | 9.8 | 49 | 49.0 | 9.6 | 52 | 53.6 | 9.4 |
| HRT ISI change | 50 | 51.0 | 10.1 | 50 | 50.8 | 9.3 | 51 | 51.3 | 10.9 |
| Variabilityb | 47 | 50.2 | 10.9 | 46 | 50.2 | 11.2 | 47 | 50.2 | 10.7 |
p<0.05 for differences by sex
HRT block change: n=542, 261 boys, 281 girls)
Variability: n=538 (257 boys, 281 girls).
3.2. In utero phthalate exposure and CPT in childhood
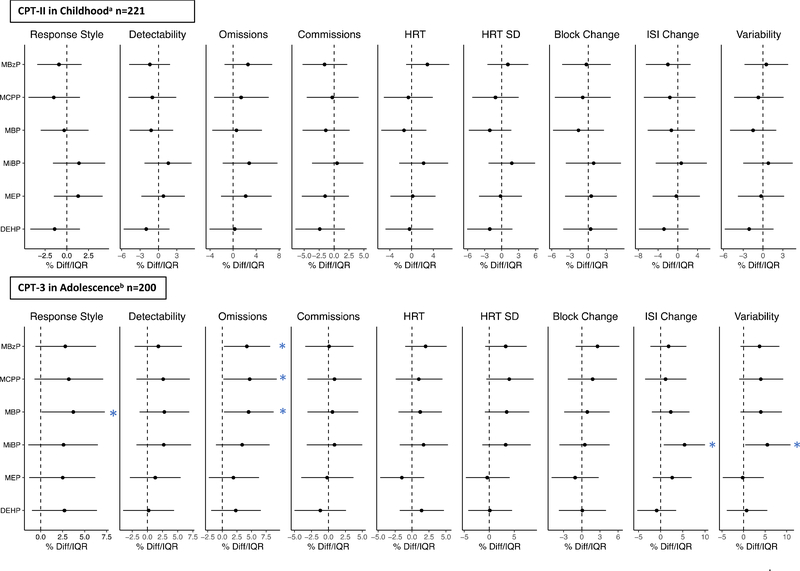
All measured phthalate metabolites were detected in greater than 90% of prenatal urine samples, as previously reported (Lewis et al. 2013). Geometric mean concentrations of individual phthalate metabolites during in utero development were not significantly associated with CPT-II scores in childhood after adjustment for child age, sex, maternal education, and specific gravity (Figure 3, Supplemental Table S3). When we additionally adjusted for gestational age at birth, birth weight, or SES score at the time of CPT administration, or considered trimester-specific phthalate metabolite concentrations, these findings did not materially change (data not shown). In addition, there were no significant exposure*sex interactions (p>0.1), indicating that the effects of in utero phthalate exposure did not differ by sex. Findings from models considering tertiles of in utero phthalate metabolite concentrations were similar to findings from continuous exposure models and provided no evidence of nonlinear associations (data not shown).
Figure 3.
Percent change in CPT-II scores at 6–12 years of age (n=221) and 9–18 years of age (n=200) per IQR increase in the geometric mean of maternal urinary phthalate metabolite concentrations across pregnancy.
*p<0.05; a Adjusted for child age at first follow-up visit, sex, maternal education, and urinary specific gravity. b Adjusted for child age at second follow-up visit, sex, years in school, maternal education, and urinary specific gravity.
3.3. In utero phthalate exposure and CPT scores in adolescence
IQR increases in in utero GM concentrations of MBzP, MCPP, and MBP were each associated with 4.2% (95%CI: 0.3, 8.2), 4.7% (95%CI: 0.2, 9.3), and 4.5% (95%CI: 0.3, 8.8) higher Omissions scores in adolescence, respectively, indicating poorer performance (Figure 3, Supplemental Table S3). In addition, in utero MBP was associated with 3.7% (95%CI: 0.4, 7.3) higher Response Style scores per IQR increase, indicating a more conservative strategy (accuracy over speed). MiBP was associated with higher ISI and Variability scores (5.4%/IQR (95%CI: 0.7, 10); 5.5%/IQR (95%CI: 0.4, 10.9), respectively), indicating inconsistent performance and attention over the course of the test. There were no significant exposure*sex interactions, and additionally adjusting for SES or taking ADHD medication in adolescence did not materially change our results (data not shown). When trimester-specific phthalate metabolite concentrations were considered, associations between phthalate metabolites and Omissions scores were attenuated (Supplemental Table S4). However, several associations between exposure specifically in the first trimester and CPT scores were now significant, although the magnitude of associations remained similar. For example, first trimester MCPP, MBP, and MiBP concentrations were associated with significantly poorer Variability scores (4.2%/IQR (95%CI: 0.3, 8.2); 4.5%/IQR (95%CI: 1.1, 8); and 5.2%/IQR (95%CI: 1, 9.6), respectively) (Supplemental Table S4). In categorical analyses, the highest tertiles of MCPP and ΣDEHP had significantly higher (more conservative) Response Style scores compared to their respective lowest tertiles, while all other findings were similar to continuous analyses (data not shown).
3.4. Adolescent phthalate exposure and CPT
Phthalate metabolites, including MCOP and MCNP which have not been previously measured in this cohort, were detected in greater than 96% of urine samples collected at the adolescent visit. The one exception was MNP, which was below the LOD (0.5 μg/L) in 99.6% of samples (Table 2). Phthalate metabolite concentrations in adolescence were generally not correlated with maternal concentrations during pregnancy (Supplemental Table S5), with the exception of MBzP, which was very weakly correlated (Spearman r= 0.21).
Table 2.
Distributions of urinary phthalate metabolite concentrations among ELEMENT adolescents (μg/L) (n=515).
| Percentiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| LOD | %>LOD | GM | GSD | 25th | 50th | 75th | 95th | Max | |
| phthalates | |||||||||
| MEHP | 1 | 93.2 | 4.15 | 2.51 | 2.30 | 4.08 | 7.94 | 17.2 | 243 |
| MEHHP | 0.1 | 99.6 | 28.4 | 2.84 | 16.0 | 29.5 | 53.0 | 120 | 3570 |
| MEOHP | 0.1 | 99.6 | 14.2 | 2.77 | 7.89 | 14.0 | 26.2 | 71.2 | 1869 |
| MECPP | 0.2 | 99.6 | 43.7 | 2.74 | 24.6 | 43.5 | 78.8 | 178 | 3512 |
| MBzP | 0.2 | 99.0 | 3.49 | 2.74 | 1.94 | 3.37 | 6.35 | 16.6 | 377 |
| MBP | 0.5 | 99.6 | 124 | 2.79 | 71.3 | 128 | 238 | 560 | 4264 |
| MiBP | 0.2, 0.1a | 99.6 | 10.4 | 2.63 | 6.17 | 11.3 | 19.2 | 48.5 | 155 |
| MCPP | 0.2 | 98.6 | 1.96 | 2.51 | 1.11 | 1.98 | 3.56 | 8.89 | 51.2 |
| MEP | 1 | 99.4 | 99.2 | 3.98 | 40.0 | 87.7 | 226 | 1100 | 8990 |
| MCOP | 0.2 | 99.6 | 4.89 | 2.81 | 2.37 | 4.85 | 9.56 | 28.1 | 150 |
| MCNP | 0.2 | 98.6 | 0.85 | 2.05 | 0.55 | 0.80 | 1.36 | 2.85 | 8.83 |
| MNP | 0.5 | 0.4 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | 0.77 |
| ΣDEHP (μmol/L) | na | na | 0.31 | 2.63 | 0.17 | 0.30 | 0.55 | 1.32 | 30.8 |
LOD=limit of detection; GM=geometric mean; GSD=geometric standard deviation; na=not applicable
LOD differed by batch;
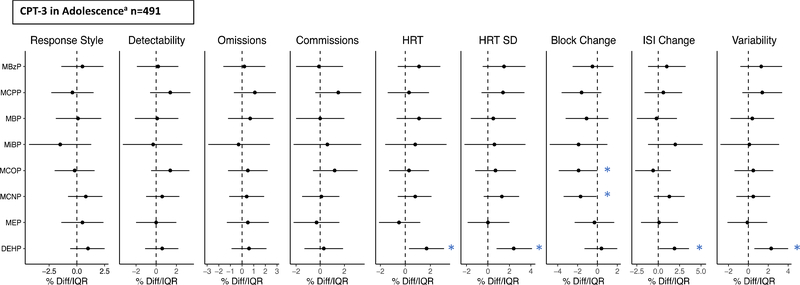
Concurrent ΣDEHP exposure in adolescence was associated with higher scores for HRT SD (2.4%/IQR (95%CI: 0.8, 4.1) and Variability (2.3%/IQR (95%CI: 0.6, 4.0), indicating poorer performance, while MCOP and MCNP were associated with lower Block Change scores (−1.9%/IQR (95%CI: −3.9, 0); −1.7%/IQR (95%CI: −3.4, −0.1), respectively), indicating better performance and sustained speed in later blocks (Figure 4, Supplemental Table S6). We did observe some significant sex*exposure interactions when considering adolescent phthalate exposure, but when stratified, there were no significant associations among either sex. When we additionally adjusted for SES or taking ADHD medication in adolescence, the results did not materially change (not shown). In categorical analyses, higher tertiles of MCPP were also associated with lower Block Change scores, again indicating better performance.
Figure 4.
Percent change in CPT-3 scores per IQR increase in urinary phthalate metabolite concentrations at 9–18 years of age (n=491).
*p<0.05; aAdjusted for child age at second follow-up visit, sex, years in school, maternal education, and urinary specific gravity.
4. Discussion
In this longitudinal study of phthalate exposure and ADHD symptoms, we found that maternal urinary phthalate levels during pregnancy were associated with higher Omissions scores on the CPT, a test of attention and impulsivity performance, at 9 to 18 years of age. Exposure to ΣDEHP during adolescence was also associated with poorer scores on several CPT measures indicating increased inattention. These findings suggest that both in utero and adolescent neurodevelopment may be sensitive periods in regard to environmental exposures.
Median t-scores for each of the CPT indices ranged from 47 to 58, within the normal range of 50, standard deviation of 10. In comparison with National Health and Nutrition Examination Survey (NHANES) participants ages 6–19 from 2013–2016, adolescents in the current study had higher urinary concentrations of DEHP metabolites, MEP, and an order of magnitude higher concentrations of MBP, but lower concentrations of MBzP, MCOP, and MCNP (CDC 2019). This pattern is similar to what was observed among a subset of this study population at ages 8–14 years (Watkins et al. 2014; Watkins et al. 2016b), although concentrations in the present study, at age 9–18 years, are slightly higher for MBP and MEP, and slightly lower for DEHP metabolites, MBzP, and MCPP.
Previous studies of in utero phthalate exposure and ADHD behaviors have reported associations between several phthalate metabolites and increased inattention, attention problems, distractability, and increased odds of ADHD diagnosis in toddlers and young children (Engel et al. 2010; Engel et al. 2018; Kobrosly et al. 2014; Ku et al. 2020). Although we did not see similar associations of in utero phthalate exposure with changes in attention in childhood, we did see associations of several phthalates with higher Omissions scores, suggesting increased inattention, in adolescence. One previous study also did not observe associations between in utero phthalate exposure and attention at ages 4 and 7 years (Gascon et al. 2015), but as there has been no additional follow-up of this population, we do not know if phthalate-related effects appeared as the children aged. Many phthalate metabolites that were associated with ADHD behaviors in previous studies of in utero exposure were also associated with increased inattention in the present study, including MBzP, MBP, and MiBP (Engel et al. 2010; Kobrosly et al. 2014; Ku et al. 2020). However, two previous studies also observed associations between in utero DEHP metabolites and ADHD behaviors in early childhood (Engel et al. 2018; Ku et al. 2020), which we did not observe here. Consistent with previous studies of in utero phthalate exposure and ADHD related behaviors, we did not see sex-specific associations between phthalate exposure and changes in attention.
We did observe cross-sectional associations between urinary ΣDEHP metabolite levels in adolescence and higher t-scores for HRT and HRT-SD, which indicate inattention, and higher t-scores for HRT-ISI Change and Variability, indicating poorer sustained attention. These findings are consistent with results from several previous cross-sectional studies of childhood and adolescent phthalate exposure and ADHD behaviors. For example, a study of NHANES participants ages 6–15 years in 2000–2004 reported that DEHP metabolites were associated with increased odds of being diagnosed with ADHD according to parent report (Chopra et al. 2014). Similarly, a study of Chinese children ages 6–13 years found significant relationships between repeated measures of urinary DEHP metabolites and increased odds of ADHD diagnosis, as well as increased inattention, hyperactivity, and externalizing behaviors (Hu et al. 2017). A series of case-control studies in South Korea reported cross-sectional relationships between DEHP metabolites and ADHD behaviors in childhood and adolescence, including ADHD diagnosis and poorer scores on the Korean version of the CPT (Park et al. 2014; Park et al. 2015). Interestingly, the Korean studies reported that associations between phthalate levels and CPT scores differed by dopamine receptor D4 (DRD4) genotype (Park et al. 2014) and methylation status (Kim et al. 2018). However, as these were case-control studies with relatively strict exclusion criteria, these findings should be repeated in other studies and populations.
Phthalate exposure may affect neurodevelopment and ADHD behaviors through a number of mechanisms. Interference with dopaminergic pathways may be one potential mechanism, as this system plays a key pathophysiological role in ADHD and brain development (Cai et al. 2021). In animal studies, DEHP exposure during gestation and puberty has been shown to alter expression of dopamine receptors 1 and 2 in mice (Hatcher et al. 2019; Wang et al. 2016), while butyl benzyl phthalate exposure decreased brain dopamine levels in fish (Deegan et al. 2019). This mechanism is also supported by the epidemiological studies in Korea in which associations between phthalate levels and CPT scores differed by dopamine receptor D4 (DRD4) genotype (Park et al. 2014) and methylation status (Kim et al. 2018).
Phthalates are also known to be anti-androgenic and weakly estrogenic (Borch et al. 2006; Chen et al. 2014; Howdeshell et al. 2008), and studies demonstrate that prenatal phthalate exposure can alter steroid hormone levels during pregnancy (Cathey et al. 2019; Sathyanarayana et al. 2017) and in offspring during childhood and adolescence (Watkins et al. 2017a, b; Wen et al. 2017). Steroid hormones are critical for neurodevelopment during gestation, the postnatal period, and adolescence, as they are involved in neurogenesis, neuronal differentiation, migration, myelination, synaptogenesis, and synaptic pruning (Cameron 2004; Schug et al. 2015). Indeed, limited studies suggest that altered androgen exposure or other disruption of the hypothalamus-pituitary-adrenal (HPA) axis during gestation may increase risk of ADHD and other neurobehavioral disorders in childhood (Kosidou et al. 2017; Miranda and Sousa 2018; Wang et al. 2017). However, steroid hormones differentially affect male and female brain development and studies of gestational phthalate exposure and attention have not consistently found sex-specific associations. Therefore, it may be unlikely that steroid hormones mediate associations between in utero and peripubertal phthalate exposure and ADHD symptoms, but further research is needed.
In addition, previous studies suggest that phthalate exposure during pregnancy can alter maternal and cord thyroid hormone levels (Huang et al. 2018; Johns et al. 2016; Romano et al. 2018). Thyroid hormone during early pregnancy is critically important for almost all aspects of neurodevelopment, including neurogenesis, neuronal migration and differentiation, glial cell differentiation, myelination, and synaptogenesis, and alterations in prenatal thyroid homeostasis have been associated with increased risk of several neurobehavioral disorders, including ADHD (Miranda and Sousa 2018). However, in a study by Engel et al., associations between urinary phthalate metabolite levels during pregnancy and childhood ADHD diagnosis in offspring were not mediated by prenatal thyroid hormone levels (Engel et al. 2018).
Changes in lipid metabolism may be another mechanism by which phthalate exposure can affect neurodevelopment. Prenatal phthalate exposure has been associated with increased lipid peroxidation during pregnancy (Ferguson et al. 2014; Ferguson et al. 2015; Holland et al. 2016) and with changes in lipid concentrations in during gestation, childhood, and adolescence (Golestanzadeh et al. 2019; Jia et al. 2015). Peripubertal phthalate exposure was associated with lower lipid levels within the current study population (Perng et al. 2017) although other studies have observed null or positive associations (Golestanzadeh et al. 2019). Lipids, specifically polyunsaturated fatty acids (PUFAs), are essential for normal neurodevelopment, as well as maintaining neurological function throughout adulthood (Pusceddu et al. 2016). PUFAs are integrated into cell membrane phospholipids particularly in the brain, and modulate neurogenesis, neurotransmission, and neuroinflammation. Several neurobehavioral disorders have been associated with low levels of omega-3 PUFAs, including ADHD, autism, and depression, (Agostoni et al. 2017; Pusceddu et al. 2016). Studies investigating the role of altered lipid profiles in phthalate exposure-related changes in neurodevelopment are needed.
Strengths of this research include multiple phthalate measurements during pregnancy to reduce exposure mis-classification and repeated CPT testing across childhood and adolescence, a period that few studies have examined in relation to in utero exposure. Limitations include the absence of phthalate exposure measurements during childhood, and only one spot measurement during adolescence. The sole use of the Connors’ CPT to characterize ADHD-related behaviors and the lack of information on ADHD diagnoses are also minor limitations. The accuracy of the CPT in characterizing attention problems varies according to common co-morbidities, such as learning disabilities or autism (Celeste et al. 2019; Lundervold et al. 2016). Use of parent or self-report of attention-related behaviors in conjunction with the CPT could perhaps provide a more robust characterization. In addition, the childhood and adolescent study visits utilized different versions of the CPT, so differences in scores between the two time points may be due to changes to the test itself. Finally, we made a number of statistical comparisons, increasing the likelihood of chance findings, and a relatively small number of women in our population had prenatal urinary phthalate measurements.
5. Conclusion
Findings from this study suggest that in utero phthalate exposure may have adverse effects on attention, but these effects may not appear until adolescence, a period of extensive neurodevelopment. In addition, exposure during adolescence itself may also result in increased inattention. Future research investigating the long-term effects of in utero and childhood phthalate exposure on attention and ADHD in adolescence, as well as identification of potential mechanisms involved, is needed.
Supplementary Material
Highlights.
Gestational phthalate exposure is associated with poorer attention in offspring.
Exposure-related effects on attention may not be apparent until adolescence.
Phthalate exposure in adolescence may also have an adverse effect on attention.
Acknowledgements
The authors acknowledge the research staff at participating hospitals and the American British Cowdray Hospital in Mexico City for providing research facilities. We thank the mothers and children for participating in the study.
Funding
This work was supported by U.S. Environmental Protection Agency (US EPA) grant RD83543601 and National Institute for Environmental Health Sciences (NIEHS) grants P20 ES018171, P01 ES02284401, and P30 ES017885. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA or the NIH. This work was also supported and partially funded by the National Institute of Public Health, Ministry of Health of Mexico.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostoni C, Nobile M, Ciappolino V, Delvecchio G, Tesei A, Turolo S, et al. 2017. The role of omega-3 fatty acids in developmental psychopathology: A systematic review on early psychosis, autism, and ADHD. Int J Mol Sci 18:2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMAI. 2000. Avances del comité de niveles socioeconómicos.Comité de Niveles Socioeconómicos. Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública, A.C. [Google Scholar]
- Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. 2006. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology 223:144–155. [DOI] [PubMed] [Google Scholar]
- Brod M, Pohlman B, Lasser R, Hodgkins P. 2012. Comparison of the burden of illness for adults with ADHD across seven countries: A qualitative study. Health Qual Life Outcomes 10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caci H, Doepfner M, Asherson P, Donfrancesco R, Faraone SV, Hervas A, et al. 2014. Daily life impairments associated with self-reported childhood/adolescent attention-deficit/hyperactivity disorder and experiences of diagnosis and treatment: Results from the European lifetime impairment survey. Eur Psychiatry 29:316–323. [DOI] [PubMed] [Google Scholar]
- Cai Y, Xing L, Yang T, Chai R, Wang J, Bao J, Shen W, Ding S, Chen G. 2021. The neurodevelopmental role of dopaminergic signaling in neurological disorders. Neurosci Lett. 2021;741:135540. [DOI] [PubMed] [Google Scholar]
- Cameron JL. 2004. Interrelationships between hormones, behavior, and affect during adolescence: Understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Introduction to part III. Ann N Y Acad Sci 1021:110–123. [DOI] [PubMed] [Google Scholar]
- Cathey AL, Watkins D, Rosario ZY, Velez C, Alshawabkeh AN, Cordero JF, et al. 2019. Associations of phthalates and phthalate replacements with CRH and other hormones among pregnant women in Puerto Rico. J Endocr Soc 3:1127–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste PM, Esteban VP, Mariana L, Maria Jose GB, Florencia B, Christy E, et al. 2019. Continuous performance test in children with intellectual disability and attention deficit hyperactivity disorder. Appl Neuropsychol Child 8:246–252. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2016. Attention-deficit / hyperactivity disorder (ADHD): Data & statistics. Available: https://www.cdc.gov/ncbddd/adhd/data.html [accessed May 11 2020].
- Centers for Disease Control and Prevention (CDC). 2019. Fourth national report on human exposure to environmental chemicals. Updated tables, January 2019, volume one. Atlanta, GA. [Google Scholar]
- Chen X, Xu S, Tan T, Lee ST, Cheng SH, Lee FW, et al. 2014. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. Int J Environ Res Public Health 11:3156–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra V, Harley K, Lahiff M, Eskenazi B. 2014. Association between phthalates and attention deficit disorder and learning disability in u.S. Children, 6–15 years. Environ Res 128:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. 2004. (CPT II) Conners’ Continuous Performance Test, Second edition. Available: https://www.wpspublish.com/store/p/2717/cp-ii-conners-continuous-performance-test-second-edition [accessed October 4 2019].
- Conners CK. 2014. Conners continuous performance test 3rd edition. Available: https://www.mhs.com/MHS-Assessment?prodname=cpt3 [accessed October 4 2019].
- Deegan AM, Steinhauer RB, Feinn RS, Moeller MC, Pylypiw HM Jr., Nabel M, Kovelowski CJ, Kaplan LAE. 2019. Modulation of brain serotonin by benzyl butyl phthalate in Fundulus heteroclitus (mummichog). Ecotoxicology. 28(9):1038–45. [DOI] [PubMed] [Google Scholar]
- de Graaf R, Kessler RC, Fayyad J, ten Have M, Alonso J, Angermeyer M, et al. 2008. The prevalence and effects of adult attention-deficit/hyperactivity disorder (ADHD) on the performance of workers: Results from the WHO world mental health survey initiative. Occup Environmen Med 65:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, et al. 2012. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry 51:990–1002.e1002. [DOI] [PubMed] [Google Scholar]
- Emser TS, Johnston BA, Steele JD, Kooij S, Thorell L, Christiansen H. 2018. Assessing ADHD symptoms in children and adults: Evaluating the role of objective measures. Behav Brain Funct 14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu CB, Silva MJ, Calafat AM, et al. 2010. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect 118:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Villanger GD, Nethery RC, Thomsen C, Sakhi AK, Drover SSM, et al. 2018. Prenatal phthalates, maternal thyroid function, and risk of attention-deficit hyperactivity disorder in the Norwegian mother and child cohort. Environ Health Perspect 126:057004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyad J, Sampson NA, Hwang I, Adamowski T, Aguilar-Gaxiola S, Al-Hamzawi A, et al. 2017. The descriptive epidemiology of DSM-IV adult adhd in the World Health Organization world mental health surveys. Atten Defic Hyperact Disord 9:47–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Cantonwine DE, Rivera-Gonzalez LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, et al. 2014. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ Sci Technol 48:7018–7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. 2015. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: A repeated measures analysis. Environ Health Perspect 123:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenberry GZ, Meeker JD, Sanchez BN, Barr DB, Panuwet P, Bellinger D, et al. 2014. Urinary 3,5,6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: Distribution, temporal variability, and relationship with child attention and hyperactivity. Int J Hyg Environ Health 217:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Valvi D, Forns J, Casas M, Martinez D, Julvez J, et al. 2015. Prenatal exposure to phthalates and neuropsychological development during childhood. Int J Hyg Environ Health 218:550–558. [DOI] [PubMed] [Google Scholar]
- Golestanzadeh M, Riahi R, Kelishadi R. 2019. Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: A systematic review and meta-analysis. Environ Sci Pollut Res Int 26:35670–35686. [DOI] [PubMed] [Google Scholar]
- Hall CL, Valentine AZ, Groom MJ, Walker GM, Sayal K, Daley D, et al. 2016. The clinical utility of the continuous performance test and objective measures of activity for diagnosing and monitoring aADHD in children: A systematic review. Eur Child Adolesc Psychiatry 25:677–699. [DOI] [PubMed] [Google Scholar]
- Hatcher KM, Willing J, Chiang C, Rattan S, Flaws JA, Mahoney MM. 2019. Exposure to di-(2-ethylhexyl) phthalate transgenerationally alters anxiety-like behavior and amygdala gene expression in adult male and female mice. Physiol Behav. 207:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland N, Huen K, Tran V, Street K, Nguyen B, Bradman A, et al. 2016. Urinary phthalate metabolites and biomarkers of oxidative stress in a mexican-american cohort: Variability in early and late pregnancy. Toxics 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Gray LE Jr. 2008. Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res 108:168–176. [DOI] [PubMed] [Google Scholar]
- Hu D, Wang YX, Chen WJ, Zhang Y, Li HH, Xiong L, et al. 2017. Associations of phthalates exposure with attention deficits hyperactivity disorder: A case-control study among Chinese children. Environ Pollut 229:375–385. [DOI] [PubMed] [Google Scholar]
- Huang HB, Kuo PL, Chang JW, Jaakkola JJK, Liao KW, Huang PC. 2018. Longitudinal assessment of prenatal phthalate exposure on serum and cord thyroid hormones homeostasis during pregnancy - Tainan Birth Cohort Study (TBCS). Sci Total Environ 619–620:1058–1065. [DOI] [PubMed] [Google Scholar]
- Jia X, Harada Y, Tagawa M, Naito H, Hayashi Y, Yetti H, et al. 2015. Prenatal maternal blood triglyceride and fatty acid levels in relation to exposure to di(2-ethylhexyl)phthalate: A cross-sectional study. Environ Health Prev Med 20:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD. 2016. Associations between repeated measures of maternal urinary phthalate metabolites and thyroid hormone parameters during pregnancy. Environ Health Perspect 124:1808–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Kim JW, Shin I, Kim BN. 2018. Interaction of DRD4 methylation and phthalate metabolites affects continuous performance test performance in ADHD. J Atten Disord. 1087054718776466. [DOI] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, et al. 2014. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ Health Perspect 122:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C, et al. 2017. Maternal polycystic ovary syndrome and risk for attention-deficit/hyperactivity disorder in the offspring. Biol Psychiatry 82:651–659. [DOI] [PubMed] [Google Scholar]
- Ku HY, Tsai TL, Wang PL, Su PH, Sun CW, Wang CJ, et al. 2020. Prenatal and childhood phthalate exposure and attention deficit hyperactivity disorder traits in child temperament: A 12-year follow-up birth cohort study. Sci Total Environ 699:134053. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, et al. 2013. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere 93:2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundervold AJ, Stickert M, Hysing M, Sørensen L, Gillberg C, Posserud MB. 2016. Attention deficits in children with combined autism and ADHD: A CPT study. J Atten Disord. 20:599–609. [DOI] [PubMed] [Google Scholar]
- Miranda A, Sousa N. 2018. Maternal hormonal milieu influence on fetal brain development. Brain Behav 8:e00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health (NIMH). 2019. Attention deficit hyperactivity disorder. Available: http://www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml [accessed March 10 2020].
- Park S, Kim BN, Cho SC, Kim Y, Kim JW, Lee JY, et al. 2014. Association between urine phthalate levels and poor attentional performance in children with attention-deficit hyperactivity disorder with evidence of dopamine gene-phthalate interaction. Int J Environ Res Public Health 11:6743–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee JM, Kim JW, Cheong JH, Yun HJ, Hong YC, et al. 2015. Association between phthalates and externalizing behaviors and cortical thickness in children with attention deficit hyperactivity disorder. Psychol Med 45:1601–1612. [DOI] [PubMed] [Google Scholar]
- Perng W, Watkins DJ, Cantoral A, Mercado-Garcia A, Meeker JD, Tellez-Rojo MM, et al. 2017. Exposure to phthalates is associated with lipid profile in peripubertal Mexican youth. Environ Res 154:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng W, Tamayo-Ortiz M, Tang L, Sanchez BN, Cantoral A, Meeker JD, et al. 2019. Early life exposure in Mexico to environmental toxicants (ELEMENT) project. BMJ Open 9:e030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. 2007. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry 164:942–948. [DOI] [PubMed] [Google Scholar]
- Pusceddu MM, Kelly P, Stanton C, Cryan JF, Dinan TG. 2016. N-3 polyunsaturated fatty acids through the lifespan: Implication for psychopathology. Int J Neuropsychopharmacol 19:pyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Eliot MN, Zoeller RT, Hoofnagle AN, Calafat AM, Karagas MR, et al. 2018. Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: The HOME study. Int J Hyg Environ Health 221:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Butts S, Wang C, Barrett E, Nguyen R, Schwartz SM, et al. 2017. Early prenatal phthalate exposure, sex steroid hormones, and newborn birth outcomes. J Clin Endocrinol Metab 102:1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Blawas AM, Gray K, Heindel JJ, Lawler CP. 2015. Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology 156:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodin O 2020. The utility of the CPT in the diagnosis of ADHD in individuals with substance abuse: A systematic review. Eur Addict Res 26:283–294. [DOI] [PubMed] [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P. 2015. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 135:e994–1001. [DOI] [PubMed] [Google Scholar]
- Turgay A, Goodman DW, Asherson P, Lasser RA, Babcock TF, Pucci ML, et al. 2012. Lifespan persistence of ADHD: The life transition model and its application. J Clin Psychiatry 73:192–201. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Chou MC, Chou WJ, Lee MJ, Lee SY, Lin PY, et al. 2017. Potential role of pre- and postnatal testosterone levels in attention-deficit/hyperactivity disorder: Is there a sex difference? Neuropsychiatr Dis Treat 13:1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Xu X, Zhu Q. 2016. Pubertal exposure to di-(2-ethylhexyl) phthalate influences social behavior and dopamine receptor D2 of adult female mice. Chemosphere. 144:1771–9. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, Tellez-Rojo MM, Ferguson KK, Lee JM, Solano-Gonzalez M, Blank-Goldenberg C, et al. 2014. In utero and peripubertal exposure to phthalates and BPA in relation to female sexual maturation. Environ Res 134c:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Fortenberry GZ, Sanchez BN, Barr DB, Panuwet P, Schnaas L, et al. 2016a. Urinary 3-phenoxybenzoic acid (3-pba) levels among pregnant women in Mexico city: Distribution and relationships with child neurodevelopment. Environ Res 147:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Peterson KE, Ferguson KK, Mercado-Garcia A, Tamayo YOM, Cantoral A, et al. 2016b. Relating phthalate and BPA exposure to metabolism in peripubescence: The role of exposure timing, sex, and puberty. J Clin Endocrinol Metab 101:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Sanchez BN, Tellez-Rojo MM, Lee JM, Mercado-Garcia A, Blank-Goldenberg C, et al. 2017a. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ Res 159:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Sanchez BN, Tellez-Rojo MM, Lee JM, Mercado-Garcia A, Blank-Goldenberg C, et al. 2017b. Impact of phthalate and BPA exposure during in utero windows of susceptibility on reproductive hormones and sexual maturation in peripubertal males. Environ health 16:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen HJ, Sie L, Su PH, Chuang CJ, Chen HY, Sun CW, et al. 2017. Prenatal and childhood exposure to phthalate diesters and sex steroid hormones in 2-, 5-, 8-, and 11-year-old children: A pilot study of the Taiwan Maternal and Infant Cohort Study. J Epidemiol 27:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.