Abstract
Background
Since the first known cases in December 2019, the disease COVID-19, caused by the novel coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), has spread to become a worldwide pandemic.
Methods
The literature in PubMed was surveyed using search terms defined by the authors. Topics important for the management of patients with COVID-19 were identified and discussed. In a structured consensus framework, recommendations and statements on these topics were formulated.
Results
Whether hospital admission is indicated for patients with COVID-19 should be decided on the basis of age, comorbidities, respiratory rate, and oxygen saturation. For every patient admitted to the hospital, a polymerase chain reaction (PCR) test shortly beforehand or immediately thereafter is mandatory. Admission to the intensive care unit is recommended for COVID-19 patients with hypoxemia (oxygen saturation < 90%) despite administration of oxygen, dyspnea, or a high respiratory rate. A treatment trial of high-flow oxygen or non-invasive ventilation is suggested in hypoxemic respiratory insufficiency, while intubation and invasive ventilation is proposed for patients with more severe hypoxemia/high respiratory rate. If additional risk factors (such as obesity, known thrombophilia, intensive care treatment, or elevated D-dimers) are present, measures to prevent thromboembolism can be intensified. Treatment with dexamethasone reduces the mortality in patients with severe COVID-19. The important protection measures are observation of hygiene and the correct wearing of personal protective equipment.
Conclusion
The most important elements of treatment in severe cases of COVID-19 are adequate oxygenation, pharmaceutical prevention of thrombosis, and administration of dexamethasone.
Cases of infection with the novel coronavirus SARS-CoV-2 were first described in China in December 2019. Since then, the virus has spread worldwide to become a pandemic. This novel coronavirus was given the official name “SARS-CoV-2,” while the symptoms and disease it causes are referred to as “COVID-19.”
In March 2020, the first S1 guideline was drawn up on critical care for patients with COVID-19 (1). This was subsequently updated and has now been extended, in the form of an S2k guideline, to encompass the entire inpatient setting. The present article provides an overview of this new S2k guideline and its key recommendations. The complete, long version is freely available on the portal of the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, AWMF) (https://www.awmf.org/leitlinien/detail/ll/113–001.html).
Method
The guideline group was made up of 21 mandate holders from the 12 participating specialty societies, the ARDS Network (ARDS, acute respiratory distress syndrome), as well as one patient representative. This S2K guideline was drawn up in accordance with AWMF criteria to provide the user with evidence-based criteria for rational decision-making. It is based on a PubMed literature search. Recommendations were graded according to the AWMF three-grade classification system:
↑↑ = Strong recommendation (should/should not).
↑ = Recommendation (ought/ought not).
-
↔ = Recommendation open (may be
considered/no specific
recommendation).
The guideline is supported by the German Robert Koch Institute.
Clinical picture
COVID-19 generally manifests as a respiratory tract infection. Common symptoms include cough, fever, and respiratory symptoms. The only symptom that is almost pathognomonic of COVID-19 is the loss of smell and taste, which occurs in approximately 21% of patients (2). The need for inpatient care depends very strongly on age. In April 2020, the average age of new infections in Germany was 52 years, while the percentage of hospitalized patients in this total group was 20%. After a decline in age to 32 years, the average age is now 45 years, and the hospitalization rate is 7% (3).
Diagnosis
Virological diagnosis
Under the current pandemic conditions, all patients should have, or undergo, an up-to-date PCR (polymerase chain reaction) test on admission (↑↑). If SARS-CoV-2 antigen detection is initially carried out on hospital admission, parallel PCR testing should be performed (↑↑). The sensitivity of antigen testing is lower compared to PCR testing, and there are significant differences in terms of performance between the various commercially available tests. A negative antigen test does not rule out infection, particularly in the case of low viral load. Also, in the case of a negative SARS-CoV-2 PCR and strong clinical suspicion, a second sample should be tested (↑↑).
Indication for inpatient admission
The indication for the hospital admission of patients with COVID-19 should be made by a physician according to clinical criteria, taking into particular account patient age, comorbidities, respiratory rate, and oxygen saturation (↑↑). Mildly affected patients with no risk factors for complications (for example, immune suppression, relevant chronic underlying diseases, advanced age) can remain in the home setting if appropriate outpatient care is ensured. The commonest comorbidities on hospital admission include cardiovascular diseases (in particular arterial hypertension), diabetes mellitus, chronic lung diseases, and obesity (4– 7).
Indication for admission to an intensive care unit
COVID-19 patients should be admitted to an intensive care unit in the case of hypoxemia (oxygen saturation < 90% below 2–4 liters oxygen/min in the absence of previous oxygen therapy) and dyspnea or increased respiratory rate (> 25/min) (↑). The disease may progress to acute respiratory distress syndrome (ARDS). One distinctive feature of this is widespread microthrombi and structural vascular changes in the pulmonary capillary bed (8). Other complications that have been described include cardiac arrhythmias, myocardial damage, thrombosis, pulmonary embolism, as well as acute kidney and multi-organ failure. The time from symptom onset to intensive care admission is approximately 10 days, while the average length of stay on an intensive care unit with invasive ventilation is 18 days (2).
Organ involvement
An analysis of 10,021 patients in German hospitals revealed that 6% of COVID-19 patients had acute kidney failure requiring dialysis; this rate was 27% for patients on mechanical ventilation (9). An abnormal urine sample in the emergency department is associated with an increased risk for requiring treatment on an intensive care unit (10). Therefore, in the case of proven “SARS-CoV-2 infection” and hospitalization, urinalysis (repeated where necessary) including determination of albuminuria, hematuria, and leukocyturia should be performed (↑). Acute cardiac involvement is common in critically ill patients with COVID-19. Echocardiography should be performed to investigate differential diagnoses in COVID-19 patients with markedly elevated troponin levels in the absence of ECG changes typical for type-1 myocardial infarction (↑). A prospective study found severe neurological complications (encephalopathy, epilepsy, stroke) in 13.5% of 4491 patients with COVID-19. These complications were associated with significantly increased in-hospital mortality (hazard ratio: 1.38) (11). In the case of suspected cerebral or spinal involvement (for example, hemorrhage or ischemia) due to COVID-19, computed tomography (CT) or magnetic resonance imaging (MRI) should be performed (↑).
Laboratory investigations
In hospitalized COVID-19 patients, the following investigations should form part of the initial diagnostic laboratory work-up and be regularly checked as required (↑):
Determination of C-reactive protein (CRP), L-lactate dehydrogenase (LDH), and aspartate aminotransferase (AST/GOT)
Differential blood count
D-dimer determination.
A systematic review article analyzed 19 studies with 2874 patients, the majority of which received inpatient care (12). Laboratory tests frequently revealed elevated CRP (58%), LDH (57%), and AST (33%). Most patients had normal procalcitonin levels, and these levels correlated with the severity of disease (13). The most frequent change in blood count was lymphocytopenia, which was present in up to 83% of patients on hospital admission (14). Elevated D-dimer levels were found in 43–60% of patients and were associated with increased mortality (15).
Imaging
Chest CT should be performed in patients with COVID-19 if there is uncertainty in the differential diagnosis, including, e.g., suspected pulmonary embolism (↑). Very early on in the course of disease, CT reveals bilateral subpleural ground-glass opacities and segmental consolidation of the lung (16, 17). However, the findings on CT are not specific for COVID-19 and can be found in other forms of viral pneumonia.
Protective measures
Hygiene
The transmission of infection by affected individuals generally occurs via droplets and aerosols, with close contact being conducive to transmission. Therefore, it is essential to ensure that basic hygiene practices (including hand hygiene), as well as protective measures for personnel, are strictly implemented. According to the Robert Koch Institute (RKI), personal protective equipment consists of:
Protective gown
Disposable gloves
Tight-fitting respirator (FFP2 or FFP3, for example when performing intubation, bronchoscopy, or other activities in which aerosols may be formed)
Safety glasses.
Treatment
Measures in acute respiratory failure with hypoxia
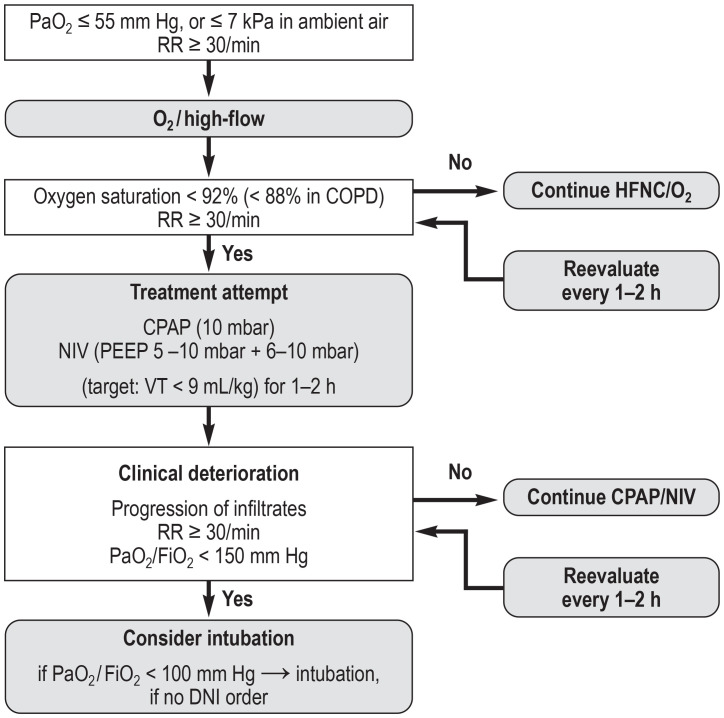
An oxygen saturation ≥ 90% (in patients with chronic obstructive pulmonary disease [COPD] > 88%) or a PaO2> 55 mm Hg should be achieved (e2) (↑). The initial focus of treatment is on oxygen administration via a nasal cannula, a Venturi mask, and high-flow nasal cannula (HFNC) oxygen therapy (figure). The latter can reduce the need for intubation compared with conventional oxygen therapy without significantly affecting mortality (18). In the case of progressive deterioration of gas exchange and increased oxygen requirements, the indication for CPAP (continuous positive airway pressure) therapy, noninvasive ventilation (NIV), or invasive ventilation needs to be assessed.
Figure.
Possible instrument-based treatment escalation in the case of acute respiratory insufficiency as a result of COVID-19 (40) (reprinted with kind permission from Thieme Verlag)
RR, respiratory rate; CPAP, continuous positive airway pressure; COPD, continuous positive airway pressure; DNI, do not intubate; NIV, noninvasive ventilation; PEEP, positive end-expiratory pressure; VT, tidal volume
In patients with COVID-19 and hypoxemic respiratory failure (PaO2/FiO2 = 100–300 mm Hg), we suggest attempting treatment with high-flow nasal cannula (HFNC) oxygen therapy or noninvasive ventilation under continuous monitoring and constant intubation (↑). The use of NIV in moderate and severe ARDS results in treatment failure in over 50% of cases. Continuous monitoring and constant readiness for intubation need to be ensured in these patients.
In patients with COVID-19 and severe hypoxemia (oxygenation index/Horovitz index: PaO2/FiO2 < 150 mm Hg) and a respiratory rate > 30/min, we suggest considering intubation and invasive ventilation; at a PaO2/FiO2 of < 100 mm Hg, intubation and invasive ventilation should, as a rule, be performed (↑).
Endotracheal intubation in patients with suspected or confirmed SARS-CoV-2 infection constitutes a high-risk intervention (19). Instrumentation of the airway in patients with COVID-19 should only be performed wearing full personal protective equipment (↑↑). This also applies to other airway procedures (bronchoscopy, open suctioning, manual ventilation, tracheostomy), which, due to the formation of aerosols, should only be performed if absolutely indicated and while implementing appropriate protective measures for the safety of medical personnel (table).
Table. Measures to minimize aerosol generation and exposure*.
| Aerosol formation | Risk minimization |
| Endotracheal intubation | – Avoid emergency intubation – Intubation to be performed by an experienced physician – Rapid sequence induction [RSI]) – Avoid bag mask ventilation – Optimal preparation and briefing – Ideally, video laryngoscopy (at arm’s length) – Endotracheal tube with stylet |
| Preoxygenation | – Tight-fitting face mask – Bi-manual mask fixation – Always FiO2 1.0 – PEEP max +5 cm H2O – 3 Min spontaneous breathing with face mask – Or 1 min, 8–12 deep breaths – Or CPAP/NIV 5/15 cm H2O |
| Fiberoptic intubation (FOI) | – Avoid where possible (aerosols) – Local anesthesia if necessary – Patient to wear oronasal mask |
| Suction | – Closed systems |
| Noninvasive ventilation (NIV) | – Only if unequivocally indicated – Non-vented mask, anti-virus filter – Ensure optimal fit of the mask |
| HFNC oxygen ctherapy | – Only if absolutely indicated – Patient to wear oronasal mask |
| Bronchoscopy | – Only if absolutely indicated |
| Tracheostomy | – To be performed by an experienced physician – Significant aerosol generation in all procedures – Postpone until patient tests negative on PCR if necessary |
| Tube disconnection | – Leave HME filter on tube – Clamp tube – Place ventilator on stand-by |
| Extubation | – Avoid suction and inflation maneuvers during extubation – Place ventilator on standby – Leave HME filter on the tube – Plastic sheet to cover patient’s face (ensure free airway) if necessary – Tight-fitting O2 mask for oxygenation – Patient to wear oronasal mask if spontaneous breathing is adequate |
*Modfied from (e1)
CPAP, continuous positive airway pressure; HME, heat and moisture exchanger;
NIV, noninvasive ventilation; PCR, polymerase chain reaction;
PEEP, positive end-expiratory pressure
Invasive ventilation and adjuvant measures
The larger observational studies that have been published showed that there were no significant differences between patients with COVID-19-related ARDS and ARDS of other causes in terms of respiratory system compliance, plateau pressure, and driving pressure (20, 21). Due to the lack of randomized studies on ventilation therapy in patients with COVID-19, the respective recommendations are derived from the most recently published guidelines on invasive ventilation in acute respiratory failure (22, 23). In mechanically ventilated patients with COVID-19 and ARDS, the tidal volume should be ≤ 6 mL/kg standard body weight and the end-inspiratory pressure ≤ 30 cm H2O (↑). The ARDS Network’s FiO2/PEEP table should be consulted for the guide settings for end-expiratory pressure (PEEP) in COVID-19. By means of close monitoring, the PEEP can be adjusted to the individual patient’s condition (↑). In the case of ARDS and a PaO2/FiO2 < 150 mm Hg, prone positioning should be consistently implemented (22). In patients with severe ARDS and treatment-refractory hypoxemia (PaO2/FiO2 quotient < 80 or 60 mm Hg), the use of veno-venous extracorporeal membrane oxygenization (ECMO) is a therapeutic option.
Cardiac arrest and cardiopulmonary resuscitation
Cardiac arrest is a not uncommon complication in hospitalized patients with COVID-19. The initial rhythm is usually electromechanical dissociation or asystole, and the likelihood of survival is correspondingly low. Since both chest compressions and airway management are likely to cause the release of aerosols, appropriate personal protective equipment is mandatory in cardiopulmonary resuscitation (24).
Thromboembolism prophylaxis/anticoagulation
Thromboembolic events are a frequent complication in COVID-19, affecting primarily the venous, but also the arterial, vascular system (25, 26). Therefore, hospitalized COVID-19 patients without contraindications should receive standard pharmacological prophylaxis for venous thromboembolism with low-molecular-weight heparin. Alternatively, fondaparinux can be used (↑↑). In the case of additional risk factors for venous thromboembolism (VTE), intensified thromboembolic prophylaxis, for example, with the half-therapeutic dose of a low-molecular-weight heparin or unfractionated heparin, can be performed if the risk of bleeding is low (↑). These risk factors include, for example:
Obesity (BMI > 35 kg/m2)
Previous VTE
Known thrombophilia
Intensive care treatment
Markedly elevated D-dimers (> 2–3 mg/L).
Drug treatment
Prophylactic antibiotic administration in laboratory-confirmed SARS-CoV-2 infection is not recommended, given that bacterial co-infections are comparatively rare at the early stage of disease (27). Numerous pharmacological treatment approaches have been investigated in the course of the pandemic. The use of the following drugs is not recommended, since, among other reasons, randomized controlled studies demonstrated no clinical benefit for patients:
Anakinra
Azithromycin
Chloroquine/hydroxychloroquine
Interferon ß-1b
Lopinavir/ritonavir
Tocilizumab.
A recently published randomized controlled study also showed no benefit for the use of convalescent plasma (28).
Remdesivir
In the randomized controlled double-blind study ACTT-1 with 1062 patients, 541 subjects received remdesivir and 521 received placebo. The administration of remdesivir reduced the time to recovery (primary endpoint) from a median of 15 to 10 days compared to the administration of placebo (risk ratio for recovery: 1.29; 95% confidence interval: [1.12; 1.49]; p < 0.001) (29). The effect was greatest in patients requiring low-flow oxygen who did not require any form of ventilation; in the subgroup requiring mechanical ventilation, no positive effect was observed.
In the remdesivir arm of the randomized SOLIDARITY study, 2743 received remdesivir (30). Death occurred in 11% of patients that received remdesivir and in 11.2% of the control group (hazard ratio 0.95; [0.81; 1.11]; p = 0.50). Thus, no benefit was seen in terms of mortality (primary endpoint). This also applied to the secondary endpoints, initiation of ventilation and duration of hospital stay.
Due to these divergent data, the guideline group made a “may be considered” recommendation: in hospitalized, non-ventilated patients with COVID-19 pneumonia requiring oxygen therapy, remdesivir treatment can be administered, preferably in the early phase of the disease (≤ 10 days after symptom onset) and at a dosage of 200 mg intravenously (IV) on day 1, and at a dosage of 100 mg IV from day 2 up to and including day 5 (↔). The WHO currently does not recommend the use of remdesivir, irrespective of the clinical stage of disease (weak or conditional WHO recommendation) (31).
Steroids
In the RECOVERY study, hospitalized COVID-19 patients were treated with dexamethasone (6 mg once daily for 10 days) or with standard treatment (32). The primary endpoint was 28-day mortality. A total of 2104 patients received dexamethasone and 4321 patients the standard treatment. Overall, 482 patients died, 22.9% in the dexamethasone group and 25.7% in the standard-treatment group (p < 0.001). The greatest benefit was seen in ventilated intensive care patients with COVID-19 (mortality, 29.3 % versus 41.4%). This mortality-reducing effect is significantly less pronounced in COVID-19 patients requiring oxygen therapy (with or without NIV) without invasive ventilation (mortality, 23.3% versus 26.2%). However, no benefit was seen in patients not requiring oxygen therapy.
A meta-analysis of seven randomized controlled studies on 1703 intensive care patients, in whom steroid therapy in severe COVID-19 infection was compared with standard treatment or placebo, showed: the administration of systemic corticosteroids is associated with a significantly lower overall 28-day mortality in patients with COVID-19 (33). Therefore, patients with severe (oxygen saturation < 90%, respiratory rate > 30/min) or critical (ARDS, sepsis, ventilation, vasopressor therapy) COVID-19 disease should be treated with dexamethasone (↑↑) (34). The dose is 6 mg dexamethasone per os (PO)/IV daily for 10 days. Alternatively, a different glucocorticoid can be used, such as hydrocortisone 50 mg IV every 8 h for 10 days.
Prognosis
Mortality in a study on 10,021 German hospitalized COVID-19 patients insured with a German statutory health insurance (AOK) was 22%, with significant differences seen between non-ventilated (16%) and ventilated patients (53%) (9). Mortality rose with age—for example, ventilated patients aged ≥ 80 years had an in-hospital mortality of 72%.
Another study analyzed the data of 1904 German patients that had been admitted to 86 hospitals with COVID-19 (35). The mortality rate was 17%, and in ventilated patients 33%. Risk factors for death included male gender, pre-existing lung disease, and advanced patient age.
According to recent figures, 87% of deaths in Germany were among those aged 70 years and older (2).
Persistent symptoms
Follow-up studies of COVID-19 sufferers showed that a significant number of affected individuals remained symptomatic far beyond the period of actual viral illness. For example, an Italian working group described 179 hospitalized patients that were followed-up at 60 days, on average, after the onset of COVID-19 symptoms (36). Of these, 87.4% complained of persistent symptoms, with dyspnea and a fatigue syndrome predominating here (37). Therefore, patients treated in hospital for COVID-19 should be followed-up at 8–12 weeks for long-term sequelae (↑).
Special aspects in pediatric patients
Compared to adults, COVID-19 infection is significantly milder in children, and severe disease is rare. In a review of 2914 pediatric patients, 47% had fever in the course of disease. The other most common symptoms included cough (48%), pharyngitis (29%), and in approximately 10% of cases also gastrointestinal symptoms involving diarrhea, nausea, and vomiting (38). From a treatment perspective, the same considerations and limitations apply to oxygen administration, high-flow oxygen therapy, noninvasive ventilation, and endotracheal intubation as in adult patients. Therapeutic approaches in childhood are based on study results and experience gained in adult medicine, given that no interventional studies in children have been published to date.
Ethical aspects
Recommendations on the distribution of intensive care resources in the context of the COVID-19 pandemic have been drawn up for the eventuality that intensive care resources in Germany—despite optimal use of the increased intensive care capacity—become insufficient to serve all patients (39).
BOX. Composition of the guideline group (collaborators).
-
Guideline authors
Prof. Dr. Stefan Kluge, Klinik für Intensivmedizin, Universitätsklinikum Hamburg-Eppendorf, Germany
Prof. Dr. Uwe Janssens, Klinik für Innere Medizin, St.-Antonius-Hospital Eschweiler, Germany
Prof. Dr. Tobias Welte, Klinik für Pneumologie, Medizinische Hochschule Hannover, Germany
Prof. Dr. Steffen Weber-Carstens, Klinik für Anästhesiologie mit Schwerpunkt operative Intensivmedizin, Charité – Universitätsmedizin Berlin, Germany
PD Dr. Gereon Schälte, Klinik für Anästhesiologie, Uniklinik RWTH Aachen, Germany
PD Dr. Christoph D. Spinner, Klinikum rechts der Isar, Klinik und Poliklinik für Innere Medizin II, Technische Universität München, Fakultät für Medizin, Munich, Germany
Dr. Jakob J. Malin, Klinik I für Innere Medizin, Klinische Infektiologie, Universitätsklinikum Köln, Cologne, Germany
Prof. Dr. Petra Gastmeier, Institut für Hygiene und Umweltmedizin, Charité – Universitätsmedizin Berlin, Germany
Prof. Dr. Florian Langer, Hämostaseologie, II. Medizinische Klinik und Poliklinik, Universitätsklinikum Hamburg-Eppendorf, Germany
PD Dr. Martin Wepler, Klinik für Anästhesiologie und Intensivmedizin, Universitätsklinikum Ulm, Germany
PD Dr. Michael Westhoff, Klinik für Pneumologie, Lungenklinik Hemer, Germany
Prof. Dr. Michael Pfeifer, Klinik und Poliklinik für Innere Medizin II, Universitätsklinikum Regensburg, Germany
Prof. Dr. Klaus F. Rabe, Abteilung für Pneumologie, LungenClinic Grosshansdorf, Germany
PD Dr. Florian Hoffmann, Kinderklinik und Kinderpoliklinik im Dr. von Haunerschen Kinderspital, LMU Klinikum, Munich, Germany
Prof. Dr. Bernd W. Böttiger, Klinik für Anästhesiologie und Operative Intensivmedizin, Universitätsklinikum Köln, Cologne, Germany
Prof. Dr. Julia Weinmann-Menke, Schwerpunkt Nephrologie, Universitätsmedizin Mainz, Germany
Dr. Alexander Kersten, Klinik für Kardiologie, Angiologie und Internistische Intensivmedizin (Medizinische Klinik I), Uniklinik RWTH Aachen, Germany
Prof. Dr. Peter Berlit, Deutsche Gesellschaft für Neurologie, Berlin, Germany
Reiner Haase, Delingsdorf, Germany
Prof. Dr. Gernot Marx, Klinik für Operative Intensivmedizin und Intermediate Care, Uniklinik RWTH Aachen, Germany
-
Prof. Dr. Christian Karagiannidis, Abteilung Pneumologie, Intensiv- und Beatmungsmedizin, Lungenklinik Köln-Merheim, Germany
Methodological guidance and neutral moderation:
Dr. Monika Nothacker, AWMF-Institut für Medizinisches Wissensmanagement Marburg/Berlin, Germany
-
Participating specialty societies and interest groups
Lead:
German Society for Medical Intensive Care Medicine and Emergency Medicine (DGIIN), Berlin, Germany
German Interdisciplinary Association for Intensive Care and Emergency Medicine (DIVI), Berlin, Germany
-
German Respiratory Society (DGP), Berlin, Germany
With the collaboration of:
German Society of Anaesthesiology and Intensive Care Medicine (DGAI), Nuremberg, Germany
German Society of Infectious Diseases (DGI), Berlin, Germany
German Society for Hygiene and Microbiology (DGHM), Münster, Germany
Society of Thrombosis and Haemostasis Research (GTH), Cologne, Germany
German Society for Pediatric and Adolescent Medicine (DGKJ), Berlin, Germany
German Resuscitation Council (GRC), Ulm, Germany
ARDS Network Germany, Berlin, Germany
German Society of Nephrology (DGfN)
German Cardiac Society (DGK)
German Society of Nephrology (DGN)
Patient representation (individual affected persons)
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interests
Prof. Kluge received research support from Ambu, Daiichi Sankyo, ETView Ltd, Fisher & Paykel, Pfizer, and Xenios. He received lecture fees from Astra, C.R. Bard, Baxter, Biotest, Cytosorbents, Fresenius, Gilead, MSD, Pfizer, Philips, and ZOLL. He received speaker’s honoraria from Bayer, Fresenius, Gilead, MSD, and Pfizer.
PD Dr. Spinner received consultancy fees, speaker’s honoraria, and/or reimbursement of travel expenses from Gilead Sciences in the context of Covid-19. He received consultancy fees from MSD, Molecular Partners, and Formycon in the context of Covid-19. In the context of COVID-19, he received research support (indirectly to his employer) from Aperion, Eli Lilly, Gilead Sciences, and Janssen-Cilag. Outside this context, he has received consultancy fees, speaker’s honoraria, and/or reimbursement of travel expenses from AbbVie, Gilead Sciences, Janssen-Cilag, MSD, and ViiV Healthcare/GSK, as well as research support (indirectly to his employer) from Gilead Sciences, GSK, Jansen-Cilag, MSD, and ViiV Healthcare.
Prof. Pfeifer received speaker’s honoraria from Astra-Zeneca, Boehringer, Chiesi, Glaxo-Smith-Kline, Novartis, and Roche. He received consultancy fees from Boehringer, Chiesi, Novartis, and Roche, as well as travel support from Boehringer.
Prof. Marx received consultancy fees and research support from Biotest, B.Braun, and Adrenomed, as well as speaker’s honoraria from B.Braun, Biotest, and Philips. He holds a patent on the modulation of the TLR4 signaling pathway (European Patent 2855519).
Prof. Karagiannidis received consultancy fees from Bayer and Xenios.
Prof. Janssens declares that no conflict of interest exists.
Clinical guidelines in the Deutsches Ärzteblatt, as in numerous other specialist journals, are not subject to a peer review procedure, since they represent texts that have already been evaluated, discussed, and broadly agreed upon multiple times by experts (peers).
References
- 1.Kluge S, Janssens U, Welte T, Weber-Carstens S, Marx G, Karagiannidis C. [Recommendations for critically ill patients with COVID-19] Medizinische Klinik, Intensivmedizin und Notfallmedizin. 2020;115:175–177. doi: 10.1007/s00063-020-00674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert Koch-Institut. Epidemiologischer Steckbrief zu SARS-CoV-2 und COVID-19. www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/ Steckbrief.html#doc13776792bodyText15. (last accessed on 10 December 2020) [Google Scholar]
- 3.Robert Koch-Institut. Aktueller Lage-/Situationsbericht des RKI zu COVID-19. www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Gesamt.html. (last accessed on 10 December 2020) [Google Scholar]
- 4.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55 doi: 10.1183/13993003.00547-2020. 2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakob CEM, Borgmann S, Duygu F, et al. First results of the „Lean European Open Survey on SARS-CoV-2-Infected Patients (LEOSS)“ Infection 2020 1-11. doi: 10.1007/s15010-020-01499-0. Epub ahead of print. doi: 10.1007/s15010-020-01499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross O, Moerer O, Weber M, Huber TB, Scheithauer S. COVID-19-associated nephritis: early warning for disease severity and complications? Lancet (London, England) 2020;395:e87–e88. doi: 10.1016/S0140-6736(20)31041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized COVID-19 patients in New York City. Neurology. 2020 doi: 10.1212/WNL.0000000000010979. 10.1212/WNL.0000000000010979 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106051. 106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 16.Antoch G, Urbach H, Mentzel HJ, Reimer P, Weber W, Wujciak D. SARS-CoV-2/COVID-19: Empfehlungen für die Radiologische Versorgung - Eine Stellungnahme der Deutschen Röntgengesellschaft (DRG) und weiterer Fachgesellschaften. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2020;192:418–421. doi: 10.1055/a-1149-3625. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rochwerg B, Einav S, Chaudhuri D, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46:2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46:2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deutsche Gesellschaft für Anästhesiologie & Intensivmedizin. S3-Leitlinie Invasive Beatmung und Einsatz extrakorporaler Verfahren bei akuter respiratorischer Insuffizienz. www.awmf.org. 2017 (last accessed on 16 December 2020) [Google Scholar]
- 23.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Empfehlungen des International Liaison Committee on Resuscitation (ILCOR), des Europäischen Rates für Wiederbelebung (ERC) und des Deutschen Rates für Wiederbelebung/German Resuscitation Council (GRC) zur CPR bei Patienten mit COVID-19. www.grc-org.de/ueber-uns/aktuelles (last accessed on 23 November 2020) [Google Scholar]
- 25.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langer F, Kluge S, Klamroth R, Oldenburg J. Coagulopathy in COVID-19 and its implication for safe and efficacious thromboprophylaxis. Hamostaseologie. 2020;40:264–269. doi: 10.1055/a-1178-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2031304. doi: 10.1056/NEJMoa2031304. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid–19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for covid-19—interim WHO Solidarity trial results. N Engl J Med. 2020 doi: 10.1056/NEJMoa2023184. doi: 10.1056/NEJMoa2023184. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rochwerg B, Agoritsas T, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370 doi: 10.1136/bmj.m3379. m3379. [DOI] [PubMed] [Google Scholar]
- 32.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. doi: 10.1056/NEJMoa2021436. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. Corticosteroids for COVID-19—living guidance. 02.09.2020. www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (last accessed on 16 December 2020) [Google Scholar]
- 35.Nachtigall I, Lenga P, Jóźwiak K, et al. Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID-19 in Germany: an observational study. Clin Microbiol Infect. 2020;26:1663–1669. doi: 10.1016/j.cmi.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ (Clinical research ed.) 2006;333 doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel NA. Pediatric COVID-19: systematic review of the literature. Am J Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102573. 102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutzmann J, Hartog C, Janssens U, et al. Entscheidungen über die Zuteilung intensivmedizinischer Ressourcen im Kontext der COVID-19-Pandemie. www.divi.de/register/aktuelle-informationen (last accessed on 23 November 2020) [Google Scholar]
- 40.Pfeifer M, Ewig S, Voshaar T, et al. Positionspapier zur praktischen Umsetzung der apparativen Differenzialtherapie der akuten respiratorischen Insuffizienz bei COVID-19. Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin e. V. (DGP). Pneumologie. 2020;74:337–357. doi: 10.1055/a-1157-9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Schälte G, Kehl F, Didion N, et al. Besonderheiten des Atemwegsmanagements bei Patienten mit vermuteter oder gesicherter COVID-19 Erkrankung und bei Patienten ohne Infektion während der Corona-Pandemie. Empfehlungen von DGAI und BDA. Anästh Intensivmed. 2020;61:S132–S136. [Google Scholar]
- E2.WHO. Clinical management of COVID-19. https://www.who.int/publications/i/item/clinical-management-of-covid-19. Version from 27.05.2020 (last accessed on 10 December 2020) [Google Scholar]