Summary
Autoantibodies related to rheumatoid arthritis (RA), such as anti‐citrullinated protein antibodies (ACPA), are often detectable in the preclinical period years before arthritis onset. However, events triggering arthritis development remain incompletely known. We aimed to determine whether ACPA isotype levels are prognostic for arthritis development in patients presenting with immunoglobulin (Ig)G ACPA and musculoskeletal pain. Study participants (n = 82) had musculoskeletal pain of any sort and duration and a positive IgG ACPA test. None of the patients had arthritis upon clinical examination at baseline, but during follow‐up (mean = 6 years), 48% developed at least one arthritic joint. IgG, IgA, IgM and secretory component (SC)‐containing ACPA was measured in longitudinally collected serum samples. Cox regression analysis was performed to test the prognostic value of baseline antibody levels and changes over time. All analysed ACPA isotype levels were associated with arthritis development in univariable Cox regression analysis. In multivariable analysis, baseline SC ACPA levels were independently prognostic for arthritis development in multivariable analysis [hazard ratio (HR) = 1·006, 95% confidence interval (CI) = 1·001–1·010, P = 0·012]. There were no significant changes in ACPA isotype levels over time, and no significant association between changes over time and arthritis development. In this prospective longitudinal study, baseline serum SC ACPA levels, but neither IgG, IgA nor IgM ACPA are prognostic for future arthritis development. Repeated measurement of ACPA isotypes do not bring additional prognostic value. The results reinforce a mucosal connection in RA development and encourage further exploration of the mechanisms underlying secretory ACPA formation as a trigger for arthritis development.
Keywords: anti‐citrullinated protein antibodies, at‐risk patients, mucosa, rheumatoid arthritis, secretory component
Smoking habits in relation to different anti‐citrullinated protein antibody (ACPA) isotypes (IgG, IgA, IgM and SC) in TIRx patients. *p‐value <0.05.

Introduction
Rheumatoid arthritis (RA) is believed to develop gradually, including a preclinical period where circulating autoantibodies, such as anti‐citrullinated protein antibodies (ACPA) and rheumatoid factor (RF), occur [1, 2] while synovial inflammation is absent [3].
In retrospective studies, approximately 40% of RA patients tested positive for circulating immunoglobulin (Ig)G ACPA before symptom onset, with increasing levels and percentage positive closer to diagnosis [4, 5]. This translates into an absolute risk of RA between 5 and 16% among asymptomatic individuals with IgG ACPA positivity, but the risk increases if joint symptoms co‐occur. Prospective observational studies reported progression to arthritis in 20–50% within a few years of IgG ACPA‐positive individuals with musculoskeletal symptoms [6, 7, 8, 9, 10, 11].
The mechanisms by which ACPA production is induced remain enigmatic, and there is still poor understanding of the early events triggering arthritis years after appearance of ACPA in the circulation. Emerging evidence suggests an important mucosal contribution in both ACPA formation and arthritis development in ACPA‐positive individuals [12]. Secretory IgA (SIgA) is the dominating antibody at mucosal linings, where it is formed upon attachment of a secretory component (SC) to dimeric IgA [13]. We previously reported secretory component (SC)‐ containing ACPAs in the circulation in recent‐onset RA patients in association with increased inflammatory markers and radiographic lung abnormalities [14, 15].
There is an apparent clinical need to improve the prediction of arthritis development among ACPA‐positive patients with arthralgia. Results from previous retrospective studies of asymptomatic individuals imply that ACPA dynamics in the circulation are relevant to monitor to improve the prognostic accuracy in this group of patients [4, 5, 16]. However, a recent prospective study on symptomatic patients could not establish an added value of assessing changes in serum IgG ACPA serum levels over time [17]. Baseline autoantibody analyses have suggested a ‘dose–response’ relationship between autoantibody load and risk of arthritis development in symptomatic at‐risk patients [10, 18] but, to our knowledge, repeated isotype‐specific ACPA analyses have not been addressed previously. SC ACPA and IgA ACPA are of particular relevance in this context, given the increasing interest in mucosal processes as triggers of ACPA‐positive RA [19]. Hence, we aimed to investigate whether levels of different ACPA isotypes, or changes therein, are prognostic for arthritis development in IgG ACPA‐positive patients with musculoskeletal symptoms.
Materials and methods
Study population
We studied 82 IgG ACPA‐positive patients without baseline clinical arthritis included in a prospective observational cohort study denoted TIRx (Swedish for ‘extra‐early rheumatology follow‐up’) at the University Hospital in Linköping, Sweden [10]. Patients referred from primary care centers between 2010 and 2013 were enrolled upon fulfilment of the inclusion criteria musculoskeletal pain and positive IgG anti‐cyclic citrullinated peptide (anti‐CCP2) serum test in clinical routine. Patients with previous inflammatory rheumatic disease or corticosteroid treatment (oral or intra‐articular) within 6 weeks prior to screening were excluded. The ethical review board in Linköping, Sweden, approved the study protocol (decision numbers M220‐09 and 2017/260‐32) and all participants signed a written informed consent.
Regular follow‐up visits, including serum sampling, were carried out during follow‐up [10], and in cases of aggravated symptoms patients were offered extra clinical examination and serum sampling. Arthritis development was defined by the findings upon clinical examination by an experienced rheumatologist. Follow‐up time was a median of 6 years [interquartile range (IQR) = 40·8–6·8].
As controls for ACPA analyses, we recruited 100 blood donors (mean age = 52 years, 50% female).
Antibody
Measurements of IgG and IgA ACPA were performed by a fluoroenzyme immunoassay with cyclic citrullinated peptide (CCP)2 as antigen on a PhaDia 250 instrument (EliA; ThermoFisher AB, Uppsala, Sweden). In the IgA ACPA assay, a mouse anti‐human IgA antibody detecting both subclasses was used as secondary antibody (ThermoFisher AB). The serum IgG‐class anti‐CCP (anti‐CCP) enzyme‐linked immunoassay (ELISA) tests (CCPlus® Immunoscan; Svar Life Science, Malmö, Sweden) were modified to analyse IgM ACPA [20] and SC ACPA [14], respectively. SC ACPA were analysed by diluting serum samples 1 : 25 in kit buffer and the secondary antibody detecting human secretory component was diluted 1 : 2000 (polyclonal goat antibody conjugated to horseradish peroxidase, GAHu/SC/PO; Nordic Biosite, Täby, Sweden). Incubation and washing were performed according to the manufacturer’s instructions. A standard curve was calculated by diluting a known serum sample with high level of SC ACPA in a series from 1 : 12·5 to 1 : 800 and then used to recalculate optical densities into arbitrary units. Interassay coefficient of variation (CV) was 9% and intra‐assay CV was 2% in the SC ACPA assay. The intra‐ and interassay variations in the IgM ACPA ELISA were 2 and 17%, respectively. In the IgM and SC ACPA assays, all samples were analysed in duplicate and reanalysed whenever the coefficient of variation between duplicates was > 20%.
ACPA isotype levels were above the upper limit of detection in 35 samples (27 for IgG ACPA, five for IgA ACPA and three for IgM ACPA), and thus further diluted 1/10 (IgG and IgA) or 1/50 (IgM) and reanalysed. Cut‐off levels were set by an accredited clinical immunology laboratory in Linköping to ≥ 7 U/ml for IgG. The cut‐off for IgA ACPA was set to > 12 U/ml (corresponding to the 99th percentile among controls). Cut‐off levels for IgM ACPA and SC ACPA were set according to the 99th percentile among 100 controls (322 and 124 AU/ml, respectively).
For investigation into ACPA isotype level changes, we used serum samples taken at inclusion and at the visit where arthritis development was confirmed. In patients who did not develop arthritis during follow‐up, we analysed serum from inclusion and the 12‐month sample. Sera were available from 31 patients from inclusion and the visit where progression to arthritis was confirmed. Baseline and 12‐month sera were available from 40 patients who did not progress to arthritis during follow‐up.
RF was analysed using nephelometry at the accredited Clinical Chemistry Laboratory at Linköping University Hospital.
Statistical analysis
Statistical analysis was performed by SPSS version 26 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism version 8 for Windows (GraphPad Software, San Diego, CA, USA). Two‐sided P‐values < 0·05 were considered statistically significant and differences between two groups were tested by Fisher’s exact test. The Mann–Whitney U‐test was used to analyse symptom duration, compare the ACPA status of different isotypes and risk factors and compare net changes (follow‐up levels subtracted from baseline levels), and relative (follow‐up levels divided by baseline levels) ACPA level change in the group progressing to clinical arthritis versus non‐progressors. Wilcoxon’s signed‐rank test was used to test differences in ACPA levels between baseline and follow‐up (i.e. when arthritis was confirmed, or after 12 months for patients not progressing). Pearson’s χ2 test was used to test the distribution of the number of ACPA isotypes when comparing more than two groups. Spearman’s signed‐rank test was used to test correlation between antibody levels. Cox regression was used to test different ACPA levels, isotype usage, risk factors and clinical variables versus progression to arthritis. Statistically significant variables in univariable Cox regression analyses were included in a multivariable Cox regression analysis.
Ethics approval
The ethical review board in Linköping, Sweden, approved the study protocol and all participants signed a written informed consent (decision numbers M220‐09 and 2017/260‐32).
Results
Baseline characteristics of the TIRx cohort are detailed in Table 1. During follow‐up, 39 patients (48%) developed clinical arthritis after a median of 6 months (IQR = 3–24 months).
Table 1.
Baseline characteristics of the TIRx cohort
| All patients (n = 82) | Developing arthritis (n = 39) | No arthritis during follow‐up (n = 43) | P‐value c | |
|---|---|---|---|---|
| Demographics | ||||
| Women, n (%) | 66 (81) | 32 (82) | 34 (79) | 0·786 |
| Age, mean (range) | 51·8 (18–76) | 55·0 (25–76) | 48·9 (18–75) | 0·089 |
| Time to arthritis, median (IQR) | 6 (3 ‐ 24) | |||
| Follow‐up time, median (IQR) | 69 (57 ‐ 77) | |||
| Symptom duration | ||||
| 0–6 months n (%) | 15 (18) | 8 (21) | 7 (16) | 0·514 |
| 6–18 months n (%) | 37 (45) | 15 (38) | 22 (51) | |
| 18+ months n (%) | 30 (37) | 16 (41) | 14 (33) | |
| Risk factors | ||||
| Ever smoker, n (%) | 39 (48) | 19 (49) | 20 (47) | 1 |
| Never smoker, n (%) | 43 (52) | 20 (51) | 23 (53) | |
| Shared epitope carrier, n (%) | 52 (64) a | 24 (62) | 28 (67) b | 0·82 |
| Antibodies | ||||
| RF positive, n (%) | 24 (30) | 16 (41) | 8 (19) | 0·031 |
| IgA ACPA‐positive, n (%) | 19 (23) | 10 (26) | 9 (21) | 0·794 |
| IgM ACPA‐positive, n (%) | 12 (15) | 9 (23) | 3 (7) | 0·06 |
| SC ACPA‐positive, n (%) | 17 (21) | 12 (31) | 5 (12) | 0·055 |
| IgG ACPA level (U/ml, mean ± s.d.) | 229 ± 489 | 340 ± 586 | 128 ± 359 | 0·213 |
| IgA ACPA level (U/ml, mean ± s.d.) | 10 ± 17 | 24 ± 65 | 7 ± 9 | 0·584 |
| IgM ACPA level AU/ml, mean ± s.d.) | 6 ± 16 | 10 ± 23 | 3 ± 2 | 0·003 |
| SC ACPA level (AU/ml, mean ± s.d.) | 89 ± 118 | 130 ± 156 | 52 ± 45 | 0·082 |
| Isotype usage | ||||
| One, n (%) | 56 (68) | 26 (67) | 30 (70) | 0·014 |
| Two, n (%) | 12 (15) | 2 (5) | 10 (23) | |
| Three, n (%) | 6 (7) | 4 (10) | 2 (5) | |
| Four, n (%) | 8 (10) | 7 (18) | 1 (2) |
Demographics analysed using Fisher’s exact test, symptom duration using Pearson’s χ2 test, risk factors using Pearson’s χ2 or Fisher’s exact tests, antibodies using Fisher’s exact test or Mann–Whitney U‐test and isotype usage using Pearson’s χ2 test.
IQR= interquartile range, RF= rheumatoid factor, ACPA= anti‐citrullinated protein antibodies, SC= secretory component‐containing; s.d. = standard deviation; Ig = immunoglobulin; TIRx = Swedish acronym for ‘extra‐early rheumatology follow‐up’.
Data from 81 patients;
data from 42 patients;
compared between the groups developing arthritis and no arthritis during follow‐up.
Occurrence of ACPA isotypes and changes in levels
Baseline status and levels of ACPA isotypes are shown in Table 1. Baseline levels of IgM ACPA were significantly (P = 0·003) higher among patients subsequently developing arthritis than among those who did not, while SC ACPA showed borderline (P = 0·082) statistical significance (Table 1). Baseline levels of SC ACPA correlated moderately with IgA ACPA levels (Supporting information, Fig. S1). Regarding proportion positive tests, only SC ACPA showed borderline significance (P = 0·055, Table 1).
Fig. 1.
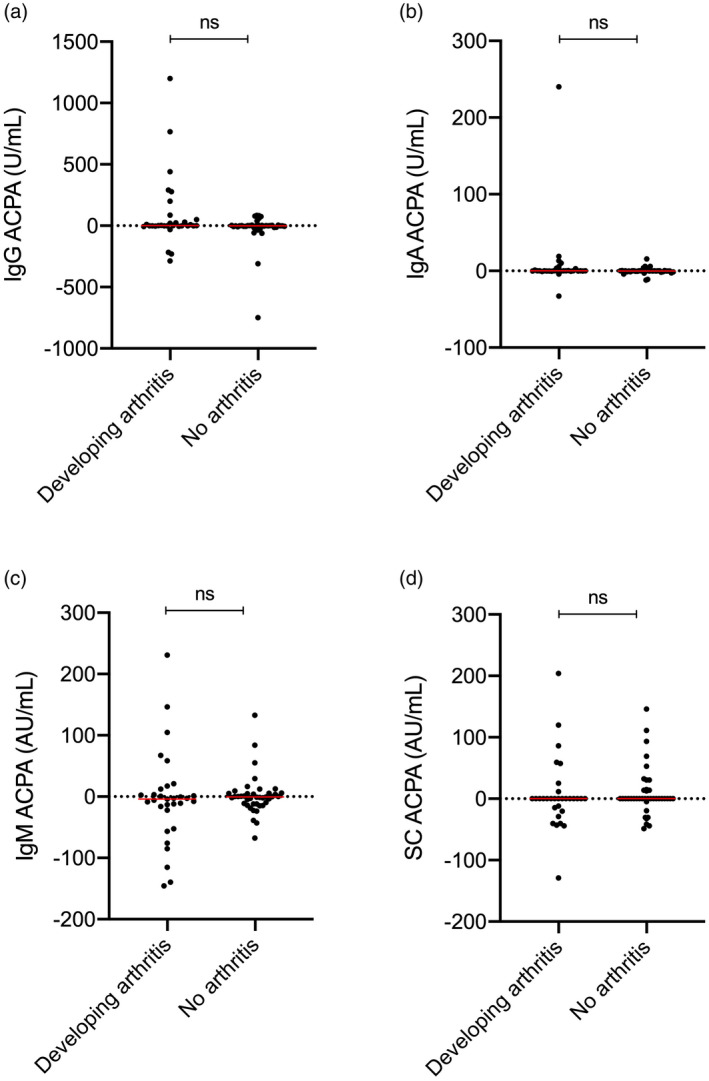
Net change (follow‐up levels subtracted from baseline levels) in levels of anti‐citrullinated protein antibodies (ACPAs) of immunoglobulin (Ig)G, IgA, IgM and secretory component‐containing (SC) isotype in patients developing (n = 31) and not developing arthritis (n = 40) during follow‐up; n.s. = not significant; dotted line represents no change and horizontal red lines = median.
Change in ACPA status was uncommon during follow‐up (at 12 months or at time of arthritis): four of the IgG‐positive patients (two of whom changed from positive to negative), two for IgA, five for IgM (two became negative) and four concerning SC ACPA (one became negative). Conversion to/from positive or negative was not associated with progression to arthritis (data not shown).
At the visit when clinical arthritis was confirmed, ACPA isotype levels were not significantly altered compared to baseline (Supporting information, Fig. S2). This was also the case among patients who did not develop arthritis during follow‐up, where baseline levels were compared to the 12‐month visit (Supporting information, Fig. S3). Furthermore, there were no significant differences in relative changes of ACPA isotypes during follow‐up (Supporting information, Table S1).
Fig. 2.
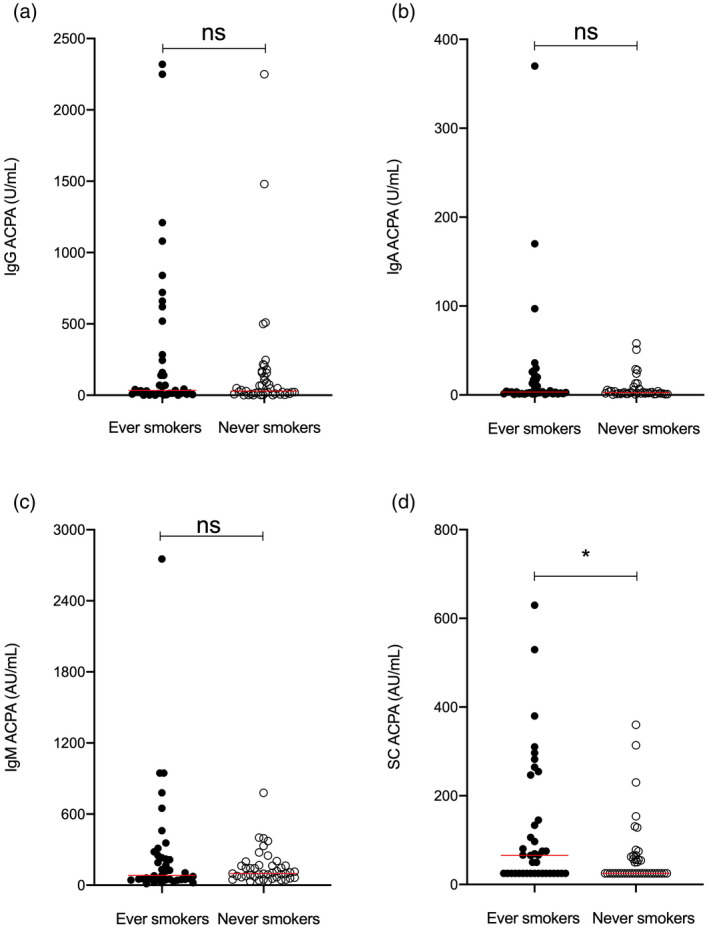
Smoking habits in relation to different anti‐citrullinated protein antibody (ACPA) isotypes [immunoglobulin (Ig)G, IgA, IgM and secretory component‐containing (SC)] in early rheumatology follow‐up [Swedish acronym for ‘extra‐early rheumatology follow‐up’ (TIRx)] patients. *P‐value < 0·05, horizontal red lines = median.
ACPA isotypes in association to smoking habits and shared epitope
Smoking habits and carriage of the human leukocyte antigen (HLA)‐DRB1 shared epitope (SE) are detailed in Table 1. Levels of baseline SC ACPA were significantly higher among ever smokers compared to never smokers (median = 66 versus 25 AU/ml, P = 0·035), while none of the other ACPA isotypes differed significantly according to smoking status (Fig. 2). The carriage of SE was not associated with altered baseline levels of any ACPA tested (Supporting information, Table S2).
Table 2.
Cox regression analyses regarding baseline factors versus arthritis development in patients at increased risk (n = 82)
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age | 1·021 (0·998–1·046) | 0·077 | ||
| Women | 1·109 (0·489–2·514) | 0·804 | ||
| Ever smoking | 1·008 (0·537–1·891) | 0·980 | ||
| Shared epitope‐positive | 0·880 (1·462–1·678) | 0·699 | ||
| RF level | 1·005 (1·003–1·007) | <0·001 | 1·000 (0·995–1·005) | 0·966 |
| IgG ACPA level (U/ml) | 1·001 (1·000–1·001) | 0·009 | 0·999 (0·998–1·001) | 0·371 |
| IgA ACPA level (U/ml) | 1·006 (1·001–1·011) | 0·011 | 0·986 (0·967–1·005) | 0·153 |
| IgM ACPA level (AU/ml) | 1·000 (1·000–1·000) | 0·001 | 1·000 (1·000–1·000) | 0·168 |
| SC ACPA level (AU/ml) | 1·005 (1·003–1·007) | <0·001 | 1·006 (1·002–1·011) | 0·008 |
| DAS28 | 1·366 (1·018–1·834)a | 0·038 | 1·225 (0·861–1·744) | 0·259 |
| CRP (mg/l) | 1·056 (1·020–1·093) | 0·002 | 1·028 (0·937–1·128) | 0·553 |
| ESR (mm/1st hour) | 1·035 (1·005–1·068)b | 0·032 | ||
P‐value < 0.05 are marked bold in the table.
HR= hazard ratio, RF= rheumatoid factor, ACP = anti‐citrullinated protein antibodies, SC = secretory component containing, DAS28 = disease activity score, CRP = C‐reactive protein, ESR = erythrocyte sedimentation rate; CI = confidence interval; Ig = immunoglobulin. Values are from baseline (a n = 77 and b n = 81). ESR are not included in multivariable analysis as being a part of disease activity score 28 (DAS28).
Antibody levels as prognostic factors for arthritis development
In univariable Cox regression analyses, baseline levels of all tested autoantibodies were associated with progression to arthritis (Table 2). As previously reported [10], baseline disease activity score 28 (DAS28), C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were also significantly prognostic for arthritis in univariable analyses, while smoking and SE were not (Table 2).
In a multivariable Cox regression analysis including variables with a P‐value < 0·05 in univariable analysis, baseline SC ACPA levels remained independently associated with progression to arthritis [hazard ratio (HR) = 1·006, 95% confidence interval (CI) = 1·002–1·011, P = 0·008]. Changes in ACPA isotype levels were not significantly different between patients who developed arthritis compared to those who did not (Fig. 1), and were not prognostic for arthritis development in Cox regression analyses (P > 0·3 for all).
In a separate Cox regression analysis evaluating isotype usage, the HR for progressing to arthritis was 1·44 per additional ACPA isotype present (95% CI = 1·07–1·94, P = 0·017).
Discussion
This is the first prospective study, to our knowledge, to address isotype‐specific ACPA responses over time in patients at increased risk of RA. We find that baseline levels of circulating SC ACPA are prognostic for arthritis development when also considering the autoantibodies in current clinical use; that is, IgG ACPA and RF. This suggests that mucosal immunization to citrullinated proteins is important for progression into clinical arthritis among patients with an already established systemic autoantibody response. In a recent study on symptom‐free first‐degree‐relatives to RA patients, we showed that SC ACPA was rare while other ACPA isotypes occurred frequently [21]. Seen together with the current findings, it could be speculated that SC ACPA formation is more related to the triggering of arthritis than to the development of a systemic ACPA response. Surprisingly, we found no significant increase in SC ACPA levels as arthritis approached. Although the patient cohort was followed long‐term, most patients developing arthritis did so within a short time‐frame from baseline (median = 6 months), and therefore the most pronounced SC ACPA increase may already have occurred prior to inclusion in the study.
The origin of circulating SC ACPA remains to be unraveled. Mucosal surfaces of the lungs could be the induction site, as we found a clear association between smoking and SC ACPA in this at‐risk population, which aligns with previous reports on early RA [14, 15]. Furthermore, SC ACPA is found in bronchoalveolar lavage fluid and serum levels associate with radiographic lung abnormalities [15]. However, other mucosal compartments may also be involved, and the relative importance of each compartment could differ between patients. For instance, it was recently shown that intestinal permeability increases before arthritis onset in both mice and humans, and reversal of this process prevented arthritis development [22]. Such increased leakage of the gut could hypothetically increase the exposure of citrullinated proteins to the local immune system and promote SC ACPA formation [23]. The oral cavity may also be of importance, as circulating SC ACPA correlates with salivary IgA ACPA and is associated with RA disease activity [24]. Thus, a limitation of the current study is that specific mucosal compartments were not addressed. Also, the selection of IgG ACPA‐positive patients, which was based on clinical practice in Sweden, may have influenced the prognostic performance of other ACPA isotypes. However, we previously showed that IgM, IgA and SC ACPA rarely occur among IgG ACPA‐negative subjects [14, 21, 25], which reduces the risk of such bias.
Previous studies have highlighted that the IgG ACPA repertoire expands prior to arthritis onset [4, 5, 16, 26] although, in symptomatic individuals, that an increasing number of ACPA reactivities predicts arthritis development remains to be shown. In the present study, we similarly found that ACPA isotype usage at baseline is prognostic for progression into arthritis, but as relatively few patients seroconverted we are unable to establish that increasing isotype usage predicts arthritis development. To this end, a larger patient cohort would be required.
Several studies, including previous data from the current cohort, have highlighted the prognostic importance of IgG ACPA levels in at‐risk patients [7, 10, 18]. Therefore, it is intriguing that the prognostic value of baseline IgG ACPA levels were no longer evident when SC ACPA was added to the multivariable model. Also, the lack of serum level increase for any of the investigated isotypes during arthritis development is surprising from a mechanistic viewpoint, but is still in agreement with a previous study concerning IgG ACPA [17].
In summary, this prospective longitudinal study on ACPA isotypes shows that baseline SC ACPA serum levels are prognostic for arthritis development, but that repeated measurements do not bring additional prognostic value in IgG ACPA‐positive symptomatic at‐risk patients. The results reinforce a mucosal connection in RA development and encourage further exploration of the mechanisms underlying secretory ACPA formation in relation to arthritis onset.
Disclosures
The authors declare no conflicts of interest.
Author contributions
K. R. L. drafted the manuscript and all authors contributed with the writing and critical revising of the manuscript. K. M. performed the IgM and SC ACPA analyses. A. K. conceived the study and was responsible for patient recruitment and characterization. All authors contributed to statistical analysis of the data. All authors approved the final version of the manuscript.
Supporting information
Fig. S1. Correlation of baseline IgA ACPA and SC ACPA levels in at‐risk patients (n = 82).
Fig. S2. Levels of anti‐citrullinated protein antibodies (ACPAs) of IgG, IgA, IgM and SC isotype at baseline and arthritis debut (n = 31). ns = not significant.
Fig. S3. Levels of anti‐citrullinated protein antibodies (ACPAs) of IgG, IgA, IgM and SC isotype at baseline and at month 12 in patients not developing arthritis during follow‐up (n = 40). ns = not significant.
Acknowledgements
This study was supported by grants from the Center for Clinical Research Dalarna, the Swedish Society of Medicine, the Swedish Research Council, Medical Research Council of Southeast Sweden, the Reinhold Sund foundation, King Gustaf V’s 80‐year foundation, the Swedish Rheumatism association and the Östergötland County Council. The authors would like to thank the Clinical Immunology Laboratory, Linköping University Hospital for analyzing IgG and IgA ACPA. We would also like to thank all patients and blood donors participating in the TIRx study.
Data Availability Statement
The data set used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1. Gerlag DM, Raza K, van Baarsen LG et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis 2012; 71:638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molendijk M, Hazes JM, Lubberts E. From patients with arthralgia, pre‐RA and recently diagnosed RA: what is the current status of understanding RA pathogenesis? RMD Open 2018; 4:e000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Hair MJ, van de Sande MG, Ramwadhdoebe TH et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol 2014; 66:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nielen MM, van Schaardenburg D, Reesink HW et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50:380–6. [DOI] [PubMed] [Google Scholar]
- 5. Rantapää‐Dahlqvist S, de Jong BA, Berglin E et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003; 48:2741–9. [DOI] [PubMed] [Google Scholar]
- 6. Bos WH, Wolbink GJ, Boers M et al. Arthritis development in patients with arthralgia is strongly associated with anti‐citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis 2010; 69:490–4. [DOI] [PubMed] [Google Scholar]
- 7. van de Stadt LA, Witte BI, Bos WH, van Schaardenburg D. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis 2013; 72:1920–6. [DOI] [PubMed] [Google Scholar]
- 8. Rakieh C, Nam JL, Hunt L et al. Predicting the development of clinical arthritis in anti‐CCP positive individuals with non‐specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis 2015; 74:1659–66. [DOI] [PubMed] [Google Scholar]
- 9. Nam JL, Hunt L, Hensor EM, Emery P. Enriching case selection for imminent RA: the use of anti‐CCP antibodies in individuals with new non‐specific musculoskeletal symptoms – a cohort study. Ann Rheum Dis 2016; 75:1452–6. [DOI] [PubMed] [Google Scholar]
- 10. Eloff E, Martinsson K, Ziegelasch M et al. Autoantibodies are major predictors of arthritis development in patients with anti‐citrullinated protein antibodies and musculoskeletal pain. Scand J Rheumatol 2020;1–9. [DOI] [PubMed] [Google Scholar]
- 11. Gerlag DM, Safy M, Maijer KI et al. Effects of B‐cell directed therapy on the preclinical stage of rheumatoid arthritis: the PRAIRI study. Ann Rheum Dis 2019; 78:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holers VM, Demoruelle MK, Kuhn KA et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol 2018; 14:542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brandtzaeg P. Secretory IgA: designed for anti‐microbial defense. Front Immunol 2013; 4:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roos K, Martinsson K, Ziegelasch M et al. Circulating secretory IgA antibodies against cyclic citrullinated peptides in early rheumatoid arthritis associate with inflammatory activity and smoking. Arthritis Res Ther 2016; 18:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roos Ljungberg K, Joshua V, Skogh T et al. Secretory anti‐citrullinated protein antibodies in serum associate with lung involvement in early rheumatoid arthritis. Rheumatology 2020; 59:852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sokolove J, Bromberg R, Deane KD et al. Autoantibody epitope spreading in the pre‐clinical phase predicts progression to rheumatoid arthritis. PLOS ONE 2012; 7:e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Beers‐Tas MH, Stuiver MM, de Koning M, van de Stadt LA, Geskus RB, van Schaardenburg D. Can an increase in autoantibody levels predict arthritis in arthralgia patients? Rheumatology 2018; 57:932–4. [DOI] [PubMed] [Google Scholar]
- 18. Ten Brinck RM, van Steenbergen HW, van Delft MAM et al. The risk of individual autoantibodies, autoantibody combinations and levels for arthritis development in clinically suspect arthralgia. Rheumatology 2017; 56:2145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mankia K, Emery P. Is localized autoimmunity the trigger for rheumatoid arthritis? Unravelling new targets for prevention. Discov Med 2015; 20:129–35. [PubMed] [Google Scholar]
- 20. Kastbom A, Roos Ljungberg K, Ziegelasch M, Wettero J, Skogh T, Martinsson K. Changes in anti‐citrullinated protein antibody isotype levels in relation to disease activity and response to treatment in early rheumatoid arthritis. Clin Exp Immunol 2018; 194:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Svard A, Roos Ljungberg K, Brink M et al. Secretory antibodies to citrullinated peptides in plasma and saliva from rheumatoid arthritis patients and their unaffected first‐degree relatives. Clin Exp Immunol 2020; 199:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tajik N, Frech M, Schulz O et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun 2020; 11:1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Menard S, Cerf‐Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol 2010; 3:247–59. [DOI] [PubMed] [Google Scholar]
- 24. Roos Ljungberg K, Borjesson E, Martinsson K, Wettero J, Kastbom A, Svard A. Presence of salivary IgA anti‐citrullinated protein antibodies associate with higher disease activity in patients with rheumatoid arthritis. Arthritis Res Ther 2020; 22:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Svärd A, Skogh T, Alfredsson L et al. Associations with smoking and shared epitope differ between IgA‐ and IgG‐class antibodies to cyclic citrullinated peptides in early rheumatoid arthritis. Arthritis Rheumatol 2015; 67:2032–7. [DOI] [PubMed] [Google Scholar]
- 26. van de Stadt LA, de Koning MH, van de Stadt RJ et al. Development of the anti‐citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum 2011; 63:3226–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Correlation of baseline IgA ACPA and SC ACPA levels in at‐risk patients (n = 82).
Fig. S2. Levels of anti‐citrullinated protein antibodies (ACPAs) of IgG, IgA, IgM and SC isotype at baseline and arthritis debut (n = 31). ns = not significant.
Fig. S3. Levels of anti‐citrullinated protein antibodies (ACPAs) of IgG, IgA, IgM and SC isotype at baseline and at month 12 in patients not developing arthritis during follow‐up (n = 40). ns = not significant.
Data Availability Statement
The data set used and/or analysed during the current study are available from the corresponding author on reasonable request.


