Abstract
Oral squamous cell carcinoma (OSCC) is one of the most prevalent cancers in India with high incidence rate in eastern region due to habits of tobacco, pan and gutkha chewing habits. In majority of OSCC, the cases were presented to clinicians at later stages of the disease which leads to increased mortality. In addition presence of minimal residual disease also significantly contributed towards disease progression. Therefore, identification of potential biomarker for prognostic stratification of patients with high risk of disease recurrence and appropriate management is utmost necessary. In this study, 80 OSCC patients were included and their tumour specimen along with cut margin (CM) was collected after surgical excision. Immunohistochemistry (IHC) was performed to check expression of TRF2 in tumour and CM of OSCC patients. Statistical analysis was carried out using SPSS based on clinical and pathological records. It was observed that 27 OSCC patients developed recurrence during the period of the study (2012–2016). It was observed that, in 34 cases (42.25%) TRF2 expression was positive in tumour, while in 46 cases (57.75%), it was negative, while it was just reverse at CM, respectively. The odds of recurrence among patients having high levels of TRF2 in CM were 2.6 times higher than the odds of recurrence among patients having lower levels of TRF2 in CM. In conclusion, this study showed that TRF2 at surgical cut margin has a prognostic significance and can be used as a molecular marker for predicting survival in OSCC patients.
Keywords: Oral squamous cell carcinoma (OSCC), TRF2, Recurrence, Prognostic marker
Introduction
Oral carcinoma is one of the most widespread cancers of the world with highest recurrence rates and low mortality mainly due to the presentation of cases at later stages and the presence of MRD (Minimal Residual Disease) [1, 2]. The prognostication of the disease still remains a significant problem owing to variables like TNM staging, histological grade and tumour thickness and involvement of several biomarkers which contribute to the risk for recurrence [3–5]. Several reports have also indicated the presence of pathological factors like lypmhovascular invasion (LVI) and perineural invasion (PNI) in poor prognosis of oral cancer patients [6–9]. PNI has also been significantly correlated with poor prognosis in solid tumour patients as well thereby suggesting PNI to be an important factor for consideration in prognostication of oral cancers [10, 11].
Telomeric repeat factor-2 (TRF2) is a telomeric shelterin protein which is involved in the maintenance of genome stability by protecting the telomeric ends of chromosomes through t-loop formation and preventing DNA repair mechanism at the telomeres [12]. Apart from being a key telomeric protein, recent reports have detailed an extra-telomeric role of TRF2 in ATM-mediated DNA damage repair at non-telomeric sites [13, 14]. Furthermore, TRF2 is also reported to be the direct target of β-catenin which in turn is reported to be involved in cancer progression in several solid tumours [15]. In a recent report, TRF2 was also reported to contribute to maintenance of colon cancer stem-like cells which may be responsible for disease recurrence [16].
In this study, we report a possible correlation of TRF2 expression with tumour recurrence. We also tried to evaluate a correlation between TRF2 expression in cut margin and tumours with the recurrence in the presence of LVI / PNI and grade of the tumour.
Materials and Methods
Clinical Sample Collection and Ethics Statement
The study was carried out on OSCC patient’s samples collected during the period 2010 to 2016 at Apollo Hospitals and Kalinga Institute of Medical Sciences, Bhubaneswar. The study was approved by the ethics committees of both institutions and conducted according to Helsinki declaration. Voluntary consent forms were duly signed by patients or their nominees prior to the participation in the study. Tissue samples were collected at the time of surgical excision. The clinical and pathological data were collected from the medical records. Cut margin (CM) and tumour tissues were collected during surgical resection of the tumour, and formalin-fixed paraffin-embedded (FFPE) block was prepared as per standard protocol [17]. A total of 80 patients were included in the study. The clinicopathological details of the OSCC patients were presented in the manuscript communicated elsewhere.
TRF2 Expression by Immunohistochemistry (IHC) Analysis
Tumour and cut margin sections (3um) of cases from both Cohorts I and II were taken on poly-L-lysine slides for immunohistochemistry. Sections were then deparaffinized, redehydrated and then probed with TRF2 antibody (Abcam; 1:500 dilution) as per laboratory established protocols [17]. The tissues were then probed with anti-TRF2 secondary antibody (Abcam, 1:1000 dilutions). Staining and detection was performed by the DAB detection system. Signals generated were observed under Leica brightfield microscope. Histoscore was obtained by multiplying percentage positive cells (1–100%) with the intensity of the positive signal (low as negative and high as positive).
Statistical Analysis
Analysis was carried out using SPSS version 25 (IBM Corp., Armonk, NY, USA). For analysis, association between level of receptors at surgical resection margin and tumour with recurrence, stage and grade of tumour was assessed using chi-square test. P value less than 0.05 were considered statistically significant. Survival has been evaluated with Kaplan-Meier survival analysis with log rank correlation.
Results
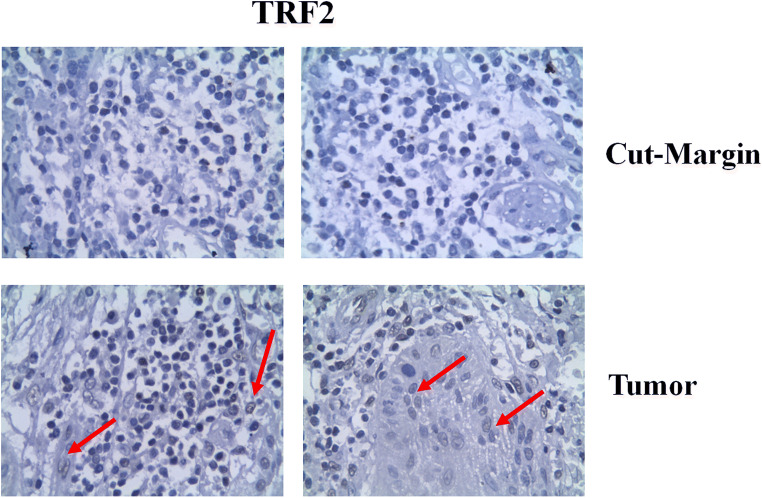
Among 80 patients, 27 patients (33.75%) developed recurrence. Table 1 shows that, in 34 cases (42.25%), TRF2 expression was positive in tumour, while in 46 cases (57.75%), it was negative, while it was just reverse at cut margin, respectively (Fig. 1). Table 2 shows the sensitivity of the marker is 88.9% when positive in cut margin, while its sensitivity is quite low 11.11% for positive in tumour. But important finding is that its negative predictive value is 91.18% and accuracy is 68.75% for TRF2 marker in cut margin. The recurrence was higher (24 out of 27 cases, 88.9%) when TRF2 was positive in cut margin. Another interesting finding in our series is that the recurrence was low (3 cases = 11.1%) in situation where TRF2 is positive in tumour and negative in cut margin (33 cases = 42.25%), while it was quite high (23 out of 27 recurrences, 85.2%) when marker is negative in tumour but positive in cut margin (in 45 cases = 56.25%) cases. In Fisher’s exact calculation with cross table linear evaluation, it was showing a significant value (P = 0.000) while comparing to the rest.
Table 1.
Expression of TRF2 at tumour and cut margin of OSCC patients
| Biomarker | Site | Number | Recurrence | Significance (P value) | |
|---|---|---|---|---|---|
| TRF2 | Tumour | + ve | 34 | 03 | 0.000 |
| − ve | 46 | 24 | |||
| Cut Margin | + ve | 46 | 24 | 0.000 | |
| − ve | 34 | 03 | |||
| Tumour + ve/cut margin − ve | 33 | 03 | 0.000 | ||
| Rest | 47 | 24 | |||
| Tumour − ve/cut margin + ve | 45 | 23 | 0.000 | ||
| Rest | 35 | 04 | |||
Fig. 1.
Immunohistochemical analysis of TRF2 in cut margin and tumour of OSCC patients
Table 2.
TRF2 as a marker for prediction of recurrence in OSCC
| Biomarker | Site | Sensitivity (%) |
Specificity (%) |
Positive predictive value (%) | Negative predictive value (%) | Accuracy (%) |
|---|---|---|---|---|---|---|
| TRF2 | Tumour | 11.11 | 41.51 | 8.82 | 47.83 | 31.25 |
| Cut Margin | 88.9 | 58.49 | 51.17 | 91.18 | 68.75 |
In multivariate analysis using Nagalkerke R2 model, as shown in Table 3, interpretation of each predictor was as follows:
LVI – The adjusted odds ratio for LVI was 0.735 with 95% confidence interval of 0.098–5.509. This indicates that in this sample, the patients who had LVI were 0.74 times less likely to have recurrence than those patients who had no LVI after controlling for other predictors in the model, and the predictor was not statistically significant.
PNI – The adjusted odds ratio for PNI was 0.849 with 95% confidence interval of 0.246–2.922. This indicates that in this sample, the patients who had PNI were 0.85 times less likely to have recurrence than those patients who had no PNI after controlling for other predictors in the model, and the predictor was not statistically significant.
Grade – The adjusted odds ratio for Grade was 1.433 with 95% confidence interval of 0.541–3.793. This indicates that in this sample, the patients who had higher Grade were 1.4 times more likely to have recurrence than those patients who had lower Grade after controlling for other predictors in the model, and the predictor was not statistically significant.
TRF2 in CM – The adjusted odds ratio for TRF2 in CM was 2.64 with 95% confidence interval of 0.070–99.183. This indicates that in this sample, the patients who had high levels of TRF2 in CM had 2.6 times more likely to have recurrence than those patients who had low levels of TRF2 in CM after controlling for other predictors in the model, and the predictor is not statistically significant (P = 0.599). (Or) The odds of recurrence among patients having high levels of TRF2 in CM was 2.6 times higher than the odds of recurrence among patients having lower levels of TRF2 in CM, after controlling for other predictors in the model.
Table 3.
Logistic regression analysis of recurrence predictors in OSCC
| Predictors | B | P Value | Exp (B) | 95% CI for Exp (B) | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| 1. | LVI | − .308 | .765 | .735 | .098 | 5.509 |
| 2. | PNI | − .164 | .795 | .849 | .246 | 2.922 |
| 3. | Grade | .360 | .469 | 1.433 | .541 | 3.793 |
| 4. | TRF2 in CM | .972 | .599 | 2.644 | .070 | 99.183 |
| 5. | TRF2 in tumour (ref) | |||||
| Nagalkerke R2 = 0.299 | ||||||
The Nagalkerke R2 value (0.299) implies that 29.9% of variation in outcome (recurrence/non-recurrence) is explained by the predictor variables included in the model.
Discussion
The College of American Pathologists guidelines state the involvement of the lymphovascular invasion (LVI), perineural invasion (PNI) and grade of the tumour along with traditional diagnostic characters like tumour size, presence of distant metastases and lymph node involvement needs to be considered for characterization of oral carcinoma [18]. Several research articles have elucidated that PNI-based characterization has a significant prognostic value in solid tumours [3, 7–9]. Furthermore, several biomarkers have been investigated for prognostication of OSCC [19–21]. TRF2 has been studied in many solid tumours and has been found to be overexpressed including head and neck cancer [22]. Its overexpression in human lung and gastric cancer suggests a role in tumour initiation and development [23, 24]. Study by Pal et al. suggested that inhibition of TRF2 expression will reduce cell proliferation and migration and induced apoptosis in renal cell carcinoma serving as a potential therapeutic target [25]. Ozden et al., based on their study in advanced cervical cancer patients, stated role of TRF2 in apoptosis and a positive correlation with distant metastasis. They suggested that TRF2 may be a factor to estimate survival for cervical cancer [26]. Ning et al. studied TRF2 role in multidrug resistant gastric cancer. Results of this study indicated that TRF2 plays an important role in DNA damage response and is involved in drug resistance of gastric cancer. Novel drugs may be generated to target TRF2 which might overcome multiple drug resistance [27]. Benhamou et al. conducted a retrospective study of 62 patients of oral squamous cell carcinoma. They assessed whether TRF2 influences tumour aggressiveness and treatment response. The study showed that TRF2 overexpression has a negative impact on survival time. On multivariate analysis they also stated that TRF2 dependent survival time was independent of tumour size [28].
In the current study, we have endeavoured to find a correlation between a previously discovered biomolecule, TRF2, as being clinically linked to prognosis [22] along with the PNI, LVI status and Grade of the tumour in question. TRF2 being a shelterin component is actively involved in the maintenance of genomic stability. Most solid tumours report genomic instability as a hallmark for oncogenesis and cancer progression. Previous in vitro analysis reports the involvement of TRF2 in cancer stemness and disease resistance and also in prognosis [22]. The present study shows increase in expression levels of TRF2 in cases with increasing stage and grade of tumour. Recurrence was more when TRF2 was positive in cut margin that is 24 out of 27 cases of recurrences (88.9%). Recurrence was also quite high (23 out of 27 recurrences, 85.2%) when marker is negative in tumour but positive in cut margin (in 45 cases = 56.25%) cases. A regression analysis of LVI, PNI, Grade of the tumour and TRF2 in cut margin was evaluated for correlation with recurrence. The patients who had higher Grade tumour were 1.4 times more likely to have recurrence than those who had lower Grade after controlling for other predictors in the model. OSCC cases with TRF2 overexpression in cut margin were 2.6 times likely to recur when compared to cases with low TRF2 in cut margin. On analysis of the LVI, PNI and grade in the given cohort, no significant correlation was observed with recurrence of tumour.
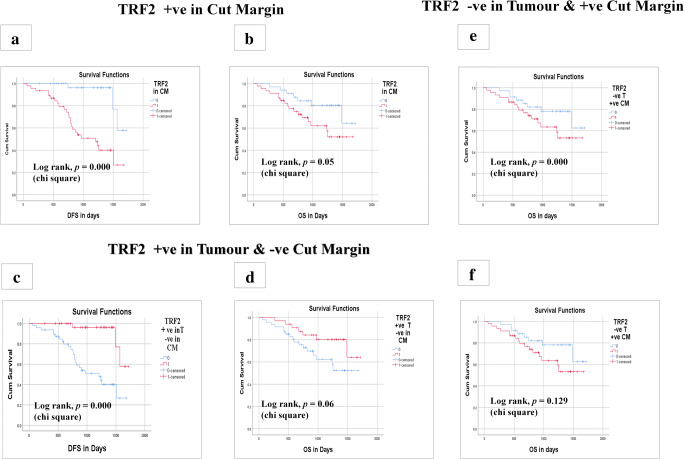
We have seen significant lower disease-free and overall survival of the patients who had positive TRF2 in cut margin as compared to negative in Kaplan-Meier survival curve with log rank correlation (P < 0.000) (Fig. 2a,b). While it has been shown better disease-free and overall survival in TRF2 positive in tumour and negative in cut margin (Fig. 2c,d). That means the disease proliferation at the periphery of the tumour has shown the aggressive nature of the disease as marked by this TRF2 molecular marker which has been elucidated by the tumorigenesis concept [29].
Fig. 2.
Kaplan-Meier curve survival analysis based on expression of TRF2 in cut margin and tumour of OSCC patients. a Kaplan-Meier curve of disease free survival (DFS) on basis of positive expression of TRF2 in cut margin. b Kaplan-Meier curve of overall survival (OS) on basis of positive expression of TRF2 in cut margin. c Kaplan-Meier curve of disease free survival (DFS) on basis of positive expression of TRF2 in tumour and negative expression in cut margin. d Kaplan-Meier curve of overall survival (OS) on basis of positive expression of TRF2 in tumour and negative expression in cut margin. e Kaplan-Meier curve of disease free survival (DFS) on basis of positive expression of TRF2 in cut margin and negative expression in tumour. f Kaplan-Meier curve of overall survival (OS) on basis of positive expression of TRF2 in cut margin and negative expression in tumour
There is an interesting revelation of our study, that there was a significant lower disease-free survival (P < 0.000) when TRF2 was positive at the cut margin but negative in tumour (Fig. 2e,f). This phenomenon has been clearly supported by the concept of epithelial cell gaining the property of stemness in the peripheral cells [30].
Conclusion
The present study showed that TRF2 at surgical cut margin has a prognostic significance as a molecular marker for predicting survival in oral cancer patients. Use of TRF2 as the molecular marker at surgical margins can predict recurrences better than histopathological analysis. Inclusion of TRF2 as a potential biomarker with existing characterization guidelines will enhance the accuracy and predictability of OSCC.
Acknowledgements
The authors acknowledge Department of Biotechnology, Government of India for providing grant and technical support of Molecular Stress and Stem Cell Biology (MSSB) group to carry out this study.
Author’s Contribution
MK carried out the surgery, planned the experiments, interpreted the clinical data and wrote the manuscript. MS performed the data analysis, interpreted the data and wrote the manuscript. SR and SP collected tissues, processed for experimental analysis, accumulated the data and helped in organizing the manuscript. BNB conceived the idea, guided through the experiments, wrote and edited the manuscript.
Funding Information
This study was supported by grant from Department of Biotechnology, Government of India, Grant No- BT/PR 17576/MED/30/1690/2016, [Virtual National Oral Cancer Institute (Understanding the Disease Biology and Epigenetic Diversity of Oral Cancer in India: Implications for New Diagnostics and Therapeutics)].
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Madhabananda Kar, Email: madhabananda@gmail.com.
Birendranath Banerjee, Email: bnbanerjee@kiitbiotech.ac.in.
References
- 1.Sproll C, Fluegen G, Stoecklein NH (2018) Minimal Residual Disease in Head and Neck Cancer and Esophageal Cancer, in Biological Mechanisms of Minimal Residual Disease and Systemic Cancer, J.A. Aguirre-Ghiso, Editor, Springer International Publishing: Cham p 55-82 [DOI] [PubMed]
- 2.Padhi S, Saha A, Kar M, Ghosh C, Adhya A, Baisakh M, Mohapatra N, Venkatesan S, Hande MP, Banerjee B. Clinico-pathological correlation of β-catenin and telomere dysfunction in head and neck squamous cell carcinoma patients. J Cancer. 2015;6(2):192–202. doi: 10.7150/jca.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S-A, Wang CC, Jiang RS, Lee FY, Lin WJ, Lin JC. Pathological features and their prognostic impacts on oral cavity cancer patients among different subsites - a singe institute's experience in Taiwan. Sci Rep. 2017;7(1):7451–7451. doi: 10.1038/s41598-017-08022-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Swiahb JN, et al. Clinical, pathological and molecular determinants in squamous cell carcinoma of the oral cavity. Future Oncol. 2010;6(5):837–850. doi: 10.2217/fon.10.35. [DOI] [PubMed] [Google Scholar]
- 5.Akhter M, et al. (2011) A study on histological grading of oral squamous cell carcinoma and its correlation with regional metastasis. Vol. 15. 168–76 [DOI] [PMC free article] [PubMed]
- 6.Viswanatha SC, Hedne N, Hasan S. Correlation between histological grading, LVI and PNI of carcinoma oral tongue to lymph node metastasis. Int J Otorhinolaryngol Head Neck Surg. 2019;5(1):2018. [Google Scholar]
- 7.Lin YT, Chien CY, Lu CT, Lou SD, Lu H, Huang CC, Fang FM, Li SH, Huang TL, Chuang HC. Triple-positive pathologic findings in oral cavity cancer are related to a dismal prognosis. Laryngoscope. 2015;125(9):E300–E305. doi: 10.1002/lary.25463. [DOI] [PubMed] [Google Scholar]
- 8.Jardim JF, Francisco AL, Gondak R, Damascena A, Kowalski LP. Prognostic impact of perineural invasion and lymphovascular invasion in advanced stage oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2015;44(1):23–28. doi: 10.1016/j.ijom.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Chen T-C, et al. (2013) The Impact of Perineural Invasion and/or Lymphovascular Invasion on the Survival of Early-Stage Oral Squamous Cell Carcinoma Patients. Vol. 20 [DOI] [PubMed]
- 10.Huh JW, Lee JH, Kim HR, Kim YJ. Prognostic significance of lymphovascular or perineural invasion in patients with locally advanced colorectal cancer. Am J Surg. 2013;206(5):758–763. doi: 10.1016/j.amjsurg.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Al-Sukhni E, et al. Lymphovascular and perineural invasion are associated with poor prognostic features and outcomes in colorectal cancer: a retrospective cohort study. Int J Surg. 2017;37:42–49. doi: 10.1016/j.ijsu.2016.08.528. [DOI] [PubMed] [Google Scholar]
- 12.Feuerhahn S, Chen LY, Luke B, Porro A. No DDRama at chromosome ends: TRF2 takes Centre stage. Trends Biochem Sci. 2015;40(5):275–285. doi: 10.1016/j.tibs.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Mao Z, et al. TRF2 is required for repair of nontelomeric DNA double-strand breaks by homologous recombination. Proc Natl Acad Sci U S A. 2007;104(32):13068–13073. doi: 10.1073/pnas.0702410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka H, et al. (2005) DNA damage-induced phosphorylation of the human telomere-associated protein TRF2, in Proc Natl Acad Sci U S A. p. 15539–44 [DOI] [PMC free article] [PubMed]
- 15.Diala I, Wagner N, Magdinier F, Shkreli M, Sirakov M, Bauwens S, Schluth-Bolard C, Simonet T, Renault VM, Ye J, Djerbi A, Pineau P, Choi J, Artandi S, Dejean A, Plateroti M, Gilson E. Telomere protection and TRF2 expression are enhanced by the canonical Wnt signalling pathway. EMBO Rep. 2013;14(4):356–363. doi: 10.1038/embor.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saha A, Padhi S, Kar M, Roy S, Maiti P, Banerjee B. Role of TRF2 in efficient DNA repair, spheroid formation and Cancer Stem Cell maintenance. Oncomedicine. 2017;2:71–79. doi: 10.7150/oncm.18373. [DOI] [Google Scholar]
- 17.Canene-Adams K (2013) Chapter Fifteen - Preparation of Formalin-fixed Paraffin-embedded Tissue for Immunohistochemistry, in Methods in Enzymology, J. Lorsch, Editor, Academic Press. p. 225–233 [DOI] [PubMed]
- 18.Ashmead MG (2013) Malignant Neoplasms of the Oral Cavity, in Encyclopedia of Otolaryngology, Head and Neck Surgery, S.E. Kountakis, Editor, Springer Berlin Heidelberg: Berlin, Heidelberg p 1558-1567
- 19.Li G, et al. Prediction of biomarkers of oral squamous cell carcinoma using microarray technology. Sci Rep. 2017;7:42105. doi: 10.1038/srep42105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedro NF, et al. Candidate biomarkers for Oral squamous cell carcinoma: differential expression of oxidative stress-related genes. Asian Pac J Cancer Prev. 2018;19(5):1343–1349. doi: 10.22034/APJCP.2018.19.5.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R, Yuan Y, Zhou Y, Zhang D, Zhang L, Zeng X, Ji N, Zhou M, Liang X, Chen Y, Geng N, Li J, Chen Q. Screening diagnostic biomarkers of OSCC via an LCM-based proteomic approach. Oncol Rep. 2018;40(4):2088–2096. doi: 10.3892/or.2018.6610. [DOI] [PubMed] [Google Scholar]
- 22.Saha A, et al. Role of Telomeric TRF2 in Orosphere formation and CSC phenotype maintenance through efficient DNA repair pathway and its correlation with recurrence in OSCC. Stem Cell Rev Rep. 2018;14(6):871–887. doi: 10.1007/s12015-018-9823-z. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi K, Kawai T, Kumaki F, Hiroi S, Mukai M, Ikeda E, Koering CE, Gilson E. Expression of mRNAs for telomeric repeat binding factor (TRF)-1 and TRF2 in atypical adenomatous hyperplasia and adenocarcinoma of the lung. Clin Cancer Res. 2003;9:1105–1111. [PubMed] [Google Scholar]
- 24.Miyachi K, Fujita M, Tanaka N, Sasaki K, Sunagawa M. Correlation between telomerase activity and telomeric-repeat binding factors in gastric cancer. J Exp Clin Cancer Res. 2002;21:269–275. [PubMed] [Google Scholar]
- 25.Pal D, Sharma U, Singh SK, Kakkar N, Prasad R. Over-expression of telomere binding factors (TRF1 & TRF2) in renal cell carcinoma and their inhibition by using SiRNA induce apoptosis, reduce cell proliferation and migration invitro. PLoS One. 2015;10:e0115651. doi: 10.1371/journal.pone.0115651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozden S, Tiber PM, Ozgen Z, Ozyurt H, Serakinci N, Orun O. Expression of TRF2 and its prognostic relevance in advanced stage cervical cancer patients. Biol Res. 2014;47(1):61. doi: 10.1186/0717-6287-47-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ning H, Li T, Zhao L, Li T, Li J, Liu J, Liu Z, Fan D. TRF2 promotes multidrug resistance in gastric cancer cells. Cancer Biol Ther. 2006;5(8):950–956. doi: 10.4161/cbt.5.8.2877. [DOI] [PubMed] [Google Scholar]
- 28.Benhamou Y, et al. Telomeric repeat-binding factor 2: A marker for survival and anti-EGFR efficacy in oral carcinoma. Oncotarget. 2016;7(28):44236–44251. doi: 10.18632/oncotarget.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6:10697–10711. doi: 10.18632/oncotarget.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]