Abstract
Weight suppression (WS) predicts future weight gain and increases in eating disorder symptoms in community and clinical samples but has received minimal attention in obesity and eating disorder prevention programs. In a sample of emerging adults (N = 364) in a randomized controlled trial evaluating two obesity and eating disorder prevention interventions versus a control condition, this study aimed to replicate the findings that WS and its interaction with baseline BMI predict increases in weight and eating disorder symptoms and test a novel hypothesis that WS would moderate the effects of the interventions on change in weight and eating disorder symptoms. Participants completed assessments at baseline, post-intervention, 6-, 12-, and 24-months. WS was calculated as the difference between highest lifetime weight and baseline weight. WS interacted with baseline BMI to predict greater weight gain over 24-months, such that those with high WS and lower baseline BMI gained weight most rapidly. WS did not predict eating disorder symptom change and did not moderate the effects of the prevention programs. Given that individuals with WS are at increased risk for weight gain, expressly targeting this high-risk population with evidence-based obesity prevention programs may be useful.
Keywords: Weight Suppression, Weight Gain, Obesity, Eating Disorders, Prevention
Weight suppression (WS), the difference between highest lifetime weight and current weight (Lowe, 1984), is associated with future increases in weight and eating disorder symptoms in community samples and clinical samples of patient with eating disorders. Being at a reduced body weight (i.e., weight suppressed) is theorized to produce slowed metabolic processes and biological pressure to regain lost weight (Carter et al., 2008; Herzog et al., 2010; Lowe et al., 2006; Rosenbaum & Leibel, 2010; Stice et al., 2011), increasing risk for subsequent weight gain. Psychological processes (e.g., increased reward-value of palatable foods) may work in tandem with biological factors to increase drive towards weight gain (Bodell & Keel, 2015; Keel et al., 2017). Research has found that WS predicts future weight gain in college students (Lowe et al., 2006; Stice et al., 2011), and samples of patients with bulimia nervosa (BN) and/or anorexia nervosa (AN) (Berner et al., 2013; Carter et al., 2008; Herzog et al., 2010; Miotto et al., 2020; Piers et al., 2019; Wildes & Marcus, 2012), though this effect did not replicate in a combined sample of patients with BN and binge eating disorder (BED) (Zunker et al., 2011). Although research on the effect of WS in weight management programs is limited, a recent study found that WS was associated with attenuated weight loss among middle-aged adults participating in a behavioral weight loss program (Call et al., 2019).
Subsequent weight gain following WS may be distressing and lead to greater effort to maintain WS through restrictive eating and compensatory behaviors. This interplay may explain the relation of WS to eating disorder symptoms reported in several studies. In college based samples, WS prospectively predicted the maintenance of bulimic symptoms at 10-year (Keel & Heatherton, 2010) and 20-year follow-up (Bodell et al., 2017). Among young women with body image concerns, WS predicted future onset of AN, BN, purging disorder, and “any eating disorder,” but not BED over three years (Stice et al., 2020). In the shorter term, WS predicted maintenance of bulimic symptoms after 18 weeks of cognitive behavioral therapy in women with BN (Butryn et al., 2006). Cross-sectional studies have also reported relations between WS and binge eating, restrictive eating, and purging behaviors in patients with BN and non-clinical samples (Burnette et al., 2017; Butryn et al., 2011; Goodman et al., 2018; Lavender et al., 2015; Lowe et al., 2007). Although WS is associated with higher levels of dietary restraint, which also may increase risk for eating pathology and weight gain, studies have demonstrated that the predictive effects of WS remain significant even after controlling for dietary restraint, highlighting the unique contribution of WS to risk for weight gain and eating pathology (Butryn et al., 2011; Stice et al., 2020).
Emerging research in patients with eating disorders also suggests that baseline body mass index (BMI) may moderate the relation between WS and weight gain or eating disorder symptoms (Gorrell et al., 2019). Among patients with AN receiving residential treatment, individuals with high WS and higher baseline BMI had greater eating disorder symptoms at discharge, and patients with high WS and lower baseline BMI had greater BMIs at discharge (Berner et al., 2013). Among patients with AN followed for 12 months after intensive treatment, WS predicted greater eating disorder symptoms when discharge BMI was higher (Bodell et al., 2016). The interaction of BMI and WS necessitates exploration in non-eating disorder samples.
Although WS has been studied in the context of eating disorder treatment, its relation to outcomes in prevention programs for eating disorders and/or obesity has received little attention. To date, only one study has examined WS in a weight gain prevention program (Wing et al., 2020), and none have examined WS in eating disorder prevention programs. In a secondary analysis of the Study of Novel Approaches to Weight Gain Prevention (SNAP) trial for young adults, greater baseline WS was associated with greater weight gain over a 3-year follow-up when all three study conditions were examined together (two intervention conditions and a self-guided control condition) (Wing et al., 2020). Notably, this study did not examine if WS moderated the effects of the obesity prevention interventions. Examining moderation effects in prevention programs could help identify whether certain interventions prevent future weight gain among those with high WS relative to those with low WS, in comparison to alternative interventions and minimal intervention control conditions. This understanding may improve the efficacy of future interventions, in part by informing inclusion and exclusion criteria.
The present study sought to replicate and extend prior research conducted in community and clinical samples by elucidating the role of WS in emerging adults enrolled in a trial comparing two obesity and eating disorder prevention programs to an educational control (Stice et al., 2018). Specifically, we aimed to examine whether: 1) baseline WS predicts greater weight gain and increases in eating disorder symptoms over 24 months; 2) baseline BMI moderates these effects; and 3) WS moderates the effects of prevention programs on change in weight and eating disorder symptoms.
Method
Participants and Procedures
This study is a secondary analysis of an experimental therapeutics randomized controlled trial comparing the efficacy of Healthy Weight, Project Health, and an educational video control in young men and women at risk for overweight/obesity and eating disorder onset. Participants (N=364) were between 17 and 23 years of age, with a BMI between 18.0 and 30.0 kg/m2 and self-reported weight concerns. The lower threshold of BMI inclusion was set at 18.0 rather than 18.5 kg/m2 (i.e., the typical lower limit for “normal weight”) because the interventions targeted individuals at high risk for either eating disorder or obesity onset and low BMI is one of the few established factors to increase risk for AN onset (Stice, Gau, Rohde, & Shaw, 2017); however, only one participant entered the study with a BMI < 18.5 kg/m2. Exclusion criteria included a current diagnosis of DSM-IV AN, BN, or BED. Measures were completed at baseline, post-intervention (6-weeks), 6-months, 12-months, and 24-months. Informed consent was obtained from all participants, and from parents in the case of minors. Study procedures were approved by the Institutional Review Board. Additional details regarding study design, recruitment, and intervention for the clinical trial are reported elsewhere (Stice et al., 2018). Briefly, results of the clinical trial demonstrated that Project Health was superior to the other conditions at preventing weight gain, and both Project Health and Healthy Weight were superior to the control condition at preventing eating disorder symptoms (Stice et al., 2018).
2.2. Measures
2.2.1. Weight and height
Weight and height were measured by research staff at all assessments using a digital scale accurate to 0.1 kg and stadiometers accurate to the nearest millimeter.
2.2.2. Weight suppression
WS was calculated as highest lifetime weight (self-reported at base line) minus baseline weight. Prior research suggests that recall of past weights is accurate (i.e., correlation between actual weight and recalled weight: r = 0.87 – 0.95) (Casey et al., 1991). Participants who weighed more at baseline than their highest past reported weight were coded as having a WS score of 0.
2.2.3. Dietary restraint
The Dutch Restrained Eating Scale (DRES (van Strien, Frijters, Bergers, et al., 1986)) assessed frequency of restrained eating behaviors on a 5-point Likert scale. The DRES has demonstrated acceptable in ternal consistency (α = 0.95) and criterion validity, and correlated with self-reported food intake, but not objectively measured caloric intake (Stice et al., 2010; van Strien, Frijters, Bergers, et al., 1986; van Strien, Frijters, van Staveren, et al., 1986).
Eating Disorder Symptoms
DSM-5 symptoms of AN, BN, and BED over the prior month were assessed with the semi-structured Eating Disorder Diagnostic Interview (EDDI; (Stice et al., 2008)). A composite score, capturing overall eating disorder pathology, and two subscale scores capturing severity of binge eating and compensatory behaviors were created. The EDDI showed good internal consistency (α = .70), inter-rater agreement (r = .90), and 1-week test-retest reliability (r = .93) in the original clinical trial (Stice et al., 2018).
Statistical Analyses
Analyses were conducted in RStudio and SPSS v. 26. At baseline, there were no missing weight, BMI, eating disorder symptom, or WS data. Across follow-up timepoints, 11.7% of weight data and 3.8% of eating disorder symptom data were missing. Multiple imputation, which is considered best-practice for missing data in weight-related research, was conducted in SPSS using MCMC algorithms known as chained equations imputation (Batterham et al., 2013). All variables included in the models were entered as predictors in the multiple imputation process to decrease bias (Van Buuren, 2018). Although there is a risk of bias when using multiple imputation for hypothesized interaction terms in multi-level models, this was not an issue as there were no missing data for the baseline variables included in interaction terms (Enders et al., 2020). Imputed values were reviewed to ensure that they fell within plausible ranges. Analyses were run in each of 20 imputed dataset and pooled using Rubin’s rule in SPSS or R using the miceadds package (Robitzsch et al., 2021). Assumptions of normality were considered; WS, overall eating disorder symptoms, binge eating severity, and compensatory behaviors severity were normalized with a square-root transformation. To identify potential confounding variables, the relation of WS to baseline characteristics was examined using Pearson bivariate correlations and independent samples T-tests. Subsequent analyses were run with and without demographic covariates (age, sex, race, ethnicity) that were related to WS; because patterns of findings did not differ, models without demographic covariates are reported.
To examine whether WS predicted change in weight, overall eating disorder symptoms, and severity of binge eating and compensatory behaviors over 24-month follow-up, linear mixed effects models were conducted. Random intercepts, accounting for individual deviations from group averages, were included because they improved model fit based on AIC and BIC. Models controlled for baseline weight and eating disorder symptoms. Time (months since baseline), baseline BMI, condition, and WS were entered as fixed effects. Categorical variables were dummy coded, time was coded such that baseline=0, and other continuous variables were centered. To test if each variable predicted weight or eating disorder symptom change over time, interaction effects of time by each predictor variable were included. To test if baseline BMI moderated the relation of WS to change in weight or eating disorder symptoms, the three-way interaction of baseline BMI*WS*time was included. To test if WS moderated the relation of condition to weight or eating disorder symptom change, separate linear mixed effects models with three-way interactions of time*condition*WS were tested. Two contrasts (Control vs. Healthy Weight, Control vs. Project Health) were included in the model; to examine the third contrast (Healthy Weight vs. Project Health), the dummy code was rotated and the model was re-run with the new contrasts (Healthy Weight vs. Project Health, Healthy Weight vs. Control). Models in which WS significantly predicted weight and eating disorder symptoms were re-run with dietary restraint as a covariate to identify if observed effects were independent of any effect of dietary restraint.
Results
Relation of WS to Baseline Characteristics
Mean baseline WS was 4.34 kg (SD=5.77). Fifty-five participants (15.1%) reported no WS (i.e., WS ≤ 0) at baseline. Table 1 displays participant characteristics and their relation to WS. At baseline, WS was positively correlated with eating disorder symptoms (r(362)=0.12, p=.02) and dietary restraint (r(362)=0.15, p=.004), but not BMI (r(362)=−0.06, p=.23) or weight (r(362)=0.01, p=.83).
Table 1.
Relations between weight suppression and participant characteristics.
| n (%) | WS, kg M (SD) | F or t | p | d | |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 261 (71.1%) | 3.60 (3.68) | 2.23 | 0.03 | 0.23 |
| Male | 103 (28.3%) | 6.20 (8.90) | |||
| Race | |||||
| Non-White | 76 (20.9%) | 4.25 (4.75) | 0.14 | 0.89 | 0.01 |
| White | 283 (77.7%) | 4.35 (6.03) | |||
| Ethnicity | |||||
| Hispanic | 40 (11.0%) | 6.94 (10.96) | 3.25 | 0.001 | 0.34 |
| Non-Hispanic | 320 (87.9%) | 3.91 (4.37) | |||
| Conditiona | |||||
| Project Health | 119 (32.7%) | 3.12 (3.44) | 5.21 | 0.006 | 0.34 |
| Healthy Weight | 122 (33.5%) | 5.33 (8.05) | |||
| Control | 123 (33.8%) | 4.54 (4.59) | |||
| M (SD) | Correlation with WS (r) | p-value | |||
| Age (years) | 19.10 (1.20) | 0.09 | 0.09 | ||
Note. WS = Weight suppression; WS was square-root transformed for analyses, but the untransformed M and SD are presented in this table. Race was collapsed into non-White and White due to small n’s for other individual racial categories.
Project Health < Healthy Weight (p = 0.01) and controls (p = 0.02).
Predictive Effect of WS on Weight Change
Table 2 displays results of the linear mixed effects models examining change in weight over 24 months. The significant time*condition interaction confirms results from the original clinical trial demonstrating that Project Health produced a slower rate of weight gain than the control group (Stice et al., 2018). The significant time*WS interaction suggests that participants with greater baseline WS experienced a steeper rate of weight gain over 24 months. This interaction is qualified by the significant time*baseline BMI*WS interaction, such that individuals with high WS experienced a steeper rate of weight gain when baseline BMI was lower (see Figure 1). A similar pattern of findings was observed when controlling for dietary restraint, which was not a significant predictor of weight change (time*dietary restraint: t(1448)=.19, p=0.85; time*WS: t(1448)=1.88, p=0.06; time*baseline BMI*WS: t(1448)=−3.89, p<0.001).
Table 2.
Results of linear mixed effects models predicting change in weight and eating disorder symptoms over 24-months.
| |
Weight (kg) |
Eating Disorder Symptoms (Composite Score) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed Effect | Estimate | SE | 95% CI | t | P | Estimate | SE | 95% CI | t | p |
| (Intercept) | 66.03 | 0.64 | 64.77–67.30 | 102.38 | <0.001 | 1.56 | 0.03 | 1.51–1.62 | 55.78 | <0.001 |
| Time (Months) | 0.11 | 0.02 | 0.08–0.14 | 7.42 | <0.001 | 0.00 | 0.00 | −0.01–0.00 | −3.16 | <0.001 |
| Condition | ||||||||||
| Healthy Weight vs. Control | 1.47 | 0.91 | −0.32–3.26 | 1.61 | 0.11 | −0.08 | 0.04 | −0.16–−0.01 | −2.12 | 0.03 |
| Project Health vs. Control | 0.52 | 0.93 | −1.30–2.34 | 0.56 | 0.58 | −0.02 | 0.04 | −0.1–0.06 | −0.55 | 0.59 |
| Baseline BMI | 2.82 | 0.14 | 2.54–3.10 | 19.75 | <0.001 | 0.02 | 0.01 | 0.01–0.03 | 3.13 | <0.001 |
| WS | 0.45 | 0.34 | −0.21–1.11 | 1.34 | 0.18 | 0.03 | 0.01 | 0.00–0.06 | 2.28 | 0.02 |
| Time*Condition | ||||||||||
| Healthy Weight vs. Control | 0 | 0.02 | −0.04–0.04 | −0.03 | 0.97 | 0.00 | 0.00 | 0.00–0.00 | −0.39 | 0.7 |
| Project Health vs. Control | −0.05 | 0.02 | −0.09–−0.01 | −2.25 | 0.02 | 0.00 | 0.00 | 0.00–0.00 | −0.04 | 0.97 |
| Time*Baseline BMI | 0 | 0 | −0.01–0.00 | −1.06 | 0.29 | 0.00 | 0.00 | 0.00–0.00 | −0.47 | 0.64 |
| Time*WS | 0.02 | 0.01 | 0.00–0.03 | 1.95 | 0.05 | 0.00 | 0.00 | 0.00–0.00 | −0.06 | 0.95 |
| Baseline BMI*WS | 0.11 | 0.12 | −0.14–0.35 | 0.85 | 0.39 | 0.00 | 0.01 | −0.01–0.01 | 0.48 | 0.63 |
| Time*Baseline BMI*WS | −0.01 | 0 | −0.02–−0.01 | −3.89 | <0.001 | 0.00 | 0.00 | 00–0.00 | −1.61 | 0.11 |
Note. WS = weight suppression; EDDI and WS scores were square-root transformed; time = 0 is baseline; other continuous variables were centered.
Fig 1.
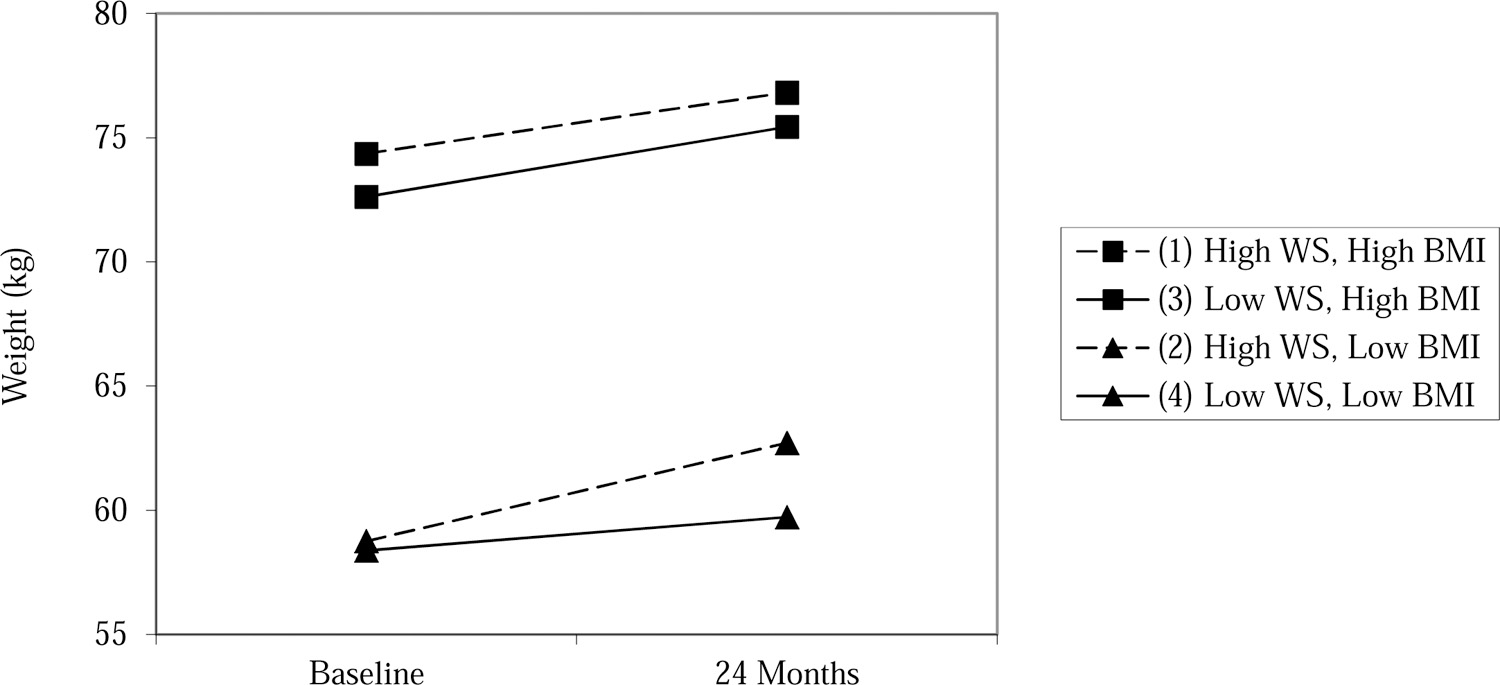
Weight change over 24 months by baseline WS and BMI.
Note. WS = weight suppression (square-root transformed); Low values are 1 SD below the mean: WS: 0.55 kg, BMI: 20.82 kg/m2; High values are 1 SD above the mean: WS: 2.89 kg, BMI: 26.11 kg/m2
Predictive Effect of WS on Eating Disorder Symptom Change
Table 2 also displays results of the linear mixed effects models examining eating disorder symptom change over 24-months. The non-significant time*WS and time*baseline BMI*WS interaction demonstrates that WS did not predict eating disorder symptom change. Results were also nonsignificant when the outcome was severity of binge eating (time*WS: t(1456)=1.12, p=0.26; time*baseline BMI*WS: t(1456)=−1.35, p=0.18) and compensatory behaviors (time*WS: t(1456)=−1.19, p=0.23; time*baseline BMI*WS: t(1456)=−0.58, p=0.56).
Moderating Effect of WS
Three-way interactions of condition*WS*time revealed that WS did not significantly moderate the relation of condition on change in weight (Project Health vs. Control: t(1502) = - 0.05, p = 0.92; Healthy Weight vs. Control: t(1502) = 0.45, p = 0.65; Project Health vs. Healthy Weight: t(1502) = −.52, p = 0.61), overall eating disorder symptoms (Project Health vs. Control: t(1787) = 0.06, p = 0.95; Healthy Weight vs. Control: t(1787) = −0.84, p = 0.40; Project Health vs. Healthy Weight: t(1787) = 0.88, p = 0.38), binge eating severity (Project Health vs. Control: t(1818) = 0.27, p = 0.79; Healthy Weight vs. Control: t(1818) = 0.60, p = 0.55; Project Health vs. Healthy Weight: t(1818) =0.29, p = 0.77), or compensatory behavior severity (Project Health vs. Control: t(1745) = −1.41, p = 0.16; Healthy Weight vs. Control: t(1745) = −0.62, p = 0.53; Project Health vs. Healthy Weight: t(1745) = −0.95, p = 0.34).
Discussion
In a sample of emerging adults enrolled in an obesity and eating disorder prevention trial, this study aimed to replicate the findings that both WS and its interaction with baseline BMI predict increases in weight and eating disorder symptoms, and to extend prior work by examining if WS moderated the effects of the prevention programs on weight and eating disorder symptoms. Although WS has been studied extensively in community samples and individuals with eating disorders, only one prior study has examined WS in an obesity prevention program, and none have examined it in eating disorder prevention programs. Additionally, this is the first study to our knowledge to examine WS as a moderator of intervention outcomes.
Consistent with one other study that examined WS in a weight gain prevention program (Wing et al., 2020), higher baseline WS in the present study predicted a steeper rate of weight gain over 24 months, although this effect was attenuated when controlling for dietary restraint. The present study adds nuance by demonstrating that individuals with high WS and lower baseline BMIs may be at particular risk for weight gain, whereas WS may pose a lesser risk for weight gain among individuals with higher baseline BMIs. Prior research has examined the interaction of WS and baseline BMI in eating disorder samples, but this is the first to our knowledge to examine it in a non-eating disorder sample. The present study’s finding is consistent with a study of individuals with AN receiving residential treatment, for whom the relation of WS to higher discharge BMI was strongest for those with low baseline BMI (Berner et al., 2013), and another study that observed this relation in a sample of patients with AN and another sample of patients with BN using relative WS (WS as a percentage of highest lifetime weight) (Piers et al., 2019). A lower BMI may exacerbate the biobehavioral changes that are theorized to drive weight gain in weight suppressed individuals. Among patients with eating disorders, higher WS correlated with lower levels of leptin, a hormone involved in energy-homeostasis that, when reduced, increases appetitive drive and decreases metabolism (Havel, 2000). Leptin is produced primarily by adipose tissue and is greater in individuals with higher BMIs (Klok et al., 2007). Individuals with high WS and low BMIs may have particularly low leptin levels, which may lead to behavioral and biological changes that increase weight.
Neither WS nor its interaction with baseline BMI predicted change in overall eating disorder symptoms or severity of binge eating or compensatory behaviors. While several studies have found a relation between WS and increases in eating pathology (Bodell et al., 2017; Butryn et al., 2006; Goodman et al., 2018; Keel & Heatherton, 2010; Lavender et al., 2015; Lowe et al., 2007), others have not. In a study of patients with BN, WS predicted weight gain but not binge eating at end of cognitive behavioral therapy (Carter et al., 2008). In a sample of college women, WS predicted increased BMI but not bulimic symptoms at 6-months (Stice et al., 2011). One explanation for these null findings is that average baseline WS was relatively low in each sample (Carter et al., 2008: 7.1 kg; Stice et al., 2011: 3.4 kg; current study: 4.3 kg). It is possible that only very high WS has a meaningful impact on change in eating disorder symptoms. In two studies that found a relation between WS and eating pathology after treatment for AN, mean WS ranged from 15 to 17 kg (Berner et al., 2013; Wildes & Marcus, 2012), levels much higher than in the present study. It is also possible that because this study’s sample had a restricted range of eating disorder symptoms, in part because individuals with a current eating disorder were excluded from the sample, there was not sufficient variability in eating disorder symptoms to observe an effect of WS.
WS did not moderate the effect of intervention on weight or eating disorder symptom change, indicating that the prevention programs were no more or less effective for individuals with high or low WS. Of note, WS differed by condition, which may have limited the ability to detect an interaction. Future research should identify key mechanisms by which WS impacts weight gain so that interventions can target these factors. Two studies in eating disorder samples suggest that individuals with higher WS find food to be more rewarding, while another study found that the drive for thinness mediated the relation between WS and eating disorder symptoms (Bodell et al., 2017; Bodell & Keel, 2015; Keel et al., 2019). These factors may be key intervention targets for individuals with high WS.
This study had many strengths, including a relatively large sample and two-year follow-up. However, several limitations, some of which are due to the fact that this study utilized previously collected data, should be noted. The sample was largely female and non-Hispanic White, similar to prior WS research (Gorrell et al., 2019; Jenkins et al., 2018). Additional research on WS in men and minoritized groups is needed. Because the present study was focused on prevention, individuals with eating disorder diagnoses at baseline were excluded, which likely restricted the range of eating pathology at later time points and may have interfered with detecting an effect of WS on eating disorder symptom change. Additionally, this study did not assess for eating disorder diagnoses that were newly recognized in DSM-5, such as night eating syndrome, limiting generalizability to newer diagnoses. Mean WS was also relatively low, with 15% of the sample reporting WS=0, which may have impacted the ability to find an effect of WS on outcomes. Finally, the measures used in this study may have introduced bias. As is the case in other WS research, highest lifetime weight was self-reported. The validity of the dietary restraint scale we used, which was included as a covariate in some analyses, along with all other self-report measures of dietary restraint is also questionable, given that prior research finds that scores on these scales do not correlate with objectively measured calorie intake (Stice et al., 2010). Despite limitations, the results of the present study suggest that future research should identify mechanisms through which WS predicts weight gain and explore possible biobehavioral mechanisms underlying the interactions between WS and BMI. Further, results suggest that it might be useful to target weight suppressed individuals with selective evidence-based weight gain prevention programs.
Highlights.
Weight suppression predicted 24-month weight gain in an obesity/eating disorder prevention trial
Weight gain was greatest in those with high weight suppression and lower initial BMI
Weight suppression did not predict eating disorder symptom change over 24 months
Weight suppression did not moderate the effects of the prevention programs
Funding and acknowledgments:
This study was supported by the National Institute of Child Health and Human Development [HD071900]. We thank the staff at Oregon Research Institute who supported the original clinical trial, as well as the participants who took part in the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.Gov Registration: NCT01680224
Declarations of interest: The authors declare no conflicts of interest.
References
- Batterham MJ, Tapsell LC, & Charlton KE (2013). Analyzing weight loss intervention studies with missing data: which methods should be used? Nutrition, 29(7–8), 1024–1029. 10.1016/j.nut.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Berner LA, Shaw JA, Witt AA, & Lowe MR (2013). The relation of weight suppression and body mass index to symptomatology and treatment response in anorexia nervosa. Journal of Abnormal Psychology, 122(3), 694–708. 10.1037/a0033930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodell LP, Brown TA, & Keel PK (2017). Weight suppression predicts bulimic symptoms at 20-year follow-up: The mediating role of drive for thinness. Journal of Abnormal Psychology, 126(1), 32–37. 10.1037/abn0000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodell LP, & Keel PK (2015). Weight suppression in bulimia nervosa: Associations with biology and behavior. Journal of Abnormal Psychology, 124(4), 994–1002. 10.1037/abn0000077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodell LP, Racine SE, & Wildes JE (2016). Examining weight suppression as a predictor of eating disorder symptom trajectories in anorexia nervosa. International Journal of Eating Disorders, 49(8), 753–763. 10.1002/eat.22545 [DOI] [PubMed] [Google Scholar]
- Burnette CB, Simpson CC, & Mazzeo SE (2017). Exploring gender differences in the link between weight suppression and eating pathology. Eating Behaviors, 27, 17–22. 10.1016/j.eatbeh.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Butryn ML, Juarascio A, & Lowe MR (2011). The relation of weight suppression and BMI to bulimic symptoms. International Journal of Eating Disorders, 44(7), 612–617. 10.1002/eat.20881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn ML, Lowe MR, Safer DL, & Agras WS (2006). Weight suppression is a robust predictor of outcome in the cognitive-behavioral treatment of bulimia nervosa. Journal of Abnormal Psychology, 115(1), 62–67. 10.1037/0021-843X.115.1.62 [DOI] [PubMed] [Google Scholar]
- Call CC, Piers AD, Wyckoff EP, Lowe MR, Forman EM, & Butryn ML (2019). The relationship of weight suppression to treatment outcomes during behavioral weight loss. Journal of Behavioral Medicine, 42(2), 365–375. 10.1007/s10865-018-9978-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter FA, McIntosh VV, Joyce PR, & Bulik CM (2008). Weight suppression predicts weight gain over treatment but not treatment completion or outcome in bulimia nervosa. Journal of Abnormal Psychology, 117(4), 936–940. 10.1037/a0013942 [DOI] [PubMed] [Google Scholar]
- Casey VA, Dwyer JT, Berkey CS, Coleman KA, Gardner J, & Valadian I (1991). Long-term memory of body weight and past weight satisfaction: a longitudinal follow-up study. American Journal of Clinical Nutrition, 53(6), 1493–1498. 10.1093/ajcn/53.6.1493 [DOI] [PubMed] [Google Scholar]
- Enders CK, Du H, & Keller BT (2020). A model-based imputation procedure for multilevel regression models with random coefficients, interaction effects, and nonlinear terms. Psychological Methods, 25(1), 88–112. 10.1037/met0000228 [DOI] [PubMed] [Google Scholar]
- Goodman EL, Baker JH, Peat CM, Yilmaz Z, Bulik CM, & Watson HJ (2018). Weight suppression and weight elevation are associated with eating disorder symptomatology in women age 50 and older: Results of the gender and body image study. International Journal of Eating Disorders, 51(8), 835–841. 10.1002/eat.22869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrell S, Reilly EE, Schaumberg K, Anderson LM, & Donahue JM (2019). Weight suppression and its relation to eating disorder and weight outcomes: a narrative review. Eating Disorders, 27(1), 52–81. 10.1080/10640266.2018.1499297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel PJ (2000). Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proceedings of the Nutrition Society, 59(3), 359–371. 10.1017/s0029665100000410 [DOI] [PubMed] [Google Scholar]
- Herzog DB, Thomas JG, Kass AE, Eddy KT, Franko DL, & Lowe MR (2010). Weight suppression predicts weight change over 5 years in bulimia nervosa. Psychiatry Research, 177(3), 330–334. 10.1016/j.psychres.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins PE, Lebow J, & Rienecke RD (2018). Weight suppression as a predictor variable in the treatment of eating disorders: A systematic review. Journal of Psychiatric and Mental Health Nursing, 25(5–6), 297–306. 10.1111/jpm.12462 [DOI] [PubMed] [Google Scholar]
- Keel PK, Bodell LP, Forney KJ, Appelbaum J, & Williams D (2019). Examining weight suppression as a transdiagnostic factor influencing illness trajectory in bulimic eating disorders. Physiology and Behavior, 208, 112565. 10.1016/j.physbeh.2019.112565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel PK, Bodell LP, Haedt-Matt AA, Williams DL, & Appelbaum J (2017). Weight suppression and bulimic syndrome maintenance: Preliminary findings for the mediating role of leptin. International Journal of Eating Disorders, 50(12), 1432–1436. 10.1002/eat.22788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel PK, & Heatherton TF (2010). Weight suppression predicts maintenance and onset of bulimic syndromes at 10-year follow-up. Journal of Abnormal Psychology, 119(2), 268–275. 10.1037/a0019190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok MD, Jakobsdottir S, & Drent ML (2007). The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obesity Reviews, 8(1), 21–34. 10.1111/j.1467-789X.2006.00270.x [DOI] [PubMed] [Google Scholar]
- Lavender JM, Shaw JA, Crosby RD, Feig EH, Mitchell JE, Crow SJ, Hill L, Le Grange D, Powers P, & Lowe MR (2015). Associations between weight suppression and dimensions of eating disorder psychopathology in a multisite sample. Journal of Psychiatric Research, 69, 87–93. 10.1016/j.jpsychires.2015.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MR (1984). Dietary concern, weight fluctuation and weight status: Further explorations of the restraint scale. Behaviour Research and Therapy, 22(3), 243–248. 10.1016/0005-7967(84)90004-4 [DOI] [PubMed] [Google Scholar]
- Lowe MR, Annunziato RA, Markowitz JT, Didie E, Bellace DL, Riddell L, Maille C, McKinney S, & Stice E (2006). Multiple types of dieting prospectively predict weight gain during the freshman year of college. Appetite, 47(1), 83–90. 10.1016/j.appet.2006.03.160 [DOI] [PubMed] [Google Scholar]
- Lowe MR, Thomas JG, Safer DL, & Butryn ML (2007). The relationship of weight suppression and dietary restraint to binge eating in bulimia nervosa. International Journal of Eating Disorders, 40(7), 640–644. 10.1002/eat.20405 [DOI] [PubMed] [Google Scholar]
- Miotto G, Chiappini I, Favaro A, Santonastaso P, & Gallicchio D (2020). Assessing the Role of Weight Suppression (WS) and Weight Loss Rate (WLR) in Eating Disorders. European Psychiatry, 41(S1), S71–S72. 10.1016/j.eurpsy.2017.01.230 [DOI] [Google Scholar]
- Piers AD, Espel-Huynh HM, & Lowe MR (2019). The independent and interacting effects of weight suppression and admission body mass index on treatment weight change in patients with anorexia nervosa or bulimia nervosa. International Journal of Eating Disorders, 52(11), 1301–1309. 10.1002/eat.23149 [DOI] [PubMed] [Google Scholar]
- Robitzsch A, Grund S, & Henke T (2021). Miceadds: Some additional multiple imputation functions, especially for mice. In R package (Version 3.11–6) http://CRAN.R-project.org/package=miceadds
- Rosenbaum M, & Leibel RL (2010). Adaptive thermogenesis in humans. International Journal of Obesity (2005), 34 Suppl 1(S1), S47–55. 10.1038/ijo.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Durant S, Burger KS, & Schoeller DA (2011). Weight suppression and risk of future increases in body mass: effects of suppressed resting metabolic rate and energy expenditure. American Journal of Clinical Nutrition, 94(1), 7–11. 10.3945/ajcn.110.010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Gau JM, Rohde P, & Shaw H (2017). Risk factors that predict future onset of each DSM–5 eating disorder: Predictive specificity in high-risk adolescent females. Journal of Abnormal Psychology, 126(1), 38. 10.1037/abn0000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Marti CN, Spoor S, Presnell K, & Shaw H (2008). Dissonance and healthy weight eating disorder prevention programs: long-term effects from a randomized efficacy trial. Journal of Consulting and Clinical Psychology, 76(2), 329–340. 10.1037/0022-006X.76.2.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Rohde P, Shaw H, & Desjardins C (2020). Weight suppression increases odds for future onset of anorexia nervosa, bulimia nervosa, and purging disorder, but not binge eating disorder. American Journal of Clinical Nutrition, 112(4), 941–947. 10.1093/ajcn/nqaa146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Rohde P, Shaw H, & Gau JM (2018). An experimental therapeutics test of whether adding dissonance-induction activities improves the effectiveness of a selective obesity and eating disorder prevention program. International Journal of Obesity (2005), 42(3), 462–468. 10.1038/ijo.2017.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Sysko R, Roberto CA, & Allison S (2010). Are dietary restraint scales valid measures of dietary restriction? Additional objective behavioral and biological data suggest not. Appetite, 54(2), 331–339. 10.1016/j.appet.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buuren S (2018). Flexible imputation of missing data. CRC press. [Google Scholar]
- van Strien T, Frijters JER, Bergers GPA, & Defares PB (1986). The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders, 5(2), 295–315. [DOI] [Google Scholar]
- van Strien T, Frijters JER, van Staveren WA, Defares PB, & Deurenberg P (1986). The predictive validity of the Dutch Restrained Eating Scale. International Journal of Eating Disorders, 5(4), 747–755. [DOI] [Google Scholar]
- Wildes JE, & Marcus MD (2012). Weight suppression as a predictor of weight gain and response to intensive behavioral treatment in patients with anorexia nervosa. Behaviour Research and Therapy, 50(4), 266–274. 10.1016/j.brat.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Russell GB, Tate DF, Espeland MA, LaRose JG, Gorin AA, Lewis CE, Jelalian E, Perdue LH, Bahnson J, Polzien K, Ferguson Robichaud E, & Study of Novel Approaches to Weight Gain Prevention Research, G. (2020). Examining Heterogeneity of Outcomes in a Weight Gain Prevention Program for Young Adults. Obesity (Silver Spring), 28(3), 521–528. 10.1002/oby.22720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunker C, Crosby RD, Mitchell JE, Wonderlich SA, Peterson CB, & Crow SJ (2011). Weight suppression as a predictor variable in treatment trials of bulimia nervosa and binge eating disorder. International Journal of Eating Disorders, 44(8), 727–730. 10.1002/eat.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]


