Abstract
Head and neck cancers (HNC) are extremely aggressive, highly recurrent, and the sixth most common cancer worldwide. Neuropeptide substance P, along with its primary receptor, neurokinin-1 (NK-1R), is overexpressed in HNC and is a central player in inflammation and growth and metastasis of several cancers. However, the precise SP-mediated signaling that promotes HNC progression remains ill defined. Using a panel of HNC lines, in this study, we investigated the effects of SP on proliferation and migration of HNC. Tumor cells were also treated with SP and alterations in inflammatory cytokines and chemokines, and their cognate receptors were analyzed by real-time PCR. Furthermore, we investigated the role of SP in inducing epithelial-mesenchymal transition (EMT), and matrix metalloproteases that promote tumor invasion. Our results showed that SP significantly increased tumor cell proliferation and migration and induced the expression of several genes that promote tumor growth, invasion, and metastasis which was suppressed by a specific NK1R antagonist L-703606. SP also activated NFκB that was suppressed on inhibiting NK1R. Collectively, our data shows that SP-NK1R-mediated inflammatory signaling comprises an important signaling axis in promoting HNC and may prove to be effective clinical target against HNC cells that are resistant to traditional therapy.
Keywords: Neuropeptide, Inflammation, Cytokines, Head neck cancer, Tumor progression
Introduction
Head and neck cancers (HNC) annually contribute to approximately 650,000 or 6% of new cancers worldwide, as well as over 300,000 deaths [1]. These cancers comprise a multitude of malignancies that develop in the upper aerodigestive epithelium after exposure to carcinogens such as tobacco and alcohol and are one of the most aggressive among solid tumors [2, 3]. HNC is known to be frequently recurrent and primarily metastatic, with the percentage of clinical metastasis ranging from 4 to 26%. Patients that have developed distant metastasis with HNC have a poor prognosis with an overall 5-year survival rate and often elude traditional therapeutic regimens [4].
Several neuropeptides have been associated with enhanced tumorigenesis of HNC cells. One such molecule that mediates inflammatory signaling cascades and is significantly involved in promotion of cancer metastasis is substance P (SP), which is central to the pathogenesis of various solid and hematological cancers [5]. SP is a mammalian undecapeptide, belonging to the tachykinin family of proteins, and its biological responses are mediated through three G protein coupled neurokinin (NK) receptors, NK1R, NK2R, and NK3R [6]. The coupling of SP and NK1R regulates significant inflammatory pathways such as the MAPK pathway and the EMT pathway [7]. It was previously shown that the SP activates inflammatory and contractile pathways in the lymphatics primarily through the NK1R, compared with its other two receptors [8]. SP concentrations are elevated in inflamed conditions and play a significant role in tumor progression [9, 10]. Furthermore, expression levels of NK1R in tumor cells have been found to correlate with the degree of malignancy [11]. SP plays a major role in modulating immune responses and a local release of SP in the lymph nodes contributes to chronic inflammatory conditions and is believed to further promote tumor migration [12, 13]. SP has been implicated as a tumor growth factor and influences the local tumor microenvironment in several cancers, such as neuroblastoma, pancreatic cancer, astrocytoma, and gliomas, by production of cytokines and direct SP inhibition causes apoptosis in breast cancer cells [14–17]. Furthermore, SP receptors are found on lymphocytes, macrophages, endothelial cells, and eosinophils; thus, they are involved in multitude of signaling mechanisms that contribute to an inflammatory microenvironment that sustains tumor progression [18]. The FDA-approved NK1R antagonist aprepitant (Emend, Merck) causes potent growth inhibition in a broad range of human tumors [19]. It is well established that tumor cells secrete a number of cytokines and chemokines that further contribute to an inflammatory tumor microenvironment and promotes tumor growth. Previous studies show that an increase in SP levels is directly associated with progression of oral squamous cell carcinoma (OSCC), and the expression of SP directly correlates with increased tumor stages of OSCC [4, 20, 21]. However, the specific molecular and cellular effects that SP has on HNCs have not yet been identified and its role in cancer-activating pathways remains unclear. Furthermore, the efficacy of NK1R inhibition by drug L-703606 in suppressing tumor enhancing pathways has not been studied in HNCs.
The primary objective of this study is to delineate the specific effects of the inflammatory neuropeptide SP on inflammatory and metastatic signaling pathways in HNCs. The proliferative and migratory effects of SP on HNCs were also investigated. Additionally, the role of the NK1R inhibitor, L-703606, in abrogating these SP-mediated effects in HNC was analyzed.
Materials and Methods
Ethics Statement
All experiments were carried out in accordance with policies and approval of Texas A&M University Institutional Biosafety Committee.
Cell Culture and Treatments
A panel of HNC cell lines, FaDu, Detroit 562, and SCC-9 cell lines were purchased from ATCC. FaDu and Detroit 562 cell lines were grown in modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, while SCC-9 cell line was grown in DMEM-F12 media supplemented with 10% FBS, 400 ng hydrocortisone, and 1% penicillin/streptomycin. The cells were maintained in humidified incubators at 37 °C and 5% CO2. Media was changed every 2–3 days dependent upon confluency, after washing with DPBS. Substance P (Sigma, MO, USA) was used at various concentrations (100 nM to 10 μM). NK1R inhibitor L-703606 (Sigma-Aldrich, MO USA) was used at a concentration of 1 μM. For inhibiting NK1R, L-703606 was added 2 h prior to the addition of SP. DMSO was used as vehicle control.
XTT Cell Proliferation Assay
FaDu, Detroit 562, and SCC-9 cell lines were grown in 96-well plates. At 70% confluency, cells were treated with 100 nM–10 μM concentrations of SP for 24 h. Cell proliferation was determined using XTT cell proliferation assay kit, (Trevigen, MD, USA) as per manufacturer’s protocol. Briefly, 24 h after SP treatment, media was removed, and cells were washed with PBS. Fifty microliters of XTT working reagent was added to the wells followed by addition of 150 μl of plain medium. The cells were incubated in 5% CO2 at 37 °C for 6 h, and absorbance was measured at 490 nm with reference wave length as 630 nm, using Spectramax 650 (Molecular devices, CA, USA) intermittently.
RNA Isolation and RT-PCR Analysis
FaDu, Detroit 562, and SCC-9 cells were grown on 12-well plates and treated with 100 nM SP, with or without 1 μM NK1R inhibitor priming as described above. Cells were lysed and total RNA was isolated using the Purelink RNA mini kit (Thermo Fisher Scientific, MA, USA) according to manufacturer’s protocol. The quantity and quality of the isolated RNA were determined using a Nanodrop (NanoDrop Technologies, Wilmington, DE). cDNA was prepared from 1 μg of RNA using Maxima™ H Minus cDNA Synthesis Master Mix (Thermo Fisher Scientific, MA, USA). Real-time PCR was performed for inflammatory markers, cytokines, chemokines, chemokine receptors, matrix metalloproteinases (MMP), and epithelial-to-mesenchymal transition (EMT) markers using SYBR Green (Applied Biosystems, CA, USA) in a real-time thermal cycler (ABI Prism 7900HT sequence detection system) (Applied Biosystems, CA, USA). All reactions were performed in triplicate. Data analysis was performed using the 2−ΔΔCt method, where RPL19 was used as the reference gene as described previously [22, 23]. Primer sequence information is available from the authors upon request.
Migration Assay
HNC cells were plated on 6-well plates, grown to at least 90% confluency, and treated with 100 nM SP, with or without 1 μM NK1R inhibitor pretreatment. Cells were then trypsinized and counted, and 1 × 105 cells were plated in 8.0-μm pore PTE standing inserts (Millipore, MA, USA). Inserts were placed in 24-well plates and respective complete media was placed in the lower chambers. Cells were allowed to migrate to the bottom of the insert for 24 h as described previously [24]. The inserts were then washed with DPBS, fixed with ice cold methanol, and stained with 0.5% crystal violet. Images were captured at × 10 magnification from five different fields using an inverted microscope (Nikon, NY, USA). The number of cells was counted using the ImageJ software, version 1.52a (National Institute of Health, https://imagej.nih.gov/ij/).
Scratch Wound Assay
HNC cells were grown in 6-well plates and respective wells were primed with or without 1 μM NK1R inhibitor for 2 h. Pipette tips were used to make 3 scratches in each well, with 1 in the upper field, 1 in the middle field, and 1 in the lower field. The cells were washed with PBS to smoothen the scratch edges. One hundred nanomolar SP was immediately added to respective wells after scratches were made. Cells were allowed to sit in complete media for 24 h, and × 4 images were captured of each scratch at 0, 12, and 24 h using an inverted microscope. Scratch wound closure or migration rate was determined using ImageJ. Migration rate was expressed as average migration rate in μm/h.
Immunofluorescence
HNC cells were plated on coverslips and grown to at least 70% confluency. Coverslips were treated with 100 nM SP and with or without 1 μM NK1R inhibitor as described above. After 24 h of treatment, coverslips were fixed with 2% paraformaldehyde and permeabilized with ice-cold methanol. Immediately afterwards, NF-κB primary antibody was placed on cover slips for 2 h. Cover slips were then incubated with goat anti-rabbit secondary antibody (AlexaFlour® 488 Green) for 1 h in the dark and washed several times. Using ProLong Gold anti-fade solution, the coverslips were mounted onto glass slides and allowed to cure overnight. Fluorescent images of NF-κB localization were captured using an inverted fluorescence microscope (Olympus, Tokyo, Japan) at × 20 magnification. NF-κB fluorescent intensity in the nucleus was calculated and plotted for each treatment group.
Statistical Analysis
All experiments were carried out with at least an N = 3. Values were averaged for each treatment group and statistically compared against each other. Using GraphPad Prism, two-way ANOVA was performed, followed by Fisher LSD to measure significance of comparisons. Values are represented as mean ± SE on each graph. Single, double, triple, and quadruple asterisks or single, double, triple, and quadruple number signs indicate p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, and p ≤ 0.0001, as compared with control or SP 100 nM, respectively.
Results
Substance P Increases the Proliferation of Head and Neck Cancer Cells
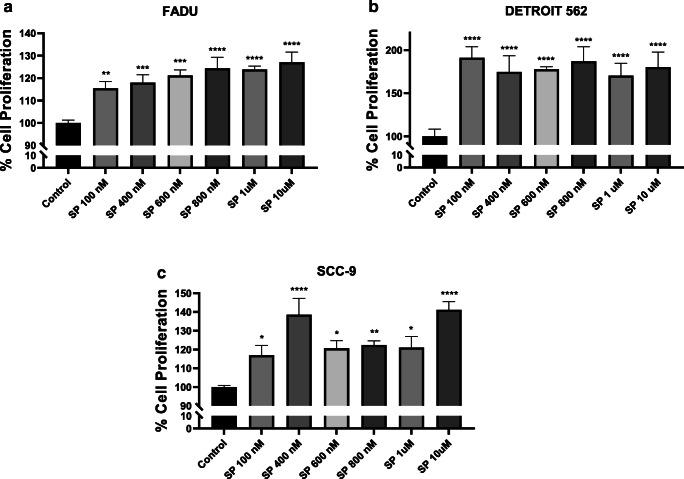
Cellular proliferation is known to play a vital role in the growth and invasion of cancer cells [21]. SP binding to NK1R, its highest affinity cognate receptor, is known to play an important role in inflammation, cell proliferation, and migration in different cancers that ultimately contributes to cancer cell invasion and metastasis. Since tumor cell proliferation is an important prerequisite for dissemination to distant sites, we first evaluated effects of SP on proliferative behavior of a panel of HNC lines. We found that SP significantly increased cell proliferation in all the three cell lines tested—FaDu (Fig. 1A), Detroit 562 (Fig. 1B), and SCC-9 (Fig. 1C). The starting 100 nM concentration tested induced 15%, 90%, and 16% increase in cell proliferation in FaDu, Detroit 562, and SCC-9 cells respectively. It was observed that all 3 cell lines had significantly increased percentage of cellular proliferation in response to SP treatment. Significant increase in cell proliferation was noted at all concentrations of SP tested.
Fig. 1.
Substance P–induced head and neck cancer (HNC) cell proliferation: After being treated with various concentrations of SP for 24 h, cellular proliferation assay of HNC cell lines (A) FaDu, (B) Detroit 562, and (C) SCC-9 was assessed by XTT assay. Values represent mean ± SE, n ≥ 3, two-way ANOVA followed by Fisher LSD for multiple comparison. Single, double, triple, and quadruple asterisks indicate p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, and p ≤ 0.0001 respectively as compared with respective control
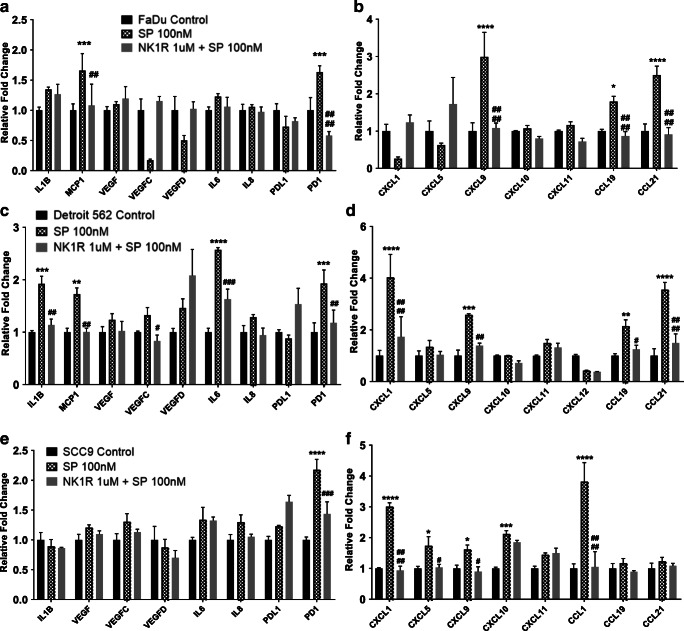
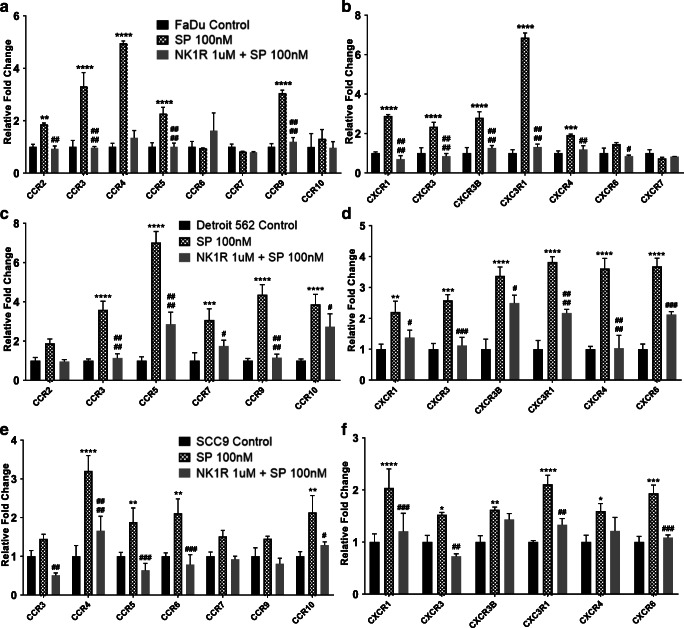
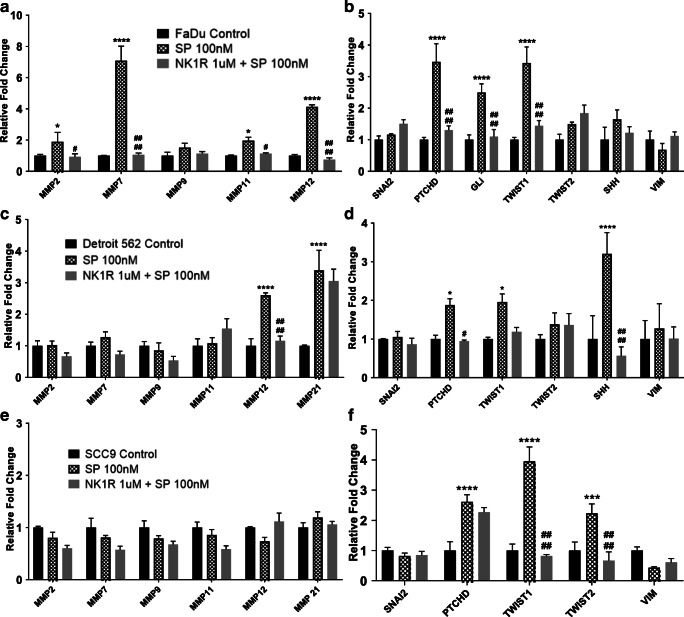
Substance P Upregulates Expression of Several Tumor-Enhancing Genes in HNC Cells and Contributes to an Inflammatory Microenvironment
SP has been shown to activate tumor-enhancing pathways in several cancers [17, 18] and that malignant cells in particular express increased levels of NK1R, further promoting SP-mediated pathways [5, 7]. To evaluate the role of SP in inflammation and metastasis in HNC cells, and the effects of NK1R inhibition on these cells, we investigated the effects of SP treatment in HNC cell lines (FaDu, Detroit 562, and SCC-9) with and without NK1R inhibitor on cytokines, chemokines, chemokine receptors, MMPs, and EMT-inducing genes by real-time PCR analysis. We found that FaDu cells, when treated with SP for 24 h, showed significant upregulation of cytokines: MCP1 and PD1 (Fig. 2A); chemokines: CXCL9, CCL19, CCL21 (Fig. 2B); cytokine receptors: CCR2, CCR3, CCR4, CCR5, CCR9 (Fig. 3A); chemokine receptors: CXCR1, CXCR3, CXCR3B, CXC3R1, CXCR4 (Fig. 3B); MMP genes: MMP2, MMP7, MMP11, MMP12 (Fig. 4A); and EMT inducing genes: PTCHD, GLI, and TWIST1 (Fig. 4B) compared with untreated control. We found that pre-treatment of these cell lines with the NK1R inhibitor abrogated most of the SP-mediated induction in gene expression (Fig. 2A–B, 3A–B and 4A–B). In order to investigate the effects of the SP-NK1R axis in other HNC lines, similarly, we treated Detroit 562 cells with SP and SP + NK1R inhibitor for 24 h and found that SP caused a significant upregulation of the following genes: cytokines: IL1β, MCP1, IL6, PD1 (Fig. 2C); chemokines: CXCL1, CXCL9, CCL19, CCL21 (Fig. 2D); cytokine receptors: CCR3, CCR5, CCR7, CCR9, CCR10 (Fig. 3C); chemokine receptors: CXCR1, CXCR3, CXCR3B, CXC3R1 CXCR4, CXCR6 (Fig. 3D); MMP genes: MMP12, MMP21 (Fig. 4C); and EMT inducing genes: PTCHD, TWIST1, SHH (Fig. 4D). NK1R inhibitor treatment of Detroit 562 cells showed a significant downregulation of the SP-mediated overexpression of these genes, except MMP21 (Fig. 4C) and TWIST1 (Fig.4D). Also in SCC-9 cells, we found that treatment with 100 nM SP for 24 h induced the cytokines: PD1 (Fig. 2E); chemokines: CXCL1, CXCL5, CXCL9, CXCL10, CCL1 (Fig. 2F); cytokine receptors: CCR4, CCR5, CCR6, CCR10 (Fig. 3E); chemokine receptors: CXCR1, CXCR3, CXCR3B, CXC3R1, CXCR4, CXCR6 (Fig. 3F); EMT-inducing genes: PTCHD, TWIST1, TWIST2 (Fig. 4F). In SCC-9 cells treated with SP (100 nM) and NK1R (1uM) for 24 h, the following genes showed downregulation: cytokines: PD1 (Fig. 2E); chemokines: CXCL1, CXCL5, CCL1 (Fig. 2F); cytokine receptors: CCR3, CCR4, CCR5, CCR6, CCR10 (Fig. 3E); chemokine receptors: CXCR1, CXCR3, CXC3R1, CXCR6 (Fig. 3F); EMT-inducing genes: TWIST1, TWIST2 (Fig.4F). Hence, our results show that SP significantly induces expression of inflammatory cytokines, chemokines, and tumor-promoting pathways across different HNC lines that is completely inhibited in the presence of NK1R inhibitor.
Fig. 2.
Substance P–induced expression of various cytokines and chemokines in HNC cells: HNC cells were treated with SP (100 nM) and NK1R inhibitor L-703606 (1 μM). mRNA expression of cytokines and chemokines were analyzed by PCR for (A and B) FaDu, (C and D) Detroit 562, and (E and F) SCC-9 cells. Values represent mean ± SE, n = 3, two-way ANOVA followed by Fisher LSD for multiple comparison. Single, double, triple, and quadruple asterisks or single, double, triple, and quadruple number signs indicate p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, and p ≤ 0.0001 respectively as compared with control or SP 100 nM
Fig. 3.
Substance P–induced expression of various chemokine receptors in HNC cells: HNC cells were treated with SP (100 nM) and NK1R inhibitor (1 μM). mRNA expression of chemokines and chemokine receptors were analyzed by real-time PCR in (A and B) FaDu, (C and D) Detroit 562, and (E and F) SCC-9 cell lines. Values represent mean ± SE, n = 3, two-way ANOVA followed by Fisher LSD for multiple comparison. Single, double, triple, and quadruple asterisks or Single, double, triple, and quadruple number signs indicate p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, and p ≤ 0.0001 respectively as compared with control or SP 100 nM
Fig. 4.
Substance P–induced expression of various MMP and EMT-inducing genes in HNC cells: HNC cells were treated with SP (100 nM) and NK1R inhibitor (1 μM). mRNA expression of various MMPs and EMT-inducing genes were analyzed by real-time PCR in (A and B) FaDu, (C and D) Detroit 562, and (E and F) SCC-9 cell lines. Values represent mean ± SE, n = 3, two-way ANOVA followed by Fisher LSD for multiple comparison. Single, double, triple, and quadruple asterisks or Single, double, triple, and quadruple number signs indicate p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, and p ≤ 0.0001 respectively as compared with control or SP 100 nM
Substance P Induces Migration of HNC Cells
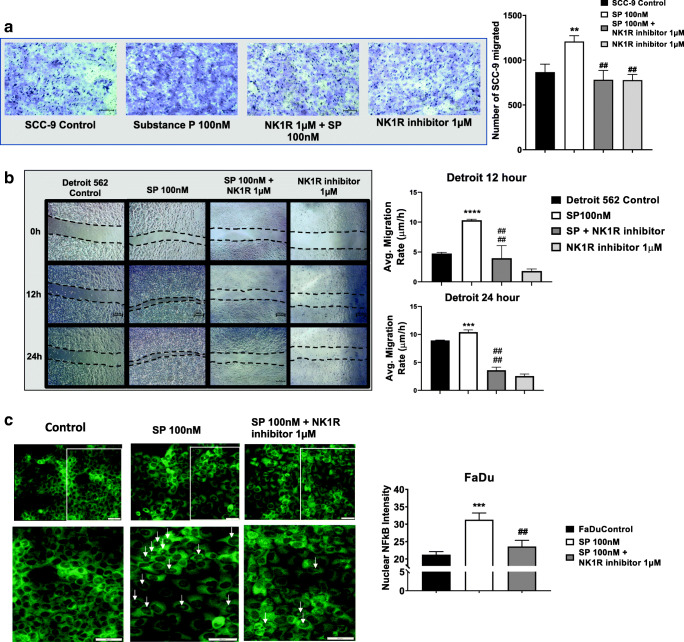
Migration of tumor cells is an important step for tumor cells to break away from the surrounding basement membrane and invade local blood and lymphatic vessels [25]. It has been noted that SP induces migration of several solid tumors and also plays key roles in wound healing and wound closure [17, 26]. However, the effect of SP and the efficacy of NK1R inhibitor, L-703606, on HNC migration have not yet been observed. In order to directly assess the role of SP in HNC cell migration, we performed modified Boyden chamber assays and scratch wound assays for two of the HNC cell lines. For the migration assay, HNC cells were primed with or without NK1R inhibitor (1 μM) for 2 h and then treated with SP (100 nM) for 24 h. We found that SP treatment significantly increased the number of tumor cells migrated (p ≤ 0.01) towards complete medium, as compared with the control cells (Fig. 5A). On the other hand, NK1R inhibition significantly decreased (p ≤ 0.01) the SP-induced migration of tumor cells, whereas NK1R inhibition alone did not induce any changes in tumor cell migration as compared with control cells (Fig. 5A). Next, to determine the migration rate of HNC cells in presence of SP, HNC cells were also treated with SP (100 nM), SP + NK1R inhibitor, and NK1R inhibitor alone and scratch wound assay was performed to measure the migration rate of cells 12 h and 24 h after treatment. We observed that SP significantly upregulated tumor cell migration, 12 h and 24 h post-treatment whereas treatment with SP + NK1R inhibitor decreased the average migration rate of the cells (Fig. 5B).
Fig. 5.
Substance P–induced NFκB nuclear translocation in HNC cells: HNC cells were treated with SP (100 nM) and with or without NK1R inhibitor for 24 h. (A) Migration of SCC-9 cells in 8-μm pores containing PTE inserts towards complete medium was assessed. Representative × 10 images were given. Graph represents number of cells migrated. (B) Rate of migration of Detroit 562 cells in the scratch wound was followed for 0 h, 2 h, 12 h, and 24 h. Representative × 4 images are shown. Graphs represent rate of migration for 12 and 24 h. (C) Immunofluorescence images of NF-kB nuclear localization in HNC cells treated with SP and its inhibitor NK1R. Fluorescent images were acquired at × 20 magnification. Bottom panel shows enlarged images of specific areas on the coverslip. The percentage of fluorescent intensity in the nucleus was calculated and plotted. Scale bar = 100 μm. Values represent mean ± SE, n ≥ 3, two-way ANOVA followed by Fisher LSD for multiple comparison. Single, double, triple, and quadruple asterisks or Single, double, triple, and quadruple number signs indicate p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, and p ≤ 0.0001 respectively as compared with control or SP 100 nM
SP Treatment Increases Nuclear Translocation of NF-kB in HNC Cells
NF-κB is a transcription factor that is central to the process of inflammation and is closely associated with increased proliferation, migration, and metastasis of several human cancers, by translocating to the nucleus of the cell and activating a cascade of inflammatory genes [17]. However, how SP regulates NF-κB to elicit inflammatory signaling and promote an inflammatory tumor microenvironment has not been studied in HNCs. Using immunofluorescence assays, we determined that SP treatment (100 nM) significantly increases the nuclear translocation of NF-κB, compared with the untreated control. Furthermore, we observed that pre-treatment with NK1R (1 μM) inhibitor significantly abrogated the effects of SP treatment, which can be seen by the decreased nuclear localization of NF-κB (Fig. 5C). The intensity of NF-κB fluorescence in the nucleus was measured and plotted (Fig. 5C). We found that tumor cells showed increased nuclear translocation of NF-KB when treated with SP compared with untreated controls. Inhibiting this signaling axis through NK1R inhibitor significantly decreased the SP-mediated induction of nuclear translocation.
Discussion
The data presented in this study indicates that SP significantly increased cellular inflammation, proliferation, and migration of a panel of head and neck cancer cell lines in an NK1R-specific manner. In addition, our data clearly shows that SP activates several inflammatory cytokines, chemokines, and chemokine receptors, as well as genes involved in tumor progression in the HNC cells. This is significant because it has been well established that the aggressiveness of HNC is primarily because of its ability to metastasize to distant organs through lymphatics and blood vessels. It is hypothesized that SP may also play an important role in perineural growth of HNC, accounting for its aggressiveness and poor patient survival rate; however, the exact role of SP in HNC development and growth has not been clearly delineated [27]. Tumor cells have co-opted several of the signaling molecules of the innate immune system, such as cytokines, chemokines, and their receptors, which facilitate their invasion, migration, and metastasis [28]. Recent studies from our lab have demonstrated that activation of specific chemokine receptors on the HNC cells and its cognate receptors on lymphatic endothelial cells enhance tumor attraction towards lymphatic vessels and hence bolster lymph node metastasis which is a prognostic factor for HNC [24]. SP influences the local tumor microenvironment in several cancers, such as neuroblastoma, gliomas, pancreatic cancers, by production of cytokines, chemokines, and other tumor-promoting pathways while direct SP inhibition causes apoptosis in breast cancer cells [14–17, 29]. Interestingly, human tumors have been shown to significantly upregulate NK1R, and it is implicated in the viability of tumor cells. Poor prognosis correlates with increased expression [30–32]. The FDA-approved NK1R antagonist aprepitant (Emend, Merck) has been shown to cause potent growth inhibition in a broad range of human tumors [19]. Thus, SP-mediated signaling in HNC cells could potentially be an important bridge between an inflammatory tumor microenvironment, immune cell modulation, and activation of tumor-promoting mechanisms, all of which contribute to HNC pathogenesis. Furthermore, the signaling axis that mediates these cytokine and chemokine responses comprises very attractive targets for cancer therapeutics. Targeting the SP-NK1R signaling will therefore be an important strategy for prevention of HNC progression.
Our studies show that SP enhances proliferation in the tumor cells and is suppressed in the presence of the NK1R antagonist. SP has been shown to significantly promote proliferation of several cancer cells and prevents apoptosis, which is a key step for the tumor cells to migrate and undergo metastasis [5]. SP significantly alters the tumor microenvironment by inducing the expression of several cytokines and chemokines that are known to be involved in cancer migration, invasion, and distant metastasis. MCP1, which is a potent monocyte-attracting chemokine and significantly induced in several metastatic cancers, was found to be increased in the HNC cells on treatment with SP indicating the activation of an inflammatory tumor microenvironment. Furthermore, levels of the programmed death 1 (PD-1), an immune checkpoint receptor, were also increased by SP in all the cell lines analyzed. This is significant as PD-1 has been shown to have cancer cell intrinsic functions in addition to its role in immune checkpoint inhibition, along with its ligand PDL1. In HNC, PD-1 monoclonal antibodies pembrolizumab and nivolumab are approved for treatment of HNC and have demonstrated significant success in clinical trials for recurrent or metastatic HNC [33]. SP induced the expression of several cytokines and chemokines in all the tumor cells such as CXCL9, CCR5 and multiple chemokine receptors such as CXCR1, CXCR3, CXCR3B, CXCR4, which are involved in various tumor promoting pathways and activate downstream signaling [34]. This SP-induced activation of a local inflammatory milieu was significantly decreased by NK1R inhibitor, which suppressed most of this elevation in gene expression. Thus SP-NK1R signaling axis has therapeutic significance as HNC is known to express several chemokines and cytokines that either enable resistance to chemotherapy and radiotherapy, or allow invasion and migration through lymphatic vessels to lymph nodes, which are key indicators of poor patient outcome [34–36].
SP was also found to induce the activation of different MMPs in a cell line–specific manner, depending on the anatomical location of HNC. MMPs play a critical role in tumor invasion and hence are significantly regulated in response to factors in the tumor microenvironment that promote tumors to invade surrounding tissues and vasculature. Interestingly, SP elicited a strong regulation of EMT-associated genes in the tumor cells. EMT is critical for early metastasis, is often induced by inflammation, and endows tumor cells with enhanced migratory and invasive properties [24]. EMT is considered to be a key event that fosters early metastasis, which is a characteristic feature of HNC. NK1R inhibitor significantly abrogated several of these upregulated EMT-associated genes, thereby initiating an anti-metastatic switch. In addition, SP increased the expression of TWIST1, TWIST2, Gli, Patched, and SHH thereby activating the EMT program in the different HNC cell lines.
Furthermore, our data demonstrates that SP increases nuclear translocation of the central inflammatory molecule NF-kB. It has been previously documented that SP modulates macrophages and fibroblasts, and promotes an inflammatory local environment by activation of NF-κB-mediated inflammatory response [37]. In murine macrophages, it has been shown that SP enhances selective inflammatory chemokine production via ERK/p38 MAPK-mediated NF-kB activation. Our data clearly documents the interplay of SP- and NF-KB-mediated inflammatory response, which is inhibited by NK1R antagonists.
Taken together, our data demonstrates that SP activates several inflammatory pathways in HNC cells by inducing chemokines, cytokines, and inflammatory markers, thereby contributing to an inflammatory tumor microenvironment and enhancing tumor proliferation and migration. SP further enhances different EMT genes and MMPs that are critical for cancer invasion and metastasis. In addition, SP induces activation of the key inflammatory molecule NF-kB. Inhibition of NK1R, with a specific receptor antagonist, abrogates several of these mechanisms and significantly suppresses SP-mediated inflammatory signaling in HNC. Thus, this data could be the basis of new therapies targeting SP-mediated events in the HNC tumor microenvironment that contributes to disease progression.
Acknowledgments
We would like to thank Dr. Lavanya Venkatasamy for the technical help.
Funding
This work was supported by the Auf-X-Grant Award from Texas A&M University Health Science Center and Research Enhancement Grant from the Department of Medical Physiology to S.C, American Heart Association grant 17SDG33670306 to S.C.
Compliance with Ethical Standards
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alsahafi E, et al. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10(8):540. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehboob R, Tanvir I, Warraich RA, Perveen S, Yasmeen S, Ahmad FJ. Role of neurotransmitter substance P in progression of oral squamous cell carcinoma. Pathol Res Pract. 2015;211(3):203–207. doi: 10.1016/j.prp.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Munoz M, Covenas R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides. 2013;48:1–9. doi: 10.1016/j.peptides.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Rameshwar P, Ganea D, Gascon P. Induction of IL-3 and granulocyte-macrophage colony-stimulating factor by substance P in bone marrow cells is partially mediated through the release of IL-1 and IL-6. J Immunol. 1994;152(8):4044–4054. [PubMed] [Google Scholar]
- 7.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty S, Nepiyushchikh Z, Davis MJ, Zawieja DC, Muthuchamy M. Substance P activates both contractile and inflammatory pathways in lymphatics through the neurokinin receptors NK1R and NK3R. Microcirculation. 2011;18(1):24–35. doi: 10.1111/j.1549-8719.2010.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jessop DS, Renshaw D, Larsen PJ, Chowdrey HS, Harbuz MS. Substance P is involved in terminating the hypothalamo- pituitary-adrenal axis response to acute stress through centrally located neurokinin-1 receptors. Stress. 2000;3(3):209–220. doi: 10.3109/10253890009001125. [DOI] [PubMed] [Google Scholar]
- 10.Chen XY, Ru GQ, Ma YY, Xie J, Chen WY, Wang HJ, Wang SB, Li L, Jin KT, He XL, Mou XZ. High expression of substance P and its receptor neurokinin-1 receptor in colorectal cancer is associated with tumor progression and prognosis. Onco Targets Ther. 2016;9:3595–3602. doi: 10.2147/OTT.S102356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swain MG, Agro A, Blennerhassett P, Stanisz A, Collins SM. Increased levels of substance P in the myenteric plexus of Trichinella-infected rats. Gastroenterology. 1992;102(6):1913–1919. doi: 10.1016/0016-5085(92)90313-N. [DOI] [PubMed] [Google Scholar]
- 12.Esteban F, Munoz M, Gonzalez-Moles MA, Rosso M. A role for substance P in cancer promotion and progression: a mechanism to counteract intracellular death signals following oncogene activation or DNA damage. Cancer Metastasis Rev. 2006;25(1):137–145. doi: 10.1007/s10555-006-8161-9. [DOI] [PubMed] [Google Scholar]
- 13.Payan DG. Neuropeptides and inflammation: the role of substance P. Annu Rev Med. 1989;40:341–352. doi: 10.1146/annurev.me.40.020189.002013. [DOI] [PubMed] [Google Scholar]
- 14.Manske JM, Hanson SE. Substance-P-mediated immunomodulation of tumor growth in a murine model. Neuroimmunomodulation. 2005;12(4):201–210. doi: 10.1159/000085652. [DOI] [PubMed] [Google Scholar]
- 15.Palma C, Maggi CA. The role of tachykinins via NK1 receptors in progression of human gliomas. Life Sci. 2000;67(9):985–1001. doi: 10.1016/S0024-3205(00)00692-5. [DOI] [PubMed] [Google Scholar]
- 16.Javid H, Mohammadi F, Zahiri E, Hashemy SI. The emerging role of substance P/neurokinin-1 receptor signaling pathways in growth and development of tumor cells. J Physiol Biochem. 2019;75(4):415–421. doi: 10.1007/s13105-019-00697-1. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Ma G, Ma Q, Li W, Liu J, Han L, Duan W, Xu Q, Liu H, Wang Z, Sun Q, Wang F, Wu E. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol Cancer Res. 2013;11(3):294–302. doi: 10.1158/1541-7786.MCR-12-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016;73(22):4249–4264. doi: 10.1007/s00018-016-2293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz M, Rosso M. The NK-1 receptor antagonist aprepitant as a broad spectrum antitumor drug. Investig New Drugs. 2010;28(2):187–193. doi: 10.1007/s10637-009-9218-8. [DOI] [PubMed] [Google Scholar]
- 20.Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35(10):5499–5502. [PubMed] [Google Scholar]
- 21.Brener S, González-Moles MA, Tostes D, Esteban F, Gil-Montoya JA, Ruiz-Avila I, Bravo M, Muñoz M. A role for the substance P/NK-1 receptor complex in cell proliferation in oral squamous cell carcinoma. Anticancer Res. 2009;29(6):2323–2329. [PubMed] [Google Scholar]
- 22.Riva G, Biolatti M, Pecorari G, Dell'Oste V, Landolfo S (2019) PYHIN Proteins and HPV: role in the pathogenesis of head and neck squamous cell carcinoma. Microorganisms 8(1) [DOI] [PMC free article] [PubMed]
- 23.Lee Y, Fluckey JD, Chakraborty S, Muthuchamy M. Hyperglycemia- and hyperinsulinemia-induced insulin resistance causes alterations in cellular bioenergetics and activation of inflammatory signaling in lymphatic muscle. FASEB J. 2017;31(7):2744–2759. doi: 10.1096/fj.201600887R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumaravel S, Singh S, Roy S, Venkatasamy L, White TK, Sinha S, Glaser SS, Safe SH, Chakraborty S. CXCL11-CXCR3 axis mediates tumor lymphatic cross talk and inflammation-induced tumor, promoting pathways in head and neck cancers. Am J Pathol. 2020;190(4):900–915. doi: 10.1016/j.ajpath.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polacheck WJ, Zervantonakis IK, Kamm RD. Tumor cell migration in complex microenvironments. Cell Mol Life Sci. 2013;70(8):1335–1356. doi: 10.1007/s00018-012-1115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suvas S. Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J Immunol. 2017;199(5):1543–1552. doi: 10.4049/jimmunol.1601751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roh J, Muelleman T, Tawfik O, Thomas SM. Perineural growth in head and neck squamous cell carcinoma: a review. Oral Oncol. 2015;51(1):16–23. doi: 10.1016/j.oraloncology.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS (2013) Chemokines in tumor progression and metastasis. Oncotarget 4(12):2171–2185. 10.18632/oncotarget.1426 [DOI] [PMC free article] [PubMed]
- 29.Blum AM, Metwali A, Crawford C, Li J, Qadir K, Elliott DE, Weinstock JV. Interleukin 12 and antigen independently induce substance P receptor expression in T cells in murine schistosomiasis mansoni. FASEB J. 2001;15(6):950–957. doi: 10.1096/fsb2fj000379. [DOI] [PubMed] [Google Scholar]
- 30.Munoz M, et al. The substance P/neurokinin-1 receptor system in lung cancer: focus on the antitumor action of neurokinin-1 receptor antagonists. Peptides. 2012;38(2):318–325. doi: 10.1016/j.peptides.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Munoz M, et al. The NK-1 receptor is expressed in human melanoma and is involved in the antitumor action of the NK-1 receptor antagonist aprepitant on melanoma cell lines. Lab Investig. 2010;90(8):1259–1269. doi: 10.1038/labinvest.2010.92. [DOI] [PubMed] [Google Scholar]
- 32.Munoz M, Rosso M, Covenas R (2019) Neurokinin-1 receptor antagonists against hepatoblastoma. Cancers (Basel) 11(9) [DOI] [PMC free article] [PubMed]
- 33.Forster MD, Devlin MJ. Immune checkpoint inhibition in head and neck cancer. Front Oncol. 2018;8:310. doi: 10.3389/fonc.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolff HA, Rolke D, Rave-Fränk M, Schirmer M, Eicheler W, Doerfler A, Hille A, Hess CF, Matthias C, Rödel RMW, Christiansen H. Analysis of chemokine and chemokine receptor expression in squamous cell carcinoma of the head and neck (SCCHN) cell lines. Radiat Environ Biophys. 2011;50(1):145–154. doi: 10.1007/s00411-010-0341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller A, et al. Chemokine receptors in head and neck cancer: association with metastatic spread and regulation during chemotherapy. Int J Cancer. 2006;118(9):2147–2157. doi: 10.1002/ijc.21514. [DOI] [PubMed] [Google Scholar]
- 36.Samara GJ, et al. CXCR4-mediated adhesion and MMP-9 secretion in head and neck squamous cell carcinoma. Cancer Lett. 2004;214(2):231–241. doi: 10.1016/j.canlet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 37.Ni T, Liu Y, Peng Y, Li M, Fang Y, Yao M. Substance P induces inflammatory responses involving NF-kappaB in genetically diabetic mice skin fibroblasts co-cultured with macrophages. Am J Transl Res. 2016;8(5):2179–2188. [PMC free article] [PubMed] [Google Scholar]