Video
Red dichromatic imaging in peroral endoscopic myotomy.
Abbreviations: DRI, dual-red imaging; ESD, endoscopic submucosal dissection; POEM, peroral endoscopic myotomy; RDI, red dichromatic imaging
A 40-year-old man presented with a 1-year history of difficulty in swallowing liquids and solids. His Eckhart’s score was 6 of 12. EGD showed dilated esophagus with liquid stasis and mild resistance noted across the gastroesophageal junction. Esophageal manometry showed elevated integrated relaxation pressure with panesophageal pressurization (achalasia cardia type II). Peroral endoscopic myotomy (POEM) was planned and was performed using the novel Evis X1 endoscopy (Olympus Corporation, Tokyo, Japan) system. The red dichromatic imaging (RDI) mode in this system is designed to enhance the visibility of deep blood vessels and bleeding sources. This article describes the utility of RDI in the identification of deep blood vessels and bleeding vessels during third-space endoscopy (Fig. 1).
Figure 1.
Red dichromatic imaging (B) clearly showing vessels in deep mucosa or submucosa compared with white-light imaging (A).
RDI mode 1 and mode 2 were used during POEM. Mucosal incision and entry into the submucosal tunnel are crucial steps in POEM. To achieve a clean mucosal incision, it is desirable to avoid deep blood vessels during the cut. Mode 2 was used to identify the deep blood vessels after local injection of saline solution with indigo carmine. A mucosal incision was made avoiding areas with deeper blood vessels using dry cut mode at 50 W on effect 3 (Erbe Elektromedizin GmbH, Tuebingen, Germany) (Fig. 2).
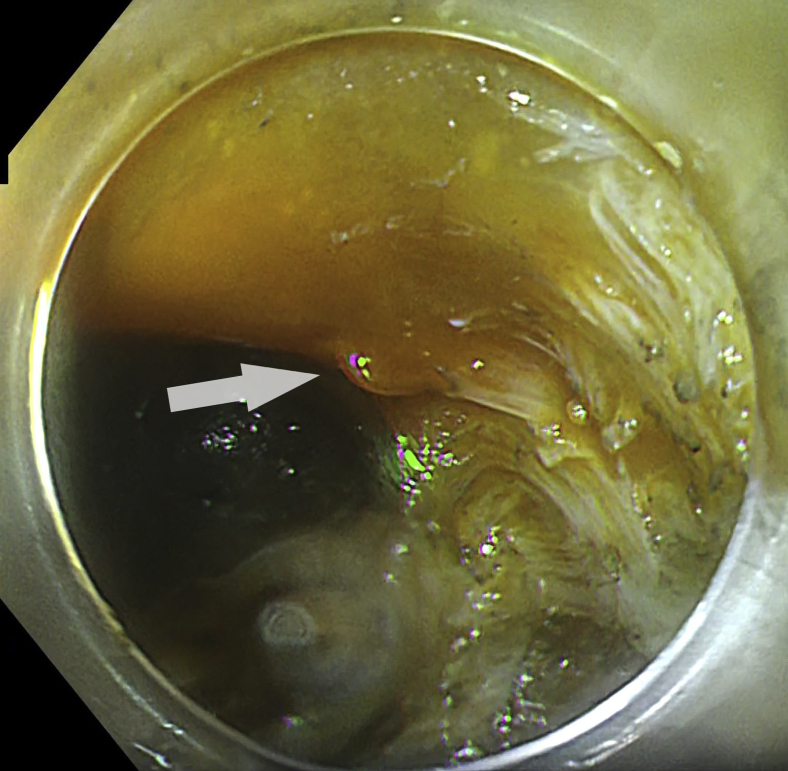
Figure 2.
Clear bloodless mucosal incision under red dichromatic imaging mode 2.
During tunneling, submucosal dissection was done using spray coagulation mode. There was an inadvertent injury to the blood vessel, leading to bleeding. White-light imaging showed pooling of blood without clear delineation of the bleeding point. Because of the differential absorption of amber light, the bleeding point was better identified with RDI. Bleeding was controlled with the use of hemostatic coagrasper forceps. Myotomy was selectively performed over inner circular muscles avoiding longitudinal muscle fibers.
There was blood spurting during myotomy because of vascular injury. Visualization was better with RDI (Fig. 3) than with white-light imaging (Fig. 4). Identification of the bleeding point is difficult with an oozing type of bleed under white-light imaging (Fig. 5). The bleeding point appears a deeper yellow (Fig. 6) than the surrounding area under RDI because amber light absorption is greater at the bleeding point, which has a higher hemoglobin concentration than the surrounding area where blood has been diluted. Complete hemostasis was achieved with coagrasper forceps. The mucosal incision was closed using hemoclips. The postprocedure period was uneventful.
Figure 3.
Red dichromatic imaging mode 1 showing blood spurting (arrow) during myotomy.
Figure 4.
White-light imaging showing blood spurting (arrow) during myotomy.
Figure 5.
White-light imaging showing oozing of blood without clear delineation of bleeding point.
Figure 6.
Bleeding point (arrow) darker compared with surrounding area under red dichromatic imaging mode 1.
Discussion
RDI is a next-generation image enhancement technique that works by using green, amber, and red wavelengths. In the past, it was known as dual-red imaging (DRI). Green light (520-550 nm) can reveal small blood vessels in superficial tissue without extending deep into the mucosa. Amber (595-610 nm) and red light (620-640 nm) can penetrate deeply into the tissue because of the low scattering property. The blood vessels in the deeper tissues absorb amber light because of a strong affinity to hemoglobin. Although red light can penetrate deeply into the tissue, it is weakly absorbed by hemoglobin. Hence, the reflected light contains red light and amber light without attenuation.
The RDI view can be enabled by mere pressing of a button on the processor. RDI contains 3 modes: mode 1, mode 2, and mode 3. As we go from mode 1 to mode 3, there is an increase in deep vessel enhancement level. Mode 1 is useful in the observation of bleeding areas: It confirms the bleeding point intraprocedurally in POEM and endoscopic submucosal dissection (ESD). Mode 2 is different in that it enhances deep blood vessels more clearly than mode 1. Mode 2 is useful in the detection of deep blood vessels during early local injection in POEM or ESD. It also predicts the depth of esophageal varices and red color signs. Mode 3 enhances both superficial and deep vessels. Deep blood vessels appear green, and shallow blood vessels appear red. Mode 3 is useful in the observation of mucosal tissue beyond the residue in colorectal screening and is helpful in the diagnosis of inflammatory activity in ulcerative colitis.
RDI enhances the visibility of deep blood vessels and bleeding sources. Hence, it leads to early identification of bleeding sources and quicker hemostasis. It also reduces the stress experienced by the endoscopist performing the procedure. RDI enhances the contrast between diluted blood and highly concentrated blood, thereby clearly revealing the bleeding source.
Yahagi et al1 showed that DRI specifically enhances thick blood vessels located at 1000 to 1500 μm. The thick blood vessels in the deep mucosa or submucosa were more visible with DRI compared with white-light imaging.1 Furuichi et al2 reported that RDI increases the visibility of esophageal varices and red color signs and also predicts the depth of esophageal varices by changing degrees of visibility. Kubosawa et al3 reported that in the presence of active bleed from a gastric ulcer, the bleeding point was better visualized with DRI and led to prompt hemostasis. DRI is useful in the presence of a spurting hemorrhage from a duodenal ulcer; bleeding point is noted as deep yellow, leading to clear identification of the bleeding point.4 A retrospective study by Yorita et al5 showed that DRI improved the visibility of blood vessels and bleeding points, especially in the presence of oozing blood and pooling of blood during gastric ESD. Maehata et al6 studied the usefulness of DRI in visualizing bleeding points and hemostasis time during ESD. Bleeding points were clearly visualized with DRI, thus facilitating hemostasis. The average hemostasis time was significantly shorter in the DRI group compared with the white-light imaging group.
POEM is a novel endoscopic technique for the treatment of achalasia and other esophageal motility disorders. It was introduced by Inoue et al in 2008.7 Thereafter, it was rapidly disseminated because of its low invasiveness, higher efficacy, and technical novelty. The steps of performing POEM include mucosal incision, submucosal tunnel creation, myotomy, and closure of the incision. Mucosotomy (2.8%) is the most common adverse event in patients undergoing POEM.8 It can result from excessive use of cautery because bleeding points could not be seen clearly with white light during active ooze. RDI will help in early recognition of bleeding points and thus prompt hemostasis. Bleeding during POEM is not very uncommon (0.5%-0.7%).9 Early recognition of bleeding points and quicker hemostasis help in decreasing adverse events. To our knowledge, the utility of RDI in the peroral endoscopic myotomy has not been studied so far. Hence, in this video (Video 1, available online at www.VideoGIE.org), we provide insight into its usefulness during POEM.
Conclusion
RDI enhances the visibility of deep blood vessels and bleeding sources. It leads to early identification of bleeding points and quicker hemostasis. It also reduces the stress experienced by the endoscopist performing POEM. However, prospective studies looking into the utility of RDI in POEM are required.
Disclosure
All authors disclosed no financial relationships.
Acknowledgments
Supported by a Robert W. Summers grant from the American Society for Gastrointestinal Endoscopy.
Footnotes
If you would like to chat with an author of this article, you may contact Dr Ramchandani at ramchandanimohan@gmail.com.
Supplementary data
Red dichromatic imaging in peroral endoscopic myotomy.
References
- 1.Yahagi N., Fujimoto A., Horii J. Dual red imaging: a novel endoscopic imaging technology visualizing thick blood vessels in the gastrointestinal wall. Endosc Int Open. 2019;7:E1632–E1635. doi: 10.1055/a-0749-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuichi Y., Gotoda T., Moriyasu F. Dual red imaging (novel advanced endoscopy) can increase visibility and can predict the depth in diagnosing esophageal varices. J Gastroenterol. 2017;52:568–576. doi: 10.1007/s00535-016-1249-2. [DOI] [PubMed] [Google Scholar]
- 3.Kubosawa Y., Mori H., Fujimoto A. Utility of dual red imaging for endoscopic hemostasis of gastric ulcer bleeding. Dig Dis. 2020;38:352–354. doi: 10.1159/000504386. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka H., Oka S., Tanaka S. Endoscopic hemostasis for spurting duodenal bleeding using dual red imaging. Dig Endosc. 2017;29:816–817. doi: 10.1111/den.12930. [DOI] [PubMed] [Google Scholar]
- 5.Yorita N., Oka S., Tanaka S. Clinical usefulness of dual red imaging in gastric endoscopic submucosal dissection: a pilot study. Clin Endosc. 2020;53:54–59. doi: 10.5946/ce.2019.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maehata T., Fujimoto A., Uraoka T. Efficacy of a new image-enhancement technique for achieving hemostasis in endoscopic submucosal dissection. Gastrointest Endosc. 2020;92:667–674. doi: 10.1016/j.gie.2020.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Inoue H., Minami H., Kobayashi Y. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 8.Haito-Chavez Y., Inoue H., Beard K.W. Comprehensive analysis of adverse events associated with per oral endoscopic myotomy in 1826 patients: an international multicenter study. Am J Gastroenterol. 2017;112:1267–1276. doi: 10.1038/ajg.2017.139. [DOI] [PubMed] [Google Scholar]
- 9.Li Q.L., Zhou P.H., Yao L.Q. Early diagnosis and management of delayed bleeding in the submucosal tunnel after peroral endoscopic myotomy for achalasia (with video) Gastrointest Endosc. 2013;78:370–374. doi: 10.1016/j.gie.2013.04.172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Red dichromatic imaging in peroral endoscopic myotomy.
Red dichromatic imaging in peroral endoscopic myotomy.