Abstract
Neck pain is very common, but most of the causes are unknown, making diagnosis and treatment extremely challenging. Current studies have found that one of the main problems in patients with neck pain is the impairment of cervical proprioception, which subsequently leads to cervical sensorimotor control disturbances. Cervical spine has a very delicate proprioceptive system that plays a crucial role in controlling posture and balance. Cervical proprioceptive impairment in neck pain occurs through a variety of mechanisms. Experimental neck muscle pain induced by injection of hypertonic saline results in inhibition of the activation of painful muscle; chronic neck pain causes structural and functional impairment of cervical muscles; excessive activation of mechanoreceptors in degenerative cervical discs and facet joints produces a large number of erroneous sensory signals. Clinical examinations to assess the link between structural pathology and neck pain have been unsuccessful, opening the way for the development of function-based tests. To date, eight neck sensorimotor control tests have been reported to evaluate patients with chronic neck pain. Although some tests may involve different subsystems (such as oculomotor system and vestibular system), all tests measure sensorimotor control in the neck, and the most commonly used is cervical joint position error (JPE) test. Current studies support the effectiveness of exercises targeting different aspects of sensorimotor function, in particular retraining aimed at improving cervical proprioception and muscle coordination. Based on the available evidence, it is recommended that patients with neck pain should be assessed and managed for cervical proprioceptive impairment and sensorimotor control disturbances.
Keywords: Cervical joint position error, Cervical proprioception, Cervical proprioceptor, Cervical sensorimotor control, Neck pain, Pathophysiology, Rehabilitation
Key Summary Points
| Neck pain is a common cause of disability worldwide, but its basic pathology and pathophysiology are still unclear. |
| Cervical spine has a very delicate proprioceptive system that plays a crucial role in controlling posture and balance. |
| One of the main problems of patients with neck pain is that the alteration of cervical proprioception leads to the disturbance of cervical sensorimotor control. |
| To date, eight neck sensorimotor control tests have been reported to evaluate patients with chronic neck pain, with the most commonly used test being the JPE test. |
| Studies support the effectiveness of exercises targeting different aspects of sensorimotor function, in particular retraining aimed at improving cervical proprioception and muscle coordination. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13379681.
Introduction
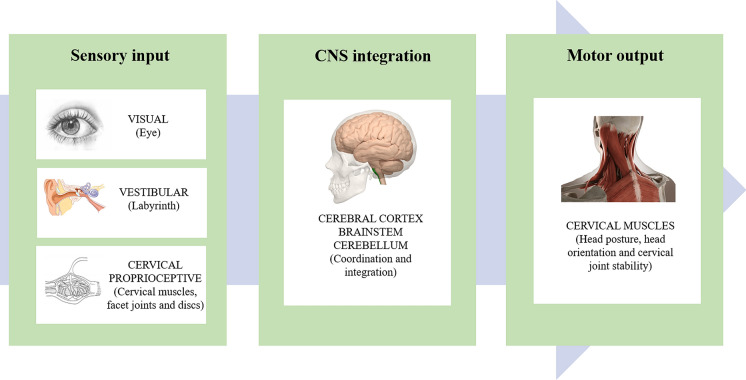
Neck pain is a common condition and one of the leading causes of disability worldwide, with mean estimates of 7.6% point prevalence (range, 5.9–38.7%), 37% annual prevalence (range, 16.7–75%), and 48.5% lifetime prevalence (range, 14.2–71%) [1]. Although neck pain can be attributed to traumatic (such as whiplash-associated) disorders, metabolic, neoplastic, inflammatory, or infectious diseases, most neck pain has no discernable cause and is considered to be idiopathic [2, 3]. As with chronic low back pain, studies have failed to explore the consistent relationship between structural pathology and neck pain [2]. Current studies have indicated that one of the main problems in patients with neck pain is the cervical proprioception impairment, which leads to cervical sensorimotor control disturbances [4–6]. Cervical sensorimotor control involves central integration and processing of all the afferent information (i.e., visual, vestibular, and cervical proprioceptive inputs), and execution of the motor program through the cervical muscles, contributing to the maintenance of head posture and balance as well as the stability of cervical joints (Fig. 1) [4–7]. The cervical sensorimotor control disturbances secondary to neck pain are considered as a protective response to limit further stimulation of the painful tissue. Such disturbances may, in the long run, further cause tissue damage, aggravate pain through peripheral and central nervous system sensitization, and promote dysfunctional motion patterns [8].
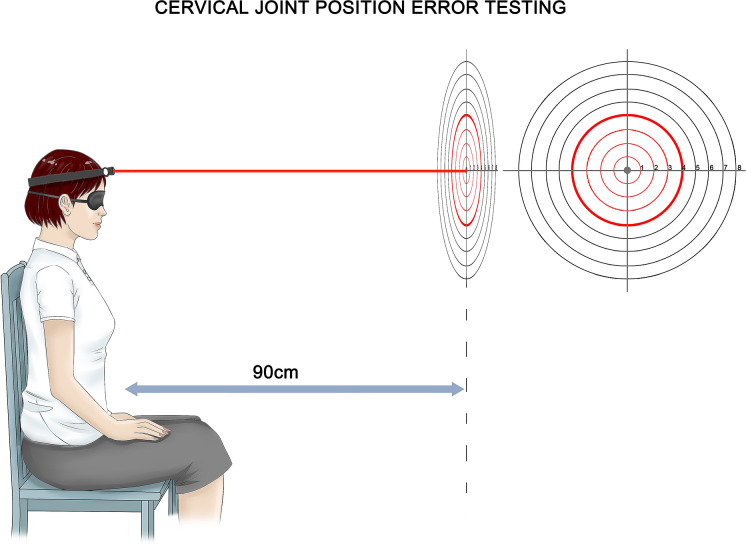
Fig. 2.
Cervical joint position error (JPE) test using a laser pointer. The patient is sitting 90 cm from the wall, and the starting point (center of target or reference point) of the laser projection is marked. The patient (blindfolded or closed) performs active neck movement and then returns to the starting position as accurately as possible. The final laser position is measured relative to the starting position (distance or angle). The errors are measured after cervical extension, flexion, lateral flexions, and rotations.
Fig. 1.
Schematic diagram of cervical sensorimotor control
The clinical practice guidelines for chronic, idiopathic neck pain do not support percutaneous or open surgical treatment, and recommend conservative treatment [9, 10]. However, as conservative treatment usually fails to maintain long-term efficacy, clinical studies begin to pay attention to the evaluation and management of proprioceptive dysfunction in patients with idiopathic neck pain [4, 5, 11–13]. Based on the existing literature, this review aims to clarify the pathophysiology, assessment methods, and potential treatments of cervical proprioceptive impairment and sensorimotor control disturbances in patients with neck pain. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Selection Methods
A comprehensive literature search was performed through PubMed and MEDLINE from the inception of the database to September 2020. The search terms included "neck pain", "cervical proprioception", "cervical sensorimotor control", "cervical joint position error", and "cervical proprioception impairment". References for this review were also identified from the personal libraries of the authors, supplemented by the reference lists of recent reviews and book chapters. Publications relevant to cervical proprioception impairment and sensorimotor control were selected based on author expertise to summarize our current understanding of the impact of neck pain on proprioception impairment. Systematic reviews and clinical trials, particularly randomized studies, were prioritized over lower-grade evidence.
Cervical Proprioceptors and Proprioception
Balance, posture, and motor coordination are common activities in daily life, which require accurate perception of the head's position in three-dimensional space [14]. The vestibular system provides information about the position of the head relative to gravity. The visual system uses external cues to identify the position of the head relative to the surrounding environment. We now know that the cervical spine has a very delicate proprioceptive system, which signals the position of the head relative to the trunk, coordinates the vestibular and visual systems and plays a crucial role in controlling posture and balance [4, 14, 15].
Distribution of Cervical Proprioceptors
Studies have shown that the spindles of the cervical muscles are the major proprioceptors of the neck and not the joint capsules [4, 16]. The spindle density of the cervical muscles is much higher than that of the shoulder and thigh muscles [17, 18]. In general, high spindle density in small muscles is associated with fine motor tasks [19]. The deep cervical muscles in humans have shown high spindle content, particularly in small suboccipital muscles [18–22]. Complex integrative mechanisms involving head–eye coordination may require complex proprioceptive inputs from the neck muscles, which may account for their high spindle content [18].
Mechanoreceptors, Ruffini corpuscles, Pacinian corpuscles, and Golgi tendon organs have been reported in human cervical facet joints [23, 24] and discs [25, 26]. As with the peripheral joints [27, 28], there are only a small number of mechanoreceptors in the lumbar and cervical facet joints [23, 24]. There was no significant difference in the distribution of receptors between the upper and lower cervical facet capsules [24]. As suggested by McLain, these findings may indicate that the role of these receptors in cervical proprioception is more limited [29]. Similarly, there are only a few proprioceptors in the cervical discs [25, 26]. However, because the cervical disc is located on the axis of cervical motion, they are in a favorable position to detect subtle changes in the direction or position of the cervical spine [25].
Density Changes of Cervical Proprioceptors
The structures of cervical spine present degenerative changes with age, but the spindle characteristics such as the spindle distribution, morphology, and density in the cervical intrinsic muscles (longus colli and multifidus) remain unchanged [19]. So far, there is still a lack of comparative studies on the distribution of mechanoreceptors in normal and osteoarthritic cervical facet joints. Cervical facet joints and peripheral joints (such as hip and knee joints) are synovial joints and are anatomically similar. Moraes et al. [28] noted that the morphology of mechanoreceptors in the hip joint capsule did not seem to differ between healthy people and patients with osteoarthritis. Overall, the density of mechanoreceptors in the joint capsule is low, but in the joint capsule of osteoarthritis patients it is lower. The density of mechanoreceptors in patients with osteoarthritis is 0.044 per mm2, and in healthy people is 0.053 per mm2 [27, 28]. Recently, a comparative study by Yang et al. [25] found that Ruffini corpuscles were obviously increased in number and deeply ingrown into inner annulus fibrosus and even into nucleus pulposus in the diseased cervical discs from cervical spondylosis patients with dizziness in comparison with the discs from patients without dizziness and control discs. The densities of Ruffini corpuscles in anterior outer annulus fibrosus are 1.02, 0.6, and 0.33 per mm2 in patients with dizziness, patients without dizziness, and normal controls, respectively [25]. In addition, the authors [30] also found that a large number of Ruffini corpuscles and substance P-positive free nerve fibers grew in the degenerative cervical discs of patients with chronic neck pain and dizziness compared with the normal control group. These patients only showed degenerative changes of the cervical disc on imaging, without cervical disc herniation or nerve root compression. The studies suggest the degenerated cervical discs play a key role in impaired cervical proprioception.
Proprioceptive information is transmitted to the central nervous system through an encoding across populations of afferent receptors, the ensemble coding, rather than the discrete units from individual receptors [31–33]. In physiological conditions, cervical proprioceptive information from cervical muscle spindles and mechanoreceptors of cervical discs and facet joints is integrated and transmitted to the central nervous system to control head position, head orientation, and full-body posture [29, 34, 35]. Any dysfunction of these cervical sensory organs or asymmetry of afferent inputs can lead to a sensory mismatch between abnormal information (e.g., from degenerative discs) and normal information (e.g., from normal spindles) [35, 36].
Pathophysiology of Cervical Proprioception Impairment in Neck Pain
Motor Control Impairment in Experimental Neck Muscle Pain
It has been reported that patients with nonspecific neck pain have motor control impairments, but the specific deficits of the underlying regulatory system remain unclear [37]. Experimental muscle pain caused by injection of chemicals such as hypertonic saline provides a way to assess changes in motor control due to changes in afferent feedback. Experimental muscle pain often results in inhibition of the activation of painful muscles. The inhibition of muscle activity induced by pain has been observed to decrease the surface electromyographic (EMG) signal amplitude [38, 39] and motor unit discharge rate [40, 41]. Since the force does not change when muscle activity decreases [38, 42], compensation mechanisms should appear in both painful and non-painful conditions to allow similar motor outputs. Muscle pain affects motor control strategies through central mechanisms [43, 44], including in the painful muscles [38] as well as in synergistic and antagonistic muscles [45]. In repetitive dynamic tasks, the nociceptive inputs from the upper trapezius by injection of hypertonic saline induces the reorganization of the coordinated activities of the three subdivisions of the trapezius muscle [46]. This is consistent with the pain adaptation model [47], and except for the decrease in painful muscle activity, the activity of the ipsilateral and contralateral non-painful muscle subdivisions increases [46].
Cervical spine is a complex biomechanical system consisting of innumerable degrees of freedom motion of each joint and at least 20 pairs of muscles, many of which perform similar functions [48]. The decrease in muscle activity due to neck pain can be compensated, for example, by reducing the activity of antagonist muscles or increasing the activity of synergistic muscles. An experimental study has shown that the local excitation of the noxious afferents in cervical muscles has an inhibitory effect on the painful muscles, which are offset at the level of the muscle group involved in the task by complex motor strategic reorganization. This change in muscle coordination is task-dependent, so motor output remains constant in the state of pain [48].
Acute neck pain induced by hypertonic saline injection at C2/3 level in the splenius capitis muscle on one side caused cervical proprioceptive disturbance with side-specific changes [49]. The clinical implications of this finding are that neck pain itself has a clear role in proprioception and neck sensorimotor control, and subsequently influence orientation. The main function of pain is to prevent further tissue damage. This corresponds to a decrease in muscle activity of the painful muscles, whose activity moves from deeper pained muscles to superficial muscles. It is likely that in neck pain, this transfer may interfere with normal proprioception [49].
Cervical Muscle Impairment in Chronic Neck Pain
A number of studies have indicated that patients with chronic neck pain may be associated with alterations in cervical motor behavior (timing and activation) [50–52], a decrease in cross-sectional area of cervical muscles [53] as well as muscular functional deficiencies in strength, endurance, precision and acuity, and range of motion [54]. Neck pain may cause maladaptive strategies, change the neck muscle coordination, and reduce the specificity of neck muscle activation, for instance, through reduced activation of the deep segmental muscles and increased activation of the superficial muscles [48, 54]. As mentioned above, muscle spindles densely packed in the deep neck muscles are the main source of proprioception afferents in the neck. These structural and functional changes in the cervical deep and superficial muscles can change the discharge of muscle spindles, which affects the afferent input and leads to alterations in proprioception [13, 55]. The results suggest that the complexity and multifaceted nature of neck muscle impairment exists in people with chronic neck pain. In addition, the effect of pain on many levels of the nervous system can alter the sensitivity of muscle spindles and change the representation and regulation of the cortex to the cervical afferent input [43, 56, 57].
Functional Impairment of The Receptors in Degenerative Cervical Discs and Facet Joints
Most chronic idiopathic neck pain is thought to arise from degenerative cervical discs and facet joints [3]. The degenerative changes in cervical discs and facet joints are always related to inflammation [58, 59]. In the inflammatory environment, the discharge characteristics of the mechanoreceptors will be excessively active, thereby producing erroneous sensory signals [25, 30].
The mechanoreceptors in the discs and facet joint capsules can monitor and control activity of paraspinal muscles. Electrical stimulation of mechanoreceptors in lumbar discs and capsules seems to induce reflex contraction of lumbar muscles. Similarly, mechanical stimulation of mechanoreceptors in the lumbar disc and facet capsule can excite the surrounding muscles [60]. Thus, the function of the cervical motor control is closely related to the cervical proprioceptive inputs from the cervical discs and facet joints, which are responsible for the optimal recruitment of cervical muscles. In the process of maintaining body balance, there are both coordination and competition among the balance organs [36, 61]. In pathological conditions, such as cervical disc degeneration or facet osteoarthritis, the erroneous proprioceptive input distorts the direct linear interaction between neck proprioception and vestibular information, resulting in subjective body orientation and spatial psychological representation, which is manifested as dizziness or subjective unstable perception [25, 62].
Relationship Between Neck Pain and Cervical Proprioception Impairment
Neck pain itself may interfere with afferent signals from the proprioceptors of the neck, leading to erroneous proprioceptive information. The system that conducts pain carries activity to motor neurons in the same spinal segment [63]. Therefore, increased pain increases muscle tension. Electrical stimulation of group III muscle afferents and intramuscular injection of hypertonic saline can significantly change the activity of leg muscle γ-motoneurons in animals [44, 64]. The algesic chemical injection of the masseter muscle preferentially affects the amplitude sensitivity of jaw muscle spindle afferents, which is consistent with the effect on static γ-motoneurons [65]. The results suggest that pain-induced modulation of spindle afferent responses is mediated by small-diameter muscle afferents and that this modulation depends in part on the transmission of nociceptive information from the trigeminal subnucleus caudalis onto trigeminal γ-motoneurons [65]. According to the pathophysiological model [66], the metabolites produced by muscle contraction activate the muscle afferent nerves of groups III and IV, which in turn activates γ-motoneurons, supplying intrafusal fibers in both homomorphous and heteromorphic muscles, thereby increasing spindle stretching sensitivity and reflex-mediated muscle stiffness. In addition, the concentration of metabolites such as potassium, lactic acid, and arachidonic acid may directly sensitize different proprioceptors, leading to erroneous proprioceptive signals. Furthermore, the erroneous proprioceptive input can trigger the increased and prolonged reflex activation of neck muscles, which may lead to neck pain over time, thus forming a vicious circle [60].
Clinical Assessment of Sensorimotor Control in Chronic Neck Pain
Neck Sensorimotor Control Tests
The vestibular, visual, and cervical proprioceptive systems have unique central and reflex connections and integration [4]. As mentioned before, the cervical sensorimotor control system in patients with idiopathic neck pain has been affected due to impaired proprioception. To date, eight neck sensorimotor control tests have been reported to evaluate patients with idiopathic neck pain: joint position error (JPE) [67], postural sway [67, 68], subjective visual vertical [69], head tilt response [70], The Fly [71], smooth pursuit neck torsion [72, 73], head steadiness [74], and rod-and-frame test (a summary of eight neck sensorimotor control tests is shown in Table 1) [14, 75, 76]. Although some tests may involve different subsystems (such as oculomotor system and vestibular system), all tests measure sensorimotor control in the neck, and the most commonly used is JPE test [77]. However, it is difficult to compare test results due to the different tasks required or the techniques used to quantify the measurements. In addition, it is not clear whether different measurement methods are equally reliable and effective, and which test is preferred [7]. A recent study [78] compared seven tests (all except the rod-and-frame test) in 50 patients with chronic idiopathic neck pain and found that different tests measured different components of sensorimotor control. The study implies that all seven tests are independent of each other. It is not possible to recommend a test battery for clinical practice.
Table 1.
Eight neck sensorimotor control tests*
| Test | Purpose | Measurement (units) | Method |
|---|---|---|---|
| JPE | Ability to reposition the head to a given position | Error from neutral head position or target (degree or cm) | Participant sits in a chair facing a target on a wall 90 cm away. A specific laser pointer is placed on top of the head and the participant is blindfolded. While starting with the laser pointer exactly in the center of the target, the participant is instructed to move their head away from the target. After returning to the center, the error between starting position and final position is assessed |
| Postural sway | Amount of movement during quiet stance | Sway area (cm2) or total sway path (mm) | Recorded during stance on a force platform in both eyes-open and eyes-closed conditions |
| Subjective visual vertical | Ability to reposition the line displayed on the screen so that it is aligned with the true vertical line | Error from true vertical (degrees) | A tilted line appears on a computer screen, disk, or virtual reality device. Participants have to use a computer mouse or control knob to reposition the line to align it with the true vertical line |
| Head tilt response | Ability to position the stripe with the true vertical line | Error from true vertical (degree) | A tilted white stripe appears in a pair of virtual reality goggles. The participant is instructed to adjust the roll angle of the head until the stripe is aligned with the gravitational vertical |
| Smooth pursuit neck torsion | Eye movements while torso is rotated relative to the head | Ratio between eye and target movement in neutral and torsion positions | Participants focus their eyes on a moving target and keep the head still, in a neutral position, and a position in which the torso is rotated relative to the head (torsion). The velocity of eye movements while following the target is recorded. The gain (ratio between eye movement and target movement) is calculated. The outcome measure (smooth pursuit neck torsion) is the difference between the gain in neutral and the average gain in torsion (right and left) |
| The Fly | Ability to track a computer-displayed target moving in a specific pattern | Error from target (cm or mm) | Participants track target (The Fly) on a computer screen by moving their head and neck. Fastrak system is used to measure the accuracy. For different difficulty levels (i.e., different fly pattens), the accuracy of the performance is reported |
| Head steadiness | Ability to hold the head still for a period of time | Angular velocity (degree/s) | Head steadiness in terms of head motion velocity is compared during two 40 second isometric neck flexion tests at a high-load test and a low-load test. Increased velocity is expected to reflect decreased head steadiness |
| Rod-and-frame test | Ability to position an offset rod into the vertical position | Vertical perception error (degree) | Rod and frame are made up of a luminescent vertical rod surrounded by a square frame. Participants are placed in a dark room where the only visual objects are the luminescent vertical rod and square frame. In the different test process, the test rod or frame offset by different degrees, and the subjects are required to use the joystick to place the rod vertically. The difference between the real vertical line and the subject's perception of the vertical line is measured |
JPE Test
Cervical joint position sense (JPS) is a major component of proprioception, and mainly reflects the ascending input (afferent) of cervical muscle, disc, capsule, and ligament receptors [79]. Abnormal cervical afferent input leads to an impaired cervical JPS, which is measured as cervical JPE [68]. Several methods exist to study cervical JPE, and the most commonly used is active motion angle reconstruction test and requires subjects to relocate a neutral head position or a predefined target head position without visual assistance selected by the researchers (Fig. 3). In the study of head position sense measurement, the measured variable is the difference between the initially determined reference point position (neutral or target position) and the position produced when the subjects try to match the target position. This difference is called JPE and the angle is in degrees (°) [80]. The test has been widely used to distinguish patients with chronic neck pain from the healthy control group [11, 12, 79].
Fig. 3.
Craniocervical flexion test. During craniocervical flexion test, the patient is positioned supine in crook lying with the neck in a neutral position (no pillow), making the line of the face horizontal. An uninflated pressure sensor is placed behind the neck so that it is close to the occiput and inflated to a baseline pressure of 20 mmHg, which is sufficient to fill the space between the table surface and the cervical lordotic curve, but does not push the neck into the lordosis. The device provides feedback and guidance to the patient to perform the five test phases required, with an additional 2 mmHg per phase and a maximum of 30 mmHg. The patient is asked to hold each position for 10 s. There is a 10-s break between the two stages. The figure shows the starting position (left) and the end position of craniocervical flexion (right). The craniocervical flexion includes the head nodding "yes" movement to keep it in contact with the supporting surface, and the flexion movement mainly occurs in the upper neck motion segments.
Revel et al. [67] firstly reported that patients with chronic neck pain showed poorer ability (6.11°) to restore the original position of the head after actively rotating the head to the maximum extent compared with healthy subjects (3.50°). They suggested changing the proprioception of the neck to account for their findings. Alahmari et al. [81] compared the JPE of 42 patients with chronic neck pain and 42 age-matched healthy people, and found that the patients had a greater error in all movement directions tested (p < 0.001). Similarly, other studies have revealed a significant greater error in patients with chronic idiopathic neck pain when compared with asymptomatic controls, despite the variability in methods used in these studies [68, 82–87]. Cervical proprioceptive errors in the patients with cervical spondylosis were also shown to be significantly larger than those of the healthy control group, indicating that the cervical proprioception in the cervical spondylosis was impaired [55]. A systematic review including 14 studies suggested that JPE was significantly higher in the neck pain group than in the control group [79]. De Zoete et al. [77] in a systematic review and meta-analysis revealed a significant difference between idiopathic neck pain and healthy groups in JPE testing, indicating that this test may be clinically useful in assessing sensorimotor control. Similarly, another systematic review and meta-analysis conducted by Stanton et al. [11] aimed to synthesize and critically evaluate the available evidence for proprioceptive dysfunction in patients with chronic idiopathic neck pain by comparing the JPE of asymptomatic controls. Pooled estimates showed that people with chronic idiopathic neck pain had moderately impaired cervical JPS during head-to-neutral repositioning tests, compared with asymptomatic controls.
Differences in JPE Test
It is difficult to quantify pain and sensorimotor control. Although pain can be described and graded subjectively, sensorimotor control is more difficult to understand. In JPE testing, since the neutral neck position is a commonly used position, the memory head position may be recalled during the test [87]. Recall of the head position in a memory may involve more than one sensory component because memory may not only rely on proprioception. Therefore, the validity of this test in evaluating sensorimotor control may be limited. In studies using a preset target in the transverse plane, recall of the head position was less likely because the head was in a less common position on the neck. The head repositioning test makes indirect evaluation of proprioception possible [88]. However, the results vary greatly. Although most studies have reported reduced sensorimotor control in patients with neck pain, other studies [88, 89] have reported that patients with subclinical neck pain have fewer relocation errors than controls. Rix et al. [86] and Palmgren et al. [90] found that people with chronic, nontraumatic neck pain were no less accurate in head repositioning than healthy controls for all movement directions except flexion. In addition, other studies have reported that there is no measurable effect on cervical proprioception in the case of cervical pain [91–93]. In general, these findings contradict the theory that the change of position sense may be due to the nociceptive input, or the pain experienced in the position-matching task provides additional feedback to improve the accuracy of position matching [29]. These contradictory results suggest the multifaceted nature of neck pain and the different mechanisms in sensorimotor control. For example, muscle fatigue and/or muscle tension and central nervous system regulation may change sensorimotor control [55]. Pain itself may interfere with muscle activity or adaptation of exercise strategies [49]. Cervical proprioception errors were significantly and positively correlated with the intensity of neck pain, indicating that the increase in pain intensity will impair proprioception [55, 94]. Different pain time courses such as acute, subacute, or persistent pain may have different effects on sensorimotor control [92]. Persistent pain may cause morphological changes in muscle components [53, 54]. In addition, an increase of age was thought to be related to the increase of cervical JPE. In elderly subjects, neck muscle strength decline, sedentary lifestyle, memory decline, and cognitive or motor impairment may result in damage to the target reproduction test [80, 81]. However, the literature on the relationship between age and JPE is contradictory [81, 83], which suggests that there may be other factors beyond age that affect the cervical proprioception [95]. Therefore, many causes may affect the proprioception and sensorimotor control of cervical spine.
Rehabilitation of Sensorimotor Control in Chronic Neck Pain
Evidence to date suggests that the management of sensorimotor control disturbances due to chronic neck pain may need to address the primary causes and secondary effects of alterations in proprioceptive activity [4, 5, 12, 13, 96]. Local treatments to relieve neck pain are not discussed here. The focus now is primarily on tailored programs that address different aspects of sensorimotor control disturbances, such as training aimed at improving proprioception and muscle coordination.
Proprioceptive Retraining
After a rehabilitation program based on eye–head coupling exercises aimed to improve proprioception in the neck, Revel et al. [12] found that the ability to reposition the head after rotation was significantly improved, and neck pain was significantly reduced. There was no significant change in JPE in the control group. Jull et al. [13] achieved similar results in a group of patients with chronic neck pain using proprioceptive training. A randomized clinical trial evaluated the impact of balance training on JPS in patients with chronic neck pain, and found that joint repositioning accuracy was improved and pain was reduced in the intervention group, while no effect was observed in the control group [97]. Another randomized control trial revealed that proprioceptive training with a gaze direction recognition exercise combined with conventional physical therapy was more effective than conventional physical therapy for patients with chronic neck pain in improving neck disability and balance [98]. In a double-blind, randomized controlled trial, Saadat et al. [99] demonstrated that sensorimotor training combined with traditional physical therapy exercises could be more effective than traditional exercise alone in improving JPS, endurance, dynamic balance, and walking speed in patients with chronic neck pain (Table 2).
Table 2.
Proprioceptive training in patients with neck pain and clinical outcomes
| Study | Study design | Participants | Interventions | Main outcome measures | Results | Main findings |
|---|---|---|---|---|---|---|
| Revel et al. [12] | RCT | Sixty participants with chronic neck pain were randomly allocated to a rehabilitation group and a control group |
The rehabilitation group received a rehabilitation program and common symptomatic treatment (e.g., nonsteroidal anti-inflammatory drugs, analgesic drugs); the control group received only symptomatic treatment without rehabilitation The rehabilitation program included head relocation practice, gaze stability, eye-follow and eye–head coordination exercises and 30–40 min at a time, twice a week for 8 weeks |
HRA; neck pain (VAS, 0–100) | Greater gain in HRA and more improved neck pain were observed in the rehabilitation group than in the control group | A rehabilitation program based on eye–head coupling should be included in most medical management of cervicalgic patients |
| Jull et al. [13] | RCT | Sixty-four female subjects with persistent neck pain and deficits in JPE were randomized into two exercise groups: proprioceptive training or craniocervical flexion training |
Proprioceptive training included head relocation practice, gaze stability, eye-follow and eye–head coordination exercises. Craniocervical flexion training included the low-load training of the craniocervical flexor muscles Less than 30 min at a time, once a week for 6 weeks |
JPE; NDI; neck pain (NRS, 0–10) | A significant decrease in JPE, neck pain intensity, and perceived disability was identified for both the proprioceptive training group and the craniocervical flexion training group. However, the proprioceptive training group had a greater reduction in JPE from right rotation compared to the craniocervical flexion training group | Both proprioceptive training and craniocervical flexion training have a demonstrable benefit on impaired cervical JPE in people with neck pain, with marginally more benefit gained from proprioceptive training |
| Beinert and Taube [97] | RCT | Thirty-four patients with subclinical neck pain were randomly assigned to balance training (intervention group) or to stay active (control group) |
The intervention consisted of three balance tasks: single leg stance, tandem stance and standing on a wobble board. Each task was performed for 20 s with a 10-s break in between 15 mins at a time, 3 times a week for 5 weeks |
NHP; RHP; neck pain (NRS,0–10) | The intervention group showed improved joint repositioning accuracy and decreased pain, whereas no effects were observed in the control group. A weak correlation was identified between reduced neck pain intensity and improved joint repositioning |
Balance training can effectively improve cervical sensorimotor function and decrease neck pain intensity |
| Duray et al. [98] | RCT | Forty patients with chronic neck pain were randomly divided into study and control groups |
The study group received conventional physical therapy and gaze direction recognition exercise for proprioceptive training; the control group received only conventional physical therapy Exercises were performed for 3 weeks with five sessions per week |
FSST; SLBT; NDI; neck pain (VAS,0–10) |
The study group tended to show higher SLBT with eyes opened and closed scores and lower neck pain intensity, FSST scores, and neck disability levels after the treatment. However, no significant differences were observed except for in pain intensity scores in the control group | Proprioceptive training should be included in physiotherapy programs to improve balance; it decreases the disability level in patients with chronic neck pain |
| Saadat et al. [99] | Double-blind, RCT | Fifty-three patients with chronic non-specific neck pain were randomized to either traditional or combined exercise groups |
The traditional group performed traditional exercises, and the combined exercise group performed sensorimotor training in addition to traditional exercises. This sensorimotor training program comprised three parts: retraining joint position and movement sense, oculomotor exercises, and balance training All patients received 12 sessions of supervised intervention 3 times per week |
JPS; neck pain (VAS,0–10), NFMET, 10-m walk test, step test, and NDI |
The combined exercise group showed significantly greater improvement compared to the traditional group in joint position sense, the 10-m walk test, and the step test. Pain intensity, muscle endurance, and disability improved in both groups |
A combination of sensorimotor training with traditional physical therapy exercises could be more effective than traditional exercises alone in improving joint position sense, endurance, dynamic balance, and walking speed |
RCT randomized controlled trial, HRA head repositioning accuracy, VAS visual analogue scale, JPE cervical joint position error, NDI Neck Disability Index, NRS Numerical rating scale, NHP neutral head position, RHP pre-rotated head positions, FSST four step square tests, SLBT single leg balance test, NFMET neck flexor muscle endurance test
Retraining of Deep Cervical Flexor Muscles
Deep muscles are important for maintaining neck posture. The support of the cervical segments mainly depends on the muscular sleeve formed by the longus colli muscle anteriorly and the semispinalis cervicis and cervical multifidus muscles posteriorly [100]. In order to stabilize the cervical segments, the deep cervical muscle activity should cooperate with the superficial muscle activity [101, 102]. Recent studies have found that the activation of deep flexors, the longus colli and longus capitis, is impaired in patients with neck pain [50, 51]. There is evidence that the strength and endurance of the craniocervical and cervical flexors decrease in the patients with neck pain, and therefore rehabilitation is required [103]. The muscle impairment determined by the craniocervical flexion test seems to be the common feature of various neck pain. These observations prompted the use of the craniocervical flexion test (Fig. 3) to retrain the deep cervical flexor muscles in the motor relearning program for patients with neck pain [104]. Craniocervical flexion training increased deep cervical flexor muscle EMG amplitude and decreased sternocleidomastoid and anterior scalene muscle EMG amplitude across all stages of the craniocervical flexion test [103]. In clinical exercise of craniocervical flexion, retraining the deep cervical flexors can improve the function of cervical proprioception, maintain neutral posture, increase the activation and endurance of the deep cervical flexors, and reduce the neck symptoms [13, 100, 105, 106].
Retraining of Deep Cervical Extensor Muscles
The deep cervical extensor muscles are anatomically capable of coordinating cervical segmental movement with the deep cervical flexors [107]. The cervical extensor muscles are considered equally important in the recovery of patients with neck pain [108]. In patients with neck pain, the activity of superficial cervical extensors is enhanced and delayed offset (relaxation) after the activity [107, 109]. Compared with healthy controls, patients with neck pain showed structural and functional changes in the deep cervical extensor muscles [110–117], emphasizing the importance of exercise in improving performance. Specific exercises of the deep neck extensors have not been extensively studied. A muscle functional magnetic resonance imaging study [118] in a group of healthy volunteers showed that both the deep and superficial extensors were activated below the C2 level when an isometric neutral head/neck extension was performed at 20% of the maximum voluntary force. Compared with the exercise in the neutral position, the same exercise in 15° of craniocervical extension increased the activity of the semispinalis capitis muscle, but not the deep extensor muscles below the C2 level. This is to be expected, because multifidus and semispinalis cervicis are the highest adherent to C2 and are therefore less affected by craniocervical extension. The results provided some preliminary insights into the impact of craniocervical orientation in response to different deep and superficial cervical extensors during performing cervical extensor exercises. O 'Leary et al. [119] then used the same method to compare the cervical extensor response patterns in patients with chronic neck pain with healthy controls, suggesting some alteration in the differential activation in cervical extensors in patients with chronic neck pain. High-load exercise is also recommended to increase muscle strength and endurance [107]. Twelve weeks of specific cervical resistance training resulted in a 34% increase in head-extension strength in healthy subjects [120]. Cross-sectional area increased by nearly 25% in splenius capitis, semispinalis capitis, semispinalis cervicis, and the multifidus, showing important hypertrophic effects [121]. A study using intramuscular EMG investigated the activity of the deep semispinalis cervicis and the superficial splenius capitis muscle at two spinal levels (C2 and C5) in healthy volunteers, and displayed the activation of the semispinalis cervicis relative to the splenius capitis when manual resistance was applied in extension over the vertebral arches (C1 and C4) [122]. A similar study in a group of women with chronic neck pain showed that localized resistance selectively activated the semispinalis cervicis muscle [123].
Etiological Management of Neck Pain
Eliminating acute neck pain and preventing it from developing into chronic neck pain are important for optimal management. If pain occurs, the cause should be analyzed and resolved during treatment. Proprioceptive input of the muscle spindles can be reduced by reducing neck pain. However, there is a lack of research in this area. The reduction in neck muscle tension caused by neck pain is important to improve postural performance. This, in turn, may lead to the normalization of proprioceptive signals in the neck muscles, reducing sensory mismatch. Cervical discogenic pain and cervical facet joint pain are the two main causes of chronic neck pain, accounting for 15–53% [124] and 40–55% [125] respectively. Management aimed at eliminating inflammation in cervical disc and facet joint is particularly important in theory. It can not only reduce the sensitivity of nociceptors but also the sensitivity of proprioceptors. The difficulty lies in the lack of specific diagnostic methods for cervical discogenic pain and facet joint pain [124, 125].
Conclusions
Neck pain is a common cause of disability worldwide, but its basic pathology and pathophysiology are still unclear. One of the main problems of patients with neck pain is that the impairment of cervical proprioception leads to the disturbance of cervical sensorimotor control. Current studies recommend training on different aspects of sensorimotor function, particularly retraining aimed at improving proprioception and muscle coordination in the neck. Overall, the recommended clinical evaluation and management of sensorimotor control disturbances are based on currently available evidence, but this is an emerging field that requires more extensive studies to refine and determine the evaluation methods and determine the best strategy for treating the disturbances in patients with chronic neck pain [4, 126].
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed equally to the manuscript.
Disclosures
Baogan Peng, Liang Yang, Yongchao Li, Tanghua Liu, and Yanqing Liu have nothing to disclose.
Compliance With Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Fejer R, Kyvik KO, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. 2006;15(6):834–848. doi: 10.1007/s00586-004-0864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans G. Identifying and treating the causes of neck pain. Med Clin North Am. 2014;98(3):645–661. doi: 10.1016/j.mcna.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SP, Hooten WM. Advances in the diagnosis and management of neck pain. BMJ. 2017;358:j3221. doi: 10.1136/bmj.j3221. [DOI] [PubMed] [Google Scholar]
- 4.Treleaven J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man Ther. 2008;13(1):2–11. doi: 10.1016/j.math.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Treleaven J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control–Part 2: case studies. Man Ther. 2008;13(3):266–275. doi: 10.1016/j.math.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Treleaven J. Dizziness, unsteadiness, visual disturbances, and sensorimotor control in traumatic neck pain. J Orthop Sports Phys Ther. 2017;47(7):492–502. doi: 10.2519/jospt.2017.7052. [DOI] [PubMed] [Google Scholar]
- 7.Michiels S, De Hertogh W, Truijen S, November D, Wuyts F, Van de Heyning P. The assessment of cervical sensory motor control: a systematic review focusing on measuring methods and their clinimetric characteristics. Gait Posture. 2013;38(1):1–7. doi: 10.1016/j.gaitpost.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 8.McCaskey MA, Schuster-Amft C, Wirth B, Suica Z, de Bruin ED. Effects of proprioceptive exercises on pain and function in chronic neck- and low back pain rehabilitation: a systematic literature review. BMC Musculoskelet Disord. 2014;15:382. doi: 10.1186/1471-2474-15-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carragee EJ, Hurwitz EL, Cheng I, et al. Treatment of neck pain: injections and surgical interventions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33(4 Suppl):S153–S169. [DOI] [PubMed]
- 10.Childs JD, Cleland JA, Elliott JM, et al. Neck pain: clinical practice guidelines linked to the International Classification of Functioning, Disability, and Health from the Orthopedic Section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2008;38(9):A1–A34. doi: 10.2519/jospt.2008.0303. [DOI] [PubMed] [Google Scholar]
- 11.Stanton TR, Leake HB, Chalmers KJ, Moseley GL. Evidence of impaired proprioception in chronic, idiopathic neck pain: systematic review and meta-analysis. Phys Ther. 2016;96(6):876–887. doi: 10.2522/ptj.20150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Revel M, Minguet M, Gregoy P, Vaillant J, Manuel JL. Changes in cervicocephalic kinesthesia after a proprioceptive rehabilitation program in patients with neck pain: a randomized controlled study. Arch Phys Med Rehabil. 1994;75(8):895–899. doi: 10.1016/0003-9993(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 13.Jull G, Falla D, Treleaven J, Hodges P, Vicenzino B. Retraining cervical joint position sense: the effect of two exercise regimes. J Orthop Res. 2007;25(3):404–412. doi: 10.1002/jor.20220. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys BK. Cervical outcome measures: testing for postural stability and balance. J Manipulative Physiol Ther. 2008;31(7):540–546. doi: 10.1016/j.jmpt.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Mergner T, Nasios G, Maurer C, Becker W. Visual object localisation in space. Interaction of retinal, eye position, vestibular and neck proprioceptive information. Exp Brain Res. 2001;141(1):33–51. [DOI] [PubMed]
- 16.Richmond FJ, Abrahams VC. What are the proprioceptors of the neck? Prog Brain Res. 1979;50:245–254. doi: 10.1016/S0079-6123(08)60825-0. [DOI] [PubMed] [Google Scholar]
- 17.Cooper S, Daniel PM. Muscle spindles in man; their morphology in the lumbricals and the deep muscles of the neck. Brain. 1963;86:563–586. doi: 10.1093/brain/86.3.563. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni V, Chandy MJ, Babu KS. Quantitative study of muscle spindles in suboccipital muscles of human foetuses. Neurol India. 2001;49(4):355–359. [PubMed] [Google Scholar]
- 19.Boyd-Clark LC, Briggs CA, Galea MP. Muscle spindle distribution, morphology, and density in longus colli and multifidus muscles of the cervical spine. Spine (Phila Pa 1976). 2002;27(7):694–701. [DOI] [PubMed]
- 20.Amonoo-Kuofi HS. The density of muscle spindles in the medial, intermediate and lateral columns of human intrinsic postvertebral muscles. J Anat. 1983;136(Pt 3):509–519. [PMC free article] [PubMed] [Google Scholar]
- 21.Amonoo-Kuofi HS. The number and distribution of muscle spindles in human intrinsic postvertebral muscles. J Anat. 1982;135(Pt 3):585–599. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JX, Thornell LE, Pedrosa-Domellöf F. Muscle spindles in the deep muscles of the human neck: a morphological and immunocytochemical study. J Histochem Cytochem. 2003;51(2):175–186. doi: 10.1177/002215540305100206. [DOI] [PubMed] [Google Scholar]
- 23.McLain RF, Pickar JG. Mechanoreceptor endings in human thoracic and lumbar facet joints. Spine (Phila Pa 1976). 1998;23(2):168–173. [DOI] [PubMed]
- 24.McLain RF. Mechanoreceptor endings in human cervical facet joints. Spine. 1994;19(5):495–501. doi: 10.1097/00007632-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Yang C, Pang X, et al. Mechanoreceptors in diseased cervical intervertebral disc and vertigo. Spine (Phila Pa 1976). 2017;42(8):540–546. [DOI] [PubMed]
- 26.Mendel T, Wink CS, Zimny ML. Neural elements in human cervical intervertebral discs. Spine (Phila Pa 1976). 1992;17(2):132–135. [DOI] [PubMed]
- 27.Tomlinson J, Zwirner J, Ondruschka B, Prietzel T, Hammer N. Innervation of the hip joint capsular complex: a systematic review of histological and immunohistochemical studies and their clinical implications for contemporary treatment strategies in total hip arthroplasty. PLoS One. 2020;15(2):e0229128. doi: 10.1371/journal.pone.0229128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moraes MR, Cavalcante ML, Leite JA, et al. The characteristics of the mechanoreceptors of the hip with arthrosis. J Orthop Surg Res. 2011;6:58. doi: 10.1186/1749-799X-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong B, McNair P, Taylor D. Head and neck position sense. Sports Med. 2008;38(2):101–117. doi: 10.2165/00007256-200838020-00002. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Chen J, Yang C, et al. Cervical intervertebral disc degeneration contributes to dizziness: a clinical and immunohistochemical study. World Neurosurg. 2018;119:e686–e693. doi: 10.1016/j.wneu.2018.07.243. [DOI] [PubMed] [Google Scholar]
- 31.Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- 32.Tuthill JC, Azim E. Proprioception. Curr Biol. 2018;28(5):R194–R203. doi: 10.1016/j.cub.2018.01.064. [DOI] [PubMed] [Google Scholar]
- 33.Riemann BL, Lephart SM. The sensorimotor system, part I: the physiologic basis of functional joint stability. J Athl Train. 2002;37(1):71–79. [PMC free article] [PubMed] [Google Scholar]
- 34.Peng B. Cervical vertigo: historical reviews and advances. World Neurosurg. 2018;109:347–350. doi: 10.1016/j.wneu.2017.10.063. [DOI] [PubMed] [Google Scholar]
- 35.Devaraja K. Approach to cervicogenic dizziness: a comprehensive review of its aetiopathology and management. Eur Arch Otorhinolaryngol. 2018;275(10):2421–2433. doi: 10.1007/s00405-018-5088-z. [DOI] [PubMed] [Google Scholar]
- 36.Brandt T, Bronstein AM. Cervical vertigo. J Neurol Neurosurg Psychiatry. 2001;71(1):8–12. doi: 10.1136/jnnp.71.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stensdotter AK, Meisingset I, Pedersen MD, Vasseljen O, Stavdahl Ø. Frequency-dependent deficits in head steadiness in patients with nonspecific neck pain. Physiol Rep. 2019;7(5):e14013. doi: 10.14814/phy2.14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graven-Nielsen T, Svensson P, Arendt-Nielsen L. Effects of experimental muscle pain on muscle activity and co-ordination during static and dynamic motor function. Electroencephalogr Clin Neurophysiol. 1997;105(2):156–164. doi: 10.1016/s0924-980x(96)96554-6. [DOI] [PubMed] [Google Scholar]
- 39.Svensson P, Arendt-Nielsen L, Houe L. Muscle pain modulates mastication: an experimental study in humans. J Orofac Pain. 1998;12(1):7–16. [PubMed] [Google Scholar]
- 40.Farina D, Arendt-Nielsen L, Merletti R, Graven-Nielsen T. Effect of experimental muscle pain on motor unit firing rate and conduction velocity. J Neurophysiol. 2004;91(3):1250–1259. doi: 10.1152/jn.00620.2003. [DOI] [PubMed] [Google Scholar]
- 41.Sohn MK, Graven-Nielsen T, Arendt-Nielsen L, Svensson P. Inhibition of motor unit firing during experimental muscle pain in humans. Muscle Nerve. 2000;23(8):1219–1226. doi: 10.1002/1097-4598(200008)23:8<1219::aid-mus10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 42.Farina D, Arendt-Nielsen L, Graven-Nielsen T. Experimental muscle pain reduces initial motor unit discharge rates during sustained submaximal contractions. J Appl Physiol (1985). 2005;98(3):999–1005. [DOI] [PubMed]
- 43.Le Pera D, Graven-Nielsen T, Valeriani M, et al. Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol. 2001;112(9):1633–1641. doi: 10.1016/s1388-2457(01)00631-9. [DOI] [PubMed] [Google Scholar]
- 44.Thunberg J, Ljubisavljevic M, Djupsjöbacka M, Johansson H. Effects on the fusimotor-muscle spindle system induced by intramuscular injections of hypertonic saline. Exp Brain Res. 2002;142(3):319–326. doi: 10.1007/s00221-001-0941-4. [DOI] [PubMed] [Google Scholar]
- 45.Ciubotariu A, Arendt-Nielsen L, Graven-Nielsen T. The influence of muscle pain and fatigue on the activity of synergistic muscles of the leg. Eur J Appl Physiol. 2004;91(5–6):604–614. doi: 10.1007/s00421-003-1026-9. [DOI] [PubMed] [Google Scholar]
- 46.Falla D, Farina D, Graven-Nielsen T. Experimental muscle pain results in reorganization of coordination among trapezius muscle subdivisions during repetitive shoulder flexion. Exp Brain Res. 2007;178(3):385–393. doi: 10.1007/s00221-006-0746-6. [DOI] [PubMed] [Google Scholar]
- 47.Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69(5):683–694. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- 48.Falla D, Farina D, Dahl MK, Graven-Nielsen T. Muscle pain induces task-dependent changes in cervical agonist/antagonist activity. J Appl Physiol (1985). 2007;102(2):601–609. [DOI] [PubMed]
- 49.Malmström EM, Westergren H, Fransson PA, Karlberg M, Magnusson M. Experimentally induced deep cervical muscle pain distorts head on trunk orientation. Eur J Appl Physiol. 2013;113(10):2487–2499. doi: 10.1007/s00421-013-2683-y. [DOI] [PubMed] [Google Scholar]
- 50.Falla DL, Jull GA, Hodges PW. Patients with neck pain demonstrate reduced electromyographic activity of the deep cervical flexor muscles during performance of the craniocervical flexion test. Spine (Phila Pa 1976). 2004;29(19):2108–2114. [DOI] [PubMed]
- 51.Falla D, Bilenkij G, Jull G. Patients with chronic neck pain demonstrate altered patterns of muscle activation during performance of a functional upper limb task. Spine (Phila Pa 1976). 2004;29(13):1436–1440. [DOI] [PubMed]
- 52.Blouin JS, Siegmund GP, Carpenter MG, Inglis JT. Neural control of superficial and deep neck muscles in humans. J Neurophysiol. 2007;98(2):920–928. doi: 10.1152/jn.00183.2007. [DOI] [PubMed] [Google Scholar]
- 53.De Pauw R, Coppieters I, Kregel J, De Meulemeester K, Danneels L, Cagnie B. Does muscle morphology change in chronic neck pain patients? A systematic review. Man Ther. 2016;22:42–49. doi: 10.1016/j.math.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Falla D. Unravelling the complexity of muscle impairment in chronic neck pain. Man Ther. 2004;9(3):125–133. doi: 10.1016/j.math.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Reddy RS, Tedla JS, Dixit S, Abohashrh M. Cervical proprioception and its relationship with neck pain intensity in subjects with cervical spondylosis. BMC Musculoskelet Disord. 2019;20(1):447. doi: 10.1186/s12891-019-2846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thunberg J, Hellström F, Sjölander P, Bergenheim M, Wenngren B, Johansson H. Influences on the fusimotor-muscle spindle system from chemosensitive nerve endings in cervical facet joints in the cat: possible implications for whiplash induced disorders. Pain. 2001;91(1–2):15–22. doi: 10.1016/s0304-3959(00)00415-2. [DOI] [PubMed] [Google Scholar]
- 57.Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;(41 Suppl):66–72. [DOI] [PubMed]
- 58.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine. 2014;70(2):185–193. doi: 10.1016/j.cyto.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holm S, Indhal A, Solomonow M. Sensorimotor control of the spine. J Electromyogr Kinesiol. 2002;12(3):219–234. doi: 10.1016/s1050-6411(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 61.Yacovino DA, Hain TC. Clinical characteristics of cervicogenic-related dizziness and vertigo. Semin Neurol. 2013;33(3):244–255. doi: 10.1055/s-0033-1354592. [DOI] [PubMed] [Google Scholar]
- 62.Karnath HO. Subjective body orientation in neglect and the interactive contribution of neck muscle proprioception and vestibular stimulation. Brain. 1994;117(Pt 5):1001–1012. doi: 10.1093/brain/117.5.1001. [DOI] [PubMed] [Google Scholar]
- 63.Carlsson CA, Pellettieri L. A clinical view of pain physiology. Acta Chir Scand. 1982;148(4):305–313. [PubMed] [Google Scholar]
- 64.Appelberg B, Hulliger M, Johansson H, Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group III muscle afferent fibres in the hind limb of the cat. J Physiol. 1983;335:275–292. doi: 10.1113/jphysiol.1983.sp014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capra NF, Hisley CK, Masri RM. The influence of pain on masseter spindle afferent discharge. Arch Oral Biol. 2007;52(4):387–390. doi: 10.1016/j.archoralbio.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johansson H, Sojka P. Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: a hypothesis. Med Hypotheses. 1991;35(3):196–203. doi: 10.1016/0306-9877(91)90233-o. [DOI] [PubMed] [Google Scholar]
- 67.Revel M, Andre-Deshays C, Minguet M. Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch Phys Med Rehabil. 1991;72(5):288–291. [PubMed] [Google Scholar]
- 68.Chen X, Treleaven J. The effect of neck torsion on joint position error in subjects with chronic neck pain. Man Ther. 2013;18(6):562–567. doi: 10.1016/j.math.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 69.Treleaven J, Takasaki H. High variability of the subjective visual vertical test of vertical perception, in some people with neck pain—should this be a standard measure of cervical proprioception? Man Ther. 2015;20(1):183–188. doi: 10.1016/j.math.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Geisinger D, Ferreira E, Suarez A, Suarez H. Head tilt response: a complementary test to the subjective visual vertical. J Vestib Res. 2010;20(5):381–389. doi: 10.3233/VES-2010-0384. [DOI] [PubMed] [Google Scholar]
- 71.Kristjansson E, Oddsdottir GL. ‘‘The Fly’’: a new clinical assessment and treatment method for deficits of movement control in the cervical spine. Spine (Phila Pa 1976).2010;35(23):E1298–E1305 [DOI] [PubMed]
- 72.Tjell C, Rosenhall U. Smooth pursuit neck torsion test: a specific test for cervical dizziness. Am J Otol. 1998;19(1):76–81. [PubMed] [Google Scholar]
- 73.Treleaven J, Jull G, LowChoy N. Smooth pursuit neck torsion test in whiplash-associated disorders: relationship to self-reports of neck pain and disability, dizziness and anxiety. J Rehabil Med. 2005;37(4):219–223. doi: 10.1080/16501970410024299. [DOI] [PubMed] [Google Scholar]
- 74.Woodhouse A, Liljebäck P, Vasseljen O. Reduced head steadiness in whiplash compared with non-traumatic neck pain. J Rehabil Med. 2010;42(1):35–41. doi: 10.2340/16501977-0484. [DOI] [PubMed] [Google Scholar]
- 75.Bagust J. Assessment of verticality perception by a rod-and-frame test: preliminary observations on the use of a computer monitor and video eye glasses. Arch Phys Med Rehabil. 2005;86(5):1062–1064. doi: 10.1016/j.apmr.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 76.Grod JP, Diakow PR. Effect of neck pain on verticality perception: a cohort study. Arch Phys Med Rehabil. 2002;83(3):412–415. doi: 10.1053/apmr.2002.29660. [DOI] [PubMed] [Google Scholar]
- 77.de Zoete RMJ, Osmotherly PG, Rivett DA, Farrell SF, Snodgrass SJ. Sensorimotor control in individuals with idiopathic neck pain and healthy individuals: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2017;98(6):1257–1271. doi: 10.1016/j.apmr.2016.09.121. [DOI] [PubMed] [Google Scholar]
- 78.de Zoete RMJ, Osmotherly PG, Rivett DA, Snodgrass SJ. Seven cervical sensorimotor control tests measure different skills in individuals with chronic idiopathic neck pain. Braz J Phys Ther. 2020;24(1):69–78. doi: 10.1016/j.bjpt.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Vries J, Ischebeck BK, Voogt LP, et al. Joint position sense error in people with neck pain: a systematic review. Man Ther. 2015;20(6):736–744. doi: 10.1016/j.math.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Alahmari KA, Reddy RS, Silvian PS, Ahmad I, Kakaraparthi VN, Alam MM. Association of age on cervical joint position error. J Adv Res. 2017;8(3):201–207. doi: 10.1016/j.jare.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alahmari KA, Reddy RS, Silvian P, Ahmad I, Nagaraj V, Mahtab M. Influence of chronic neck pain on cervical joint position error (JPE): Comparison between young and elderly subjects. J Back Musculoskelet Rehabil. 2017;30(6):1265–1271. doi: 10.3233/BMR-169630. [DOI] [PubMed] [Google Scholar]
- 82.Cheng CH, Wang JL, Lin JJ, Wang SF, Lin KH. Position accuracy and electromyographic responses during head reposition in young adults with chronic neck pain. J Electromyogr Kinesiol. 2010;20(5):1014–1020. doi: 10.1016/j.jelekin.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 83.Edmondston SJ, Chan HY, Ngai GC, et al. Postural neck pain: an investigation of habitual sitting posture, perception of 'good' posture and cervicothoracic kinaesthesia. Man Ther. 2007;12(4):363–371. doi: 10.1016/j.math.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Elsig S, Luomajoki H, Sattelmayer M, Taeymans J, Tal-Akabi A, Hilfiker R. Sensorimotor tests, such as movement control and laterality judgment accuracy, in persons with recurrent neck pain and controls. A case-control study. Man Ther. 2014;19(6):555–561. doi: 10.1016/j.math.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 85.Grip H, Sundelin G, Gerdle B, Karlsson JS. Variations in the axis of motion during head repositioning—a comparison of subjects with whiplash-associated disorders or non-specific neck pain and healthy controls. Clin Biomech (Bristol, Avon) 2007;22(8):865–873. doi: 10.1016/j.clinbiomech.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 86.Rix GD, Bagust J. Cervicocephalic kinesthetic sensibility in patients with chronic, nontraumatic cervical spine pain. Arch Phys Med Rehabil. 2001;82(7):911–919. doi: 10.1053/apmr.2001.23300. [DOI] [PubMed] [Google Scholar]
- 87.Kristjansson E, Dall'Alba P, Jull G. A study of five cervicocephalic relocation tests in three different subject groups. Clin Rehabil. 2003;17(7):768–774. doi: 10.1191/0269215503cr676oa. [DOI] [PubMed] [Google Scholar]
- 88.Lee HY, Teng CC, Chai HM, Wang SF. Test-retest reliability of cervicocephalic kinesthetic sensibility in three cardinal planes. Man Ther. 2006;11(1):61–68. doi: 10.1016/j.math.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 89.Lee HY, Wang JD, Yao G, Wang SF. Association between cervicocephalic kinesthetic sensibility and frequency of subclinical neck pain. Man Ther. 2008;13(5):419–425. doi: 10.1016/j.math.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Palmgren PJ, Andreasson D, Eriksson M, Hägglund A. Cervicocephalic kinesthetic sensibility and postural balance in patients with nontraumatic chronic neck pain—a pilot study. Chiropr Osteopat. 2009;17:6. doi: 10.1186/1746-1340-17-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teng CC, Chai H, Lai DM, Wang SF. Cervicocephalic kinesthetic sensibility in young and middle-aged adults with or without a history of mild neck pain. Man Ther. 2007;12(1):22–28. doi: 10.1016/j.math.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 92.Woodhouse A, Vasseljen O. Altered motor control patterns in whiplash and chronic neck pain. BMC Musculoskelet Disord. 2008;9:90. doi: 10.1186/1471-2474-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Armstrong BS, McNair PJ, Williams M. Head and neck position sense in whiplash patients and healthy individuals and the effect of the cranio-cervical flexion action. Clin Biomech (Bristol, Avon) 2005;20(7):675–684. doi: 10.1016/j.clinbiomech.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 94.Knapstad MK, Goplen FK, Ask T, Skouen JS, Nordahl SHG. Associations between pressure pain threshold in the neck and postural control in patients with dizziness or neck pain—a cross-sectional study. BMC Musculoskelet Disord. 2019;20(1):528. doi: 10.1186/s12891-019-2922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uthaikhup S, Jull G, Sungkarat S, Treleaven J. The influence of neck pain on sensorimotor function in the elderly. Arch Gerontol Geriatr. 2012;55(3):667–672. doi: 10.1016/j.archger.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 96.Röijezon U, Björklund M, Bergenheim M, Djupsjöbacka M. A novel method for neck coordination exercise–a pilot study on persons with chronic non-specific neck pain. J Neuroeng Rehabil. 2008;5:36. doi: 10.1186/1743-0003-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beinert K, Taube W. The effect of balance training on cervical sensorimotor function and neck pain. J Mot Behav. 2013;45(3):271–278. doi: 10.1080/00222895.2013.785928. [DOI] [PubMed] [Google Scholar]
- 98.Duray M, Şimşek Ş, Altuğ F, Cavlak U. Effect of proprioceptive training on balance in patients with chronic neck pain. Agri. 2018;30(3):130–137. doi: 10.5505/agri.2018.61214. [DOI] [PubMed] [Google Scholar]
- 99.Saadat M, Salehi R, Negahban H, Shaterzadeh MJ, Mehravar M, Hessam M. Traditional physical therapy exercises combined with sensorimotor training: the effects on clinical outcomes for chronic neck pain in a double-blind, randomized controlled trial. J Bodyw Mov Ther. 2019;23(4):901–907. doi: 10.1016/j.jbmt.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 100.Falla D, Jull G, Russell T, Vicenzino B, Hodges P. Effect of neck exercise on sitting posture in patients with chronic neck pain. Phys Ther. 2007;87(4):408–417. doi: 10.2522/ptj.20060009. [DOI] [PubMed] [Google Scholar]
- 101.Blomgren J, Strandell E, Jull G, Vikman I, Röijezon U. Effects of deep cervical flexor training on impaired physiological functions associated with chronic neck pain: a systematic review. BMC Musculoskelet Disord. 2018;19(1):415. doi: 10.1186/s12891-018-2324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jull G, Falla D. Does increased superficial neck flexor activity in the craniocervical flexion test reflect reduced deep flexor activity in people with neck pain? Man Ther. 2016;25:43–47. doi: 10.1016/j.math.2016.05.336. [DOI] [PubMed] [Google Scholar]
- 103.Jull GA, Falla D, Vicenzino B, Hodges PW. The effect of therapeutic exercise on activation of the deep cervical flexor muscles in people with chronic neck pain. Man Ther. 2009;14(6):696–701. doi: 10.1016/j.math.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 104.Jull GA, O'Leary SP, Falla DL. Clinical assessment of the deep cervical flexor muscles: the craniocervical flexion test. J Manipul Physiol Ther. 2008;31(7):525–533. doi: 10.1016/j.jmpt.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 105.Jull G, Trott P, Potter H, et al. A randomized controlled trial of exercise and manipulative therapy for cervicogenic headache. Spine (Phila Pa 1976). 2002;27(17):1835–1843. [DOI] [PubMed]
- 106.Falla D, O'Leary S, Farina D, Jull G. The change in deep cervical flexor activity after training is associated with the degree of pain reduction in patients with chronic neck pain. Clin J Pain. 2012;28(7):628–634. doi: 10.1097/AJP.0b013e31823e9378. [DOI] [PubMed] [Google Scholar]
- 107.Schomacher J, Falla D. Function and structure of the deep cervical extensor muscles in patients with neck pain. Man Ther. 2013;18(5):360–366. doi: 10.1016/j.math.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 108.O'Leary S, Falla D, Elliott JM, Jull G. Muscle dysfunction in cervical spine pain: implications for assessment and management. J Orthop Sports Phys Ther. 2009;39(5):324–333. doi: 10.2519/jospt.2009.2872. [DOI] [PubMed] [Google Scholar]
- 109.Johnston V, Jull G, Souvlis T, Jimmieson NL. Neck movement and muscle activity characteristics in female office workers with neck pain. Spine (Phila Pa 1976). 2008;33(5):555–563. [DOI] [PubMed]
- 110.Fernández-de-las-Peñas C, Albert-Sanchís JC, Buil M, Benitez JC, Alburquerque-Sendín F. Cross-sectional area of cervical multifidus muscle in females with chronic bilateral neck pain compared to controls. J Orthop Sports Phys Ther. 2008;38(4):175–180. doi: 10.2519/jospt.2008.2598. [DOI] [PubMed] [Google Scholar]
- 111.Uhlig Y, Weber BR, Grob D, Müntener M. Fiber composition and fiber transformations in neck muscles of patients with dysfunction of the cervical spine. J Orthop Res. 1995;13(2):240–249. doi: 10.1002/jor.1100130212. [DOI] [PubMed] [Google Scholar]
- 112.Cagnie B, Cools A, De Loose V, Cambier D, Danneels L. Differences in isometric neck muscle strength between healthy controls and women with chronic neck pain: the use of a reliable measurement. Arch Phys Med Rehabil. 2007;88(11):1441–1445. doi: 10.1016/j.apmr.2007.06.776. [DOI] [PubMed] [Google Scholar]
- 113.Jordan A, Mehlsen J, Ostergaard K. A comparison of physical characteristics between patients seeking treatment for neck pain and age-matched healthy people. J Manipul Physiol Ther. 1997;20(7):468–475. [PubMed] [Google Scholar]
- 114.Lee H, Nicholson LL, Adams RD. Neck muscle endurance, self-report, and range of motion data from subjects with treated and untreated neck pain. J Manipul Physiol Ther. 2005;28(1):25–32. doi: 10.1016/j.jmpt.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 115.Peolsson A, Kjellman G. Neck muscle endurance in nonspecific patients with neck pain and in patients after anterior cervical decompression and fusion. J Manipul Physiol Ther. 2007;30(5):343–350. doi: 10.1016/j.jmpt.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 116.Schomacher J, Farina D, Lindstroem R, Falla D. Chronic trauma-induced neck pain impairs the neural control of the deep semispinalis cervicis muscle. Clin Neurophysiol. 2012;123(7):1403–1408. doi: 10.1016/j.clinph.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 117.Lindstrøm R, Schomacher J, Farina D, Rechter L, Falla D. Association between neck muscle coactivation, pain, and strength in women with neck pain. Man Ther. 2011;16(1):80–86. doi: 10.1016/j.math.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 118.Elliott JM, O'Leary SP, Cagnie B, Durbridge G, Danneels L, Jull G. Craniocervical orientation affects muscle activation when exercising the cervical extensors in healthy subjects. Arch Phys Med Rehabil. 2010;91(9):1418–1422. doi: 10.1016/j.apmr.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 119.O'Leary S, Cagnie B, Reeve A, Jull G, Elliott JM. Is there altered activity of the extensor muscles in chronic mechanical neck pain? A functional magnetic resonance imaging study. Arch Phys Med Rehabil. 2011;92(6):929–934. doi: 10.1016/j.apmr.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 120.Conley MS, Stone MH, Nimmons M, Dudley GA. Resistance training and human cervical muscle recruitment plasticity. J Appl Physiol (1985). 1997;83(6):2105–2111. [DOI] [PubMed]
- 121.Conley MS, Stone MH, Nimmons M, Dudley GA. Specificity of resistance training responses in neck muscle size and strength. Eur J Appl Physiol Occup Physiol. 1997;75(5):443–448. doi: 10.1007/s004210050186. [DOI] [PubMed] [Google Scholar]
- 122.Schomacher J, Erlenwein J, Dieterich A, Petzke F, Falla D. Can neck exercises enhance the activation of the semispinalis cervicis relative to the splenius capitis at specific spinal levels? Man Ther. 2015;20(5):694–702. doi: 10.1016/j.math.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 123.Schomacher J, Petzke F, Falla D. Localised resistance selectively activates the semispinalis cervicis muscle in patients with neck pain. Man Ther. 2012;17(6):544–548. doi: 10.1016/j.math.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 124.Onyewu O, Manchikanti L, Falco FJ, et al. An update of the appraisal of the accuracy and utility of cervical discography in chronic neck pain. Pain Physician. 2012;15(6):E777–E806. [PubMed] [Google Scholar]
- 125.Falco FJ, Datta S, Manchikanti L, et al. An updated review of the diagnostic utility of cervical facet joint injections. Pain Physician. 2012;15(6):E807–E838. [PubMed] [Google Scholar]
- 126.Sremakaew M, Jull G, Treleaven J, Barbero M, Falla D, Uthaikhup S. Effects of local treatment with and without sensorimotor and balance exercise in individuals with neck pain: protocol for a randomized controlled trial. BMC Musculoskelet Disord. 2018;19(1):48. doi: 10.1186/s12891-018-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.