Abstract
We aimed to evaluate SIGLEC1 (CD169) as a biomarker in multiple sclerosis (MS) and Neuromyelitis optica spectrum disorder (NMOSD) and to evaluate the presence of SIGLEC1+ myeloid cells in demyelinating diseases. We performed flow cytometry-based measurements of SIGLEC1 expression on monocytes in 86 MS patients, 41 NMOSD patients and 31 healthy controls. Additionally, we histologically evaluated the presence of SIGLEC1+ myeloid cells in acute and chronic MS brain lesions as well as other neurological diseases. We found elevated SIGLEC1 expression in 16/86 (18.6%) MS patients and 4/41 (9.8%) NMOSD patients. Almost all MS patients with high SIGLEC1 levels received exogenous interferon beta as an immunomodulatory treatment and only a small fraction of MS patients without interferon treatment had increased SIGLEC1 expression. In our cohort, SIGLEC1 expression on monocytes was—apart from those patients receiving interferon treatment—not significantly increased in patients with MS and NMOSD, nor were levels associated with more severe disease. SIGLEC1+ myeloid cells were abundantly present in active MS lesions as well as in a range of acute infectious and malignant diseases of the central nervous system, but not chronic MS lesions. The presence of SIGLEC1+ myeloid cells in brain lesions could be used to investigate the activity in an inflammatory CNS lesion.
Subject terms: Neuroimmunology, Neurological disorders
Introduction
Multiple sclerosis (MS) and Neuromyelitis optica spectrum disorder (NMOSD) are both chronic, demyelinating inflammatory diseases of the central nervous system that are a significant cause of disability in the young. While certain aspects of the pathophysiology of both diseases have been uncovered, many aspects towards a complete understanding remain to be elucidated1.
SIGLEC1 (Sialic acid-binding immunoglobulin-type lectins-1, CD169) is a sialic acid binding cell-surface protein, exclusively expressed on monocytes and macrophages2. Its expression is upregulated upon contact with type I interferons and to a lesser degree, other activatory stimuli such as LPS3,4. As such, SIGLEC1 expression has been used as a surrogate marker for type I interferon activity in autoimmune diseases like Systemic lupus erythematosus5 or primary Sjögren syndrome6 as well as interferonopathies7 and viral infections8,9.
In the healthy brain, SIGLEC1 is only expressed by some perivascular and choroid plexus macrophages, but not by microglia10. In mice, mechanical insult to the brain led to an accumulation of SIGLEC1+ myeloid cells in the damaged area10. The cause of this accumulation, however, is unclear—either the contact of serum components or inflammatory signals with resident myeloid cells induces SIGLEC1 expression or blood-derived SIGLEC1+ myeloid cells infiltrate the CNS.
The role of type I interferons and SIGLEC1 in the pathophysiology of MS and NMOSD is not yet clear. In relapsing–remitting MS (RRMS), type I interferons are used as an immunomodulatory treatment that reduces the rate of relapses11. It does, however, not protect against clinical progression in the progressive forms of MS (primary progressive MS (PPMS) and secondary progressive MS (SPMS))11. In a recent report, SIGLEC1 positive myeloid cells were found within MS lesions and specific ablation of SIGLEC1 expressing cells in the experimental autoimmune encephalitis (EAE) model of MS led to an amelioration of disease12. Additionally, plasmacytoid dendritic cells, the main type I interferon-producing cells, were found in increased frequencies in the cerebrospinal fluid of MS patients during a flare13. Two studies found SIGLEC1 expression to be increased on blood monocytes of MS patients, especially those with a progressive type of MS12,14. Taken together, there is conflicting evidence on the role of type I interferons and the interferon-induced expression of SIGLEC1 in MS as being protective or pathogenic. This is likely due to the heterogeneity of patients and disease stages and potentially also the confounding effect of interferon therapy.
In NMOSD, there is some evidence that type I interferons play a role in its pathogenesis: many patients with NMOSD have an overlap with additional, type I interferon-dependent diseases, such as SLE15 and a recent report describes a series of patients with increased levels of endogenous or exogenous interferon α who went on to develop a seropositive NMOSD16. Interferon treatment does not prevent NMOSD relapses17 and anecdotally even increases the disease activity18. Transcriptomic profiling of blood from NMOSD patients identified a “interferon high” signature in 16 of 38 (42.1%) of NMOSD patients19. This signature included the transcript for SIGLEC1 and was associated with higher disease severity. Additionally, we recently described the presence of low-density granulocytes in MS and NMOSD patients20, a subset of granulocytes that produce high levels of type I interferons in SLE21. Thus, we hypothesized that type I interferons could also play a role in the pathogenesis of NMOSD.
In this work we investigated expression of SIGLEC1 on monocytes of patients with MS and NMOSD and correlated the expression with clinical parameters. In addition, we analysed human brain tissue sections of active, inflammatory and chronic MS lesions as well as other neurological and systemic diseases.
Results
SIGLEC1 expression is increased in a subset of patients with multiple sclerosis
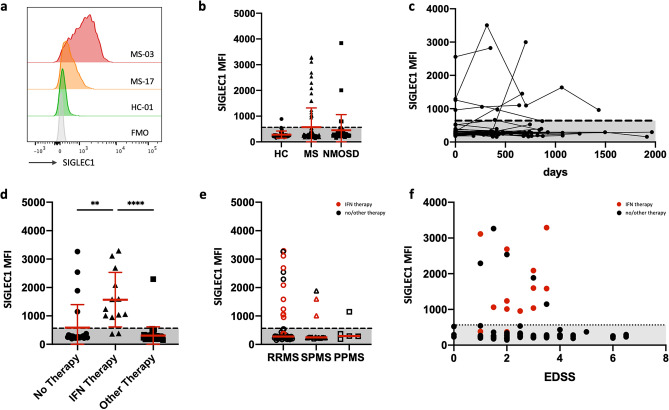
We investigated the SIGLEC1 expression on CD14+ monocytes in the peripheral blood of MS and NMOSD patients as well as in healthy controls. In most of the samples, monocyte SIGLEC1 expression was barely above the negative (fluorescence minus one) control (Fig. 1a,b). We defined a physiologic range of SIGLEC1 expression as two standard deviations from the mean SIGLEC1 expression in healthy controls (normal MFI range 0–564). Accordingly, 16/86 (18.6%) MS patients, 4/41 (9.8%) NMOSD patients and 1/31 (3.2%) healthy controls had increased SIGLEC1 levels. Almost all individuals with available longitudinal samples remained in their respective SIGLEC1 high or low category over a follow-up period of up to 1965 days. (Fig. 1c).
Figure 1.
(a) Representative histograms of SIGLEC1 fluorescence on CD14high monocytes, FMO: fluorescence minus one control. (b) Cross-sectional analysis of SIGLEC1 expression on CD14high monocytes of 31 healthy controls, 86 MS patients and 41 NMOSD patients. If multiple samples of the same individual were analysed, the mean of the measurements is displayed. (c) Longitudinal analysis of SIGLEC1 expression of 52 individuals with up to five measurements. (d) Comparison of SIGLEC1 expression in MS patients, receiving no immunomodulatory treatment (n = 24), interferon beta (n = 13) or a non-interferon treatment (n = 49). Treatment with interferon with significantly associated with higher levels of SIGLEC1 expression: Kruskal–Wallis test with Dunn’s correction, **p < 0.01, ****p < 0.0001. (e) Comparison of SIGLEC1 expression in patients with RRMS (n = 63), SPMS (n = 15) and PPMS (n = 5). Red symbols indicate interferon treatment. (f) Scatter plot of the EDSS disability score against SIGLEC1 expression, no significant correlation was detected.
Treatment with type 1 interferon explains almost all increased
Next, we aimed to identify parameters that were associated with increased SIGLEC1 in MS patients. One obvious explanation would be exogenous type 1 interferon as treatment. 11/16 MS patients (68.6%) with increased SIGLEC1 expression levels received interferon beta treatment at the time of measurement (Fig. 1d). While a previous report found increased SIGLEC1 levels to be more prevalent in MS patients with a progressive form of MS, we found only 2/20 patients primary or secondary progressive MS with increased SIGLEC1 levels that were not explained by interferon treatment (Fig. 1e). SIGLEC1 expression on monocytes did not correlate with the Expanded Disability Status Scale (EDSS) (Fig. 1f), nor had it a temporal association with relapses. In the NMOSD patients, one of the four patients with increased SIGLEC1 expression had the additional diagnosis of a mixed connective tissue disease (MCTD), a disease that is also associated with increased type 1 interferon activity22.
SIGLEC1 expression on brain-infiltrating myeloid cells
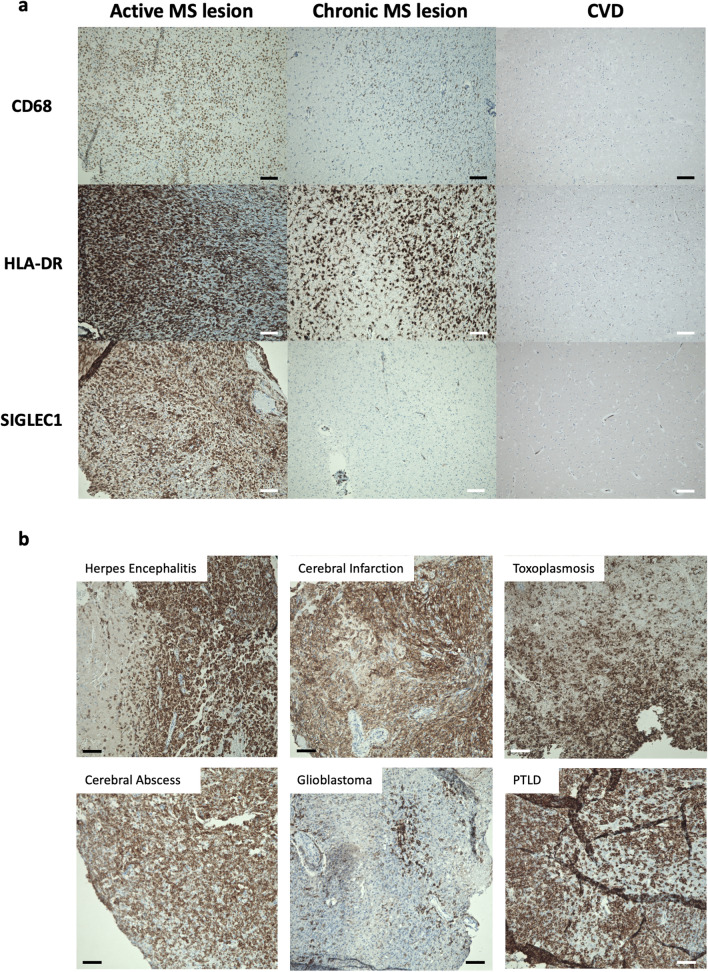
To investigate the presence and specificity of SIGLEC1+ myeloid cells in inflammatory MS lesion, we analysed brain tissue sections from 4 patients with MS lesions that had clinical, radiological and histological signs of activity (gadolinium uptake on MRI imaging, presence of myelin-laden macrophages), 5 patients with secondary-progressive MS (SPMS) who died of acute non-neurologic causes and with histologically classified chronic lesions and 6 patients who died of cardiovascular or multi-organ failure. In all control samples, SIGLEC1 positivity was limited to cells in the leptomeninges as well as perivascular macrophages, as previously reported10. In all four samples from patients with active MS lesions as well as in one patient with the Marburg variant of MS who died of the disease 14 days after onset, we found a dense infiltrate of CD68+HLA-DR+ myeloid cells that stained predominantly positive for SIGLEC1 (Fig. 2a). In contrast, in five samples from patients with secondary-progressive MS (SPMS) with histologically classified chronic lesions, we found CD68+HLA-DR+ infiltrates of varying density, but almost no SIGLEC1 expression (Fig. 2a). This indicates that SIGLEC1 expression on myeloid cells could be used to distinguish active inflammatory lesions from chronic lesions.
Figure 2.
(a) Representative immunohistochemical stainings of CD68, HLA-DR and SIGLEC1 (CD169) in brain tissue from a patient with active inflammatory multiple sclerosis lesion, a chronic lesion from a patient with secondary-progressive multiple sclerosis (SPMS) and a control patient who died of cardiovascular disease (CVD). Both RRMS and SPMS show infiltrates with CD68+ and HLA-DR+ myeloid cells, but only in the active lesion, these express SIGLEC1. Nuclei are stained blue and the respective marker antigens in brown. The scale bar indicates 100 µm. These results are representative of 4 RRMS, 5 SPMS and 7 control samples that were examined. (b) Immunohistochemical stainings of SIGLEC1 in brain tissue from patients with Herpes simplex viral encephalitis (n = 2), cerebral infarction with inflammatory changes (n = 2), cerebral toxoplasmosis (n = 1), cerebral abscess (n = 2), glioblastoma (n = 2) and post-transplant lymphoproliferative disease (PTLD, n = 1). Infiltrates of SIGLEC1+ myeloid cells were observed in all samples.
To corroborate this hypothesis, we studied the SIGLEC1 expression in the brain tissue of 8 patients with other inflammatory neurological diseases. SIGLEC1+ myeloid infiltrates were present in patients with glioblastoma (n = 2), herpes simplex encephalitis (n = 2) and cerebral infarction (n = 2), as well as toxoplasmosis (n = 1), cerebral abscess (n = 2) and post-transplant lymphoproliferative disorders (n = 1, PTLD, Fig. 2b). No increase in SIGLEC1+ cells was noted in the brain of a patient who died of amyotrophic lateral sclerosis (ALS).
In summary, SIGLEC1+ is expressed on brain-infiltrating myeloid cells in a broad range of active inflammatory lesions, but not in chronic MS lesions.
Discussion
Here, we report that, after accounting for interferon treatment, patients with multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) did not have increased expression of SIGLEC1 on monocytes in the peripheral blood.
We identified SIGLEC1+ on CD68+ HLA-DR+ myeloid cells in active inflammatory MS lesions and a range of other inflammatory, infectious or malignant brain lesions. SIGLEC1+ expression was low on myeloid cells in chronic MS lesions of SPMS patients, indicating that SIGLEC1+ myeloid cells could serve as a marker of inflammatory activity. These findings are in line with a previous report that found SIGLEC1+ MHC-II+ myeloid cells to be present in mice after retinal transplantation, but not in the healthy retina or retinal degeneration23. Future studies will be needed to investigate, whether the increase in SIGLEC1+ cells in inflammatory brain lesions is due to the infiltration of SIGLEC1+ cells from the peripheral blood or an upregulation of SIGLEC1 in tissue-resident microglia, as well as define the inflammatory signals leading to such an upregulation. We propose that SIGLEC1+ can serve as a marker to differentiate active from inactive MS lesions, which will require additional validation for use in routine histopathological diagnostics.
Our results on SIGLEC1 expression on blood monocytes are in contrast to two previous reports which identified increased levels of SIGLEC1 in cohorts of 44 MS patients12,14. The study by Malhotra et al.14 focussed on untreated MS patients and identified modest increases in SIGLEC1 expression in MS patients (approximately twofold increase of MFI between MS patients and HC) and especially those with progressive disease. A second study by Bogie et al.12 again identified small (approximately twofold) increases in SIGLEC1 expression on monocytes in 57 MS patients and found that these increases were independent of progressive disease and also of interferon treatment, an observation that is at odds with our own findings and biological plausibility.
This disparity might be due to different reasons: it could be that the authors mostly observed small differences between individuals that would have all been classified as “SIGLEC1 low expressors” in our study, as the approximately twofold-differences they describe are significantly smaller compared to the five–tenfold increases in SIGLEC1 MFI in “SIGLEC1 high expressors” in our study. Different studies investigated the SIGLEC1 expression using different antibody clones which could also explain some of the differences; in this study, we used the same clone as previous studies that investigated SIGLEC1 in rheumatologic diseases5,6.
Additionally, our data are at odds with a previous report that found a type 1 interferon signature in NMOSD patients that was conducted with patients from the same patient cohort19. While 42% of NMOSD cohorts in the study by Agasing et al. showed an “high type 1 interferon” signature, we found < 10% of NMOSD patients had increased type 1 interferon activity as measured by SIGLEC1 expression; a result that is neither significantly different from healthy controls, nor likely of clinical consequence. As the results of whole blood bulk transcriptomic data are often subject to unforeseen external influences and changes in the cellular composition of the blood, they should usually be validated by protein level data.
Even though our data are not in agreement with multiple, previously published results, the validity of our assay is supported by the internal plausibility control of interferon treated MS patients. However, one significant weakness of our own study is the relative clinical quiescence of the patients enrolled; most did not have a relapse within the last six months before the measurement and most did not have a change in EDSS in longitudinal sampling. Additionally, most patients were under immunosuppressive treatment. Future studies in MS and NMOSD patients during a clinical relapse could thus still discover the presence of SIGLEC1 expressing monocytes in the peripheral blood.
In summary, our data indicate that type I interferons or SIGLEC1 expressing cells in the peripheral blood do not play a major role in the pathogenesis of most patients with stable NMOSD or MS, however SIGLEC1+ myeloid cells in the brain are present in active inflammatory MS lesions as well as in other inflammatory neurological diseases of the CNS.
Methods
Cohort
We analysed frozen peripheral blood mononuclear cells (PBMCs) from a total of 86 MS patients, 41 NMOSD patients and 31 healthy controls (HCs) that were included in observational cohort studies for MS and NMOSD at the NeuroCure Clinical ResearchCenter, Charité—Universitätsmedizin Berlin. PBMCs from all patients with sufficient available material were included in the study with no prior selection according to disease activity, treatment, age or sex. All MS patients fulfilled the 2017 revised McDonald criteria24, while the NMOSD patients fulfilled the 2015 Wingerchuck international consensus diagnostic criteria25. Further patient characteristics are provided in Table 1.
Table 1.
Epidemiological data.
| Healthy controls | MS patients | NMOSD patients | |
|---|---|---|---|
| Number of individuals | 31 | 86 | 41 |
| Age (median, IQR) | 31 (27–34) | 45 (34–52) | 57 (45–64) |
| Sex (n female) | 15 (48.4%) | 53 (61.6%) | 37 (90.2%) |
| Subtype (n) | – |
RRMS: 63 (73.3%) SPMS: 15 (17.4%) PPMS: 5 (5.9%) |
AQP4+: 23 (56.1%) MOG+: 7 (17.1%) Seronegative: 3 (7.3%) Unknown: 8 (19.5%) |
| Additional immune-mediated diseases (n) | Autoimmune thyroiditis (2), asthma bronchiale (1) | Autoimmune thyroiditis (10), asthma bronchiale (2), psoriasis (1), idiopathic myocarditis (1), celiac disease (1) | Autoimmune thyroiditis (3), myasthenia gravis (1), Sjögren syndrome (1), MCTD (1) |
| Immunosuppressive medication (n) | – |
None: 24 (27.9%) Dimethyl fumarate: 15 (17.4%), glatiramer acetate: 14 (16.3%), interferon beta: 13 (15.1%), fingolimod: 7 (8.1%), teriflunomide: 5 (5.8%), methylprednisolone: 2 (2.3%), daclizumab: 2 (2.3%), rituximab: 1 (1.2%), ocrelizumab: 1 (1.2%), natalizumab 1 (1.2%) |
Rituximab: 19 (46.3%) Azathioprine: 9 (22.0%) None: 6 (14.6%) Prednisolone: 3 (7.3%) Mycophenolate: 2 (4.9%) Glatiramer acetate: 1 (2.4%) Unknown: 1 (2.4%) |
IQR interquartile range, RRMS relapse-remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis, PPMS primary progressive multiple sclerosis, AQP4+ aquaporin-4 antibody positive, MOG+ myelin oligodendrocyte glycoprotein antibody positive, MCTD mixed connective tissue disease).
Longitudinal samples were available for 28/86 MS patients, 12/41 NMOSD patients and 12/31 healthy controls. Follow-up periods were up to 1965 days and included 2 to 5 time points.
PBMC isolation and flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA anticoagulated blood. Blood and phosphate-buffered saline supplemented with 0.5% bovine serum albumin (PBS/BSA) were mixed at a 1:1 ratio and 35 ml were layered onto 15 ml Ficoll-Paque PLUS gradient (GE Healthcare). PBMCs were isolated according to the manufacturer’s protocol and stored in liquid nitrogen until the analysis.
For analysis batches of 20–35 samples were thawed and washed in RPMI 1640 cell medium (Gibco). In each batch, a mix of samples from healthy controls and MS/NMOSD patients were included. Cells were stained with antibodies against CD14 (DRFZ, clone TM1), CD169 (SIGLEC1, clone 7–239, BioLegend Cat# 346004, RRID:AB_2189029) and a dead cell stain (eBioscience Fixable Viability Dye eFluor 780). For each batch, a fluorescence minus one (FMO) control for SIGLEC1 was measured and FMO measurements remained stable over multiple batches. The samples were acquired on a FACSCanto cytometer (BD Biosciences) and all cytometry experiments were performed according to published standards26. Using the FlowJo software (Version 10.4.1 for Mac, FlowJo LLC), we identified monocytes and excluded doublets based on scatter parameters. We then gated on CD14high, living cells and analysed the median fluorescence intensity (MFI) for SIGLEC1 (Fig. 1a).
Histology of human brain tissue
We investigated archived cryo- and formalin preserved biopsy and autopsy tissue from patients who had been diagnosed in the Department of Neuropathology, Charité—Universitätsmedizin Berlin with inflammatory demyelination of the central nervous system (CNS) consistent with multiple sclerosis or other inflammatory, malignant or infectious diseases of the CNS (other neurological disease, OND). Tissue that was categorized as active MS lesions stemmed from patients with clinical and MRI evidence of active disease with gadolinium contrast agent uptake and/or perifocal edema in T2-weighted sequences. These tissue samples were all derived from diagnostic biopsies that were performed to diagnose an unclear tumefactive lesion that was diagnosed as inflammatory demyelinating lesion. After follow-up, these patients were then clinically diagnosed with MS. Histologically these lesions showed all active demyelination with myelin laden macrophages. In contrast, the patients with lesions categorized as chronic MS lesions were patients diagnosed with SPMS that had died of a non-neurologic cause and where only chronically active or inactive lesions with lack of myelin-laden macrophages were analysed27. Patients who died of cardiovascular cases or multi-organ failure served as controls. All patients with multiple sclerosis fulfilled clinical diagnosis criteria according to the 2017 revised McDonald criteria24 and none of the patients were treated with interferon.
Immunohistology of human brain tissue
All stains were performed on 8 μm cryomicrotome sections or 4-μm-thick FFPE tissue sections according to standard procedures. Stains included Hematoxylin and eosin (H&E), CD68 (EBM11, Dako #M0718, 1:100), HLA-DR (CR3/43; Dako #M0775, 1:200) and SIGLEC1 (HSn7D2, Novus Biologicals, 1:200). Immunohistochemical stainings were performed on a Benchmark XT autostainer (Ventana Medical Systems, Tuscon, AZ, USA) with standard antigen retrieval methods for the FFPE sections (CC1 buffer, pH8.0, Ventana Medical Systems, Tuscon, AZ, USA). The presence of SIGLEC1 perivascular and leptomeningeal staining served as an internal quality control. Microscopy was performed on a BZ-9000 BioRevo microscope (Keyence, Neu-Isenburg, Germany). Positively stained cells were defined by presence of a nucleus and cytoplasmic staining.
Data analysis
Statistical analyses were performed using Python 3.7.6 with the Numpy (v1.19.0)28 and Pandas (v1.1.0)29 packages as well as GraphPad Prism (v8.4.3. for Mac). A normal range for SIGLEC1 expression in FACS expression was calculated as two standard deviations from the mean SIGLEC1 expression in healthy controls. When multiple measurements of the same individual were available, the mean of the measurements was calculated and used for comparative analysis. The Chi-squared test or Kruskal–Wallis test with Dunn’s correction was used to compare between the different groups for binary and continuous variables respectively.
Ethics approval and consent to participate
The analysis of blood was approved by the ethics committee of the Charité—Universitätsmedizin Berlin (MS: EA1/163/12; NMOSD: EA1/041/14) and informed consent was obtained from all participants in the study. The study of brain histology was approved approved by the ethics committee of the Charité—Universitätsmedizin (EA1/078/16). This study was performed in accordance with all relevant guidelines and regulations, such as the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonization.
Acknowledgements
The authors are most grateful to the patients and/or their relatives consenting to research on their tissue or blood samples. We are also indebted to Peggy Mex, Ralf Uecker and Petra Matylewski for excellent technical assistance and advice.
Author contributions
L.O., R.A.N., A.E.H. and H.R. designed the experiments; L.O., P.D., R.B., A.D., V.S., J.B.S. and H.R. performed experiments, cared for the patients and collected the data. L.O., P.D., R.B., K.R., D.S., W.S., R.A.N., A.E.H., F.P. and H.R. analysed and interpreted the data. L.O. wrote the first draft of the manuscript and designed the figures. All authors provided intellectual input into the revision of the manuscript. H.R. supervised the work.
Funding
Open Access funding enabled and organized by Projekt DEAL. LO was supported by the BIH-MD Promotionsstipendium of the Charité Universitätsmedizin Berlin and the Berlin Institute of Health and was a member of the Leibniz Graduate School for Chronic Inflammation.
Data availability
All datasets used and analysed during the current study are available from the corresponding authors on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lennard Ostendorf, Email: lennard.ostendorf@charite.de.
Helena Radbruch, Email: helena.radbruch@charite.de.
References
- 1.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97:742–768. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 3.Izquierdo-Useros N, et al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012;10:e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biesen R, et al. Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis Rheum. 2008;58:1136–1145. doi: 10.1002/art.23404. [DOI] [PubMed] [Google Scholar]
- 5.Rose T, et al. IFNα and its response proteins, IP-10 and SIGLEC-1, are biomarkers of disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 2013;72:1639–1645. doi: 10.1136/annrheumdis-2012-201586. [DOI] [PubMed] [Google Scholar]
- 6.Rose T, et al. SIGLEC1 is a biomarker of disease activity and indicates extraglandular manifestation in primary Sjögren’s syndrome. RMD Open. 2016;2:e000292. doi: 10.1136/rmdopen-2016-000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orak B, et al. SIGLEC1 (CD169) as a potential diagnostical screening marker for monogenic interferonopathies. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2020 doi: 10.1111/pai.13400. [DOI] [PubMed] [Google Scholar]
- 8.Bedin A-S, et al. Monocyte CD169 expression as a biomarker in the early diagnosis of COVID-19. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michlmayr D, et al. Comprehensive immunoprofiling of pediatric zika reveals key role for monocytes in the acute phase and no effect of prior dengue virus infection. Cell Rep. 2020;31:107569. doi: 10.1016/j.celrep.2020.107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry VH, Crocker PR, Gordon S. The blood-brain barrier regulates the expression of a macrophage sialic acid-binding receptor on microglia. J. Cell Sci. 1992;101(Pt 1):201–207. doi: 10.1242/jcs.101.1.201. [DOI] [PubMed] [Google Scholar]
- 11.Filippi M, et al. Multiple sclerosis. Nat. Rev. Dis. Primer. 2018;4:43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 12.Bogie JF, et al. CD169 is a marker for highly pathogenic phagocytes in multiple sclerosis. Mult. Scler. J. 2018;24:290–300. doi: 10.1177/1352458517698759. [DOI] [PubMed] [Google Scholar]
- 13.Longhini ALF, et al. Plasmacytoid dendritic cells are increased in cerebrospinal fluid of untreated patients during multiple sclerosis relapse. J. Neuroinflamm. 2011;8:2. doi: 10.1186/1742-2094-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malhotra S, et al. SIGLEC1 and SIGLEC7 expression in circulating monocytes of patients with multiple sclerosis. Mult. Scler. J. 2013;19:524–531. doi: 10.1177/1352458512458718. [DOI] [PubMed] [Google Scholar]
- 15.Jarius S, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J. Neuroinflamm. 2012;9:14. doi: 10.1186/1742-2094-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams J, et al. Neuromyelitis optica in patients with increased interferon alpha concentrations. Lancet Neurol. 2020;19:31–33. doi: 10.1016/S1474-4422(19)30445-4. [DOI] [PubMed] [Google Scholar]
- 17.Uzawa A, Mori M, Hayakawa S, Masuda S, Kuwabara S. Different responses to interferon beta-1b treatment in patients with neuromyelitis optica and multiple sclerosis: response to IFN beta-1b treatment in NMO and MS. Eur. J. Neurol. 2010;17:672–676. doi: 10.1111/j.1468-1331.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- 18.Palace J, Leite MI, Nairne A, Vincent A. Interferon beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch. Neurol. 2010;67:1016–1017. doi: 10.1001/archneurol.2010.188. [DOI] [PubMed] [Google Scholar]
- 19.Agasing AM, et al. Transcriptomics and proteomics reveal a cooperation between interferon and T-helper 17 cells in neuromyelitis optica. Nat. Commun. 2020;11:2856. doi: 10.1038/s41467-020-16625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostendorf L, et al. Low-density granulocytes are a novel immunopathological feature in both multiple sclerosis and neuromyelitis optica spectrum disorder. Front. Immunol. 2019;10:2725. doi: 10.3389/fimmu.2019.02725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denny MF, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds JA, et al. Type I interferon in patients with systemic autoimmune rheumatic disease is associated with haematological abnormalities and specific autoantibody profiles. Arthritis Res. Ther. 2019;21:147. doi: 10.1186/s13075-019-1929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sancho-Pelluz J, et al. Sialoadhesin expression in intact degenerating retinas and following transplantation. Investig. Opthalmol. Vis. Sci. 2008;49:5602. doi: 10.1167/iovs.08-2117. [DOI] [PubMed] [Google Scholar]
- 24.Thompson AJ, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 25.Wingerchuk DM, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cossarizza A, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition) Eur. J. Immunol. 2019;49:1457–1973. doi: 10.1002/eji.201970107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metz I, et al. Pathologic heterogeneity persists in early active multiple sclerosis lesions: pathologic heterogeneity in MS Ann. Neurol. 2014;75(728):738. doi: 10.1002/ana.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris CR, et al. Array programming with NumPy. Nature. 2020;585:357–362. doi: 10.1038/s41586-020-2649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinney, W. Data structures for statistical computing in Python. In Python in Science Conference 56–61. 10.25080/Majora-92bf1922-00a (2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets used and analysed during the current study are available from the corresponding authors on request.