Abstract
Gastric cancer (GC) is a serious fatal cancer on a global scale because of its presentation at advanced stage. The expressions of vascular endothelial growth factor (VEGF), E-cadherin, and matrix metalloproteinases (MMPs) in other cancers have been reported. However, its expression and underlying mechanisms are little known in gastric cancer in Indian context. In this study, we detected mRNA expression of VEGF, E-cadherin, and MMPs (MMP-1, MMP-2, and MMP-9) in 73 gastric cancer tissues and 27 normal controls by reverse-transcriptase polymerase chain reaction (RT-PCR). Receiver operator characteristics analysis was done for determining the diagnostic utility of VEGF, MMPs and E-cadherin with respect to the sensitivity and specificity. The association of VEGF, MMPs, and E-cadherin expression with the clinicopathological characteristics and the prognosis was subsequently analyzed. The mRNA expression results showed that E-cadherin was significantly downregulated in 47.9% of GC in comparison to control. There was no change in VEGF expression observed in 90.4% GC cases. MMP-1, MMP-2, and MMP-9 were overexpressed in 13.7%, 28.8%, and 11% of GC, respectively, with significant change in MMP-2 (p ≤ 0.0001) and MMP-9 (p = 0.027) in comparison to control. Our results strengthen the necessity of more studies to elucidate the prophetic role of these genes in the development of gastric cancer.
Keywords: Gastric cancer, VEGF, E-cadherin, MMPs, Diagnostic utility
Introduction
Gastric cancer (GC) is second most fatal cancer worldwide [1]. The prognosis in metastatic GC is still very poor despite recent advances. Multiple factors such as environmental and genetic factors play role in causation of GC [2]. The incidence rate varies with the variation in geographical region. The disease is symptomatic in late stage. Diagnosis of early gastric cancer is a problem and routine screening is not feasible because of its cost [3]. Surveillance Epidemiology and End Results Database (SEER) report suggested that the tumor directly spread outside the stomach or regional lymph nodes in 31%, and the tumor metastasized to distant organs in 34% of GC cases [4].
Recent studies have shown that interactions between tumor cells and activated stromal cells create a unique microenvironment which influence tumor growth, vascularization, invasion, and metastasis [5]. Vascular endothelial growth factor (VEGF) is one of the most potent angiogenic factors which promote endothelial cell proliferation and migration, induce angiogenesis, and increase the vascular permeability to plasma and its proteins [6, 7].
E-cadherin is a member of the family of transmembrane glycoproteins and normally expressed in epithelial cells and is responsible for calcium-dependent cell-to-cell adhesion critical to the maintenance of tissue structure and morphogenesis [8, 9]. Abrogation of the E-cadherin function induces loss of adherens junctions and impairment of cell adhesiveness and cell proliferation signaling pathways [10]. E-cadherin is involved in several signaling pathways, such as the Wnt/β-catenin, Rho GTPase, and EGF/EGFR pathways, which are activated in carcinogenesis and play a role in GC [11]. Functional loss of E-cadherin has frequently been associated with poor prognosis and survival in patients of various malignancies [9].
Matrix metalloproteinases (MMPs) are a family of extracellular zinc-dependent neutral endopeptidases capable of degrading all extracellular matrix components [12]. MMPs not only play an important role in tissue remodeling but are also involved in many pathological conditions such as tumor invasion and metastasis [13]. MMP1 is an interstitial collagenase and various evidences have shown that aberrant expression of MMP1 has been implicated in the progression of human cancers like bladder cancer, prostate carcinoma, and gastric cancer [14–16]. MMP-2 and MMP-9 are also having collagenase and gelatinase activity involved in tumor invasion and metastasis [17].
The objective of this study was to look at the expression of VEGF, E-cadherin, and MMPs in GC and to correlate with different clinic-pathological parameters. The expressions of VEGF, E-cadherin, and MMPs in other cancers have been reported. Some studies have also been conducted in gastric cancer. But such type of work has not been done earlier in Indian context. This study will help in exploiting the potential therapeutic application of anti-angiogenic factors and MMP inhibitors in gastric cancer.
Material and Methods
Study Design
The present study was performed to examine the expression levels of VEGF, MMPs, and E-cadherin in gastric cancer patients by reverse-transcriptase polymerase chain reaction (RT-PCR).
Setting
This study has been carried out in Institute of Medical Sciences, Banaras Hindu University, Varanasi, in collaboration with the Department of Molecular and Human Genetics, BHU. The histopathology of the samples was performed in the Department of Pathology, BHU. The study included gastric cancer patients and normal samples. The study was approved by the institute’s ethics committee.
Subjects
Newly diagnosed cases of gastric cancer were included in the study. Biopsies of seventy-three cases of gastric cancer and twenty-seven normal samples were collected after obtaining informed consent. Samples were collected following surgery or upper GI endoscopy. Control group included who underwent endoscopy for non-malignant pathology and endoscopic biopsies were taken from normal-looking mucosa. Collected samples were immediately snap frozen in liquid nitrogen and then stored at − 80 °C deep freezer.
Isolation of RNA, cDNA Preparation, and RT-PCR
RNA isolation was carried out by using TRI Reagent® (Ambion® by life technologies) and quantified by Nanodrop spectrophotometer. cDNA was synthesized by using high-capacity cDNA synthesis kit (Applied Biosystems). cDNA was amplified with the help of PCR by using gene-specific primers for VEGF, E-cadherin, MMP-1, MMP-2, MMP-9, and β-actin.
Densitometric analysis was carried out by Alpha Imager 2000 software (Alpha Innotech Corp, San Leandra, CA, USA), and the integrated density value (IDV) of the samples was calculated. The ratio of IDV of the above mentioned genes (VEGF, E-cadherin, MMP-1, MMP-2, MMP-9) to that of β-actin was used for relative expression estimation in each sample. The up and downregulated expression was determined by comparing the average value ± 2 × SD of the normal to the tumor samples.
Statistical Analysis
The statistical analysis was performed using the statistical software SPSS statistics version 17.0 for windows (US). Mann-Whitney U test was used for non-parametric variables. A p value of less than 0.05 (p < 0.05) was considered as statistically significant. The reciprocal/receiver operating characteristic curve (ROC) was constructed to evaluate the diagnostic utility and cut-off value of the test.
Results
Patient Demographics
The patient’s demographic details are given in Table 1. A total of 73 patients of GC (40 male and 33 female) with a mean age of 53 years were enrolled. The most common type of growth was ulcer proliferative type (50.7%), and site of the growth was distal in 64.3% of cases. Most of the patients belong to stage III (64.3%), followed by stage II (30.1%). Intestinal type of tumor was present in 65.8% of GC cases (Table 1).
Table 1.
Clinicopathological features of gastric cancer patients
| Characteristics | Gastric cancer n (%) |
|---|---|
| Mean age | 53 years |
| Gender | |
| Male | 40 (54.8%) |
| Female | 33 (45.2%) |
| Type of growth | |
| Type I (polypoid) | 8 (5.5%) |
| Type II (ulcero proliferative growth) | 37 (50.68%) |
| Type III (ulcero infiltrative growth) | 20 (27.4%) |
| Type IV (diffuse infiltration) | 8 (10.96%) |
| Site of growth | |
| Proximal | 10 (13.6%) |
| Body | 10 (13.6%) |
| Diffuse | 6 (8.2%) |
| Distal | 47 (64.3%) |
| Stage | |
| I | 1 (1.39%) |
| II | 22 (30.13%) |
| III | 40 (54.79%) |
| IV | 10 (13.69%) |
| Histological type | |
| Intestinal | 48 (65.8%) |
| Diffuse | 25 (34.2%) |
Expression Pattern of VEGF, E-Cadherin, MMP-1, MMP-2, MMP-9
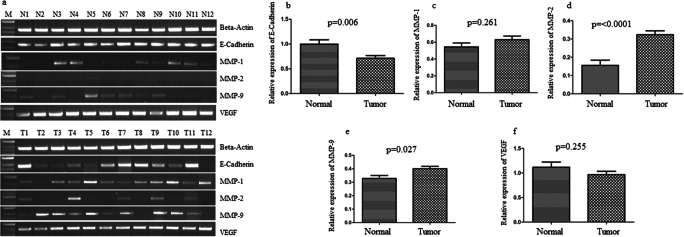
The IDV of VEGF, E-cadherin, MMP-1, MMP-2, and MMP-9 to β-actin in gastric cancer was calculated. The results for the mRNA expressions of VEGF, E-cadherin, MMP-1, MMP-2, and MMP-9 in tumor and normal tissues are detailed in Fig. 1 and Table 2. The mean IDV ratio of VEGF in GC was 0.97 ± 0.6 and that in controls was 0.87 ± 0.49. It was observed that VEGF expression was overexpressed in 7 (9.6%) patients of GC. No change in expression was found in 66 (90.4%) cases. Thus, no significant difference was observed (p > 0.05).
Fig. 1.
Expression of VEGF, E-cadherin, MMP-1, MMP-2, and MMP-9 mRNA level in gastric carcinoma as compared to normal gastric biopsy. a Gel pictures showing expression pattern of VEGF, E-cadherin, MMP-1, MMP-2, and MMP-9 mRNA level in gastric tumor biopsy in comparison to gastric mucosa. The expression of beta-actin is shown as an internal control. b Normalized expression of E-cadherin at mRNA level. c Normalized expression of MMP-1 at mRNA level. d Normalized expression of MMP-2 at mRNA level. e Normalized expression of MMP-9 at mRNA level. f Normalized expression of VEGF at mRNA level
Table 2.
Expression of E-cadherin, VEGF, MMPs (MMP-1, MMP-2, MMP-9) in gastric cancer
| S. no. | Genes | Gastric cancer n (%) |
Normal control n (%) |
p value |
|---|---|---|---|---|
| 1. | VEGF | |||
|
Upregulated Downregulated No change |
7 (9.6%) 0 66 (90.4%) |
3 (11.1%) 0 24 (88.9%) |
> 0.05 | |
| 2. | E-cadherin | |||
|
Upregulated Downregulated No change |
10 (13.7%) 35 (47.9%) 28 (38.4%) |
4 (14.8%) 22 (81.5%) 1 (3.7%) |
< 0.01* | |
| 3. | MMP-1 | |||
|
Upregulated Downregulated No change |
10 (13.7%) 0 63 (86.3%) |
3 (11.1%) 0 24 (88.9%) |
> 0.05 | |
| 4. | MMP-2 | |||
|
Upregulated Downregulated No change |
21 (28.8%) 0 52 (71.2%) |
1 (3.7%) 0 26 (96.3%) |
< 0.01* | |
| 5. | MMP-9 | |||
|
Upregulated Downregulated No change |
8 (11%) 0 65 (89%) |
3 (11.1%) 0 24 (88.9%) |
> 0.05 | |
The mean IDV ratio of E-cadherin in GC was 0.69 ± 0.41, whereas in the controls, it was 0.84 ± 0.16. E-cadherin was downregulated in 38.4% of gastric cancer cases and found to be statistically significant (p = 0.006; Fig. 1b). In this study, mean IDV ratio of MMP-1 was 0.74 ± 0.37 and 0.52 ± 0.23 in GC and controls, respectively. Overexpression of MMP-1 was detected in 13.7% of GC cases, while it was 11.1% in control, showing no difference in significance (p > 0.05).
In case of gene MMP-2, the mean IDV ratio was 0.32 ± 0.1 in GC and 0.15 ± 0.15 in control. The expression analysis showed that MMP-2 was significantly overexpressed in 28.8% of gastric cancer patients than controls (< 0.01; Fig. 1d). The mean IDV ratio of MMP-9 to β-actin was 0.36 ± 0.16, whereas in the controls, it was 0.34 ± 0.3. MMP-9 was upregulated in 11% of cases with no significant difference between the groups (p > 0.05; Fig. 1e).
The evaluation of mRNA expression of VEGF, E-cadherin, MMP-1, MMP-2, and MMP-9 genes did not show any significant association with any of the clinicopathological parameters (Table 3).
Table 3.
Association of expression of E-cadherin, VEGF, MMPs (MMP1, MMP2, MMP9) in gastric cancer with different clinical parameters
| Genes | E-cadherin | VEGF | ||||||
| Clinical features | +Ve | −Ve | No change | p value | +Ve | −Ve | No change | p value |
| Serosal invasion | ||||||||
|
Present Absent |
5 (50%) 5 (50%) |
14 (50%) 14 (50%) |
20 (57.1%) 15 (42.9%) |
0.83 |
3 (42.9%) 4 (57.1%) |
0 0 |
36 (54.5%) 30 (45.5%) |
> 0.05 |
| Lymph node metastasis | ||||||||
|
Present Absent |
8 (80%) 2 (20%) |
19 (67.9) 9 (32.1%) |
25 (71.4%) 10 (28.6%) |
0.76 |
6 (85.7%) 1 (14.3%) |
0 0 |
46 (69.7%) 20 (30.3%) |
0.37 |
| Liver metastasis | ||||||||
|
Present Absent |
2 (20%) 8 (80%) |
4 (14.3%) 24 (85.7%) |
5 (14.3%) 30 (85.7%) |
0.89 |
1 (14.3%) 6 (85.7%) |
0 0 |
10 (15.2%) 56 (84.6%) |
0.95 |
| Stage | ||||||||
|
I + II III + IV |
1 (10%) 9 (90%) |
12 (42.6%) 16 (57.1%) |
10 (28.6%) 25 (71.4%) |
0.13 |
1 (14.3%) 6 (85.7%) |
0 0 |
22 (33.3%) 44 (66.7%) |
0.3 |
| Genes | MMP-1 | MMP-2 | ||||||
| Serosal invasion | ||||||||
|
Present Absent |
7 (70%) 3 (30%) |
0 0 |
32 (50.8%) 31 (49.2%) |
0.25 |
9 (42.9%) 12 (57.1%) |
0 0 |
30 (57.7%) 22 (42.3%) |
0.25 |
| Lymph node metastasis | ||||||||
|
Present Absent |
8 (80%) 2 (20%) |
0 0 |
44 (69.8%) 19 (30.2%) |
0.51 |
13 (61.9%) 8 (38.1%) |
0 0 |
39 (75%) 13 (25%) |
0.26 |
| Liver metastasis | ||||||||
|
Present Absent |
3 (30%) 7 (70%) |
0 0 |
8 (12.7%) 55 (87.3%) |
0.15 |
5 (23.8%) 16 (76.2%) |
0 0 |
6 (11.5%) 46 (88.5%) |
0.18 |
| Stage | ||||||||
|
I + II III + IV |
7 (70%) 3 (30%) |
0 0 |
16 (25.4%) 47 (74.6%) |
0.06 |
12 (57.8%) 9 (42.9%) |
0 0 |
11 (21.2%) 41 (78.8%) |
0.3 |
| Genes | MMP-9 | |||||||
| Clinical features | +Ve | −Ve | No change | p value | ||||
| Serosal Invasion | ||||||||
|
Present Absent |
4 (50%) 4 (50%) |
0 0 |
35 (53.8%) 30 (46.2%) |
0.83 | ||||
| Lymph node metastasis | ||||||||
|
Present Absent |
4 (50%) 4 (50%) |
0 0 |
48 (73.8%) 17 (26.2%) |
0.16 | ||||
| Liver metastasis | ||||||||
|
Present Absent |
2 (25%) 6 (75%) |
0 0 |
9 (13.8%) 56 (86.2%) |
0.4 | ||||
| Stage | ||||||||
|
I + II III + IV |
3 (37.5%) 5 (62.5%) |
0 0 |
20 (30.8%) 45 (69.2%) |
0.69 | ||||
Statistical significance at p < 0.05
+ve upregulated, −ve downregulated
ROC Analysis
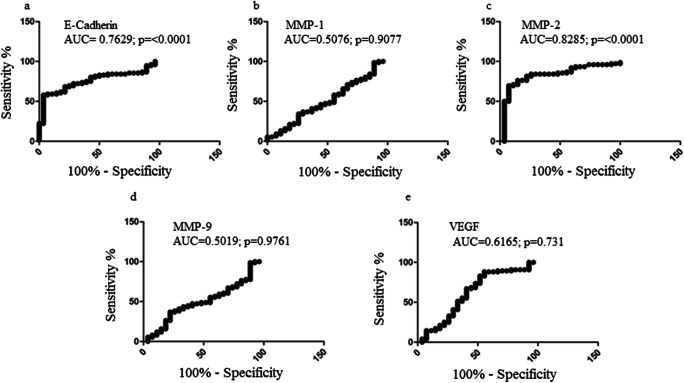
In order to determine the diagnostic utility of VEGF, E-cadherin, MMP-1, MMP-2, and MMP-9, ROC analysis was applied which best segregates diseased from non-diseased condition (Fig. 2). Threshold densitometry value of VEGF mRNA was determined by the maximum sum of sensitivity and specificity and was found to be 70.3. The area under ROC curve of VEGF was 0.6165 (standard error ± 0.06923, 95% CI 0.4807 to 0.7522, p = 0.07316), corresponding to the sensitivity of 40.79% (95% CI 29.65 to 52.67) and specificity 70.37% (95% CI 49.82 to 86.25%) (Fig. 2e). Threshold densitometry value of E-cadherin mRNA was determined by the maximum sum of sensitivity and specificity and was found to be 38.3. The area under ROC curve of E-cadherin was 0.7629 (standard error ± 0.04701, 95% CI 0.6708 to 0.8551, p ≤0.0001), corresponding to the sensitivity of 60.53% (95% CI 48.65 to 71.56) and specificity 85.71% (95% CI 67.33 to 95.97 (Fig. 2a). Threshold densitometry value of MMP-1 mRNA was determined by the maximum sum of sensitivity and specificity and was found to be 50. The area under ROC curve of MMP-1 was 0.5076 (standard error ± 0.06529, 95% CI 0.3796 to 0.6355, p = 0.9075), corresponding to the sensitivity of 40.79% (95% CI 29.65 to 52.67) and specificity 62.96% (95% CI 42.37 to 80.60 (Fig. 2b). Threshold densitometry value of MMP-2 mRNA was determined by the maximum sum of sensitivity and specificity and was found to be 69.2. The area under ROC curve of MMP-2 was 0.8285 (standard error ± 0.04693, 95% CI 0.7365 to 0.9205, p≤0.0001), corresponding to the sensitivity of 69.74% (95% CI 58.13 to 79.75) and specificity of 92.59% (95% CI 75.71 to 99.09) (Fig. 2c). Threshold densitometry value of MMP-9 mRNA was determined by the maximum sum of sensitivity and specificity and was found to be 45.3. The area under ROC curve of MMP-9 was 0.5019 (standard error ± 0.06378, 95% CI 0.3769 to 0.6270, p = 0.9761), corresponding to the sensitivity of 40.79% (95% CI 29.65% to 52.67%) and specificity 70.37% (95% CI 49.82 to 86.25 (Fig. 2d).
Fig. 2.
Receiver operator characteristics (ROC) curve of mRNA in terms of sensitivity and specificity (a). ROC curve of E-cadherin mRNA in terms of sensitivity and specificity. b ROC curve of MMP-1 mRNA in terms of sensitivity and specificity. c ROC curve of MMP-2 mRNA in terms of sensitivity and specificity. d ROC curve of MMP-9 mRNA in terms of sensitivity and specificity. e ROC curve of VEGF mRNA in terms of sensitivity and specificity
Discussion
Gastric cancer is the second leading cause of cancer-related death in the world despite the intensive decrease in mortality rate [18, 19]. The present study depicts the expression pattern of E-cadherin, VEGF, and MMPs (MMP-1, MMP-2, MMP-9) in gastric cancer.
VEGF has been known as the key player in angiogenesis [20]. VEGF strongly stimulate the growth of endothelial cells leading to the formation of new blood vessels, thus providing essential nutrients for tumor growth. Therefore, VEGF-based antiangiogenesis therapy may be of therapeutic benefit against solid tumors and has been tested in several tumors. In a study, VEGF expression level was significantly enhanced in 81% of GC in comparison to adjacent non-cancerous tissues and normal gastric mucosa [10]. This suggested an important role of VEGF in GC development. In this report, we observed that high expression of VEGF was present in only 7 cases of GC while it was present in only 3 cases of normal control with no significant difference. Furthermore, on comparison with different clinical parameters, it did not show any significant difference between the groups. However, we observed positive expression of VEGF in a higher percentage of late-stage tumors (85.7%) than early-stage tumors (Table 3). The findings of this study are contrasting but a major relevance of the same could be attributed to the fact that angiogenesis is caused by the interaction between the various angiogenesis activators and inhibitors, other than VEGF [21]. Though, further studies are needed to explain it in detail.
The E-cadherin also played an important role in tumor angiogenesis. Zhou et al. (2010) observed a reduced expression of E-cadherin in 78% of GC compared to normal gastric mucosa and found to be correlated with poor differentiation and tumor invasion. We observed E-cadherin expression was downregulated in 47.9% of GC cases (p < 0.0001). Similar results have been reported in other study that showed negative E-cadherin expression in 27% of the GC cases [22]. Other study demonstrated a 38% rate of abnormal E-cadherin expression by immunohistochemistry (IHC) in GC patients [23]. It was reported by various studies that E-cadherin expression frequencies vary from 46 to 82% in GC [24–27]. The reduced expression of E-cadherin strongly favors its involvement in GC pathogenesis through different molecular mechanisms [28]. There was no statistically significant correlation between E-cadherin expression and clinical parameters found in this study. The association between E-cadherin expression and different clinical parameters are ambiguous. Studies showed a significant correlation between abnormal E-cadherin expression and high-grade tumors, location and lymph node involvement [29, 30], venous invasion, and lymph node metastasis [31]. However, other researchers described lack of correlation between the E-cadherin expression with lymph node involvement and lymph node metastasis [25, 27].
MMPs are a family of enzymes that proteolytically degrade various components of the extracellular matrix (ECM). MMP-1 and MMP-2 are the main components of the MMP family and are thought to be mainly associated with tumor invasion and metastasis [32]. A study on GC found overexpression of MMP-1 and MMP-2 in 80% and 76% of GC, respectively [10]. The MMP-2 and MMP-9 have been shown to play critical roles in the “angiogenic switch,” and tumor cells could synthesize and secrete large amounts of MMP-2 and MMP-9 in a paracrine and/or autocrine manner to stimulate angiogenesis and increase VEGF release. Increased mRNA expression of MMP-2 and MMP-9 was observed in human head and neck squamous cell carcinoma (HNSCC) tumors [33]. The high expression of MMP1 was related with occurrence, and development of head and neck squamous cell carcinoma has been reported [34], which can be used as an independent risk factor and has a great clinical significance. MMP-1 expression was found to be related to breast tumor progression and poor prognosis in a study [35]. MMP-1 expression has been reported to inversely correlate with survival in advanced cancers. Some recent studies reported that MMP1 upregulation is associated with more aggressive phenotype of breast cancer [36]. In this study, MMP1 was found to be upregulated in GC cases but it was not statistically significant. Studies have reported that a large heterogeneity is present in MMP expression among different cancers [37]. Their analysis supported some MMPs are mostly upregulated in many cancers while other MMPs are more specific to certain cancers. Hence, from this point, we can also explain the variation in MMP expression in this study [37].
The most common enzymes for type IV collagen degradation are MMP-2 and MMP-9 and play significant roles in tumor angiogenesis, invasion, and metastasis [38, 39]. Several researches reported association of MMP-2 and MMP-9 expression with poor prognosis of GC [40–42]. In the present report, MMP-2 was significantly upregulated 28.8% of the GC patients (p < 0.01) in comparison to control. In case of MMP-1 and MMP-9, the mRNA expression was upregulated in 13.7% and 11% cases of GC, respectively, with no significant difference (p > 0.05). In this study, the expression of MMP-1, MMP-2, and MMP-9 was not found to be associated with any clinical parameters like tumor stage, serosal invasion, liver metastasis, and lymph node metastasis. MMP-1 expression did not correlate with any of the other clinicopathological features evaluated, namely, depth of tumor invasion, lymphatic invasion, venous invasion, lymph node metastasis, and Dukes’ classification [43]. In this study, MMP1 expression was not associated with any clinical parameters.
Recent study observed that positive expression of MMP-9, VEGF, and negative expression of E-cadherin were involved in tumor invasion and metastasis and can be used as malignant markers for GC [44]. Previous study concluded that MMP-2, MMP-9, and VEGF are closely involved in the growth, angiogenesis, and progression of gastric carcinomas [45]. Guo et al. (2007) showed that MMP2 and MMP9 mRNA expression was correlated with small cell carcinoma histologic type of lung cancer. We speculate that MMP-1 and MMP-2 are involved in the degradation of E-cadherin, which has an important role in the GC development [46].
In conclusion, abnormal expressions of E-cadherin, VEGF, and MMP-2 may represent the molecular changes in the development of gastric cancer. In this study, we explored its diagnostic potential through ROC analysis. We identified these markers as a potential tool for early detection of gastric cancer at the mRNA level. However, at mRNA level, E-cadherin and MMP-2 show diagnostic utility. At present, the most conventional treatment strategy for gastric cancer is surgery; however, some patients diagnosed with gastric cancer at later stages are not eligible for surgery. Hence, the early identification of disease is important and our study may be useful in the improvement of the understanding of early diagnosis. It is a need of the hour to have deep understanding of the molecular factors involved in GC development which further help in identification of new biomarkers of gastric cancer for early diagnosis and effective therapy. An increase in the expression of mRNA does not necessarily mean that a protein is overexpressed at a level sufficiently high. Thus, further protein level and functional studies in large number of samples are required to provide benefit to the medical community. Further studies are needed to identify and validate diagnostic, prognostic, predictive, and therapeutic application of these genes.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration ethical standards and approved by the Institutional Ethical Committee of Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. The study was approved by the Institutional Ethical Committee of Institute of Medical Sciences, Banaras Hindu University.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murugesan CS, Manickavasagam K, Chandramohan A, Jebaraj A, Jameel ARA, Jain MS, Venkataraman J. Gastric cancer in India: epidemiology and standard of treatment. Updat Surg. 2018;70:233–239. doi: 10.1007/s13304-018-0527-3. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK. SEER cancer statistics review, 1975-2008. Bethesda: National Cancer Institute; 2011. [Google Scholar]
- 5.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858–863. doi: 10.1002/(SICI)1097-0142(19960301)77:5<858::AID-CNCR8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.Shiozaki H, Oka H, Inoue M, Tamura S, Monden M. E-cadherin mediated adhesion system in cancer cells. Cancer. 1996;77:1605–1613. doi: 10.1002/(SICI)1097-0142(19960415)77:8+<1605::AID-CNCR4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11–22. doi: 10.1016/j.critrevonc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Li G, Wu J, Zhang Z, Wu Z, Fan P, Hao T, Zhang X, Li M, Zhang F, Li Q, Lu B, Qiao L. Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumour Biol. 2010;31(6):549–558. doi: 10.1007/s13277-010-0068-y. [DOI] [PubMed] [Google Scholar]
- 11.Bure IV, Nemtsova MV, Zaletaev DV. Roles of E-cadherin and noncoding RNAs in the epithelial-mesenchymal transition and progression in gastric cancer. Int J Mol Sci. 2019;20(12):E2870. doi: 10.3390/ijms20122870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubben FJGM, Sier CFM, van Duijn W, Griffioen G, Hanemaaijer R, van de Velde CJ, van Krieken JH, Lamers CB, Verspaget HW. Matrix metalloproteinase-2 is a consistent prognostic factor in gastric cancer. Br J Cancer. 2006;94:1035–1040. doi: 10.1038/sj.bjc.6603041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahari VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999;31:34–45. doi: 10.3109/07853899909019260. [DOI] [PubMed] [Google Scholar]
- 14.Shin DH, Dier U, Melendez JA, Hempel N. Regulation of MMP-1 expression in response to hypoxia is dependent on the intracellular redox status of metastatic bladder cancer cells. Biochim Biophys Acta. 2015;1852:2593–2602. doi: 10.1016/j.bbadis.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozden F, Saygin C, Uzunaslan D, Onal B, Durak H, Aki H. Expression of MMP-1, MMP-9 and TIMP-2 in prostate carcinoma and their influence on prognosis and survival. J Cancer Res Clin Oncol. 2013;139(8):1373–1382. doi: 10.1007/s00432-013-1453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai QW, Li J, Li XQ, Wang JQ, Huang Y. Expression of STAT3, MMP-1 and TIMP-1 in gastric cancer and correlation with pathological features. Mol Med Rep. 2012;5:1438–1442. doi: 10.3892/mmr.2012.849. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V, Abbas A, Fausto N, Robbins C (2010) Pathologic basis of disease, 8th edn. Elsevier Printed in China, Copyright, pp 62–63 82, 206–209,302–303, 328–331
- 18.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 20.Shibuya M. VEGF-VEGFR system as a target for suppressing inflammation and other diseases. Endocr Metab Immune Disord Drug Targets. 2015;15(2):135–144. doi: 10.2174/1871530315666150316121956. [DOI] [PubMed] [Google Scholar]
- 21.Park SH, Lee SS. The relationship between serum VEGF concentration and prognosis of lung cancer. Korean J Intern Med. 2003;18(4):207–211. doi: 10.3904/kjim.2003.18.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schizas D, Moris D, Michalinos A, Kanavidis P, Oikonomou D, Papalampros A, Machairas A, Liakakos T. E-cadherin in gastric carcinomas: relations with histological parameters and its prognostic value. J BUON. 2017;22(2):383–389. [PubMed] [Google Scholar]
- 23.Czyzewska J, Guzinska-Ustymowicz K, Ustymowicz M, Pryczynicz A, Kemona A. The expression of E-cadherin- catenin complex in patients with advanced gastric cancer: role in formation of metastasis. Folia Histochem Cytobiol. 2010;48:37–45. doi: 10.2478/v10042-010-0017-z. [DOI] [PubMed] [Google Scholar]
- 24.Stanculescu D, Margaritescu C, Stepan A, Mitrut AO. E-cadherin in gastric carcinomas related to histological prognostic parameters. Romanian J Morphol Embryol. 2011;52(3 Suppl):1107–1112. [PubMed] [Google Scholar]
- 25.Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, Yang JM. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8:987–993. doi: 10.3748/wjg.v8.i6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan AO, Lam SK, Wong BC, Wong WM, Yuen MF, Yeung YH, Hui WM, Rashid A, Kwong YL. Promoter methylation of E-cadherin gene in gastric mucosa associated with helicobacter pylori infection and in gastric cancer. Gut. 2003;52:502–506. doi: 10.1136/gut.52.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzman P, Araya J, Villaseca M, Roa I, Melo A, Muñoz S, Roa J. Immunohistochemical expression of the E-cadherin-catenin complex in gastric cancer. Rev Med Chil. 2006;134:1002–1009. doi: 10.4067/S0034-98872006000800009. [DOI] [PubMed] [Google Scholar]
- 28.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anbiaee R, Mojir Sheibani K, Torbati P, Jaam H. Abnormal expression of e-cadherin in gastric adenocarcinoma, and its correlation with tumor histopathology and helicobacter pylori infection. Iran Red Crescent Med J. 2013;15:218–222. doi: 10.5812/ircmj.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karayiannakis AJ, Syrigos KN, Chatzigianni E, Papanikolaou S, Karatzas G. E-cadherin expression as a differentiation marker in gastric cancer. Hepatogastroenterology. 1998;45:2437–2442. [PubMed] [Google Scholar]
- 31.Ohno T, Aihara R, Kamiyama Y, Mochiki E, Asao T, Kuwano H. Prognostic significance of combined expression of MUC1 and adhesion molecules in advanced gastric cancer. Eur J Cancer. 2006;42:256–263. doi: 10.1016/j.ejca.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial–mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail upregulation. Clin Cancer Res. 2008;14:4743–4750. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 33.Hauff SJ, Raju SC, Orosco RK, Gross AM, Diaz-Perez JA, Savariar E, Nashi N, Hasselman J, Whitney M, Myers JN, Lippman SM, Tsien RY, Ideker T, Nguyen QT. Matrix-metalloproteinases in head and neck carcinoma-cancer genome atlas analysis and fluorescence imaging in mice. Otolaryngol Head Neck Surg. 2014;151(4):612–618. doi: 10.1177/0194599814545083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu LQ, Chen ZW, Yi SJ. association between the expression of MMP1 gene and prognosis in head and neck squamous cell carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;32(4):287–291. doi: 10.13201/j.issn.1001-1781.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Boström P, Soderstrom M, Vahlberg T, Soderstrom KO, Roberts PJ, Carpen O, Hirsimäki P. MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer. 2011;11:348. doi: 10.1186/1471-2407-11-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen CH, Senfter D, Basilio J, Holzner S, Stadler S, Krieger S, et al. NF-kappaB contributes to MMP1 expression in breast cancer spheroids causing paracrine PAR1 activation and disintegrations in the lymph endothelial barrier in vitro. Oncotarget. 2015;6(36):39262–39275. doi: 10.18632/oncotarget.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gobin E, Bagwell K, Wagner J, Mysona D, Sandirasegarane S, Smith N, Bai S, Sharma A, Schleifer R, She JX. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer. 2019;19(1):581. doi: 10.1186/s12885-019-5768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo HY, Huang YS, Tseng CH, Chen YC, Chang YW, Shih HM, Wu CW. PML represses lung cancer metastasis by suppressing the nuclear EGFR-mediated transcriptional activation of MMP2. Cell Cycle. 2014;13:3132–3142. doi: 10.4161/15384101.2014.949212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Ding Z, Jian H, Shen L, Zhu L, Lu S. Prognostic value of MMP9 activity level in resected stage I B lung adenocarcinoma. Cancer Med. 2016;5:2323–2331. doi: 10.1002/cam4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Mingo M, Moran A, Sanchez-Pernaute A, Iniesta P, Diez-Valladares L, Perez-Aguirre E, de Juan C, García-Aranda C, Díaz-López A, García-Botella A, Martín-Antona E, Benito M, Torres A, Balibrea JL. Expression of MMP-9 and TIMP-1 as prognostic markers in gastric carcinoma. Hepatogastroenterology. 2007;54:315–319. [PubMed] [Google Scholar]
- 41.Elnemr A, Yonemura Y, Bandou E, Kinoshita K, Kawamura T, Takahashi S, Tochiori S, Endou Y, Sasaki T. Expression of collagenase-3 (matrix metalloproteinase-13) in human gastric cancer. Gastric Cancer. 2003;6:30–38. doi: 10.1007/s101200300004. [DOI] [PubMed] [Google Scholar]
- 42.Sier CF, Kubben FJ, Ganesh S, Heerding MM, Griffioen G, Hanemaaijer R, van Krieken JH, Lamers CB, Verspaget HW. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br J Cancer. 1996;74:413–417. doi: 10.1038/bjc.1996.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunami E, Tsuno N, Osada T, Saito S, Kitayama J, Tomozawa S, Tsuruo T, Shibata Y, Muto T, Nagawa H. MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. Oncologist. 2000;5(2):108–114. doi: 10.1634/theoncologist.5-2-108. [DOI] [PubMed] [Google Scholar]
- 44.Gao H, Lan X, Li S, Xue Y. Relationships of MMP-9, E-cadherin, and VEGF expression with clinicopathological features and response to chemosensitivity in gastric cancer. Tumour Biol. 2017;39(5):1010428317698368. doi: 10.1177/1010428317698368. [DOI] [PubMed] [Google Scholar]
- 45.Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26(5A):3579–3583. [PubMed] [Google Scholar]
- 46.Guo CB, Wang S, Deng C, Zhang DL, Wang FL, Jin XQ. Relationship between matrix metalloproteinase 2 and lung cancer progression. Mol Diagn Ther. 2007;11(3):183–192. doi: 10.1007/BF03256240. [DOI] [PubMed] [Google Scholar]