Abstract
Objective
To examine the association between delay of antibiotic treatment and 28‐day mortality in a study of septic patients identified by the Sepsis‐3 criteria.
Methods
A prospective observational cohort study of patients (≥ 18 years) with sepsis admitted to a Danish emergency department between October 2017 and March 2018. The interval between arrival to the ED and first delivery of antibiotics was used as time to antibiotic treatment (TTA). Logistic regression was used in the analysis of the association between TTA and mortality adjusted for potential confounding.
Results
A total of 590 patients, median age 74.2 years, were included. Overall 28‐day mortality was 14.6% (95% confidence interval [CI], 11.8–17.7). Median TTA was 4.7 hours (interquartile range 2.7–8.1). The mortality in patients with TTA ≤1 hour was 26.5% (95% CI, 12.8–44.4), and 15.3% (95% CI, 9.8–22.5), 10.5% (95% CI, 6.6–15.8), and 12.8 (95% CI, 7.3–20.1) in the timespans 1–3, 3—6, and 6–9 hours, respectively, and 18.8% (95% CI, 12.0–27.2) in patients with TTA >9 hours. With patients with lowest mortality (TTA timespan 3–6 hours) as reference, the adjusted odds ratio of mortality was 4.53 (95% CI, 1.67–3.37) in patients with TTA ≤1 hour, 1.67 (95% CI, 0.83–3.37) in TTA timespan 1–3 hours, 1.17 (95% CI, 0.56–2.49) in timespan 6–9 hours, and 1.91 (95% CI, 0.96–3.85) in patient with TTA >9 hours.
Conclusions
The adjusted odds of 28‐day mortality were lowest in emergency department (ED) patients with sepsis who received antibiotics between 1 and 9 hours and highest in patients treated within 1 and >9 hours after admission to the ED.
Keywords: Cohort study, emergency department, infection, sepsis, antibiotics, treatment delay, mortality
1. INTRODUCTION
1.1. Background
Empirical antimicrobial therapy of patients with sepsis is recommended within 1 hour of presentation at the emergency department (ED) independently of the severity. 1 However, the recommended early administration of antibiotics (AB) has been debated for several years. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10
Currently, it is only retrospective, observational, and non‐randomized controlled trials 9 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 that have concluded survival benefit of early administration of antibiotics during hospital stay. However, in the study by Husabø et al, 12 it was found that patients who started antibiotic treatment between 2 and 3 hours after admission had the lowest mortality.
A systematic review and meta‐analysis from 2015 including 11 observational studies reported no significant mortality benefit of administering antibiotics within 3 hours of ED triage or within 1 hour of shock recognition in severe sepsis and septic shock. 22 A recently published review and meta‐analysis of mainly retrospective studies showed no difference in mortality between immediate (0 to 1 hour after onset) and early (1 to 3 hours after onset) antibiotics in patients with severe sepsis or septic shock. 23 In a recent review of 20 observational studies that evaluated timely antibiotic administration in the ED, it was concluded that early and effective antibiotic is important for survival, especially for patients with septic shock. 24 Included in the review, 24 a large retrospective study of 35,000 ED sepsis patients from the United States found that the adjusted odds for hospital mortality increased 9% per elapsed hour between ED registration and AB administration, and the effect was largest for patients treated within the first hour and in patients with septic shock. 14 In another large retrospective sepsis study of 10,811 ED patients from the United States, it was found that adjusted odds for 1‐year mortality increased with 10% per elapsed hour from ED arrival to delivery of antibiotics. 13 A single randomized controlled trial (RCT) of the effect of prehospital administration of antibiotics compared to usual hospital care did not show improved survival regardless of illness severity. 25
1.2. Importance
There are limited data from prospective studies of sepsis patients investigating the impact of time to treatment with antibiotic (TTA) on outcome in sepsis patients identified by the updated sepsis criteria (Sepsis‐3). 26 In addition, studies adjusting for confounding factors affecting mortality are sparse.
1.3. Goals of this investigation
The aim of this study was to examine the association between TTA and 28‐day mortality in a prospective study of septic patients identified by the Sequential Organ Failure Assessment (SOFA) 26 score after adjusting for potential confounding factors.
The Bottom Line
Despite guidelines recommending prompt administration of antibiotics in sepsis, the relationship between time to antibiotics and outcome remains controversial. In this secondary analysis of a prospective cohort study that included 590 patients, the adjusted odds of 28‐day morality were lowest among patients who received antibiotics between 1 and 9 hours of emergency department arrival, though potential for significant confounding exists.
2. METHODS
2.1. Study design and setting
This study is a secondary analysis of a prospective cohort study of the prognostic accuracy of quickSOFA (qSOFA) among all adult patients with an infection admitted to the ED of Slagelse Hospital between October 2017 and March 2018. 27 The ED has 26,500 visits annually from an uptake area of 198,000 adults. The Danish healthcare system is universal and based on free and equal access to health care for all citizens. All acutely hospitalized patients are admitted through the ED of a public hospital. Most patients are referred to the hospitals by a general practitioner or arrive to the ED by an emergency ambulance without any preceding contact with the ED. Privately funded Danish hospitals have no walk‐in clinics for acute care. 28
2.2. Selection of participants
Every working‐day during the study period all electronic records of ED patients (N = 12,092) were screened for infection defined as documented or suspected infection diagnosed by the emergency physician and if either intravenous or oral antibiotics were delivered within 24 hours from arrival (N = 3176). A total of 1066 were excluded in the primary study leaving an infection cohort of 2112 ED patients (Figure 1). 27 Among patients determined to have infection, we included patients with ≥ 2 SOFA score on admission and treated with intravenous antibiotics for at least 48 hours after arrival to the ED. Patients (n = 19) with unknown TTA were excluded (Figure 1).
FIGURE 1.
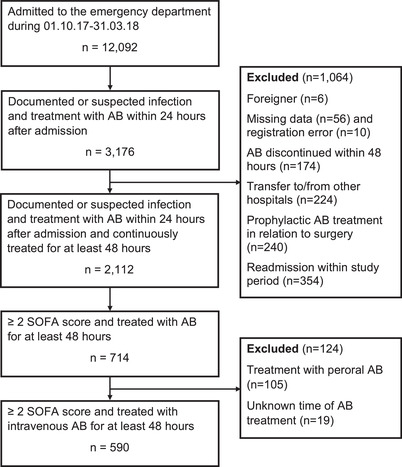
Flow chart
AB, antibiotics; SOFA, sequential organ failure assessment
2.3. Definitions
We have calculated the TTA as the time between arrival to the ED and the time for the delivery of first dose of intravenous antibiotics.
Sepsis was defined as an increase of 2 or more of the total SOFA score compared to the patient's individual baseline value. The calculations of the SOFA scores were done retrospectively. The definitions of qSOFA and Systemic Inflammatory Response Syndrome (SIRS) followed the original guidelines. 26 , 29 , 30
We have adjusted the baseline SOFA score for chronic diseases. Patients with chronic diseases (respiratory, kidney, liver) were classified according to the Charlson Comorbidity Index (CCI). 31 The baseline SOFA score assigned a value from 1 to 4 depending on an assessment of the severity of the chronic disease after consensus between 2 of the authors (SMOBA, RHS). This assessment was based on a combination of information on the degree of chronicity (mild, moderate, or severe kidney and liver disease) from the CCI classification and the creatinine and bilirubin values upon arrival to the ED. We defined 3 comorbidity levels: low (CCI score 0), moderate (CCI score 1 or 2), or high (CCI score ≥3). The adjustment for chronic pulmonary disease was based on information on pulmonary disease according to the CCI classification and if different grades of decreased arrival PaO2 values at the ED were considered to be chronically reduced. Patients with known dementia had a baseline SOFA score of one. Patients without known chronic disease had a baseline SOFA value of 0.
Altered mental state was defined as either a Glasgow Coma Scale (GCS) value <15 or AVPU (Alert, Verbal, Pain, Unresponsive) other than A.
A positive blood culture was defined as at least 1 positive blood culture during the stay in the ED (coagulase negative staphylococci excluded).
2.4. Management of patients with infection
During the study period, the ED nurses performed triage and electronic registration of all patients according to a standardized procedure. This included information regarding chief complaints and an assessment of vital parameters.
The standard sepsis screening protocol in the ED was as follows: Patients who had a qSOFA score of at least 2 or fulfilled the SIRS criteria for sepsis, or in other cases where nurses, independently of qSOFA or SIRS, suspected sepsis, were given priority for prompt medical examination by an ED physician. If a physician suspected sepsis a standard treatment protocol was recommended to be initiated within 1 hour from recognition of sepsis irrespective of disease severity. This included oxygen administration, arterial blood gas analysis with measurement of lactate, blood tests, an ECG, treatment with intravenous fluids (rapid administration of 30 mL/kg crystalloid for hypotension) and intravenous broad‐spectrum AB after blood cultures were drawn, and identification and management of the source of sepsis. Foci of the infection were specified by bacterial culturing of possibly infected tissues and body fluids. As required, other examinations were performed: X‐ray, ultrasound, computed tomography, gynecological examinations, etc. The treatment protocol was based on Danish national guidelines for early recognition and treatment of patients with increased risk of developing life‐threatening infectious diseases 32 and the recommendations in the international guidelines for management of severe sepsis and septic shock. 33
If the patients required hospitalization for >48 hours after the initial treatment, they were transferred to a medical ward. Deteriorating or critical patients despite adequate treatment were transferred to the ICU.
2.5. Data collection
We used a standardized form for data collection in the primary study. 27 Information on medical history, vital parameters on admission, the time (date, hours, minutes) of initiating intravenous AB treatment, laboratory tests on admission, results of other examinations, and information about transfers to ICU were obtained by experienced data abstractors (SMOBA, RHS) from the triage forms and electronic records. The admission ECG was analyzed for the occurrence of atrial fibrillation (AF) and registered either as a history of AF before admission or new‐onset AF (episodes of AF documented on a 12‐lead ECG on admission and without a history of prior AF). Information on sources of infections was based on a review of all records at discharge with specific information on infectious source diagnosed and documented in the records by the physicians during hospital stay. Information about death was obtained from the Danish Civil Registration System. 34
We performed a 3‐day pilot study before the initiation of the study to ensure the collection of all data defined in the study protocol. 27 Finally, the collected data were entered in an electronic database. The data collection and data entry process were randomly controlled by the authors (SMOBA, RHS). The researchers met regularly to discuss and clarify disputes regarding the collected data and analyses.
2.6. Analysis
The primary outcome was all‐cause 28‐day mortality. Categorical data were reported as counts and percentages with 95% confidence intervals (CI). Continuous data were presented as medians with interquartile ranges (IQR). We have compared groups by using differences within medians within 95% CI and exact differences of proportions with 95% CI, the Wilcoxon rank sum test, and the chi‐square test. Differences were assumed significant if the 95% CI for the median difference or the 95% CI for the difference of proportions did not include zero and if P values were < 0.05.
Logistic regression models were used in the analysis of the association between the time to treatment with AB and the outcome adjusted for potential confounding. TTA was categorized in 5‐time intervals (≤ 1 hour, and the timespans 1–3 hours, 3–6 hours, 6–9 hours, and > 9 hours). The potential confounders were chosen based on existing knowledge of the association between the variables and mortality or if the variables in the crude analysis were associated with death. We have adjusted for the following variables in the regression analyses of the association between TTA and mortality: age, gender, CCI, systolic blood pressure (SBP), mental status, temperature (TP), lactate, and creatinine. The TTA group (timespan 3–6 hours) with the lowest mortality was used as a reference group. We used multiple imputation by the Markov Chain Monte Carlo procedure under missing at random assumptions to impute missing variables (SBP, TP, lactate) in the regression analyses. 35 The imputation model using 10 imputed datasets included the outcome variable (death), exposure of interest (TTA), and the covariates: age, gender, CCI score, temperature, systolic blood pressure, lactate, creatinine, and mental status.
Stata 15.1 (StataCorp, College Station, Texas, USA) was used for all analyses.
3. RESULTS
3.1. Population
A total of 2112 patients were admitted with infectious diseases (Figure 1) and 714 (33.8%) fulfilled the criteria for sepsis (SOFA ≥ 2). A total of 124 (17.4%) were excluded (Figure 1) leaving 590 patients (55.6% male) with a median age of 74.2 years (IQR 65.8‐83.8). A total of 499 (84.6%) patients met either the qSOFA (n = 124) or SIRS criteria (n = 375).
3.2. Baseline characteristics
Baseline characteristics according to survival status are shown in Table 1. The 28‐day mortality and in‐hospital mortality were 14.6% (95% CI, 11.8–17.7) and 8.0% (95% CI, 5.9–10.5), respectively.
TABLE 1.
Baseline characteristics according to 28‐day mortality in septic patients identified by the Sequential Organ Failure Assessment (SOFA) score
| Non‐survivors n = 86 (14.6%) | Survivors n = 504 (85.4%) | Difference (95% CI) | P value | |
|---|---|---|---|---|
| Female gender; n (%) | 31 (36.1) | 231 (45.8) | 9.7 (÷1.3–20.7) | 0.10 |
| Age; years (IQR) | 79.2 (70.3–86.5) | 73.5 (64.7–83.6) | 4.8 (2.2–7.4) | 0.002 |
| CCI score; n (%) | ||||
| 0 | 7 (8.1) | 141 (28.0) | 19.9 (12.9–26.9) | <0.001 |
| 1‐2 | 42 (48.8) | 241 (47.8) | 1.0 (÷12.4–10.4) | 0.90 |
| 3+ | 37 (43.0) | 122 (24.2) | 18.8 (7.6–30.0) | <0.001 |
| History of sepsis; n (%) | 35 (40.7) | 146 (29.0) | 11.7 (0.6–22.8) | 0.03 |
| Atrial fibrillationa | ||||
| None, n (%) | 67 (77.9%) | 389 (77.2) | 0.7 (÷8.8–10.2) | 0.50 |
| New‐onset atrial fibrillation; n (%) | 9 (10.5%) | 22 (4.4) | 6.1 (÷0.6–12.8) | 0.03 |
| History of atrial fibrillation; n (%) | 10 (11.6) | 93 (18.4) | 6.8 (÷0.8–14.4) | 0.07 |
| Severity of disease | ||||
| SBP; mmHg, median (IQR)b | 121 (99–145) | 123.5 (107–145) | 4 (÷3–11) | 0.27 |
| SBP < 90; n (%) | 11 (12.8) | 24 (4.8) | 8 (0.7–15.3) | 0.01 |
| Respiratory rate; min‐1, median (IQR) | 20 (17–24) | 20 (18–25) | 1 (0–2) | 0.31 |
| Heart rate; min‐1, median (IQR) | 91.5 (77–109) | 94 (79.5–110) | 3 (÷3–8) | 0.35 |
| O2‐saturation; %, median (IQR) | 96 (92–98) | 95 (93‐97) | 0 (÷1–1) | 0.72 |
| Core temperature; oCelsius, median (IQR)c | 37.0 (36.5–37.9) | 37.5 (36.8‐38.5) | 0.5 (0.2–0.7) | < 0.001 |
| Altered mental state; n (%) | 33 (38.4) | 128 (25.4) | 13.0 (2.0–24.0) | 0.01 |
| qSOFA score ≥ 2 on admission; n (%) | 24 (27.9%) | 100 (19.8) | 8.1 (÷2.0–18.2) | 0.11 |
| SIRS criteria ≥ 2 on admission; n(%) | 50 (58.1) | 325 (64.5) | 6.4 (÷4.8–17.6) | 0.27 |
| Laboratory results | ||||
| CRP; median (IQR) | 91 (27–160) | 89 (35.9–160) | 3 (÷20–14) | 0.71 |
| WBC; x109/L, median (IQR) | 12.7 (8.0–18.6) | 12.1 (9.1–16.7) | 0.1 (÷1.4–1.6) | 0.89 |
| Creatinine; μmol/L, median (IQR) | 131 (71–204) | 110.5 (77–166) | 11 (÷31–6) | 0.17 |
| Bilirubin; mmol/L, median (IQR) | 11 (8–19) | 10 (7–17) | 1 (÷1–1) | 0.31 |
| Platelets; x109/L, median (IQR) | 227 (147–296) | 209 (147–287) | 5 (÷31–21) | 0.66 |
| Lactate; mmol/L, median (IQR)d | 1.8(1.0–3.4) | 1.3(0.9–2.1) | 0.3(÷0.7–0) | 0.02 |
| Lactate > 2 mmol/L; n (%) | 21 (38.9) | 80 (26.8) | 12.1 (1.1–23.1) | 0.06 |
| Glucose; mmol/L, median (IQR) | 7.4 | 7.2 | 0.1 (÷0.5–0.4) | 0.67 |
| Admission to ICU; n (%) | 16 (18.6) | 73 (14.5) | 4.1 (÷4.7–12.9) | 0.32 |
| Vasopressor; n (%) | 7 (8.1) | 9 (1.8) | 6.3 (0.4–12.2) | 0.004 |
| Mechanical ventilation; n (%) | 5 (5.8) | 29 (5.8) | 0 | 1.00 |
| Dialysis; n (%) | 1 (1.2) | 2 (0.4) | 0.8 (÷1.6–3.2) | 0.37 |
| Positive blood cultures; n (%)e | 15 (17.4) | 58 (11.5) | 5.9 (÷2.5–14.4) | 0.15 |
| Source of infection | ||||
| Pulmonary; n (%) | 56 (65.1) | 312 (61.9) | 3.2 (÷7.7–14.2) | 0.63 |
| Urine; n (%) | 21 (24.4) | 94 (18.7) | 5.7 (÷4.0–15.4) | 0.23 |
| Abdominal; n (%) | 6 (7.0) | 54 (10.7) | 3.7 (÷2.3–9.7) | 0.34 |
| Central nervous system; n (%) | 1 (1.2) | 4 (0.8) | 0.4 (÷2.0–2.8) | 0.54 |
| Unknown; n(%) | 6 (7.0) | 42 (8.3) | 1.3 (÷7.2–4.6) | 0.83 |
CCI, Charlson Comorbidity Index; CI, confidence interval; CRP, C‐reactive protein; IQR, interquartile range; qSOFA, quick Sequential Organ Failure Assessment; SBP, systolic blood pressure; SIRS, Systemic Inflammatory Response Syndrome; WBC, white blood cell.
aA total of 42 patients had missing information on atrial fibrillation on admission.
b7 patients with missing information on blood pressure.
c13 patients without core temperature measurements.
dA total of 32 (37.2%) non‐survivors and 205 (40.7%) survivors did not have lactate measured on admission.
eA total of 50 (60.0%) non‐survivors and 341 (67.7%) survivors had blood cultures taken on admission.
Twenty‐eight‐day mortality was significantly associated with age, the comorbidity score, if previously hospitalized with sepsis, hypotension, altered mental status, lactate level > 2 mmol/L, and low core temperature on admission (Table 1). Non‐survivors admitted to the ICU were more often treated with vasopressors (Table 1). The number of patients with a new‐onset AF on admission and a qSOFA score ≥2 was increased among non‐survivors (Table 1).
3.3. Source of infection
The most common sites of infections were the lungs (62.4%), urinary tract (19.5%), and abdomen (10.2%). A total of 48 (8.1%) patients had unknown sites of infection (Table 1).
3.4. Time to antibiotic treatment
Median TTA was 4.7 hours (IQR 2.7–8.1). The distribution of patients according to hourly intervals of TTA is shown in Table 2. A total of 34 (5.8%) patients were treated with AB within 1 hour, 137 (23.2%) within 1–3 hours, 190 (32.2%) within 3–6 hours, 117 (19.8%) within 6–9 hours, and 112 (19.0%) >9 hours after admission.
TABLE 2.
Unadjusted and adjusted odds ratio for 28‐day mortality among septic patients identified by the Sequential Organ Failure Assessment (SOFA) score
| Time to AB treatment (TTA) (hours) | Number of patients (%) | Mortality N (%; 95% CI) | Unadjusted odds ratio(95% CI) | Adjusted a odds ratio(95% CI) |
|---|---|---|---|---|
| ≤ 1 | 34 (5.8) | 9 (26.5; 12.8–44.4) | 3.06 (1.25–7.46) | 4.53 (1.67–3.37) |
| 1 < TTA ≤3 | 137 (23.2) | 21 (15.3;9.8–22.5) | 1.54 (0.80–2.97) | 1.67 (0.83–3.37) |
| 3 < TTA ≤6 | 190 (32.2) | 20 (10.5; 6.6–15.8) | Reference | Reference |
| 6 < TTA ≤9 | 117 (19.8) | 15 (12.8; 7.3–20.1) | 1.25 (0.61–2.55) | 1.17 (0.56–2.49) |
| > 9 | 112 (19.0) | 21 (18.8; 12.0–27.2) | 1.96 (1.01–3.80) | 1.91 (0.96–3.85) |
AB, antibiotic; CI, confidence interval; TTA, time to antibiotic treatment.
Adjusted for age, systolic blood pressure <90 mmHg on admission, Charlson Comorbidity Index, altered mental state, lactate, creatinine, and temperature on admission.
Patients with either hypotension (SBP < 90 mmHg) or a lactate level > 2 mmol/L on admission (n = 122) had a significantly lower TTA (4.1 vs 5.0 hours; 95% CI for median difference 0.1–1.5; P = 0.024) compared to patients with either higher SBP or lower lactate levels. TTA was significantly lower among patients with a qSOFA score ≥ 2 (3.4 vs 5.0 hours) or SIRS criteria ≥ 2 (3.9 vs 6.2 hours) on admission (Table 3).
TABLE 3.
Time to antibiotic treatment among septic patients admitted to an emergency department
| Timea to antibiotic treatment, Median hours (IQR) | Median difference (95% CI) | P value | |
|---|---|---|---|
| All septic patients (n = 590) | 4.7 (2.7–8.1) | ||
| qSOFA score on admission | |||
| < 2 (n = 466) | 5.0 (3.0–8.2) | 1.2 (0.6–1.8) | 0.005 |
| ≥ 2 (n = 124) | 3.4 (2.2–6.7) | ||
| SIRS criteria on admission | |||
| < 2 (n = 215) | 6.2 (4.0–9.2) | 2.1 (1.5–2.7) | < 0.001 |
| ≥ 2 (n = 375) | 3.9 (2.2–7.0) |
CI; confidence interval. IQR; interquartile ranges. qSOFA; quick Sequential Organ Failure Assessment. SIRS; Systemic inflammatory Response Syndrome.
Time from admission to the ED and administration of intravenous antibiotics (door‐to‐needle).
3.5. Mortality
The median TTA was not significantly different between survivors and non‐survivors (4.6 vs 5.2 hours; median difference 0.15 (95% CI, ÷1.1–0.8); P = 0.758). The mortality (26.5%; 95% CI, 12.8–44.4) was highest in patients treated with AB within 1 hour after admission to the ED and declining to 15.3% (9.8–22.5) in the TTA timespan 1–3 hours, 10.5% (6.6–15.8) in the timespan 3–6 hours, 12.8% (7.3–20.1) in the timespan 6–9 hours and 18.8% (12.0–27.2) in patients treated >9 hours after admission (Table 2).
After adjustment for potential confounding, and with patients with the lowest mortality (TTA timespan 3–6 hours) as reference group, our analyses showed that odds ratio (OR) for mortality was highest (OR 4.53; 1.67–3.37) in patients treated with antibiotics within 1 hour (Table 2). OR for mortality in the timespan 1–3 hours, 6–9 hours, and > 9 hours was 1.67 (0.83–3.37), 1.17 (0.56–2.49) and 1.91 (0.96–3.85), respectively (Table 2).
4. LIMITATIONS
There are some limitations of our study. First, the high mortality rates among patients treated early with antibiotics may be explained by confounding by indication. Furthermore, when using arrival time as time zero and time for study entry, and prescription of first antibiotic dose within a varying time period after study entry as exposure to treatment, immortal time bias 36 , 37 may also affect the validity. Second, increased workload and bustle in the ED could potentially have caused incorrect and delayed time registrations. However, the risk of misclassification of TTA was assumed approximately equal among the outcome groups being compared. The risk of misclassification was therefore classified as non‐differential, which will bias toward the null. 38 Fourth, our method used to calculate SOFA scores after correction for chronic diseases has not been validated. We cannot exclude a risk of misclassification of septic patients. However, we assume that misclassification may occur similarly in the compared groups. Therefore, the bias is toward the null. Fifth, the study was performed as a single‐center study. Sixth, a total of 19 patients were excluded because of missing values to calculate the SOFA score. However, we believe that the small number of excluded patients did not introduce any bias into our analyses. Finally, the sample size was relatively small. Sample sizes in other studies 9 , 11 , 12 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 25 , 39 , 40 , 41 , 42 , 43 , 44 vary considerably. In this context, our study has included a relatively small number of patients, which is reflected in our estimates. A larger sample size could have made our estimates more precise.
5. DISCUSSION
The main finding of our study was that adjusted odds of 28‐day mortality were lowest in patients who received antibiotics between 1 and 9 hours. The highest odds of mortality were found in patients treated early and >9 hours after admission to the ED.
The observational study design used in our study and other studies may be subject to bias of different types. Because the decision to initiate treatment with antibiotics is guided by the physician's expectations of the prognosis and the expected effect of the treatment on outcomes, confounding by indication may be a potential threat to the internal validity. When acute medical action and treatment with antibiotics is guided by the expectation of an increased risk of death in septic patients if not treated, the therapy provided to those at assumed high risk will appear less effective. Our analyses were adjusted for the severity of disease on admission. However, we do not know all the factors that are included in the determination of the prognosis. Therefore, it may be difficult to perform adequate adjustments of the analyses. Confounding by indication therefore has been described as an intractable and stubborn bias. 45 , 46
When using arrival time as time zero and time for study entry, and prescription of first antibiotic dose within a varying time period after study entry as exposure to treatment, immortal time bias 36 , 37 , 47 may affect the validity of the effect estimates. The time between time zero and first dose of antibiotics is “immortal.” Patients exposed for antibiotic treatment had to be free of events and survive until first administration of antibiotics. A method to handle immortal time bias is to replace the time‐fixed time of sepsis presentation (ED arrival time) with the exact time for presentation of signs of sepsis. However, sepsis is difficult to define and identify. Expert clinicians often disagree on whether sepsis is present or absent despite the application of common definitions, 10 , 48 and there is poor agreement between abstractors for identifying sepsis time zero. 49
RCTs of time to treatment with antibiotics in patients with sepsis are limited. The Prehospital Antibiotics Against Sepsis (PHANTASi) trial, 25 an RCT comparing the effect of giving antibiotics in the ambulance, did not lead to improved 28‐day survival, regardless of illness severity, compared with usual care. The results of this study are supported by previous published systematic reviews and meta‐analyses. 22 , 23
The results of our study and other observational studies that compare outcomes of different time intervals of antibiotic treatments should be viewed in the light of the risk of bias. Inspired by the PHANTASi trial, 25 we suggest that similar large multicenter prehospital RCTs should be performed among well‐defined sepsis populations fulfilling updated sepsis criteria with relevant and precise definitions of time zero and randomized into treatment arms with varying degrees of disease severity.
Until more solid data are available, a framework for timing of antimicrobials should be based on simultaneously evaluation of the likelihood of infection and an assessment of the severity of the illness. 50 For the sickest patients, broad‐spectrum antibiotics should be administered within 1 hour and then narrowed or discontinued as new data on microbial findings or alternative diagnoses are presented. 50 Less ill and clinically stable patients can await additional data to confirm the diagnosis before prescription of antibiotics. 50
In summary, our study found that adjusted odds of 28‐day mortality were lowest in ED patients with sepsis who received antibiotics between 1 and 9 hours. The highest odds of mortality were found in patients treated early and >9 hours after admission to the ED. Because of the observational design, bias may be considered as potential threats to internal validity. It is recommended to use study design approaches with low risk of bias in future research of the impact of TTA on outcome in septic patients.
CONFLICTS OF INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
KS: Design of study, interpretation of data, manuscript preparation. : SMOBA: Collection of data, interpretation of data, manuscript preparation. RHS: Collection of data, interpretation of data, manuscript preparation. FEN: Conceived the idea and designed the study, analysis of data, interpretation of data, manuscript preparation. All authors approved the final version of the manuscript.
ETHICS
The study was reported to The Danish Data Protection Agency (REG‐105‐2017). On May 16, 2017 the study was defined as a quality project by the Secretariat of The Committee on Health Research Ethics of Region Zealand and therefore it is not covered by Committee Act and is not obligated to report for the ethics committee system.
Siewers K, Abdullah SMOB, Sørensen RH, Nielsen FE Time to administration of antibiotics and mortality in sepsis. JACEP Open. 2021;2:e12435. 10.1002/emp2.12435
Supervising Editor: Nicholas Johnson, MD
Funding and support: This project received financial support from Region Zealand Health Research Foundation and Naestved, Slagelse, and Ringsted Hospitals Research Fund.
REFERENCES
- 1. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925‐928. [DOI] [PubMed] [Google Scholar]
- 2. Kalantari A, Rezaie SR. Challenging the one‐hour sepsis bundle. West J Emerg Med. 2019;20(2):185‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singer M. Antibiotics for sepsis: does each hour really count, or is it incestuous amplification?. Am J Respir Crit Care Med. 2017;196(7):800‐802. [DOI] [PubMed] [Google Scholar]
- 4. Marik PE, Farkas JD, Spiegel R, Weingart S. POINT: should the surviving sepsis campaign guidelines be retired? Yes. Chest. 2019;155(1):12‐14. [DOI] [PubMed] [Google Scholar]
- 5. Patel JJ, Bergl PA, Disselkamp M, Coz Yataco AO, Simpson SQ. COUNTERPOINT: should broad‐spectrum antibiotics be routinely administered to all patients with sepsis as soon as possible? No. Chest. 2019;156(4):645‐647. [DOI] [PubMed] [Google Scholar]
- 6. Disselkamp M, Coz Yataco AO, Simpson SQ. POINT: should broad‐spectrum antibiotics be routinely administered to all patients with sepsis as soon as possible? Yes. Chest . 2019;156(4):645‐647. [DOI] [PubMed] [Google Scholar]
- 7. Schinkel M, Nannan Panday RS, Wiersinga WJ, Nanayakkara PWB. Timeliness of antibiotics for patients with sepsis and septic shock. J Thorac Dis. 2020;12(Suppl 1):S66‐S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mi MY, Klompas M, Evans L. Early administration of antibiotics for suspected sepsis. N Engl J Med. 2019;380(6):593‐596. [DOI] [PubMed] [Google Scholar]
- 9. Ferrer R, Martin‐Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline‐based performance improvement program*. Crit Care Med. 2014;42(8):1749‐1755. https://journals.lww.com/ccmjournal/Fulltext/2014/08000/Empiric_Antibiotic_Treatment_Reduces_Mortality_in.1.aspx. [DOI] [PubMed] [Google Scholar]
- 10. Rhee C, Chiotos K, Cosgrove SE, et al. Infectious Diseases Society of America Position Paper: recommended revisions to the National Severe Sepsis and Septic Shock Early Management Bundle (SEP‐1) Sepsis Quality Measure. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2021;72(4):541‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock*. Crit Care Med. 2006;34(6). https://journals.lww.com/ccmjournal/Fulltext/2006/06000/Duration_of_hypotension_before_initiation_of.1.aspx. [DOI] [PubMed] [Google Scholar]
- 12. Husabø G, Nilsen RM, Flaatten H, et al. Early diagnosis of sepsis in emergency departments, time to treatment, and association with mortality: an observational study. PLoS One. 2020;15(1):e0227652‐e0227652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peltan ID, Brown SM, Bledsoe JR, et al. ED door‐to‐antibiotic time and long‐term mortality in sepsis. Chest. 2019;155(5):938‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu VX, Fielding‐Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235‐2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seymour CW, Kahn JM, Martin‐Gill C, et al. Delays from first medical contact to antibiotic administration for sepsis. Crit Care Med. 2017;45(5):759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andersson M, Ostholm‐Balkhed A, Fredrikson M, et al. Delay of appropriate antibiotic treatment is associated with high mortality in patients with community‐onset sepsis in a Swedish setting. Eur J Clin Microbiol Infect Dis. 2019;38(7):1223‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal‐directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045‐1053. [DOI] [PubMed] [Google Scholar]
- 19. Joo YM, Chae MK, Hwang SY, et al. Impact of timely antibiotic administration on outcomes in patients with severe sepsis and septic shock in the emergency department. Clin Exp Emerg Med. 2014;1(1):35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruce HR, Maiden J, Fedullo PF, Kim SC. Impact of nurse‐initiated ED sepsis protocol on compliance with sepsis bundles, time to initial antibiotic administration, and in‐hospital mortality. J Emerg Nurs. 2015;41(2):130‐137. [DOI] [PubMed] [Google Scholar]
- 21. Yokota PKO, Marra AR, Martino MDV, et al. Impact of appropriate antimicrobial therapy for patients with severe sepsis and septic shock–a quality improvement study. PLoS One. 2014;9(11):e104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta‐analysis. Crit Care Med. 2015;43(9):1907‐1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rothrock SG, Cassidy DD, Barneck M, et al. Outcome of immediate versus early antibiotics in severe sepsis and septic shock: a systematic review and meta‐analysis. Ann Emerg Med. 2020;76(4):427‐441. [DOI] [PubMed] [Google Scholar]
- 24. Nauclér P, Huttner A, van Werkhoven CH, et al. Impact of time to antibiotic therapy on clinical outcome in patients with bacterial infections in the emergency department: implications for antimicrobial stewardship. Clin Microbiol Infect. 2021. [DOI] [PubMed] [Google Scholar]
- 25. Alam N, Oskam E, Stassen PM, et al. Prehospital antibiotics in the ambulance for sepsis: a multicentre, open label, randomised trial. Lancet Respir Med. 2018;6(1):40‐50. [DOI] [PubMed] [Google Scholar]
- 26. Singer M, Deutschman CS, Seymour CW, et al. The third International Consensus Definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315(8):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. SMO Bin Abdullah, Sørensen RH, Dessau RBC, Sattar SMRU, Wiese L, Nielsen FE. Prognostic accuracy of qSOFA in predicting 28‐day mortality among infected patients in an emergency department: a prospective validation study. Emerg Med J. 2019;36(12):722‐728. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250‐1256. [DOI] [PubMed] [Google Scholar]
- 30. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644‐1655. [DOI] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 32. The Danish Society for Patient Safety . Patientsikkert Sygehus. Tidlig Opsporing Af Sepsis [In Danish]. Version 2013‐06‐10. (Downloaded from https://docplayer.dk/14119250).
- 33. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580‐637. [DOI] [PubMed] [Google Scholar]
- 34. Schmidt M, Pedersen L, Sørensen HT. The danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541‐549. [DOI] [PubMed] [Google Scholar]
- 35. Pedersen AB, Mikkelsen EM, Cronin‐Fenton D, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. 2017;9:157‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2008;167(4):492‐499. [DOI] [PubMed] [Google Scholar]
- 37. Abrahami D, Hudson M, Suissa S. Statins and lower mortality in rheumatic diseases: an effect of immortal time bias?. Semin Arthritis Rheum. 2020;51(1):211‐218. [DOI] [PubMed] [Google Scholar]
- 38. Fletcher RH, Fletcher SW, Fletcher GS. Clinical Epidemiology: The Essentials. Fifth edit. Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 39. Seok H, Song J, Jeon JH, et al. Timing of antibiotics in septic patients: a prospective cohort study. Clin Microbiol Infect. 2020;26(11):1495–1500. [DOI] [PubMed] [Google Scholar]
- 40. Peltan ID, Bledsoe JR, Oniki TA, et al. Emergency department crowding is associated with delayed antibiotics for sepsis. Ann Emerg Med. 2019;73(4):345‐355. [DOI] [PubMed] [Google Scholar]
- 41. Stoneking LR, Winkler JP, DeLuca LA, et al. Physician documentation of sepsis syndrome is associated with more aggressive treatment. West J Emerg Med. 2015;16(3):401‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferrer R, Artigas A, Suarez D, et al. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009;180(9):861‐866. [DOI] [PubMed] [Google Scholar]
- 43. Ryoo SM, Kim WY, Sohn CH, et al. Prognostic value of timing of antibiotic administration in patients with septic shock treated with early quantitative resuscitation. Am J Med Sci. 2015;349(4):328‐333. [DOI] [PubMed] [Google Scholar]
- 44. Bloos F, Thomas‐Rüddel D, Rüddel H, et al. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi‐center study. Crit Care. 2014;18(2):R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Giordano SH, Kuo Y‐F, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112(11):2456‐2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bosco JLF, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63(1):64‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yadav K, Lewis RJ. Immortal time bias in observational studies. JAMA. 2021;325(7):686‐687. [DOI] [PubMed] [Google Scholar]
- 48. Rhee C, Kadri SS, Danner RL, et al. Diagnosing sepsis is subjective and highly variable: a survey of intensivists using case vignettes. Crit Care. 2016;20:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rhee C, Brown SR, Jones TM, et al. Variability in determining sepsis time zero and bundle compliance rates for the centers for medicare and medicaid services SEP‐1 measure. Infect Control Hosp Epidemiol. 2018;39(8):994‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prescott HC, Iwashyna TJ. Improving sepsis treatment by embracing diagnostic uncertainty. Ann Am Thorac Soc. 2019;16(4):426‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]


