Abstract
Telomerase reverse transcriptase gene promoter (TERTp) mutation is a potential candidate for pathogenesis and therapeutic target of tonsillar squamous cell carcinomas (TSCCs) in association with human papillomavirus (HPV). Their clinical relevance has not been validated under the new 8th American Joint Committee on Cancer (AJCC) staging system. We analyzed real-time peptide nucleic acid–mediated PCR and sequencing methods (TERTp mutation) and real-time PCR-based assay (HPV) in 80 surgically resected TSCCs. The 8th edition staging system improved the stratification of the early and advanced stages and between T or N categories for overall survival over the 7th edition. TERTp mutation was found in 7.5%, and HPV in 80.0% of the patients. The majority (83.3%) of TERTp mutation cases were HPV-positive TSCCs. Applying the 8th edition staging system, TERTp mutation was an independent factor of poor prognosis for disease-free survival (DFS) in TSCC patients, supporting the clinical significance of TERTp mutation in tonsil cancer. TERTp mutations were also negatively correlated with overall survival and DFS in HPV-negative TSCCs. Conclusively, TERTp mutation provides negative prognostic impact on survival of surgically managed tonsil cancers staged with the AJCC 8th edition.
Keywords: Tonsil, Squamous cell carcinoma, Human papillomavirus, Head and neck cancer, Prognosis
Introduction
Tonsillar squamous cell carcinomas (TSCCs) account for 70–80% of the oropharyngeal cancers most prevalent for human papillomavirus (HPV) [1–3]. In oropharyngeal squamous cell carcinomas (SCCs), HPV is associated with better prognosis and response to radiochemotherapy compared with HPV-negative oropharyngeal SCCs [3, 4]. Tonsils are the subsite of oropharyngeal cancers with the highest HPV-positive rate [2, 3]. The 5-year survival in early cases has been reported to be > 90%, which decreases to < 20% in advanced tonsil cancer [1–3, 5]. Both primary surgery and radiotherapy/chemotherapy are effective treatments for TSCCs [4]; however, treatment failures can develop unexpectedly. The 8th edition of the American Joint Committee on Cancer (AJCC) staging system for oropharyngeal cancer incorporates HPV infection and extranodal extension [6], but it is unclear whether those result in better stratification of Korean patients, as the area has relatively low incidence of oropharyngeal cancer [7–9]. Because risk stratification, prognosis prediction, treatment selection, and follow-up strategies often depend on the AJCC system, validation of staging is crucial.
The TERT promoter (TERTp), a critically important regulatory element for telomerase expression harboring binding sites for a number of transcriptional activators and repressors, contributes to increased telomerase activity that leads to immortalization of cells, which is one of the hallmarks of cancer [10, 11]. Two mutually exclusive G–A mutations at nucleotide − 124 and − 146 within the core promoter region of TERT gene occur as a pathogenic mutation in human malignancies [12], which are also driver mutations in head and neck SCCs [13–17]. The TERTp mutation, alone and with the HPV oncogenes, plays an important role in oral and uterine cervical SCCs [14, 18, 19]. Two viral oncogenes, E6 and E7, expressed by high-risk HPV-associated cancers, affect the oncogenic pathway related to cellular immortalization, typically activating telomerase expression [18, 20, 21]. Although previous studies examined multiple subsites of oral or oropharyngeal cancers, the frequencies of TERTp mutation in tonsil cancers and the association to HPV have rarely been investigated in the series of their studies [22, 23]. Furthermore, the revised 8th AJCC staging system in terms of oropharyngeal cancers with or without HPV has not been validated in the Far East Asian cohort data, specifically on tonsil cancers.
Here, we investigated TERTp mutation, HPV infection, and clinicopathological characteristics in 80 Korean primary TSCC patients. We sought to validate the 8th edition of the AJCC staging system compared with the 7th edition, with variation in patient location and therapy.
Patients and Methods
Patients
Formalin-fixed, paraffin-embedded (FFPE) tissues were obtained from 80 TSCC patients who underwent primary resection, with no prior treatment and complete medical records at our institution between 1997 and 2018. Clinical information was analyzed using medical records and radiological results. Heavy smoking was defined as > 20 pack-years [6]. Alcohol consumption was defined as > 14 drinks/week [6]. Of these 80 patients, 11 patients underwent postoperative radiotherapy, 2 patients had chemotherapy, and 39 patients had chemoradiotherapy following the surgical resection. The remaining 28 patients were treated with surgery alone. Radiation doses ranged from 5040 to 7200 cGy/36 fractions over 8 weeks.
Diagnosis and histological differentiation were evaluated according to the World Health Organization classification [1]. Patients were re-staged according to the 8th editions of the AJCC/UICC TNM classification [6]. The study protocol was approved by Sacred Heart Hospital Institutional Review Board (No. 14-2-57) and performed in accordance with the relevant guidelines and regulations (Declaration of Helsinki). Informed consent was obtained from the patients and from the next of kin (deceased patients) before enrollment in the study.
DNA Extraction and Detection of TERT Promoter Mutation
Genomic DNA was extracted from 10-μm-thick sections of 10% neutral FFPE tumor tissue blocks using Maxwell 16 FFPE Tissue LEV DNA Purification Kit for DNA (Promega, USA). TERTp mutations (C250 and C228) were identified using the PNAClamp™ TERT mutation detection kit (PANAGENE, Daejeon, South Korea [10]. Subsequently, the TERTp mutation analyses were also confirmed by directional sequencing of PCR fragments amplified from genomic DNA. The primers used for TERTp were as follows [24]: forward, 5′-AGTGGATTCGCGGGCACAGA-3′, and reverse, 5′-AGCACCTCGCGGTAGTGG-3′, which amplified a 346 bp fragment. PCR amplification was carried out in a reaction volume of 30 μl containing 100 ng of template DNA, 10× PCR buffer, 0.25 mM dNTPs, 10 pmol primers, and 1.25 U Taq DNA polymerase (Solgent, Korea). The thermal cycling conditions were as follows: denaturation at 95 °C for 3 min, followed by 10 cycles of 95 °C denaturation for 30 s, 60 °C annealing for 30 s, and 68 °C elongation for 1 min. This was followed by 30 cycles under the same settings, with the elongation step modified to continue for an additional 5 s each cycle. PCR was completed with final elongation at 68 °C for 7 min. PCR products were electrophoresed on 2% agarose gels and purified with a Solgent PCR purification kit (Solgent). All amplification products were sequenced bidirectionally using an automated sequencer (ABI 3130xl; Applied Biosystems, Foster City, CA, USA) using the BigDye Terminator v1.1 kit (Applied Biosystems) and the appropriate forward and reverse primers.
Detection of HPV
HPV status was evaluated by PANA RealTyper HPV genotyping kit and PANA RealTyper HPV screening kit (PANAGENE). This kit, approved for clinical use in Korea, detects a total of 40 HPV genotypes including 20 high-risk genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 69, 70, 73, and 82), 2 low-risk genotypes (6 and 11), and 18 other genotypes. Briefly, real-time PCR assays were performed in a 25 μl reaction mixture containing 19 μl of HPV mix, 1 μl of Taq DNA polymerase, and 5 μl of extracted DNA, positive control, or negative control. PCR was performed using the following conditions: 1 cycle of incubation at 50 °C for 2 min and Taq activation at 95 °C for 15 min; 45 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 45 s, and extension at 72 °C for 15 s; and a melting curve step at 95 °C for 5 min, 35 °C for 5 min, followed by increase in temperature from 35 °C to 80 °C for 5 min, with a gradual increment of 0.5 °C (every 5 s) to achieve fluorescence in all four channels (FAM, HEX or VIC, ROX, and Cy5).
Statistical Analysis
Correlations between the TERTp mutation and clinicopathological variables were assessed using the Chi-squared test or two-tailed Fisher’s exact test. Factors found to be significant in univariate analysis were included in subsequent binary logistic regression analysis to identify independent variables associated with TERTp mutation. Survival analyses were performed using the Kaplan-Meier method and were compared using a log-rank test. Overall survival (OS) was defined as the interval from the first day of surgery until death. Disease-free survival (DFS) was defined as the interval from the first day of surgery until tumor recurrence. OS and DFS were analyzed until February 2019. Univariate and multivariate analyses using the Cox proportional hazard regression model were applied to determine the hazard ratio (HR) and 95% confidence intervals (CI) for specific variables related to OS and DFS. SPSS version 18 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. P values < 0.05 were considered statistically significant.
Results
Comparisons Between the AJCC 8th and 7th Edition Staging Systems
According to the 8th edition staging system, 18 (22.5%) tumors were classified as T1, 31 (38.8%) as T2, 20 (25.0%) as T3, and 11 (13.7%) as T4. Of the 80 patients, 17 (21.3%) were categorized as N0, 37 patients (46.3%) as N1, 11 (13.7%) as N2, and 15 (18.7%) as N3. Combining the T and N categories, the overall stages of 31 patients were diagnosed as stage I (38.8%), 18 (22.5%) as stage II, 11 (13.7%) as stage III, and 20 (25.0%) as stage IV. The median follow-up period was 64 months (range, 3–136 months). The 5-year OS and DFS rates were 53.2% and 43.8%, respectively.
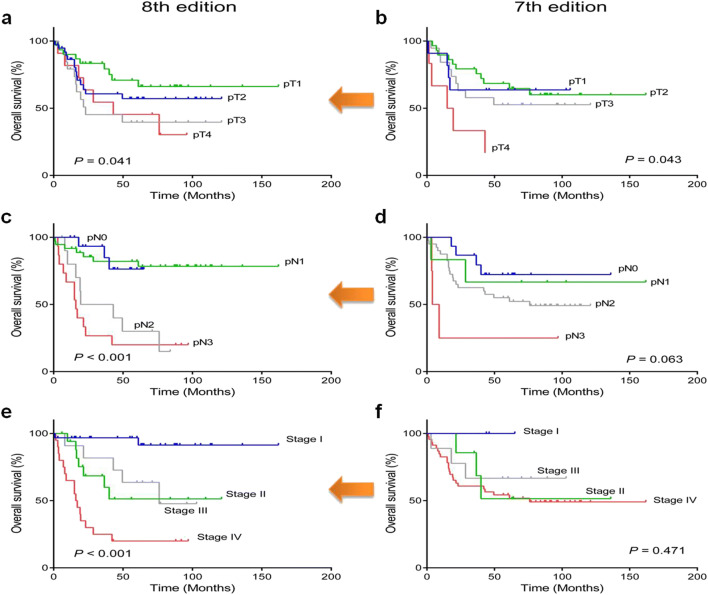
We performed Kaplan–Meier survival analyses of OS in 80 patients who had previous TNM information according to AJCC 8th vs. AJCC 7th staging system (Fig. 1). We compared the T category, N category, and overall stages assigned by the 8th and 7th editions of the AJCC staging system. As shown in Fig. 3, the 7th edition of the AJCC staging system performed poorly with respect to the discrimination and stratification of N category and overall stages (P = 0.063 and P = 0.471, respectively). Only the T category was well discriminated according to clinical outcomes (P = 0.043). In contrast, the 8th edition provided statistically significant stratification for the T category, N category, and overall staging (P = 0.041, P < 0.001, and P < 0.001, respectively). Therefore, the 8th edition was applied in our study.
Fig. 1.
Overall survival analyses of tonsillar squamous cell carcinomas according to newly revised pT category (a), pN category (c), and AJCC stage 8th (e) compared with previous pT (7th edition) (b), pN (7th edition) (d), and AJCC stage 7th (f)
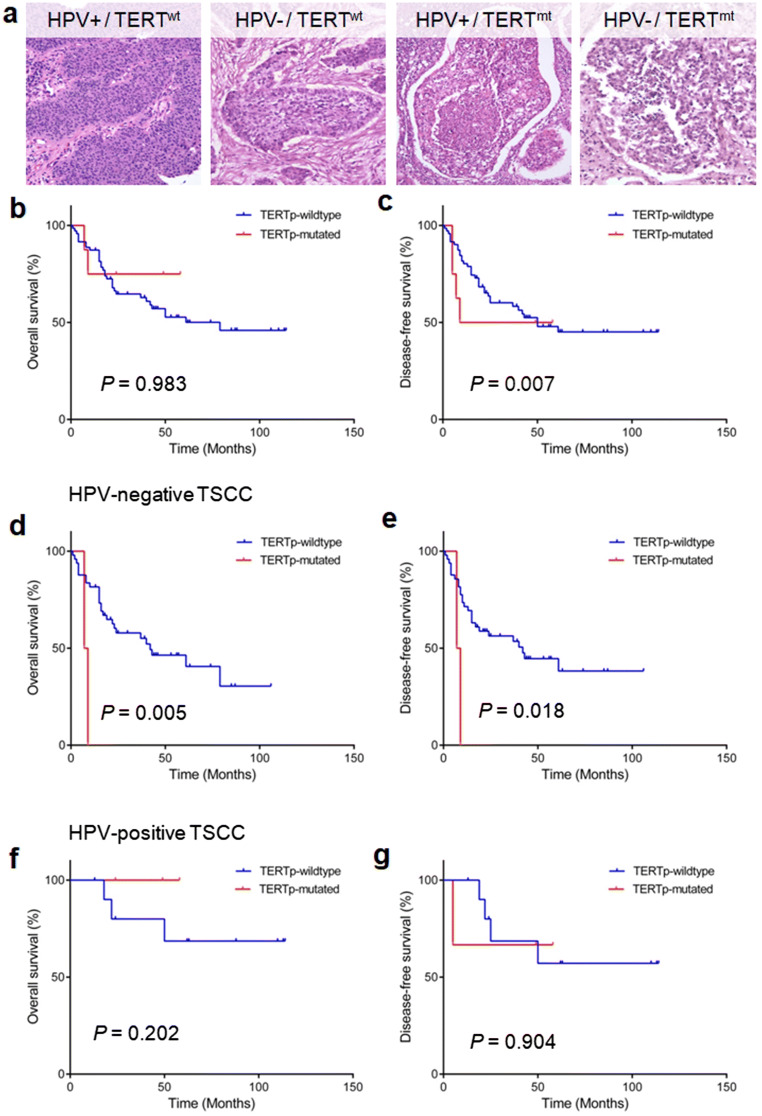
Fig. 3.
a Representative H&E images according to HPV and TERTp mutation (magnifications, × 200). TERTp mutation is not related to overall survival (b), whereas it has a strong prognostic impact on shorter disease-free survival (c). In HPV-negative tumors, TERTp mutation is associated with worse overall survival (d) and disease-free survival (e). However, there are no survival correlations in terms of overall (f) and disease-free (g) survivals in HPV-positive tumors
HPV and TERTp Mutation
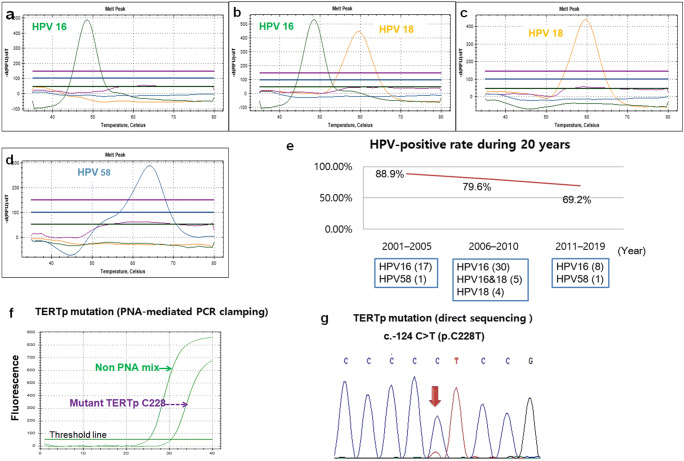
HPV was identified in 64 tumors (80.0%) analyzed by real-time HPV genotyping kit. All 80 cases were interpretable. There were only high-risk HPV genotypes including HPV 16 (54/64, 84.4%), HPV 18 (4/64, 6.2%), HPV 58 (1/64, 1.6%), and concurrent HPV 16 and HPV 18 (5/64, 7.8%) (Fig. 2a–d). We also analyzed the incidence of HPV in TSCCs during the last 20 years (Fig. 2e). The HPV-positive rates in those tonsil cancers decreased from 88.9% (16/18) in 2001–2005 to 79.6% (29/49) in 2006–2010 and 69.2% (9/13) in 2011–2019.
Fig. 2.
(a–c) HPV genotypes detected in tonsillar squamous cell carcinomas: HPV 16 (a), concurrent HPV 16 and HPV 18 (b), and HPV 18 (c). d HPV-negative tonsil cancers. e The periodic incidence of HPV in tonsillar squamous cell carcinomas during 20 years. f PNA clamp real-time PCR detected TERTp mutation on C228 in tonsillar squamous cell carcinomas. g Sequence chromatography demonstrated TERTp c.-124 C > T (p. C228T) mutation
The real-time quantitative PCR with PNA-mediated clamping method identified six mutations at position − 124 within TERTp in 80 TSCC patients (Fig. 2f). No TERTp mutation at position − 146 was identified. To confirm these results, direct sequencing was repeatedly performed and consistently identified TERTp mutations in 6 (7.5%) of 80 tumor samples. The observed point mutations were hot spot nucleotide changes G to A at position − 124 within TERTp in 6 cases (Fig. 2g).
We analyzed the associations of HPV or TERTp mutation with clinical and pathological features of 80 TSCCs (Table 1). The presence of HPV was more frequently associated with younger age (≤ 60 years) (P = 0.010), low alcohol consumption (P = 0.021), pN-positive status (P < 0.001), lower AJCC stage (P = 0.020), and presence of ipsilateral lymph node metastasis (P = 0.004). HPV positivity was not associated with TERTp mutation. There were no statistical associations between TERTp mutation and clinicopathologic features of TSCCs.
Table 1.
Association between HPV and TERTp mutation and patient characteristics
| Parameter | Total | HPV | TERTp | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | P | Mutated | Wildtype | P | ||
| N = 80 (%) | n = 64 (80.0%) | n = 16 (20.0%) | n = 6 (7.5%) | n = 74 (92.5%) | |||
| Sex | 1.000 | 1.000 | |||||
| Male | 70 (87.5) | 56 (87.5) | 14 (87.5) | 6 (100) | 64 (86.5) | ||
| Female | 10 (12.5) | 8 (12.5) | 2 (12.5) | 0 (0) | 10 (13.5) | ||
| Age (year) | 0.010* | 1.000 | |||||
| ≤ 60 | 52 (65.0) | 46 (71.9) | 6 (37.5) | 4 (66.7) | 48 (64.9) | ||
| > 60 | 28 (35.0) | 18 (28.1) | 10 (62.5) | 2 (33.3) | 26 (35.1) | ||
| Smoking | 0.263 | 0.392 | |||||
| Light | 33 (41.3) | 32 (50.0) | 5 (31.2) | 1 (16.7) | 32 (43.2) | ||
| Heavy | 47 (58.7) | 32 (50.0) | 11 (68.8) | 5 (83.3) | 42 (56.8) | ||
| Alcohol | 0.021* | 0.667 | |||||
| Light | 50 (62.5) | 44 (68.8) | 6 (37.5) | 3 (50.0) | 47 (63.5) | ||
| Heavy | 30 (37.5) | 20 (31.2) | 10 (62.5) | 3 (50.0) | 27 (36.5) | ||
| Tumor location | 0.173 | 0.651 | |||||
| Right side | 47 (58.7) | 40 (62.5) | 7 (43.8) | 3 (50.0) | 44 (59.5) | ||
| Left side | 33 (41.3) | 24 (37.5) | 9 (56.2) | 3 (50.0) | 30 (40.5) | ||
| pT category | 0.088 | 0.556 | |||||
| T1-T2 | 49 (61.3) | 36 (56.3) | 13 (81.3) | 3 (50.0) | 46 (62.2) | ||
| T3-T4 | 31 (38.7) | 28 (43.7) | 3 (18.7) | 3 (50.0) | 28 (37.8) | ||
| pNodal status | < 0.001* | 0.333 | |||||
| N0 | 17 (21.3) | 8 (12.5) | 9 (56.2) | 0 (0) | 17 (23.0) | ||
| N1–3 | 63 (78.7) | 56 (87.5) | 7 (43.8) | 6 (100) | 57 (77.0) | ||
| pAJCC stage (8th) | 0.020* | 0.624 | |||||
| I–III | 60 (75.0) | 52 (81.2) | 8 (50.0) | 4 (66.7) | 56 (75.7) | ||
| IV | 20 (25.0) | 12 (18.8) | 8 (50.0) | 2 (33.3) | 18 (24.3) | ||
| HPV status | – | 1.000 | |||||
| Positive | 64 (80.0) | – | – | 5 (83.3) | 59 (79.7) | ||
| Negative | 16 (20.0) | – | – | 1 (16.7) | 15 (20.3) | ||
| BOT invasion | 0.263 | 0.407 | |||||
| Present | 43 (53.8) | 32 (50.0) | 5 (31.2) | 4 (66.7) | 33 (44.6) | ||
| Absent | 37 (46.2) | 32 (50.0) | 11 (68.8) | 2 (33.3) | 41 (55.4) | ||
| Soft palate invasion | 0.154 | 0.423 | |||||
| Present | 28 (35.0) | 25 (39.1) | 3 (18.7) | 3 (50.0) | 25 (33.8) | ||
| Absent | 52 (65.0) | 39 (60.9) | 13 (81.3) | 3 (50.0) | 49 (66.2) | ||
| Ipsilateral LN meta | 0.004* | 1.000 | |||||
| Present | 58 (72.5) | 51 (79.7) | 7 (43.8) | 5 (83.3) | 53 (71.6) | ||
| Absent | 22 (27.5) | 13 (20.3) | 9 (56.2) | 1 (16.7) | 21 (28.4) | ||
| Contralateral LN meta | 1.000 | 1.000 | |||||
| Present | 12 (15.0) | 10 (15.6) | 2 (12.5) | 1 (16.7) | 11 (14.9) | ||
| Absent | 68 (85.0) | 54 (84.4) | 14 (87.5) | 5 (83.3) | 63 (85.1) | ||
| ENE | 0.485 | 0.409 | |||||
| Present | 51 (63.8) | 42 (65.6) | 9 (56.2) | 5 (83.3) | 46 (62.2) | ||
| Absent | 29 (36.2) | 22 (34.4) | 7 (43.8) | 1 (16.7) | 28 (37.8) | ||
HPV human papillomavirus, p pathologic, LN lymph node, BOT base of tongue, AJCC American Joint Committee on Cancer, ENE extranodal extension
*Statistically significant, P < 0.05
Coexistence of HPV and TERTp mutation occurred in 5 cases (6.3%) of 80 TSCCs: a total of 5 cases (83.3%) among the 6 TERTp mutations were in HPV 16-positive tonsil cancers and one TERTp mutated tumor was HPV-negative. TSCCs showed the most frequently HPV-positive/TERTp-wild-type (n = 59, 73.7%), followed by HPV-negative/TERTp-wild-type (n = 15, 18.8%), HPV-positive/TERTp-mutated (n = 5, 6.2%), and HPV-negative/TERTp-mutated (n = 1, 1.3%) (Fig. 3a).
Prognostic Correlation of TERTp Mutation
In Kaplan–Meier survival analyses, TERTp mutation was found to be statistically associated with shorter DFS rates than those of TERTp wild-type (P = 0.007), whereas no statistical difference was observed for OS between TERTp mutation and TERTp wild-type (P = 0.983) (Fig. 3b, c). We further analyzed the prognostic impact of TERTp mutations on OS and DFS according to HPV status. TERTp mutations were strongly correlated with decreased OS and DFS in patients with HPV-negative TSCCs (P = 0.005 and P = 0.018, respectively) (Fig. 3d, e). However, there were no prognostic correlations of TERTp mutations with OS or DFS TERTp mutations in patients with HPV-positive TSCCs (P = 0.202 and P = 0.904, respectively) (Fig. 3f, g).
We analyzed the OS and DFS through univariate and multivariate analyses (Table 2). In the univariate analyses, older age (P = 0.006), higher T category (P = 0.007), and base of tongue (BOT) invasion (P = 0.015) were associated with shorter OS rates, while TERTp mutation (P = 0.007), higher T category (P = 0.004), BOT invasion (P = 0.002), and soft palate invasion (P = 0.005) were associated with shorter DFS rates. Multivariate analyses confirmed that older age and higher T category were independent negative prognostic factors for shorter OS in patients with TSCCs (P < 0.001, HR: 4.467, 95% CI: 2.037–9.793; P = 0.016, HR: 3.152, 95% CI: 1.244–7.988, respectively). TERTp mutation was identified to be the only independent prognostic factor for DFS in tonsil cancers (P = 0.021, HR: 3.216, 95% CI: 1.197–8.644).
Table 2.
Univariate and multivariate analyses of overall survival and disease-free survival of patients with tonsillar squamous cell carcinoma by univariate and multivariate analyses
| Overall survival | Disease-free survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| TERTp | 0.984 | 0.983 | 3.879 | 0.007* | 3.216 | 0.021* | ||
| Wildtype vs. mutated | (0.234–4.146) | (1.449–10.388) | (1.197–8.644) | |||||
| HPV | 0.580 | 0.195 | 0.802 | 0.567 | ||||
| Absent vs. present | (0.255–1.321) | (0.377–1.707) | ||||||
| Sex | 0.454 | 0.282 | 0.719 | 0.533 | ||||
| Male vs. female | (0.108–1.910) | (0.255–2.029) | ||||||
| Age (year) | 2.744 | 0.006* | 4.467 | < 0.001* | 1.813 | 0.075 | ||
| < 60 vs. ≥ 60 | (1.327–5.674) | (2.037–9.793) | (0.943–3.488) | |||||
| Tonsil side | 1.396 | 0.363 | 0.917 | 0.794 | ||||
| Rt vs. Lt | (0.680–2.863) | (0.478–1.758) | ||||||
| Alcohol | 1.371 | 0.394 | 1.064 | 0.852 | ||||
| Light vs. heavy | (0.663–2.834) | (0.554–2.045) | ||||||
| Smoking | 1.853 | 0.105 | 1.358 | 0.353 | ||||
| Light vs. heavy | (0.878–3.908) | (0.712–2.591) | ||||||
| pT category | 2.745 | 0.007* | 3.152 | 0.016* | 2.549 | 0.004* | 1.556 | 0.257 |
| T1–2 vs. T3–4 | (1.321–5.708) | (1.244–7.988) | (1.340–4.850) | (0.725–3.341) | ||||
| pN category | 2.651 | 0.110 | 2.821 | 0.050 | ||||
| N0 vs. N1–3 | (0.801–8.773) | (0.998–7.968) | ||||||
| BOT invasion | 2.579 | 0.015* | 1.770 | 0.222 | 2.951 | 0.002* | 1.823 | 0.159 |
| Absent vs. present | (1.206–5.513) | (0.707–4.426) | (1.504–5.791) | (0.790–4.206) | ||||
| Soft palate invasion | 1.775 | 0.116 | 2.513 | 0.005* | 1.445 | 0.356 | ||
| Absent vs. present | (0.867–3.630) | (1.323–4.771) | (0.662–3.156) | |||||
HR hazard ratio, CI confidence interval, HPV human papillomavirus, Rt right, Lt left, p pathologic, BOT base of tongue
*Statistically significant, P value < 0.05
Discussion
In the present study, the new staging system improved stratification of early and advanced stages and between T or N category concerning overall survival based on Korean population. Under the new AJCC 8th edition, the prognostic implication of TERTp mutation in tonsil cancers has been rarely reported. Applying the new AJCC 8th edition, we confirmed the negative prognostic impact of TERTp mutation on survival of surgically managed tonsil cancers staged with the new AJCC 8th edition, especially on HPV-negative TSCCs. TERTp mutation has been reported as a predictor of poor prognosis in laryngeal cancers but not in oral SCCs [17, 25]. A meta-analysis based on published articles concluded that TERTp mutation serves as an adverse prognostic factor in any cancer regardless of organ [26]. Overexpression of TERT by its promoter mutation representing late events of the oncogenic process may increase the self-renewal capacity of cancer stem cells and induce poor clinical outcomes [27, 28]. The non-canonical functions of TERTp mutation might biologically sustain how TERTp mutation is related to poor prognosis.
Vinagre et al. [29] divided various tumors into those with a high frequency of mutations (≥ 5%) and tumors with no mutations or with a very low frequency of TERTp mutations (< 5%) because mutations affecting the telomerase coding region are very uncommon in the cancer setting [11]. We observed 7.5% TERTp mutation in TSCCs, which is a relatively high frequency according to Vinagre et al. [29]. The frequency of TERTp mutation is variable in head and neck SCCs, which could be because the TERTp mutation can also result from environmental factors such as ultraviolet radiation and chemical carcinogens [29, 30]. Thus, oral cavity SCCs have a wide range of the frequency of 0–65% for the TERTp mutation [14, 17, 22, 23]; this mutation is also common for tongue area (63.6%) and laryngeal SCCs (27%) [22, 25].
The lack of specific clinicodemographic features related to TERTp mutation, found in the present study, has been mainly described in SCCs in head and neck or other sites including skin, lung, and uterine cervix [14, 22, 25, 31]. The majority (83.3%) of TERTp mutations occurred exclusively in the HPV16-positive TSCCs, although there was no statistical association between TERTp mutation and HPV. The considerable presence of TERTp mutation in HPV-positive tumors but a lack of statistical association between them have been previously described in the oral, oropharyngeal, and uterine cervical cancers, where all HPV16-positive oral SCCs and 70% of HPV-positive uterine cervical cancers harbored TERTp mutations [14, 22]. Some previous studies assumed that HPV-induced TERTp activations would be functionally different from the consequences of TERTp mutation in the absence of HPV [11, 18–20].
This study thoroughly investigated HPV status and genotypes in clinical specimens and stratified patients according to HPV status, with long-term follow-up data. In the present study, only high-risk HPV was dominated (80%) with the majority (92.2%) of HPV16. High-risk HPVs are the cause of approximately 31% of oropharyngeal SCCs [2, 9]. The prevalence of HPV infections in TSCCs is 37–80% in western countries and 35–73% in Korea [3, 7, 21, 32, 33].
HPV16 is the dominant virus in oropharyngeal SCC accounting for 82–87% of all HPV-positive cases [9]. The fraction of oropharyngeal cancer attributable to HPV is similarly the highest (> 40%) in developed countries including Europe, North America, Australia, New Zealand, Japan, and Republic of Korea [9, 34]. HPV infection was not an independent prognostic factor for OS and DFS in the 8th edition in the present study, which may be because of the incorporation of HPV into T and N categories of the staging system, and the effect of HPV might have been overshadowed by the impact of the staging modification because our study analyzed all together with HPV-positive and HPV-negative tumors.
In conclusion, the present study reveals that the TERTp mutation is present in a subpopulation of patients with TSCC and emphasizes the negative prognostic impact of TERTp mutation in tonsil cancers under the newly 8th edition of the AJCC staging system for oropharyngeal cancers, especially on HPV-negative TSCCs. This may be utilized to determine clinical aggressiveness of TSCCs.
Abbreviations
- TSCC
Tonsillar squamous cell carcinoma
- HPV
Human papillomavirus
- SCC
Oropharyngeal squamous cell carcinoma
- AJCC
American Joint Committee on Cancer
- TERTp
TERT promoter
- OS
Overall survival
- DFS
Disease-free survival
- BOT
Base of tongue
Author Contributions
MJK and HK designed the study, interpreted the data, wrote the manuscript, and drafted of the manuscript; ESN, SJC, K-WM, and MH participated in study coordination and pathologic data analysis; BP, HGC, ESK, HSH, TK, and HJK collected clinical samples and analyzed clinical data. All authors read and approved the final manuscript.
Funding
This research was supported by the Hallym University Research Fund (HURF-2018-64) and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT of Korea government (NRF-2019R1C1C1004463) granted to MJ Kwon.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Compliance with Ethical Standards
Declarations
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
The surgical materials were collected and used according to the instructions of Kangdong Sacred-Heart Hospital Institutional Ethics Committee (No. 14-2-57). Specimens and data were stored anonymously.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Naggar AK, JKC C, Rubin Grandis J, Takata T, Slootweg PJ, International Agency for Research on Cancer . WHO classification of head and neck tumours. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 2.Taberna M, Mena M, Pavon MA, Alemany L, Gillison ML, Mesia R. Human papillomavirus-related oropharyngeal cancer. Ann Oncol. 2017;28(10):2386–2398. doi: 10.1093/annonc/mdx304. [DOI] [PubMed] [Google Scholar]
- 3.Luginbuhl A, Sanders M, Spiro JD. Prevalence, morphology, and prognosis of human papillomavirus in tonsillar cancer. Ann Otol Rhinol Laryngol. 2009;118(10):742–749. doi: 10.1177/000348940911801010. [DOI] [PubMed] [Google Scholar]
- 4.Kuo YY, Chu PY, Chang SY, Wang YF, Tsai TL, Yang MH, Wang LW, Tai SK. Treatment selection for tonsillar squamous cell carcinoma. J Chin Med Assoc. 2013;76(4):211–217. doi: 10.1016/j.jcma.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Olaleye O, Moorthy R, Lyne O, Black M, Mitchell D, Wiseberg J A 20-year retrospective study of tonsil cancer incidence and survival trends in South East England: 1987-2006. Clin Otolaryngol 36(4):325–335 [DOI] [PubMed]
- 6.Amin MB, American Joint Committee on Cancer., American Cancer Society (2017) AJCC cancer staging manual. 8th Edition, American Joint Committee on Cancer, Springer: Chicago IL
- 7.Song JS, Kim MS, Park JW, Lee YS, Kang CS. Expression of human papillomavirus-related proteins and its clinical implication in tonsillar squamous cell carcinoma. Korean J Pathol. 2012;46(2):177–186. doi: 10.4132/KoreanJPathol.2012.46.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park K, Cho KJ, Lee M, Yoon DH, Kim J, Kim SY, Nam SY, Choi SH, Roh JL, Han MW, Lee SW, Song SY, Back JH, Kim SB. p16 immunohistochemistry alone is a better prognosticator in tonsil cancer than human papillomavirus in situ hybridization with or without p16 immunohistochemistry. Acta Otolaryngol. 2013;133(3):297–304. doi: 10.3109/00016489.2012.741327. [DOI] [PubMed] [Google Scholar]
- 9.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HS, Kwon MJ, Song JH, Kim ES, Kim HY, Min KW. Clinical implications of TERT promoter mutation on IDH mutation and MGMT promoter methylation in diffuse gliomas. Pathol Res Pract. 2018;214(6):881–888. doi: 10.1016/j.prp.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 12.Arita H, Narita Y, Takami H, Fukushima S, Matsushita Y, Yoshida A, Miyakita Y, Ohno M, Shibui S, Ichimura K. TERT promoter mutations rather than methylation are the main mechanism for TERT upregulation in adult gliomas. Acta Neuropathol. 2013;126(6):939–941. doi: 10.1007/s00401-013-1203-9. [DOI] [PubMed] [Google Scholar]
- 13.Dogan S, Xu B, Middha S, Vanderbilt CM, Bowman AS, Migliacci J, Morris LGT, Seshan VE, Ganly I. Identification of prognostic molecular biomarkers in 157 HPV-positive and HPV-negative squamous cell carcinomas of the oropharynx. Int J Cancer. 2019;145:3152–3162. doi: 10.1002/ijc.32412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinothkumar V, Arunkumar G, Revathidevi S, Arun K, Manikandan M, Rao AK, Rajkumar KS, Ajay C, Rajaraman R, Ramani R, Murugan AK, Munirajan AK. TERT promoter hot spot mutations are frequent in Indian cervical and oral squamous cell carcinomas. Tumour Biol. 2016;37(6):7907–7913. doi: 10.1007/s13277-015-4694-2. [DOI] [PubMed] [Google Scholar]
- 15.Ghantous Y, Bahouth Z, Abu El-Naaj I. Clinical and genetic signatures of local recurrence in oral squamous cell carcinoma. Arch Oral Biol. 2018;95:141–148. doi: 10.1016/j.archoralbio.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Barczak W, Suchorska WM, Sobecka A, Bednarowicz K, Machczynski P, Golusinski P, Rubis B, Masternak MM, Golusinski W. hTERT C250T promoter mutation and telomere length as a molecular markers of cancer progression in patients with head and neck cancer. Mol Med Rep. 2017;16(1):441–446. doi: 10.3892/mmr.2017.6590. [DOI] [PubMed] [Google Scholar]
- 17.Chang KP, Wang CI, Pickering CR, Huang Y, Tsai CN, Tsang NM, Kao HK, Cheng MH, Myers JN. Prevalence of promoter mutations in the TERT gene in oral cavity squamous cell carcinoma. Head Neck. 2017;39(6):1131–1137. doi: 10.1002/hed.24728. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Roberts J, Dakic A, Zhang Y, Schlegel R. HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function. Virology. 2008;375(2):611–623. doi: 10.1016/j.virol.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HY, Kim G, Cho H, Kim S, Lee D, Park S, Park KH, Lee H. Diagnostic performance of HPV E6/E7, hTERT, and Ki67 mRNA RT-qPCR assays on formalin-fixed paraffin-embedded cervical tissue specimens from women with cervical cancer. Exp Mol Pathol. 2015;98(3):510–516. doi: 10.1016/j.yexmp.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Dakic A, Chen R, Disbrow GL, Zhang Y, Dai Y, Schlegel R. Cell-restricted immortalization by human papillomavirus correlates with telomerase activation and engagement of the hTERT promoter by Myc. J Virol. 2008;82(23):11568–11576. doi: 10.1128/JVI.01318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Koo BS, Kang S, Park K, Kim H, Lee KR, Lee MJ, Kim JM, Choi EC, Cho NH. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120(7):1418–1425. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- 22.Annunziata C, Pezzuto F, Greggi S, Ionna F, Losito S, Botti G, Buonaguro L, Buonaguro FM, Tornesello ML. Distinct profiles of TERT promoter mutations and telomerase expression in head and neck cancer and cervical carcinoma. Int J Cancer. 2018;143(5):1153–1161. doi: 10.1002/ijc.31412. [DOI] [PubMed] [Google Scholar]
- 23.Boscolo-Rizzo P, Giunco S, Rampazzo E, Brutti M, Spinato G, Menegaldo A, Stellin M, Mantovani M, Bandolin L, Rossi M, Del Mistro A, Tirelli G, Dei Tos AP, Guerriero A, Niero M, Da Mosto MC, Polesel J, De Rossi A. TERT promoter hotspot mutations and their relationship with TERT levels and telomere erosion in patients with head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2020;146(2):381–389. doi: 10.1007/s00432-020-03130-z. [DOI] [PubMed] [Google Scholar]
- 24.Kwon MJ, Kang SY, Cho H, Lee JI, Kim ST, Suh YL (2019) Clinical relevance of molecular subgrouping of gliomatosis cerebri per 2016 WHO classification: a clinicopathological study of 89 cases. Brain Pathol [DOI] [PMC free article] [PubMed]
- 25.Qu Y, Dang S, Wu K, Shao Y, Yang Q, Ji M, Shi B, Hou P. TERT promoter mutations predict worse survival in laryngeal cancer patients. Int J Cancer. 2014;135(4):1008–1010. doi: 10.1002/ijc.28728. [DOI] [PubMed] [Google Scholar]
- 26.Yuan P, Cao JL, Abuduwufuer A, Wang LM, Yuan XS, Lv W, Hu J. Clinical characteristics and prognostic significance of TERT promoter mutations in cancer: a cohort study and a meta-analysis. PLoS One. 2016;11(1):e0146803. doi: 10.1371/journal.pone.0146803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He TC, He Y, Hruban RH, Jallo GI, Mandahl N, Meeker AK, Mertens F, Netto GJ, Rasheed BA, Riggins GJ, Rosenquist TA, Schiffman M, Shih Ie M, Theodorescu D, Torbenson MS, Velculescu VE, Wang TL, Wentzensen N, Wood LD, Zhang M, McLendon RE, Bigner DD, Kinzler KW, Vogelstein B, Papadopoulos N, Yan H. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong DE, Woo SR, Nam H, Nam DH, Lee JH, Joo KM. Preclinical and clinical implications of TERT promoter mutation in glioblastoma multiforme. Oncol Lett. 2017;14(6):8213–8219. doi: 10.3892/ol.2017.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinagre J, Pinto V, Celestino R, Reis M, Populo H, Boaventura P, Melo M, Catarino T, Lima J, Lopes JM, Maximo V, Sobrinho-Simoes M, Soares P. Telomerase promoter mutations in cancer: an emerging molecular biomarker? Virchows Arch. 2014;465(2):119–133. doi: 10.1007/s00428-014-1608-4. [DOI] [PubMed] [Google Scholar]
- 30.Populo H, Boaventura P, Vinagre J, Batista R, Mendes A, Caldas R, Pardal J, Azevedo F, Honavar M, Guimaraes I, Manuel Lopes J, Sobrinho-Simoes M, Soares P. TERT promoter mutations in skin cancer: the effects of sun exposure and X-irradiation. J Invest Dermatol. 2014;134(8):2251–2257. doi: 10.1038/jid.2014.163. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Gong R, Wang R, Pan Y, Cai D, Pan B, Li Y, Xiang J, Li H, Zhang J, Sun Y, Chen H. Recurrent TERT promoter mutations in non-small cell lung cancers. Lung Cancer. 2014;86(3):369–373. doi: 10.1016/j.lungcan.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Mellin H, Friesland S, Auer G, Dalianis T, Munck-Wikland E. Human papillomavirus and DNA ploidy in tonsillar cancer--correlation to prognosis. Anticancer Res. 2003;23(3C):2821–2828. [PubMed] [Google Scholar]
- 33.Lee M, Kim SB, Lee SW, Roh JL, Choi SH, Nam SY, Kim SY, Cho KJ. Human papillomavirus prevalence and cell cycle related protein expression in tonsillar squamous cell carcinomas of Korean patients with clinicopathologic analysis. Korean J Pathol. 2013;47(2):148–157. doi: 10.4132/KoreanJPathol.2013.47.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gayatree A, Tanveer N, Arora VK, Arora V (2020) Are histomorphological features predictive of p16 immunopositivity different for oral and oropharyngeal squamous cell carcinoma? Indian Journal of Surgical Oncology 11(2):248–255 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.