Selpercatinib is a RET tyrosine kinase inhibitor (TKI) recently approved for treating RET-altered non-small cell lung cancer and thyroid cancers.1 Secondary RET mutations and MET amplification have been identified as mechanisms of resistance to selpercatinib.2–5 However, other mechanisms of selpercatinib resistance may exist.
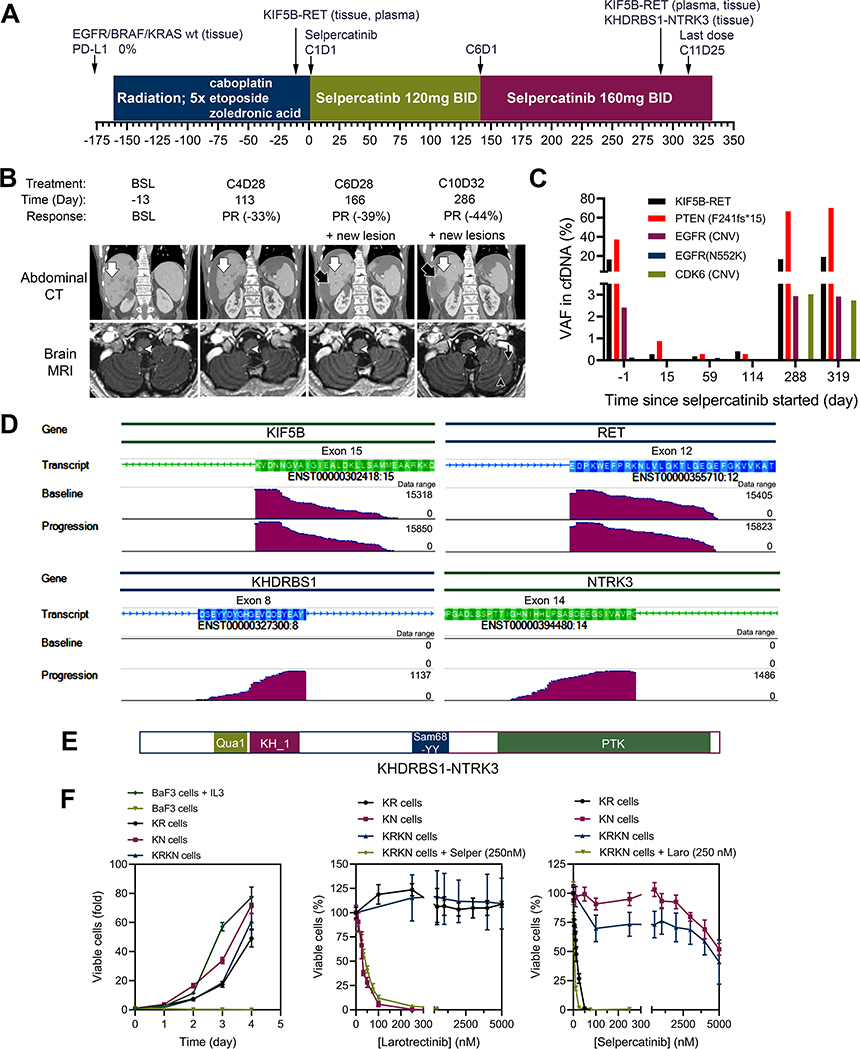
A 62-year-old man with high-grade neuroendocrine carcinoma of thoracic origin developed skin, liver, and intracranial metastases (Supplementary Information, Supplementary Figure S1). He received whole brain radiation, and chemotherapy with carboplatin and etoposide (Figure 1A). Post 5 cycles evaluation showed disease progression (Figure 1B, Supplementary Figure S2). Cell-free DNA (cfDNA) analysis (Guardant) revealed KIF5B-RET fusion (variant allele frequency [VAF] 8.4%) and PTEN F241fs mutation (VAF 17.8%). He was then enrolled on the selpercatinib LIBRETTO-001 trial (NCT03157128) starting at 120 mg orally twice daily. Within 2 weeks of starting treatment he showed an overall improved performance status and the serum bilirubin was normalized. At Cycle 4, CT imaging indicated a confirmed partial response per RECIST V1.1 (39% tumor reduction, Figure 1B). His dose of selpercatinib was increased to 160 mg orally twice daily, with subsequent deepening of tumor response to 44% tumor reduction. After 10 months of treatment, CT imaging showed increased liver lesions and MRI revealed innumerable new brain metastases (Figure 1B), the patient’s clinical performance status worsened and he developed rapidly rising hyperbilirubinemia, and the patient and family elected to hospice care.
Figure 1.
Treatment history and responses to selpercatinib. (A) Treatment history of a patient with metastatic high-grade neuroendocrine carcinoma of thoracic origin. (B) Serial coronal reformations from a contrast-enhanced abdominal CT (top row) and axial images from contrast-enhanced brain MRI (bottom row) in a 62-year-old man. There are innumerable hepatic and brain lesions, which decrease in size on therapy (examples indicated by white arrow in the liver and white arrowhead in the medulla oblongata). New lesions appear in the liver during cycle 6 (black arrow) and in the brain in cycle 7 (black arrowhead and black thin arrow) and continue to grow on follow-up scans. (C) Levels of variant alleles detected in cfDNA during the course of selpercatinib treatment. (D) Genome browser images illustrating alignments of fusion supporting sequencing reads of cDNA from baseline and progression tissue samples. (E) Schematic presentation of the KHDRBS1-NTRK3 chimeric protein. (F) The parental BaF3 cells required interleukin-3 (IL3) whereas BaF3/KIF5B-RET (KR), BaF3/KDHRBS1-NTRK3 (KN), and BaF3/KIF5B-RET/KDHRBS1-NTRK3 (KRKN) were IL3-independent (left). Cell viability assay of KR, KN, and KRKN cells treated with selpercatinib (Selper), larotrectinib (Laro), or combination of the two drugs.
cfDNA analysis from blood samples collected serially during treatment revealed a KIF5B-RET fusion (16.3% VAF), PTEN F241fs*15 mutation (37.2% VAF), and EGFR copy number variation (CNV: 2.4) at screening (Figure 1C, Supplementary Table S1). During treatment with selpercatinib, these variant alleles decreased by ~98% in two weeks, and remained low for 4 months. At the time of disease progression, all three co-variants again increased to higher levels. No RET kinase domain mutations were detected in cfDNA at any time point.
NGS analysis (TST-170, Illumina, Inc.) was performed on the pre-treatment tumor biopsy (skin) and a resistant tumor biopsy (liver). A KHDRBS1-NTRK3 fusion (K8;N14) and the KIF5B-RET fusion (K15;R12) were detected in the resistant tumor sample, while only the KIF5B-RET was detected in the pre-treatment sample (Figure 1D, E; Supplementary Figure S3). The KHDRBS1-NTRK3 fusion was not detected in cfDNA at any time point because the Guardant assay was not designed to detect NTRK3 fusions.
In cell cultures, KHDRBS1-NTRK3 transformed BaF3 cells into interleukin-3 independence, which was inhibited by the Trk kinase inhibitor larotrectinib but not by selpercatinib, indicating that KHDRBS1-NTRK3 is an oncogenic tyrosine kinase (Figure 1F, Supplementary Figure S4). As expected, KIF5B-RET-transformed BaF3 cells were sensitive to selpercatinib but not to larotrectinib. Importantly, BaF3 cells co-expressing KIF5B-RET and KHDRBS1-NTRK3 were resistant to selpercatinib. Co-treatment of these cells with selpercatinib and larotrectinib suppressed active ERK1/2 and AKT, and induced apoptosis of these cells (Figure 1F, Supplementary Figure 4).
Together, these results highlight the importance of real-time incorporation of molecular profiling results into clinical care, and identify a novel, targetable oncogenic fusion as a new resistance mechanism in KIF5B-RET fusion cancer.
Supplementary Material
Acknowledgments
Funding
The clinical trial was funded by Loxo Oncology, a subsidiary of Eli Lilly. This study was supported by National Institutes of Health grant R01CA242845 (to B.H.M.M., V.S. and J.W.), the Oklahoma Tobacco Settlement Endowment Trust (to the Stephenson Cancer Center), the Cancer Prevention and Research Institute of Texas (RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy, 1U01 CA180964, NCATS Grant UL1 TR000371 (Center for Clinical and Translational Sciences), and the MD Anderson Cancer Center Support Grant (P30 CA016672). The shared resources at the University of Oklahoma Health Sciences Center were supported by NIH/National Institute of General Medical Sciences grant P20GM103639, and the NIH/National Cancer Institute Grant P30CA225520.
Disclosure
V. Subbiah: Research funding/ Grant support for clinical trials: Roche/ Genentech, Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint medicines, Loxo oncology, Medimmune, Altum, Dragonfly therapeutics, Takeda and, National Comprehensive Cancer Network, NCI-CTEP and UT MD Anderson Cancer Center, Turning point therapeutics, Boston Pharmaceuticals; Travel: Novartis, Pharmamar, ASCO, ESMO, Helsinn, Incyte; Consultancy/ Advisory board: Helsinn, LOXO Oncology/Eli Lilly, R-Pharma US, INCYTE, QED pharma, Medimmune, Novartis. Other: Medscape.
S. M. Rothenberg: past employee of and stock options from Loxo Oncology; current employee of and stock options from Pfizer, Inc. K. Ebata were employees of Loxo Oncology at Lilly, a subsidiary of Eli Lilly.
All remaining authors have declared no conflicts of interest.
REFERENCES
- 1.Drilon A, Oxnard GR, Tan DSW et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon BJ, Tan L, Lin JJ et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J Thorac Oncol 2020;15:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin JJ, Liu SV, McCoach CE et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann Oncol 2020;31:1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subbiah V, Shen T, Terzyan SS et al. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann Oncol 2021;32:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen EY, Johnson ML, Clifford SE et al. Overcoming MET-Dependent Resistance to Selective RET Inhibition in Patients with RET Fusion-Positive Lung Cancer by Combining Selpercatinib with Crizotinib. Clin Cancer Res 2021;27:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.