Abstract
Liver, bones, and brain are common sites for breast cancer metastasis. We report here a rare scenario of metastasis to pancreatic head from breast cancer after a disease free interval of 7 years. A 60-year old breast cancer survivor noticed upper abdominal pain for 2 weeks, and her investigations revealed a pancreatic head mass lesion. Computed tomography imaging revealed a solitary pancreatic mass lesion with portal cavernoma formation and a guided biopsy yielded adenocarcinoma on histopathological examination. Immunohistochemistry processing demonstrated estrogen receptor, cytokeratin 7, and GATA 3 positivity which confirmed it to be a metastasis. Therapy was initiated with palbociclib and exemestane. Later, everolimus was started in view of failure of hormonal therapy. The patient is still alive 21 months after diagnosing the recurrence.
Keywords: Pancreatic metastasis, Breast cancer, Palbociclib, Estrogen receptor
Introduction
Advances in oncology have made it possible to put a strong defense against breast cancer. Consequently, the survival of breast cancer patients has increased. But, there still exists a possibility of developing distant recurrences even after many years [1]. Liver, bones, and brain are the usual sites where breast cancer metastasizes [2]. Pancreas is an unusual site for metastasis [3]. We report here a solitary pancreatic metastasis from a breast primary after 7 years of primary treatment. Evaluation of such unusual case scenarios may provide us insights into the hallmark process of tissue invasion and metastasis[4] and its management.
Case Report
A 60-year old breast cancer survivor presented with complaints of new onset upper abdominal pain for 2 weeks. She was on treatment for hypertension and hypothyroidism. She did not have pallor, icterus, clubbing, lymphadenopathy, or pedal edema. Her abdominal examination did not reveal any abdominal lump. Her complete blood count, kidney function tests, and liver function tests were within normal limits. Ultrasonography (USG) revealed a mass in the body of pancreas of largest dimension 2.4 cm and an enlarged peripancreatic node of largest dimension 1.2 cm. The rest of the USG examination was normal.
Computed tomography (CT) scan of the patient revealed a likely neoplastic diffuse hypoenhancing lesion of size 4.3 × 2.8 cm in the head region and uncinate process of pancreas infiltrating into the portal vein with portal cavernoma formation. There was long segment abutment of the common hepatic artery. (Figs. 1 and 2) No distant metastases were identified.
Fig. 1.
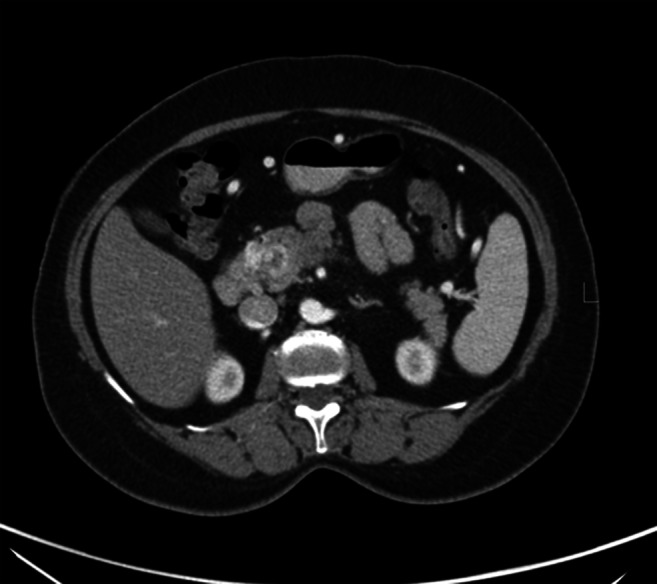
Axial CT image showing head of pancreas mass with multiple venous collaterals due to encasement of portal vein
Fig. 2.

Coronal CT image showing pancreatic head mass with abutment of common hepatic artery
The lady has been treated for left breast cancer 7 years ago. She had undergone modified radical mastectomy and adjuvant chemotherapy. The histology was infiltrating duct carcinoma. Estrogen receptor (ER) was positive while progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) were negative. She also had completed her hormonal therapy for 5 years in view of ER positive breast cancer. Examination of the opposite breast, axillae, and neck was normal.
The dilemma was whether the lesion was a primary pancreatic malignancy or metastasis from breast cancer. Patient underwent a CT-guided percutaneous transgastric trucut biopsy of the pancreatic mass. Biopsy report revealed adenocarcinoma which could be primary pancreatic malignancy or metastasis. Hence, the sample was processed for immunohistochemistry (IHC). Tumor cells were cytokeratin (CK) 7; ER and GATA 3 (GATA binding protein 3) positive; and Ca19.9, CK 20, and synaptophysin IHC markers were negative. Final impression was metastasis of ductal carcinoma in known case of breast cancer. (Figs. 3 and 4) The metastasis was ER positive, PR negative, and HER2 negative. Absence of any symptoms and signs suggestive of widespread disease; normal blood investigation reports; likelihood of good prognostic disease in view of hormone receptor positivity and long disease-free interval (DFI); and avoidance of the trouble and cost of repeat imaging were the reasons to avoid a staging positron emission tomography contrast-enhanced CT scan (PETCECT).
Fig. 3.
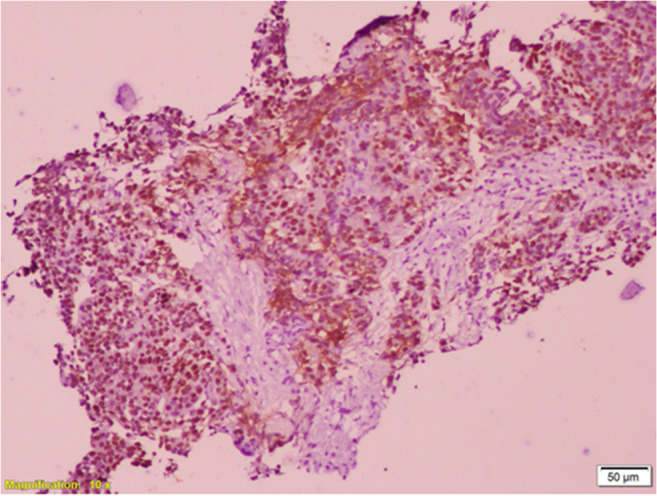
Photomicrograph showing ER positivity of tumor cells by immunohistochemistry
Fig. 4.
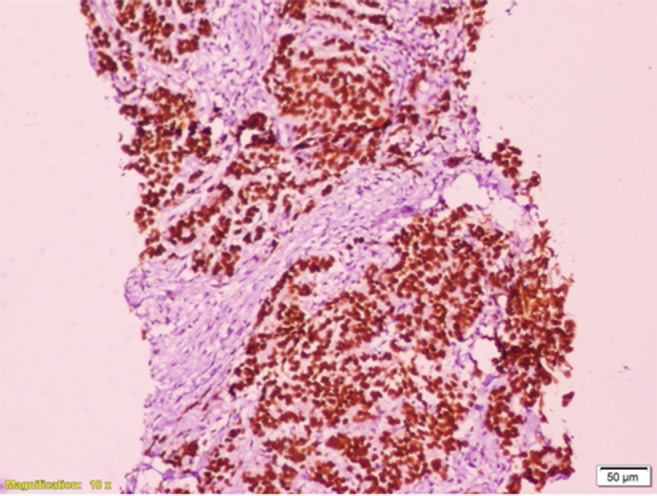
Photomicrograph showing GATA3 positivity of tumor cells by immunohistochemistry
The case was discussed in a multidisciplinary tumor board meeting. Treatment options of hormonal therapy along with palbociclib, local ablative therapy like stereotactic body radiotherapy (SBRT) and surgery were discussed. Presence of portal cavernoma and abutment of hepatic artery precluded surgical resection. Systemic therapy was initiated with cyclin-dependent kinase (CDK) 4 inhibitor, palbociclib, and exemestane, to assess biology of the disease, address micrometastatic disease, and reserve local ablative therapy like SBRT for consolidation. A response assessment PETCECT done 3 months after initiation of therapy demonstrated stable disease. But unfortunately, an increase in the size of pancreatic mass lesion and two liver metastases were identified on response PETCECT done 6 months after initiation of therapy. Due to unequivocal progression of disease and no visceral crisis, therapy was changed to palbociclib and fulvestrant. After 6 months, PETCECT scan showed progression of the pancreatic head mass and regression of the liver lesions. Everolimus with exemestane therapy was started. Clinicoradiological evaluation 3 months later revealed stable disease, but PETCECT scan done 6 months later revealed liver metastases. Hence, the patient has been advised for chemotherapy with paclitaxel. The patient is alive 21 months since diagnosis.
Discussion
Twenty eight cases of pancreatic metastases from breast cancer seem to have been reported so far [5]. A case of solitary pancreatic metastasis from breast cancer has been reported previously from India few decades ago [6]. Disease-free interval of 7 years and solitary pancreatic head metastasis from ductal carcinoma of breast leading to portal cavernoma formation without jaundice are the noteworthy features of this case. With advances in oncology, patients with oligometastatic breast cancer can be offered hope for cure and good quality of life. [7] Reporting of more such cases will definitely help in generating evidence, understanding the management aspects in detail, guiding therapy, and offering hope in such rare case scenarios. It has been noted that the head region has been a more common site of pancreatic metastasis from breast cancer and jaundice as a frequent presenting feature.[5] In this case metastasis has spared the common bile duct with resultant delayed clinical presentation without obstructive jaundice. IHC played a key role in identifying the metastatic nature of the pancreatic lesion. Tissue was obtained by percutaneous transgastric CT-guided trucut biopsy of the head of pancreas lesion. It is a safe technique and obviates the need of endoscopic procedure [8]. Hence, it also seems more cost effective. CK 7 and ER positivity by IHC pointed to the diagnosis of metastasis from breast cancer and resolved the dilemma.[9] In this case the primary malignancy was infiltrating ductal carcinoma which is less common than lobular carcinoma in having pancreatic metastasis [5].
A well-defined strategy for management of oligometastatic breast cancer is still not available due to dearth of evidence with regard to this case scenario [10, 11]. The mass was unresectable due to the radiological findings of portal cavernoma formation and long segment abutment of the common hepatic artery. Irresectability of the mass and availability of efficacious and safe systemic therapy for hormone receptor positive metastatic breast cancer were important factors guiding management strategy [12–14]. Palbociclib is an inhibitor of CDK4 as well as CDK 6. Treatment with palbociclib and letrozole as a first line therapy offers improvement in the progression-free survival of patients with metastatic ER positive and HER2 negative breast cancer. Hence, treatment was started with palbociclib and exemestane. Portal vein encasement and formation of portal cavernoma likely contributed to early spread of the disease to the liver while on first line therapy. A change in therapy to fulvestrant helped achieve a response. Later, progression on palbociclib and fulvestrant was challenged with everolimus and exemestane on the basis of the results of the BOLERO-2 trial [15].
Hormone receptor positivity and solitary metastasis at presentation are likely to confer a good prognosis to the patient [16, 17]. It is also worth noting that prognosis of patients with pancreatic metastatic disease is usually better than for patients with primary pancreatic tumors [18]. If the lesion would have been resectable, a pancreaticoduodenectomy might have proved to be a curative therapy for this lady [19, 20]. If the disease had remained stable to hint of good disease biology, SBRT would have been an option available in the armamentarium for the possibility of achieving prolonged remission and cure [21, 22]. Whether SBRT should have been offered in the very beginning is debatable. A definite strategy for sequencing of therapies in oligorecurrent breast cancer to achieve a prolonged response is not known due to lack of robust evidence in the form of randomized controlled trials.
Conclusion
Multidisciplinary approach helped us to achieve a diagnosis and offer appropriate therapy to a rare case of solitary pancreatic metastasis from breast cancer. With further publications of such cases, better treatment strategies might be devised to tackle pancreatic metastasis from breast cancer. This case emphasizes the need to maintain an index of suspicion for metastasis of breast cancer in a long-term follow-up case having new onset symptoms. It also demonstrates the necessity of regular follow-up.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Parag Ingle, Email: paragringle@gmail.com.
Pravin Khandare, Email: pravin.gmc@gmail.com.
Manjit Rajput, Email: drmanjit.rajput@strandls.com.
Megha Tiwari, Email: drmeghatiwari@hcgel.com.
Mangesh Patil, Email: drmangeshp@gmail.com.
Ajay Mehta, Email: drajay.mehta@hcgel.com.
References
- 1.Hellman, S. & Harris, J. R. in Diseases of the breast (eds Harris, J. R., Lippman, M. E., Morrow, M. & Osborne, C. K.), 407–423 (Lippincott Williams & Wilkins, Philadelphia, 2000). Describes the clinical behaviour of untreated breast cancer, including the incidences of regional lymphnode and distant metastasis
- 2.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura E, Shimizu M, Itoh T, Manabe T. Secondary tumors of the pancreas: clinicopathological study of 103 autopsy cases of Japanese patients. Pathol Int. 2001;51(9):686–690. doi: 10.1046/j.1440-1827.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Apodaca-Rueda M, Chaim FHM, Garcia MDS, Saito HPA, Gestic MA, Utrini MP et al (2017) Solitary pancreatic metastasis from breast cancer: case report and review of literature. Sao Paulo Med J [DOI] [PMC free article] [PubMed]
- 6.Mehta SA, Jagannath P, Krishnamurthy SC, De Souza LG. Isolated pancreatic metastasis from locally controlled breast cancer: a case report. Indian J Cancer. 1991;28(1):48–50. [PubMed] [Google Scholar]
- 7.Kobayashi T, Ichiba T, Sakuyama T, Arakawa Y, Nagasaki E, Aiba K, Nogi H, Kawase K, Takeyama H, Toriumi Y, Uchida K. Possible clinical cure of metastatic breast cancer: lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer. 2012;19(3):218–237. doi: 10.1007/s12282-012-0347-0. [DOI] [PubMed] [Google Scholar]
- 8.Tseng HS, Chen CY, Chan WP, Chiang JH. Percutaneous transgastric computed tomography-guided biopsy of the pancreas using large needles. World J Gastroenterol. 2009;15(47):5972–5975. doi: 10.3748/wjg.15.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaha DC. Significance of immunohistochemistry in breast cancer. World Journal of Clinical Oncology. 2014;5(3):382–392. doi: 10.5306/wjco.v5.i3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rastogi S, Gulia S, Bajpai J, Ghosh J, Gupta S. Oligometastatic breast cancer: a mini review. Indian journal of medical and paediatric oncology: official journal of Indian Society of Medical & Paediatric Oncology. 2014;35(3):203. doi: 10.4103/0971-5851.142035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makhlin I, Fox K. Oligometastatic breast cancer: is this a curable entity? A contemporary review of the literature. Curr Oncol Rep. 2020;22(2):15. doi: 10.1007/s11912-020-0867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network NCCN practice guidelines for pancreatic cancer, version 1. Published 2020
- 13.Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, Giorgetti C. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 14.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Campone M, Piccart M, Burris HA, III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieto Y, Nawaz S, Jones RB, Shpall EJ, Cagnoni PJ, McSweeney PA, Barón A, Razook C, Matthes S, Bearman SI. Prognostic model for relapse after high-dose chemotherapy with autologous stem-cell transplantation for stage IV oligometastatic breast cancer. J Clin Oncol. 2002;20(3):707–718. doi: 10.1200/JCO.2002.20.3.707. [DOI] [PubMed] [Google Scholar]
- 17.Yoo GS, Yu JI, Park W, Huh SJ, Choi DH. Prognostic factors in breast cancer with extracranial oligometastases and the appropriate role of radiation therapy. Radiation oncology journal. 2015;33(4):301–309. doi: 10.3857/roj.2015.33.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonapasta SA, Gregori M, Lanza R, Sangiorgi E, Menghi A, Scarpini M, Modesti M. Metastasis to the pancreas from breast Cancer: difficulties in diagnosis and controversies in treatment. Breast Care (Basel) 2010;5(3):170–173. doi: 10.1159/000314249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crippa S, Angelini C, Mussi C, Bonardi C, Romano F, Sartori P, Uggeri F, Bovo G. Surgical treatment of metastatic tumors to the pancreas: a single center experience and review of the literature. World J Surg. 2006;30(8):1536–1542. doi: 10.1007/s00268-005-0464-4. [DOI] [PubMed] [Google Scholar]
- 20.Pérez Ochoa A, Sáez Hernáez F, Cajigas Fernández C, Pérez de la Lastra L, Ondiviela Gracia R, García de Polavieja Carrasco M. Pancreatic metastases from ductal and lobular carcinomas of the breast. Clin Transl Oncol. 2007;9(9):603–605. doi: 10.1007/s12094-007-0110-8. [DOI] [PubMed] [Google Scholar]
- 21.Milano MT, Zhang H, Metcalfe SK, Muhs AG, Okunieff P. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat. 2009;115(3):601–608. doi: 10.1007/s10549-008-0157-4. [DOI] [PubMed] [Google Scholar]
- 22.Possanzini M, Greco C. Stereotactic radiotherapy in metastatic breast cancer. Breast. 2018;41:57–66. doi: 10.1016/j.breast.2018.06.011. [DOI] [PubMed] [Google Scholar]


