Summary
Dedicator of cytokinesis 2 (Dock2), an atypical guanine exchange factor, is specifically expressed on immune cells and mediates cell adhesion and migration by activating Rac and regulates actin cytoskeleton remodeling. It plays a crucial role in the migration, formation of immune synapses, cell proliferation, activation of T and B lymphocytes and chemotaxis of pDCs and neutrophils. However, in‐vivo physiological functions of Dock2 have been relatively seldom studied. Our previous studies showed that Dock2−/− mice were highly susceptible to colitis induced by Citrobacter rodentium infection, and in early infection, Dock2−/− mice had defects in macrophage migration. However, the specific roles of Dock2 in the migration and functions of macrophages are not clear. In this study, we found that the expression of chemokines such as chemokine (C‐C motif) ligand (CCL)4 and CCL5 and chemokine receptors such as chemokine (C‐C motif) receptor (CCR)4 and CCR5 in bone marrow‐derived macrophages (BMDM) of Dock2−/− mice decreased after infection, which were supported by the in‐vivo infection experimental results; the Transwell experiment results showed that Dock2−/− BMDM had a defect in chemotaxis. The bacterial phagocytic and bactericidal experiment results also showed that Dock2−/− BMDM had the defects of bacterial phagocytosis and killing. Furthermore, the adoptive transfer of wild‐type BMDM alleviated the susceptibility of Dock2−/− mice to C. rodentium infection. Our results show that Dock2 affects migration and phagocytic and bactericidal ability of macrophages by regulating the expression of chemokines, chemokine receptors and their responses to chemokine stimulation, thus playing an essential role in the host defense against enteric bacterial infection.
Keywords: chemokine, Citrobacter rodentium, colitis, Dock2, macrophage
Dock2 is an atypical guanine exchange factor to mediate cell adhesion and migration by activating Rac in various immune cells. However, the specific roles of Dock2 in the migration and functions of macrophages are not clear. Our results show that Dock2 regulates the expression of chemokines/chemokine receptors, the responses to chemokine stimulation, and the phagocytic and bactericidal ability of macrophages, thus playing an essential role in the host defense against enteric bacterial infection.
Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a recurrent intestinal inflammation with unclear etiology. Its lesions mainly involve ileum, rectum and colon, and its clinical manifestations include diarrhea, abdominal pain and hematochezia, etc.; therefore, IBD seriously affects the life quality of patients [1, 2]. IBD has become a global disease, generally more prevalent in developed countries. However, the last decade of data has also shown an increasing incidence in newly industrialized countries [3]. So far, there have been many types of IBD animal models, among which the colitis induced by Citrobacter rodentium infection is involved in both innate and acquired immune responses [4, 5, 6]. However, the specific roles of many host genes or proteins in regulating immune cell function during C. rodentium infection remain largely unclarified.
Dedicator of cytokinesis (Dock2), described initially as KIAA0209, is a member of the Drosophila melanogaster myoblast city (CDM) family of proteins [7]. Dock2 is a guanine nucleotide exchange factor (GEFs), which can mediate GTP–guanosine diphosphate (GDP) exchange and specifically activate the small G protein Rac1, regulating the cytoskeleton formation [8]. It is very conservative in the evolutionary process and can play different roles by the interaction of its structural domains with other molecules [9, 10]. First, the Dock homology region (DHR)‐1 domain promotes the translocation and polarization of Dock2 to the cell membrane by inducing polarized accumulation of F‐actin and phosphatidylinositol 3,4,5‐triphosphate (PIP3) [11]. Secondly, the DHR2 domain has guanine nucleotide exchange activity to activate Rac [12]. Thirdly, the Src homology3 (SH3) domain can bind to the carboxyl‐terminal of engulfment and cell motility protein 1 (ELMO1), making SH3 unable to inhibit DHR2 and enhancing the activation of Rac and maintaining the protein level of Dock2 [13, 14].
Dock2 can regulate the migration of pDCs and neutrophils by activating Rac [15, 16, 17, 18, 19]. Dock2 can also regulate T and B cell development and proliferation through affecting the formation of immune synapses and the migration of T and B cells under the stimulation of chemokines [20, 21, 22]. Dock2 plays a vital role in Rac activation induced by activation of chemokine receptor and antigen receptors, regulating migration and activation of various immune cells [23]. However, it is not clear whether Dock2 regulates the migration and activation of macrophages.
Our previous study showed that Dock2−/− mice were more susceptible to C. rodentium infection than controls. Compared with wild‐type (WT) mice, the bacteria in Dock2−/− mice were more likely to spread to the whole‐body organs, and their ability to recruit immune cells was reduced. Also, more bacterial adhesion in intestinal mucosa at the early stage of infection and less macrophage migration were observed in Dock2−/− mice [24], suggesting the role of Dock2 in innate immunity against enteric bacterial infection.
During intestinal inflammation, the chemotactic effect of macrophages in inflammatory sites is mainly mediated by chemokine signaling. Studies have shown that Rac1 induced polarization and directional movement of macrophages through the phosphatidylinositol 3‐kinase (PI3K) activation mediated by C‐C motif chemokine ligand (CCL) 4 and CCL5 [25, 26]. The binding of chemokines to chemokine receptors also leads to signal transduction and intracellular activation events which, in turn, induce macrophages to change shape and continue to migrate into inflammatory areas, and therefore a large number of activated macrophages continually migrating from the circulatory system to the mucosal layer [27]. CDM family protein is considered as a regulator of cytoskeleton dynamics by acting on the upstream of Rac during phagocytosis and cell migration [28, 29, 30], but it is still not clear which chemokine effects were mediated by Dock2 during macrophage migration.
In this study, we show that Dock2 deficiency led to decreased expression of chemokines CCL4 and CCL5 and their chemokine receptors C‐C motif chemokine receptor (CCR)4 and CCR5 on macrophages and reduced cell responsiveness to chemokine stimulation, affecting macrophage migration. Dock2 also mediates phagocytic and bactericidal functions of macrophages. Transfer of WT macrophages into Dock2−/− mice increased the host resistance to C. rodentium infection. This suggests that Dock2 may regulate the migration and phagocytosis and bactericidal function of macrophages, becoming a potential therapeutic target for the treatment of IBD.
Methods
Mice
Dock2−/− mice have been described previously [31], and C57BL/6 mice were used as WT control. Mice were reared in specific pathogen‐free (SPF) facilities at the Experimental Animal Center at Gannan Medical University, Ganzhou, Jiangxi, China. Animal experiments were carried out according to the standards of the Ethics Committee of Gannan Medical University.
The preparation of bone marrow‐derived macrophage (BMDM)
Mice femurs were separated and removed under sterile conditions. The bone marrow inside the femur was flushed repeatedly with a 1‐ml syringe and cells were washed three times with phosphate‐buffered saline (PBS) solution. The bone marrow was put into the cell culture medium containing Iscove’s modified Dulbecco’s medium (IMDM), 10% fetal bovine serum (gibco), 30% L929 cell culture supernatant, 1% penicillin–streptomycin (gibco) and 1% non‐essential amino acid (Solarbio, Beijing Solarbio Science and Technology Co. Ltd, Beijing, China) for 5 days. The morphological changes and growth of the cells were observed under light microscope.
Bacterial infection
C. rodentium (ATCC no. 51459) was grown in Luria–Bertani (LB) broth at 37℃ overnight in a shaker and subcultured the next day. Bacteria with optical density (OD)600 between 0·6 and 0·8 were used for infection. Mice were fasted for 4 h prior to infection with 1 × 1010 CFU C. rodentium per mouse by oral gavage. Fecal pellets were collected on days 4, 7 and 10 after infection. Mice were euthanized on days 4 and 10, and colon tissues were taken. Bacterial counts from homogenized feces were determined with serial dilution and incubated on MacConkey agar plates at 37°C for 24 h. For BMDM, cells were stimulated with 20 multiplicity of infection (MOI) of C. rodentium, 20 MOI of Salmonella typhmurium or 1 μg/ml lipopolysaccharide (LPS) for 0, 2, 4 or 8 h.
Histological analysis
The colon was fixed in 10% formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E), as described previously [32]. Anti‐mouse F4/80 rabbit antibody (ServiceBio, Wuhan, China; cat. no. GB11027, 1 : 500 dilution) was used for the immunohistochemistry analysis of tissue macrophages.
Reverse transcription–quantitative polymerase chain reaction (RT–qPCR)
RNA was extracted from intestinal tissue or BMDMs with Trizol and reverse‐transcribed into cDNA. RT–qPCR was performed on an QuantStudio 7 Flex real‐time PCR instrument with SYBR Green kit (ThermoFisher, Fremont, CA, USA) using corresponding primers (the sequences are shown in Supporting information, Table S1). β‐actin was selected as the internal reference and the relative expression of target genes in different groups was expressed as 2−ΔΔCT.
Enzyme‐linked immunosorbent assay (ELISA) analysis
Colon tissues or BMDM were homogenized in RIPA buffer supplemented with protease and phosphatase inhibitors (Roche, Basel, Switzerland). Protein levels of chemokines in colon homogenates and cell supernatant were determined by multiplex ELISA, according to the manufacturer’s instructions (Elabscience, Wuhan, China).
Flow cytometry analysis
WT and Dock2−/− mouse BMDM were infected with 20 MOI of C. rodentium. After 8 h, cells were stained by fluorescent labeled antibodies against CCR1, CCR2, CCR4 and CCR5 (BioLegend, San Diego, CA, USA) at 4℃ incubation for 30 min. Cell stainings were analyzed by flow cytometry (BD FACSCantoⅡ; BD Biosciences, San Jose, CA, USA).
Transwell assay
WT or Dock2−/− BMDM (2 × 105 cells) were added into the Transwell upper chamber (Costar, London, UK), and chemokines, including CCL2, CCL4, CCL5 and SDF‐1β (Peprotech, Rocky Hill, NJ, USA), were added into the lower chamber for overnight culture. The pore size was 8 μm. Then, cells were fixed with 4% paraformaldehyde for 30 min, stained with 0·1% crystal violet for 20 min and counted under the microscope.
Phagocytic and bactericidal analysis
WT and Dock2−/− BMDM were infected with 10 MOI C. rodentium or S. typhmurium, and 50 μg/ml gentamicin was added 1 h later to kill extracellular bacteria. At 2 (detection of phagocytic capacity) and 20 h (detection of bactericidal capacity) after infection, cells were lysed and cultured on agar plates after a series of dilutions to count the number of live intracellular bacteria to determine phagocytic and bactericidal activities.
Adoptive transfer of macrophages
The suicidal liposome technique has been used to deplete macrophages [33]. Macrophage depletion was performed using the tail injection of 100 μl of clodronate liposomes (CL)/macrophage scavenging agent (MSA) (Yeasen, Shanghai, China) on days 1 and 2 before BMDM injection; 1 × 106 WT and Dock2−/− BMDM were injected through the tail vein to Dock2−/− mice on days 0, 4 and 7. C. rodentium infection was performed at 12 h after macrophage transfer.
Statistical analysis
GraphPad Prism version 8.0 was used for data analysis. The data were expressed as mean ± standard error of the mean (s.e.m.). Analysis of variance (anova) with Sidak’s post‐hoc method was used for comparison among multiple groups, and the t‐test was used for comparison between two groups. *P < 0·05 was considered a significant difference.
Results
Dock2 regulates the expression of chemokines in macrophages after C. rodentium infection
Previous studies have shown that Dock2 regulates the migration of macrophages after C. rodentium infection in mice [24]. Chemokines such as MCP‐1/CCL2, CCL4, CCL5, CCL22 and stromal cell‐derived factor‐1β (SDF‐1β)/CXC chemokine receptor 12 (CXCR12) were involved in the migration of macrophages [34, 35]. To investigate whether Dock2 participates in migration of macrophages by regulating chemokine expression, WT and Dock2−/− BMDM were cultured. C. rodentium was used to stimulate macrophages and cells were collected after 0, 2, 4 and 8 h, respectively. The gene and protein expression of chemokines were detected by RT–qPCR and ELISA, respectively. The mRNA levels of chemokines, including CCL4, CCL5 and SDF‐1β in Dock2−/− BMDM were significantly lower than those in WT BMDM after 8 h of stimulation with C. rodentium (Fig. 1a–c). In contrast, the mRNA levels of CCL2 and CCL22 were not significantly different between WT and Dock2−/− BMDM (Fig. 1d,e). Similarly, the protein levels of these chemokines in WT and Dock2−/− BMDM after stimulation were consistent with their mRNA results (Fig. 1f–j). These results suggest that Dock2 may regulate the gene and protein expression of chemokines CCL4, CCL5, SDF‐1β in macrophages, affecting the migration of macrophages.
Fig. 1.
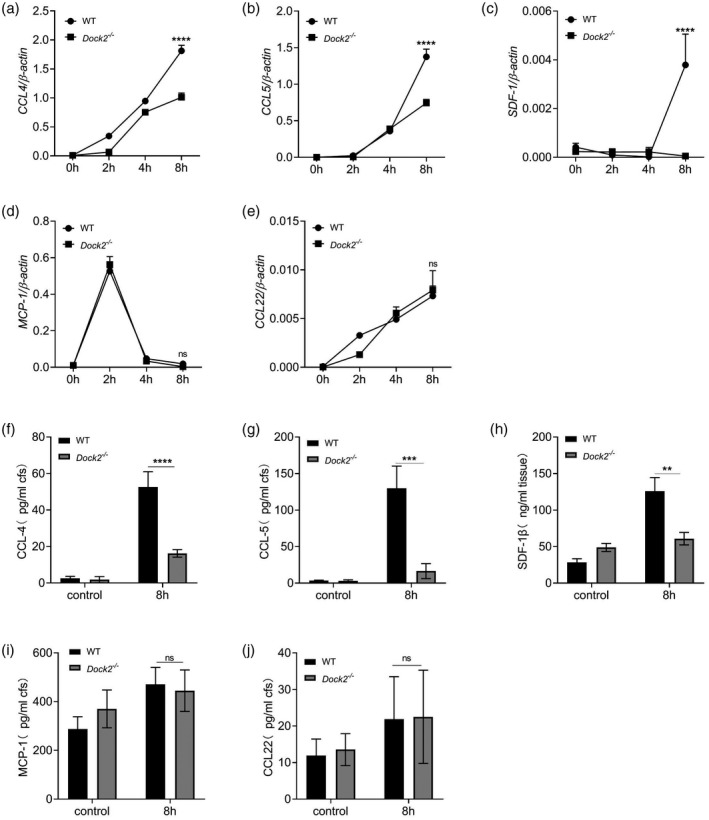
Dedicator of cytokinesis 2 (Dock2) regulates the expression of chemokines in macrophages after Citrobacter rodentium infection. (a–e) Reverse transcription–quantitative polymerase chain reaction (RT–qPCR) analysis of the gene expression of chemokine (C‐C motif) ligand (CCL)4, CCL5, stromal cell‐derived factor (SDF‐1β), monocyte chemoattractant protein (MCP)‐1 and CCL22 in wild‐type (WT) and Dock2−/− bone marrow‐derived macrophages (BMDM) that were either uninfected or infected with C. rodentium for 2, 4 and 8 h. (f–j) Enzyme‐linked immunosorbent assay (ELISA) analysis of the protein levels of CCL4, CCL5, SDF‐1β, MCP‐1 and CCL22 in the supernatants from WT and Dock2−/− BMDM that were either uninfected or infected with C. rodentium for 8 h. Data were representative of two independent experiments [mean ± standard error of the mean (s.e.m.)]. Data were analyzed by two‐way analysis of variance (anova) and two‐tailed t‐test at each time‐point. **P < 0·01; ***P < 0·001; ****P < 0·0001; n.s. = not statistically significant.
In order to test whether the secretion of chemokines CCL4, CCL5 and SDF‐1β regulated by Dock2 is limited to C. rodentium, we stimulated WT and Dock2−/− BMDM with LPS or 20 MOI of S. typhmurium and measured chemokines via ELISA methods in the cell supernatants at 0, 2, 4 and 8 h after stimulation. The results showed that the protein levels of CCL4 and CCL5 were not significantly different between WT and Dock2−/− BMDM after LPS or S. typhmurium infection, although SDF‐1β expression in Dock2−/− BMDM was lower than that in WT BMDM at 8 h after S. typhmurium infection (Supporting information, Fig. S1a–c). These results indicate that Dock2 plays a role in regulating CCL4 and CCL5 secretion during C. rodentium infection, but not LPS stimulation or S. typhmurium infection.
Dock2 regulates the expression of chemokine receptors in macrophages after C. rodentium infection
Chemokine function is induced by the binding of chemokines to their specific chemokine receptors. One chemokine can bind to many chemokine receptors, and multiple ligands may activate one chemokine receptor. Previous studies have shown that B‐type chemokines such as CCL2, CCl4 and CCL5 induce the aggregation and selective activation of macrophages in inflammatory sites [26, 36]. Therefore, we detected the expression of corresponding chemokine receptors such as CCR1 (ligands CCL4, CCL5), CCR2 (ligands CCL2), CCR4 (ligands CCL22, CCL5) and CCR5 (ligands CCL4, CCL5) [37, 38, 39], and explore whether Dock2 also affects the expression of chemokine receptors in macrophages. WT and Dock2−/− BMDM were collected at 0, 2, 4 and 8 h after C. rodentium infection and the expression of chemokine receptors was detected by RT–qPCR and flow cytometry. The mRNA levels of CCR1 and CCR2 were not significantly different between WT and Dock2−/− BMDM (Fig. 2a,b). In contrast, the mRNA levels of chemokine receptors, including CCR4 and CCR5 in Dock2−/− BMDM were significantly lower than those in WT BMDM after 8 h of stimulation with C. rodentium (Fig. 2c,d). Flow cytometry results also demonstrated that CCR4 and CCR5 protein levels of Dock2−/− BMDM significantly decreased after infection with C. rodentium for 8 h, while CCR1 and CCR2 were not statistically significantly different (Fig. 2e,f). The expression of CCR1, CCR2, CCR4 and CCR5 was similar between WT and Dock2−/− BMDM before C. rodentium infection (data not shown). These results suggest that Dock2 may regulate the gene and protein expression of chemokine receptors CCR4 and CCR5 in macrophages, together with chemokine, participating in the regulation of macrophage migration.
Fig. 2.
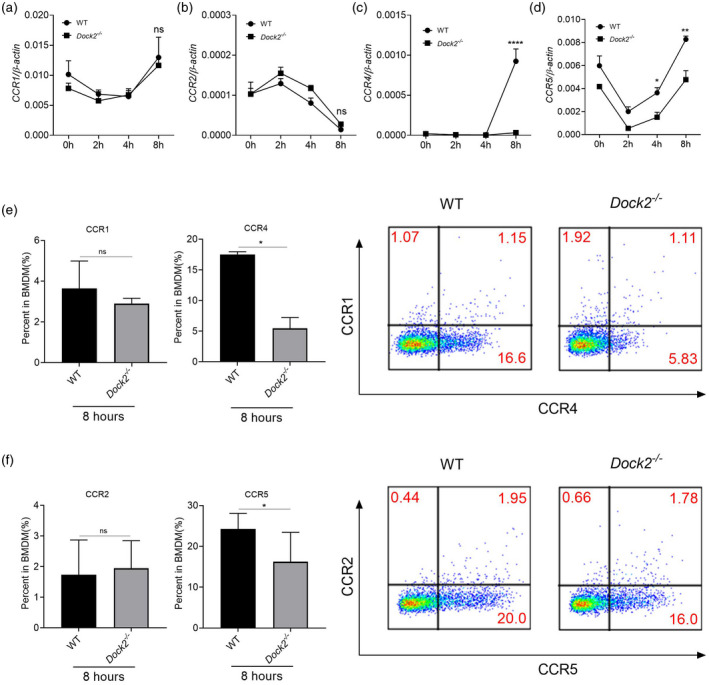
Dedicator of cytokinesis 2 (Dock2) regulates the expression of chemokine receptors in macrophages after Citrobacter rodentium infection. (a–d) Reverse transcription–quantitative polymerase chain reaction (RT–qPCR) analysis of the gene expression chemokine (C‐C motif) receptor (CCR)1, CCR2, CCR4 and CCR5 in wild‐type (WT) and Dock2−/− bone marrow‐derived macrophages (BMDM) that were either uninfected or infected with C. rodentium for 2, 4 and 8 h. (e–f) Flow cytometry analysis of the percentages of CCR1‐, CCR2‐, CCR4‐ and CCR5‐positive cells in WT and Dock2−/− BMDM that were either uninfected or infected with C. rodentium for 8 h. Data were representative of two independent experiments [mean ± standard error of the mean (s.e.m.)]. Data were analyzed by two‐way analysis of variance (anova) and two‐tailed t‐test at each time‐point. *P < 0·05; **P < 0·01; ****P < 0·0001; n.s. = not statistically significant.
Dock2 regulates the expression of colonic chemokines and their receptors after C. rodentium infection in mice
To investigate whether Dock2 regulates the expression of chemokines and their receptors in vivo, we detected the expression of chemokines and their receptors in colon tissue of mice after C. rodentium infection using RT–qPCR or ELISA. On day 4 after C. rodentium infection, there was no difference in mRNA or protein levels of CCL4, CCL5, SDF‐1β, MCP‐1 and CCL22 between WT and Dock2−/− mice (Fig. 3a–f, Supporting information, Fig. S2a,b). On day 10 after infection, the mRNA and protein levels of CCL4 and CCL5, but not SDF‐1β, MCP‐1 or CCL22, in the colon tissues of Dock2−/− mice, in comparison with WT mice, were significantly decreased (Fig. 3a–f, Supporting information, Fig. S2a,b). In addition, the mRNA levels of CCR1, CCR2, CCR4 and CCR5 were also not significantly different at day 4 after infection (Fig. 3g,h; Supporting information, Fig. S2c,d). The mRNA levels of chemokines CCL4 and CCL5 and corresponding chemokine receptors CCR4 and CCR5, but not CCR1 and CCR2, were also significantly decreased in Dock2−/− mice at day 10 after infection (Fig. 3g,h; Supporting information, Fig. S2c,d). These results suggest that Dock2 can participate in the expression of chemokines and their receptors in vivo, which may affect the migration of immune cells, especially macrophages.
Fig. 3.
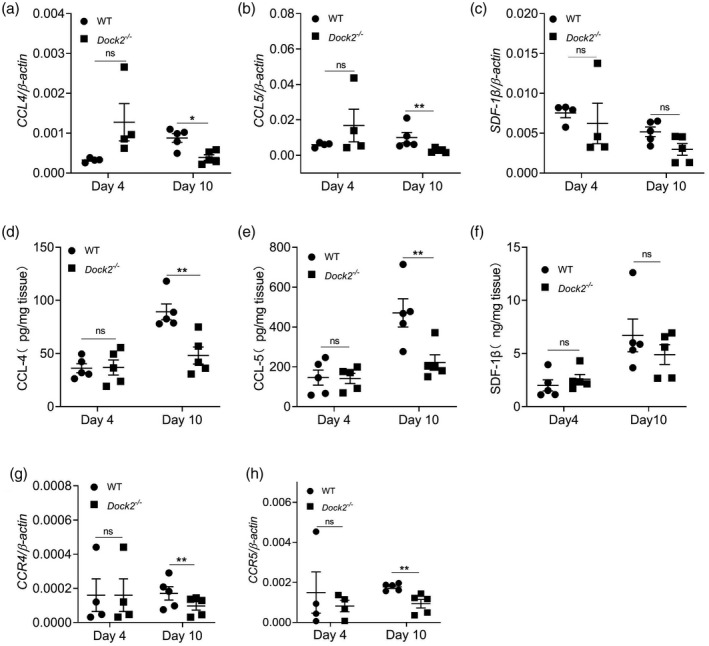
Dedicator of cytokinesis 2 (Dock2) regulates the expression of colonic chemokines and their receptors after Citrobacter rodentium infection in mice. The colonic levels of chemokine (C‐C motif) ligand (CCL)4, CCL5, stromal cell‐derived factor (SDF‐1β), (a–c) mRNAs, (d–f) proteins and chemokine (C‐C motif) receptor (CCL)4, CCR5 (g,h) mRNAs in uninfected WT and Dock2−/− mice which were either uninfected or infected with C. rodentium. Data wererepresentative of two independent experiments [mean ± standard error of the mean (s.e.m.)]. Data were analyzed by two‐tailed t‐test at each time‐point. *P < 0·05; **P < 0·01; n.s. = not statistically significant.
Dock2 affects the responsiveness of macrophages to chemokines
To investigate whether Dock2 directly regulates macrophage responsiveness to chemokines in addition to regulating the expression of chemokines and their receptors, we used the Transwell assay to test whether Dock2 directly affects chemokine‐mediated macrophage migration. We established CCL2, CCL4, CCL5 and SDF‐1β concentration gradients to induce the migration of WT and Dock2−/− BMDM. Compared to WT BMDM, the average cell mobility of Dock2−/− BMDM showed a significant reduction under stimulation at various concentrations of CCL4, but not CCL2, and the difference reached the maximum at a concentration of 200 ng/ml (Fig. 4a,b). Under stimulation of 75, 150 and 300 ng/ml CCL5, the average cell mobility of Dock2−/− BMDM was significantly reduced and the difference reached the maximum at a concentration of 75 ng/ml (Fig. 4c). Under stimulation of 25, 50 and 100 ng/ml SDF‐1β, the average cell migration of Dock2−/− BMDM was significantly reduced, and the difference reached the maximum at concentration of 100 ng/ml (Fig. 4d). These results suggest that Dock2 regulates macrophage migration by affecting responsiveness to CCL4, CCL5 and SDF‐1β stimulation.
Fig. 4.
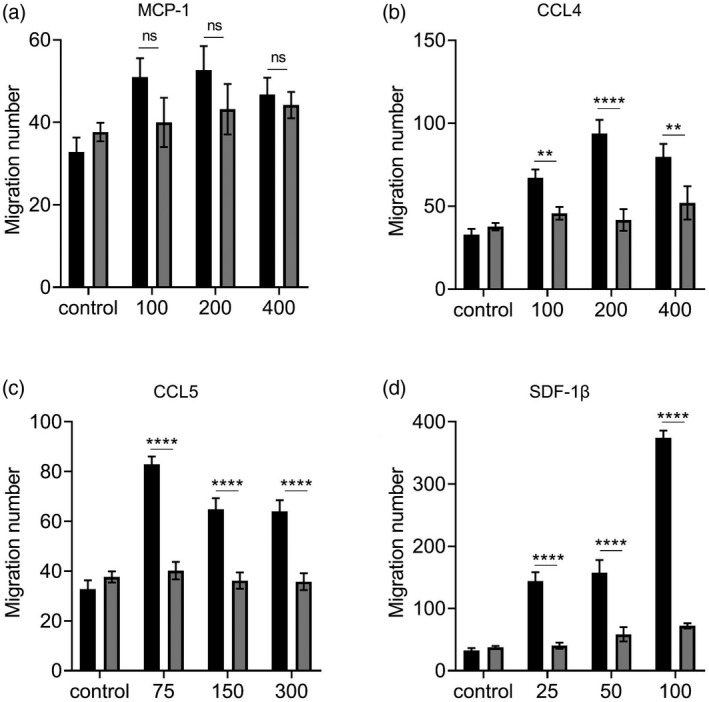
Dedicator of cytokinesis 2 (Dock2) affects the responsiveness of macrophages to chemokines. Wild‐type (WT) and Dock2−/− bone marrow‐derived macrophages (BMDM) (2 × 105) were activated with monocyte chemoattractant protein (MCP)‐1 (a), chemokine (C‐C motif) ligand (CCL)4, (b), CCL5 (c) and stromal cell‐derived factor (SDF‐1β) (d). The migration abilities of WT and Dock2−/− BMDM in response to MCP‐1, CCL4, CCL5 and SDF‐1β were countered in Transwell chemotaxis assays. The migrated cells were counted under microscope, and data were expressed as the numbers of migrated cells. Data were representative of two independent experiments [mean ± standard error of the mean (s.e.m.)]. Data were analyzed by two‐way analysis of variance (anova) at each dose‐point. **P < 0·01; ****P < 0·0001; n.s. = not statistically significant.
Effect of Dock2 on the phagocytic and bactericidal function of macrophages
Macrophages are the major type of phagocytes in the first‐line host defense against bacterial infection. The anti‐infection ability of macrophages is related not only to whether they migrate to the infection sites, but also to macrophage ability to engulf and kill bacteria. S. typhmurium is a good model for studying the intestinal epithelial barrier against bacterial pathogens [40]. Similar to C. rodentium infection,S. typhmurium infection can also induce colitis that mimics human ulcerative colitis [41, 42], therefore we used it as a control to show phagocytic defects. To further study the impact of Dock2 on the phagocytic and bactericidal function of macrophages, we co‐cultured the WT and Dock2−/− BMDM with C. rodentium or S. typhmurium for 2 or 20 h to test the phagocytic and the bactericidal ability, respectively. The results showed that, at 2 h after incubation, the ability of Dock2−/− BMDM to phagocytose C. rodentium and S. typhmurium was significantly lower than that of WT BMDM (Fig. 5a), and at 20 h after infection the bactericidal ability of Dock2−/− BMDM was significantly lower than that of WT BMDM (Fig. 5b), indicating that Dock2 could regulate the phagocytic and bactericidal function of macrophages.
Fig. 5.
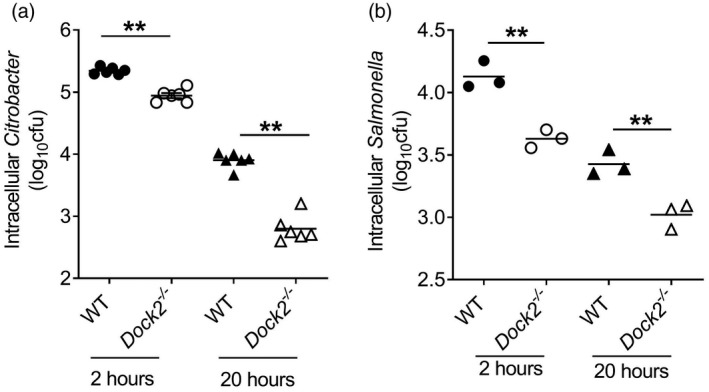
Effect of dedicator of cytokinesis 2 (Dock2) on the phagocytic and bactericidal function of macrophages. Bone marrow‐derived macrophages (BMDM) (106) were infected with 10 multiplicity of infection (MOI) Citrobacter rodentium or Salmonella typhmurium. Wild‐type (WT) and Dock2−/− BMDM were lysed at 2 and 20 h after infection, and the numbers of intracellular C. rodentium and S. typhmurium were determined using the colony‐forming unit (CFU) method (a,b). Data were representative of two independent experiments [mean ± standard error of the mean (s.e.m.)]. Data were analyzed by two‐tailed t‐test at each time‐point. **P < 0·01.
WT macrophages protect Dock2 −/− mice against C. rodentium‐induced colitis
Dock2 can regulate the migration and phagocytosis and bactericidal ability of macrophages. To further test whether macrophage function regulated by Dock2 could affect host defense against C. rodentium infection, we transferred WT or Dock2−/− macrophages into Dock2−/− mice after infection. First, we injected macrophage scavenging agent to deplete original macrophages in Dock2−/− mice. Immunohistochemistry analysis showed that the numbers of F4/80‐positive cells in the colon, spleen and liver were significantly reduced after the injection of macrophage scavenging agent (Supporting information, Fig. S3). Then, we infected macrophage‐depleted Dock2−/− mice with C. rodentium and injected WT or Dock2−/− BMDM via the tail vein on days 0, 4 and 7 after infection (Fig. 6a). Mice were euthanized on day 10 of infection, and colon length and C. rodentium load were analyzed. Dock2−/− mice receiving WT BMDM had significantly reduced C. rodentium load and less severe colon shortening in comparison with Dock2−/− mice receiving Dock2−/− BMDM (Fig. 6b,c), suggesting that the Dock2−/− mice receiving WT macrophages had considerably enhanced resistance against C. rodentium infection. Consistently, histological analysis of colon tissues showed that Dock2−/− mice receiving WT BMDM had significantly reduced tissue damage (Fig. 6d). Furthermore, the protein levels of CCL4 and CCL5 in colon tissues of Dock2−/− mice receiving WT BMDM were higher than controls (Fig. 6e). These results suggest that Dock2 plays a crucial protective role in the early stage of C. rodentium infection by regulating the functions of macrophages.
Fig. 6.

Wild‐type (WT) macrophages protect Dock2−/− mice against Citrobacter rodentium‐induced colitis. (a) Scheme of the macrophage adoptive transfer experiments. (b) C. rodentium colony‐forming unit (CFU) in fecal samples. (c) Colon lengths on day 10 after infection. (d) Hematoxylin and eosin (H&E) staining of colon tissues; scale bar = 50 μm. (e) The colonic protein levels of chemokine (C‐C motif) ligand (CCL)4 and CCL5; (b,c,e) n = 5 for WT and Dock2−/− mice. Data are representative of two independent experiments [mean ± standard error of the mean (s.e.m.)]. Data were analyzed by two‐tailed t‐test. *P < 0·05.
Discussion
We have previously demonstrated that Dock2−/− mice were susceptible to C. rodentium infection and that their macrophage migration from the colonic submucosa to the lamina propria was defective during C. rodentium infection [24]. Although Dock2 can regulate the migration and function of various types of immune cells by activating Rac, the role of Dock2 in macrophage migration and bactericidal function is not well characterized.
Our study shows that Dock2 plays an important role in chemokine‐induced macrophage migration. The mRNA and protein expression of CCL4 and CCL5, and their corresponding receptors CCR4 and CCR5 were significantly decreased in Dock2−/− macrophages in comparison with WT macrophages upon C. rodentium infection. Also, the protein expression of SDF‐1β were significantly decreased in Dock2−/− macrophages in comparison with WT macrophages upon S. typhmurium infection. Using Transwell experiment analysis, we found that Dock2−/− macrophages had defects in their responsiveness to the same concentration of chemokines as WT macrophages. In addition, Dock2 deficiency can also severely impair the phagocytic and bactericidal ability of macrophages. Macrophages play a variety of functions through phagocytosis, such as clearing invading pathogens, eliminating inflammation and maintaining tissue homeostasis. Therefore, Dock2 can enhance the host defense against infection by the phagocytic and bactericidal ability.
We have shown that Dock2 regulates macrophages’ phagocytosis, but it is not clear whether the chemokine secretion defects in Dock2−/− macrophages are due to the reduced antigen load in the cells. We stimulated macrophages with LPS and S. typhmurium and measured chemokine expression profiles.
The results showed that LPS and S. typhmurium stimulation did not induce the defect in the protein expression of chemokines CCL4 and CCL5 in Dock2−/− macrophages compared with WT macrophages. Therefore, it seems that chemokine secretion defect of Dock2−/− BMDM may not be due to LPS stimulation.
As shown in Fig. 5, Dock2−/− BMDM exhibited defective phagocytosis and thus had less antigen load in both C. rodentium and Salmonella infection. However, only C. rodentium, but not S. typhmurium infection, induce the defect in the protein expression of chemokine CCL4 and CCL5. Therefore, it seems that chemokines secretion defect of Dock2−/− BMDM may not be due to reduced antigen load in cells.
Finally, the in‐vivo macrophage depletion and adoptive transfer experiments demonstrated that Dock2 was directly involved in host defense against infection in vivo. These results suggest that Dock2 could regulate migration and bactericidal ability of macrophages, conferring host resistance to enteric bacterial infection, indicating that Dock2 may be a new strategic target for the treatment of IBD.
Studies have found that Dock2 affected lymphocyte migration in response to chemokines CCL21 (B cells), CXCL12 (T cells and B cells) and CXCL13 (T cells) in a dose‐dependent manner [23]. In the absence of Dock2, chemokine‐induced F‐actin polymerization failed to induce normal chemokine‐induced gradient migration in vitro [23]. Short‐term homing of T and B cells lacking Dock2 was severely impaired, despite similar surface expression levels of chemokine receptors [43, 44]. Also, under the induction of chemokine CXCL18, neutrophils activated Rac2 through PI3K and SRC‐Elmo‐Dock2 pathways to co‐regulate neutrophil chemotaxis [45]. In short, Dock2, as a downstream molecule of chemokine receptors, plays a vital role in inducing the migration of immune cells. Therefore, we explored which chemokine signalings regulated by Dock2 affect the migration and functions of macrophages.
Although our study showed that chemokine stimuli such as CCL4 and CCL5 in macrophages could induce the activation of Dock2 in vitro and in vivo, the specific signaling pathways are not well understood. A previous study found that the interaction of CCL4 and CCL5 with their receptors CCR1 and CCR5 led to GTPase activation of the Rho family and Rac activation, which was critical for CCR1‐ and CCR5‐triggered signaling cascade and further mediated chemokine‐induced actin cytoskeleton remodeling [26]. This process was also essential for effective recruitment and activation of macrophages in inflammatory sites. Another study found that Rac1 and p21‐activated kinase 2 (PAK2) were activated in a Gi‐ and PI3Kγ‐dependent manner through the binding of chemokine CCL5 to its receptors CCR1 and CCR5, ultimately controlling the chemotactic response of macrophages [25]. The evidence in this study showed the mechanisms how Dock2 regulated the migratory activity of macrophages.
Dock2−/− BMDM was defective in the expression of chemokine receptors such as CCR4 and CCR5 after C. rodentium infection. It would be interesting to know how the down‐regulation of these chemokine receptors contributes to the migration defect of Dock2−/− BMDM upon stimulation of CCL4 and CCL5. However, as these chemokine receptors are membrane‐bound molecules, it is difficult to manipulate the expression of these receptors using agonist or other small molecules.
The signaling pathways and functions induced by the binding of CCL5 to CCR4 and CCL4/5 to CCR5 could be different, which deserves further investigation. In addition, it is not clear whether CCL4 can partially compensate for CCL5 and whether CCR4 can compensate for CCR5. Furthermore, it is also needed to determine which pathway Dock2 could regulate the migration of colonic macrophages in mice during the early stage of C. rodentium infection.
Disclosures
We declare that the authors do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Author Contributions
Z. L. designed the study, generated the hypothesis, analyzed the data and revised the manuscript. L. J. performed the experiments, interpreted the results and wrote the manuscript. Y. C. and L. X. performed part of the experiments.
Supporting information
Fig. S1. Dock2 regulates the protein expression of chemokines in macrophages after LPS or S. typhmurine infection. ELISA analysis of the protein levels of CCL4, CCL5 and SDF‐1β (a‐c) in the supernatants from WT and Dock2−/− BMDM that were either not stimulated or stimulated with 1 ug/ml LPS, 20 MOI S. typhmurine, or C. rodentium for 2, 4, or 8 hours. Data were representative of two independent experiments (Mean ± SEM). Data were analyzed by two‐way ANOVA. *P < 0·05; ***P < 0·001; ****P < 0·0001; NS, not statistically significant.
Fig. S2. The colonic chemokine expression profile of WT and Dock2−/− mice after C. rodentium infection. The colonic expression levels of MCP‐1, CCL22 proteins (a‐b) and CCR4, CCR5(c‐d) mRNAs in WT and Dock2−/− mice which were either uninfected or infected with C. rodentium. Data were representative of two independent experiments (Mean ± SEM). Data were analyzed by Two‐tailed t‐test. NS, not statistically significant.
Fig. S3. The effect of macrophage depletion by macrophage scavenging agents (MSA). The mice were treated with PBS or MSA via intravenous injections, macrophages in colon, liver and spleen were stained with anti‐mouse F4/80 antibody using immunohistochemistry methods. N = 5, scale bar, 50 μm.
Table S1. Primer sequences of chemokine and chemokine receptors.
Acknowledgements
This work was supported by funds from the Natural Science Foundation of Jiangxi Province (20151BAB205061 and 20171ACB20024), the National Natural Science Foundation of China (31560260) and the Scientific and Technological Innovation Team Project of Gannan Medical University (TD201703) (to Z. L.).
Data Availability Statement
Data will be made available upon reasonable request.
References
- 1. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007; 448:427–34. [DOI] [PubMed] [Google Scholar]
- 2. Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med 2008; 263:597–606. [DOI] [PubMed] [Google Scholar]
- 3. Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: East meets West. J Gastroenterol Hepatol 2020; 35:380–9. [DOI] [PubMed] [Google Scholar]
- 4. Collins JW, Keeney KM, Crepin VF et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol 2014; 12:612–23. [DOI] [PubMed] [Google Scholar]
- 5. Deng W, Li Y, Vallance BA, Finlay BB. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect Immun 2001; 69:6323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol 2005; 7:1697–706. [DOI] [PubMed] [Google Scholar]
- 7. Nishikimi A, Kukimoto‐Niino M, Yokoyama S, Fukui Y. Immune regulatory functions of DOCK family proteins in health and disease. Exp Cell Res 2013; 319:2343–9. [DOI] [PubMed] [Google Scholar]
- 8. Miyamoto Y, Yamauchi J. Cellular signaling of Dock family proteins in neural function. Cell Signal 2010; 22:175–82. [DOI] [PubMed] [Google Scholar]
- 9. Meller N, Merlot S, Guda C. CZH proteins: a new family of Rho‐GEFs. J Cell Sci 2005; 118:4937–46. [DOI] [PubMed] [Google Scholar]
- 10. Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol 2002; 3:1150–5. [DOI] [PubMed] [Google Scholar]
- 11. Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science 1995; 268:863–5. [DOI] [PubMed] [Google Scholar]
- 12. Gadea G, Blangy A. Dock‐family exchange factors in cell migration and disease. Eur J Cell Biol 2014; 93:466–77. [DOI] [PubMed] [Google Scholar]
- 13. Ye Z, Liu Z, Henderson A et al. Increased CYP4B1 mRNA is associated with the inhibition of dextran sulfate sodium‐induced colitis by caffeic acid in mice. Exp Biol Med 2009; 234:605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008; 9:503–10. [DOI] [PubMed] [Google Scholar]
- 15. Janardhan A, Swigut T, Hill B, Myers MP, Skowronski J. HIV‐1 Nef binds the DOCK2‐ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis. PLOS Biol 2004; 2:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang H, Pan F, Erickson LM et al. Deletion of DOCK2, a regulator of the actin cytoskeleton in lymphocytes, suppresses cardiac allograft rejection. J Exp Med 2005; 202:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wen Y, Elliott MJ, Huang Y et al. DOCK2 is critical for CD8(+) TCR(–) graft facilitating cells to enhance engraftment of hematopoietic stem and progenitor cells. Stem Cells 2014; 32:2732–43. [DOI] [PubMed] [Google Scholar]
- 18. Nishikimi A, Fukuhara H, Su W et al. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science 2009; 324:384–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kunisaki Y, Nishikimi A, Tanaka Y et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol 2006; 174:647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J, Xu L, Shaheen S et al. Growth of B cell receptor microclusters is regulated by PIP(2) and PIP(3) equilibrium and Dock2 recruitment and activation. Cell Rep 2017; 21:2541–57. [DOI] [PubMed] [Google Scholar]
- 21. Das SR, Jameel S. Biology of the HIV Nef protein. Ind J Med Res 2005; 121:315–32. [PubMed] [Google Scholar]
- 22. Jing Y, Kang D, Liu L et al. Dedicator of cytokinesis protein 2 couples with lymphoid enhancer‐binding factor 1 to regulate expression of CD21 and B‐cell differentiation. J Allergy Clin Immunol 2019; 144:1377–90.e4. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y, Meng F, Wang B, He L, Liu Y, Liu Z. Dock2 in the development of inflammation and cancer. Eur J Immunol 2018; 48:915–22. [DOI] [PubMed] [Google Scholar]
- 24. Liu Z, Man SM, Zhu Q et al. DOCK2 confers immunity and intestinal colonization resistance to Citrobacter rodentium infection. Sci Rep 2016; 6:27814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weiss‐Haljiti C, Pasquali C, Ji H et al. Involvement of phosphoinositide 3‐kinase gamma, Rac, and PAK signaling in chemokine‐induced macrophage migration. J Biol Chem 2004; 279:43273–84. [DOI] [PubMed] [Google Scholar]
- 26. Di Marzio P, Dai WW, Franchin G, Chan AY, Symons M, Sherry B. Role of Rho family GTPases in CCR1‐ and CCR5‐induced actin reorganization in macrophages. Biochem Biophys Res Comm 2005; 331:909–16. [DOI] [PubMed] [Google Scholar]
- 27. MacDermott RP, Sanderson IR, Reinecker HC. The central role of chemokines (chemotactic cytokines) in the immunopathogenesis of ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis 1998; 4:54–67. [DOI] [PubMed] [Google Scholar]
- 28. Côté JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180‐related proteins with guanine nucleotide exchange activity. J Cell Sci 2002; 115:4901–13. [DOI] [PubMed] [Google Scholar]
- 29. Reif K, Cyster J. The CDM protein DOCK2 in lymphocyte migration. Trends Cell Biol 2002; 12:368–73. [DOI] [PubMed] [Google Scholar]
- 30. Brugnera E, Haney L, Grimsley C et al. Unconventional Rac‐GEF activity is mediated through the Dock180–ELMO complex. Nat Cell Biol 2002; 4:574–82. [DOI] [PubMed] [Google Scholar]
- 31. Fukui Y, Hashimoto O, Sanui T et al. Haematopoietic cell‐specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 2001; 412:826–31. [DOI] [PubMed] [Google Scholar]
- 32. Man SM, Zhu Q, Zhu L et al. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell 2015; 162:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. You Y, Zhou C, Li D et al. Sorting nexin 10 acting as a novel regulator of macrophage polarization mediates inflammatory response in experimental mouse colitis. Sci Rep 2016; 6:20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamon M, Mbemba E, Charnaux N et al. A syndecan‐4/CXCR4 complex expressed on human primary lymphocytes and macrophages and HeLa cell line binds the CXC chemokine stromal cell‐derived factor‐1 (SDF‐1). Glycobiology 2004; 14:311–23. [DOI] [PubMed] [Google Scholar]
- 35. Kanda H, Tateya S, Tamori Y et al. MCP‐1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116:1494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hori M, Nobe H, Horiguchi K, Ozaki H. MCP‐1 targeting inhibits muscularis macrophage recruitment and intestinal smooth muscle dysfunction in colonic inflammation. Am J Physiol Cell Physiol 2008; 294:C391–401. [DOI] [PubMed] [Google Scholar]
- 37. Zaitseva M, Blauvelt A, Lee S et al. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med 1997; 3:1369–75. [DOI] [PubMed] [Google Scholar]
- 38. Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein‐1. Cytokine Growth Factor Rev 2002; 13:455–81. [DOI] [PubMed] [Google Scholar]
- 39. Izumi K, Mizokami A. Suppressive role of androgen/androgen receptor signaling via chemokines on prostate cancer cells. J Clin Med 2019; 8:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nilsson OR, Kari L, Steele‐Mortimer O. Foodborne infection of mice with Salmonella typhimurium . PLOS ONE 2019; 14:e0215190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deriu E, Liu JZ, Pezeshki M et al. Probiotic bacteria reduce Salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe 2013; 14:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harris JC, Dupont HL, Hornick RB. Fecal leukocytes in diarrheal illness. Ann Intern Med 1972; 76:697–703. [DOI] [PubMed] [Google Scholar]
- 43. Nombela‐Arrieta C, Lacalle RA, Montoya MC et al. Differential requirements for DOCK2 and phosphoinositide‐3‐kinase gamma during T and B lymphocyte homing. Immunity 2004; 21:429–41. [DOI] [PubMed] [Google Scholar]
- 44. Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG. Cutting edge: differential roles for phosphoinositide 3‐kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J Immunol 2004; 173:2236–40. [DOI] [PubMed] [Google Scholar]
- 45. Sai J, Raman D, Liu Y, Wikswo J, Richmond A. Parallel phosphatidylinositol 3‐kinase (PI3K)‐dependent and Src‐dependent pathways lead to CXCL8‐mediated Rac2 activation and chemotaxis. J Biol Chem 2008; 283:26538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Dock2 regulates the protein expression of chemokines in macrophages after LPS or S. typhmurine infection. ELISA analysis of the protein levels of CCL4, CCL5 and SDF‐1β (a‐c) in the supernatants from WT and Dock2−/− BMDM that were either not stimulated or stimulated with 1 ug/ml LPS, 20 MOI S. typhmurine, or C. rodentium for 2, 4, or 8 hours. Data were representative of two independent experiments (Mean ± SEM). Data were analyzed by two‐way ANOVA. *P < 0·05; ***P < 0·001; ****P < 0·0001; NS, not statistically significant.
Fig. S2. The colonic chemokine expression profile of WT and Dock2−/− mice after C. rodentium infection. The colonic expression levels of MCP‐1, CCL22 proteins (a‐b) and CCR4, CCR5(c‐d) mRNAs in WT and Dock2−/− mice which were either uninfected or infected with C. rodentium. Data were representative of two independent experiments (Mean ± SEM). Data were analyzed by Two‐tailed t‐test. NS, not statistically significant.
Fig. S3. The effect of macrophage depletion by macrophage scavenging agents (MSA). The mice were treated with PBS or MSA via intravenous injections, macrophages in colon, liver and spleen were stained with anti‐mouse F4/80 antibody using immunohistochemistry methods. N = 5, scale bar, 50 μm.
Table S1. Primer sequences of chemokine and chemokine receptors.
Data Availability Statement
Data will be made available upon reasonable request.


