Abstract
Study Design:
Retrospective clinical review.
Objective:
To assess the use of intraoperative computed tomography (CT) image-guided navigation (IGN) and robotic assistance in posterior lumbar surgery and their relationship with patient radiation exposure and perioperative outcomes.
Methods:
Patients ≥18 years old undergoing 1- to 2-level transforaminal lateral interbody fusion in 12-month period were included. Chart review was performed for pre- and intraoperative data on radiation dose and perioperative outcomes. All radiation doses are quantified in milliGrays (mGy). Univariate analysis and multivariate logistic regression analysis were utilized for categorical variables. One-way analysis of variance with post hoc Tukey test was used for continuous variables.
Results:
A total of 165 patients were assessed: 12 IGN, 62 robotic, 56 open, 35 fluoroscopically guided minimally invasive surgery (MIS). There was a lower proportion of women in open and MIS groups (P = .010). There were more younger patients in the MIS group (P < .001). MIS group had the lowest mean posterior levels fused (P = .015). Total-procedure radiation, total-procedure radiation/level fused, and intraoperative radiation was the lowest in the open group and highest in the MIS group compared with IGN and robotic groups (all P < .001). Higher proportion of robotic and lower proportion of MIS patients had preoperative CT (P < .001). Estimated blood loss (P = .002) and hospital length of stay (P = .039) were lowest in the MIS group. Highest operative time was observed for IGN patients (P < .001). No differences were observed in body mass index, Charlson Comorbidity Index, and postoperative complications (P = .313, .051, and .644, respectively).
Conclusion:
IGN and robotic assistance in posterior lumbar fusion were associated with higher intraoperative and total-procedure radiation exposure than open cases without IGN/robotics, but significantly less than MIS without IGN/robotics, without differences in perioperative outcomes. Fluoro-MIS procedures reported highest radiation exposure to patient, and of equal concern is that the proportion of total radiation dose also applied to the surgeon and operating room staff in fluoro-MIS group is higher than in IGN/robotics and open groups.
Keywords: radiation, intraoperative CT, image-guided navigation, robotic surgery, minimally invasive surgery
Introduction
As rates of lumbar spinal instrumentation and minimally invasive surgery (MIS) continue to increase, methods to improve accuracy and reduce radiation burden have concurrently adapted. Pedicle screws are used to improve fusion rates but their placement can be challenging, and misplacement can lead to neurologic damage, vascular and visceral structure damage, persistent pain, revision surgeries, and higher overall costs.1,2 Utilization of 2-dimensional fluoroscopic navigation, which has been the mainstay of multiple medical disciplines since gaining popularity in the 1980s, has been shown to reduce the number of misplaced screws.3-6 Despite this, however, pedicle screw misplacement has been estimated to occur between 5% and 30% of spine procedures involving freehand and fluoroscopically guided placement.7-13 New technologies have been increasingly used to improve the accuracy of pedicle screw positioning and patient safety, including robotic navigational guidance and intraoperative computed tomography (CT) image-guided navigation (IGN).14-20
A major topic of discussion regarding novel methods of navigational assistance in spine surgery is radiation exposure, chiefly the balance of who is exposed and to what dosage. Radiation exposure in spinal procedures, especially in MIS, is of concern to everyone involved in the surgery, including the patient, surgical team, and operating room staff. Dosimetry readings during fluoroscopically guided spinal surgery have been shown to be 10 to 12 times greater than those in nonspine orthopedic procedures, based on measured fluoroscopy times.21 Other than the patient, surgeons generally receive the highest dose due to their proximity to radiation scatter6,22-25 (Figure 1). Thus, it is crucial to consider the radiation dosages given not only to the patient perioperatively but also to the operating room staff.
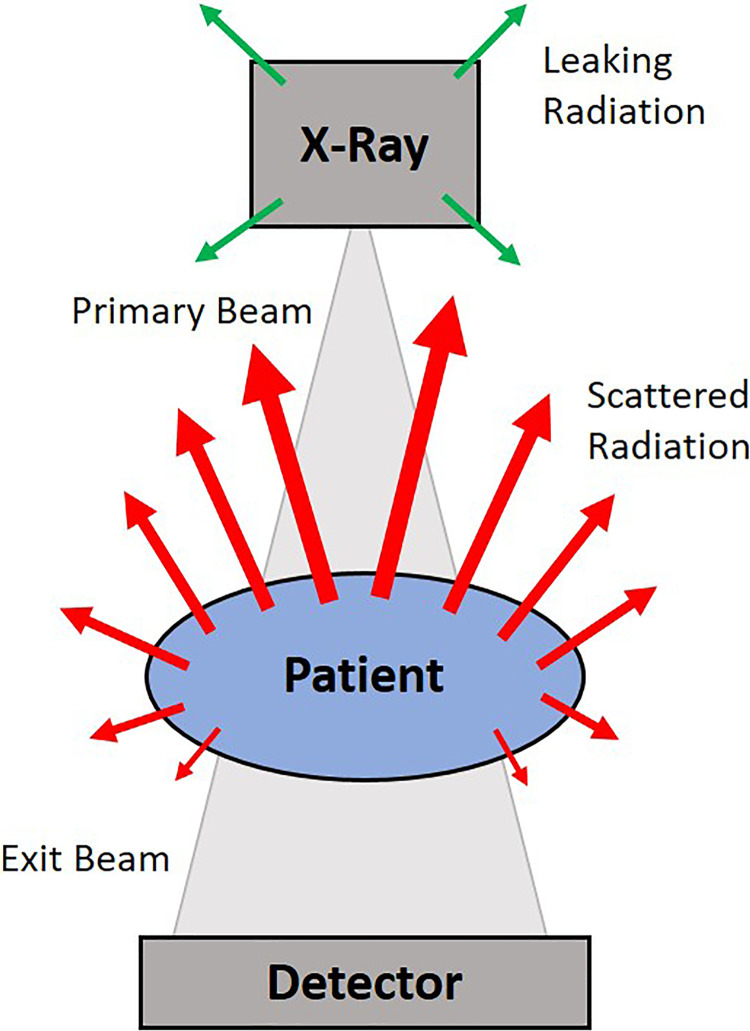
Figure 1.
Scattered radiation during intraoperative fluoroscopy of the lumbar spine. Primary beam: x-ray beam prior to any interaction with the patient, grid, table or image intensifier. Exit beam: beam that interacts with the detector. Leakage radiation (green): leakage from the x-ray tube housing that does not contribute significantly to staff dose. Scattered radiation (red): radiation resulting from Compton scattering in the patient that contributes most to staff radiation dose. Backscatter of radiation is more pronounced toward the source side.
Several studies have raised concerns over radiation exposure with both robotic assistance and IGN, particularly in weighing the reduction of intraoperative radiation exposure to operative staff against potentially increased exposure to the patient.26-28 Radiation exposure for spine surgeons using conventional fluoroscopy may approach or exceed the annual cumulative exposure acceptable for established lifetime dose equivalent limits.24,29 However, a single acquisition required for IGN has also been shown to expose the patient to the equivalent of 4.35 years’ background radiation.1 The purpose of this study was to assess the use of IGN and robotic assistance in 1- or 2-level transforaminal lateral interbody fusion (TLIF) surgeries, along with their relationship to patient radiation exposure and perioperative outcomes. This study is, to our knowledge, the first to compare this across IGN, robotic assistance, fluoroscopically guided MIS (fluoro-MIS), and open TLIF.
Materials and Methods
Data Source and Inclusion Criteria
A retrospective review from January 2018 to December 2018 was conducted for adult patients (≥18 years) undergoing 1- or 2-level TLIF at a single academic institution. 19 spine surgeons were involved in the study, all of whom were fellowship trained in either orthopedic surgery or neurosurgery. All surgeons were required to perform radiation safety courses as part of hospital accreditation. Patients were grouped by type of guidance used for pedicle screw insertion: intraoperative CT IGN, robotic assistance, fluoro-MIS, and open surgery. IGN was utilized by 2 different surgeons, who each primarily performed IGN cases. Robotic assistance was used by 7 different surgeons, one of whom performed more than half of all robotic cases. Sixteen different surgeons performed open cases and 9 different surgeons performed fluoro-MIS cases (Table 1). In all cases, a radiation technician was present to operate the fluoroscopy as per hospital radiology guidelines.
Table 1.
Total Number of Cases Performed by Surgeon.
| Surgeon | Total Number of Cases Performed |
|---|---|
| 1 | 2 |
| 2 | 7 |
| 3 | 4 |
| 4 | 2 |
| 5 | 7 |
| 6 | 1 |
| 7 | 35 |
| 8 | 1 |
| 9 | 14 |
| 10 | 1 |
| 11 | 23 |
| 12 | 8 |
| 13 | 5 |
| 14 | 11 |
| 15 | 11 |
| 16 | 1 |
| 17 | 11 |
| 18 | 12 |
| 19 | 9 |
Robotic Guidance System
The robotic guidance system used at our institution is the ExcelsiusGPS system (Globus Medical Inc, Audubon, PA, USA). The system was used for open and mini-open cases, as well MIS cases in lieu of conventional fluoroscopic guidance during pedicle screw insertion. Patients who underwent preoperative CT scanning outside the operating room had their images uploaded to the proprietary software prior to surgery. After fiducial marker placement, all patients underwent intraoperative fluoroscopic registration, and these images were merged with preoperative CT data. This method allowed the robotic arm to be stereotactically positioned in order to guide incision marking, drilling, and pedicle screw insertion. Final fluoroscopic imaging was performed to confirm implant positioning (Figure 2).
Figure 2.
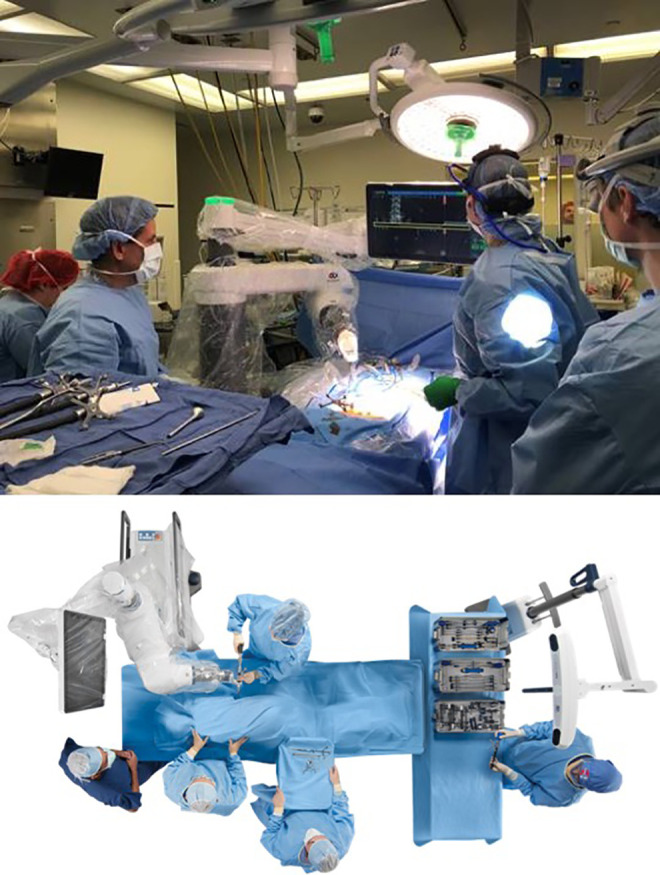
Operating room setup of intraoperative robotic assistance system for transforaminal lateral interbody fusions (TLIFs) at our institution.
Intraoperative CT Image-Guided Navigation
The IGN system used at our institution is the Airo mobile intraoperative CT scanner (BrainLab, Munich, Germany). The system was used for open instrumented cases. With this system, a radiology technician executes scans intraoperatively with a mobile CT scanner after placement of a fiducial marker on a known anatomical landmark. Images were transferred automatically to the navigation workstation, which enabled the surgeon to guide screw placement via simultaneous axial, sagittal, and coronal views displayed on the navigation system. Postinstrumentation imaging was performed via 2D fluoroscopy or intraoperative CT depending on surgeon preference (Figure 3).
Figure 3.
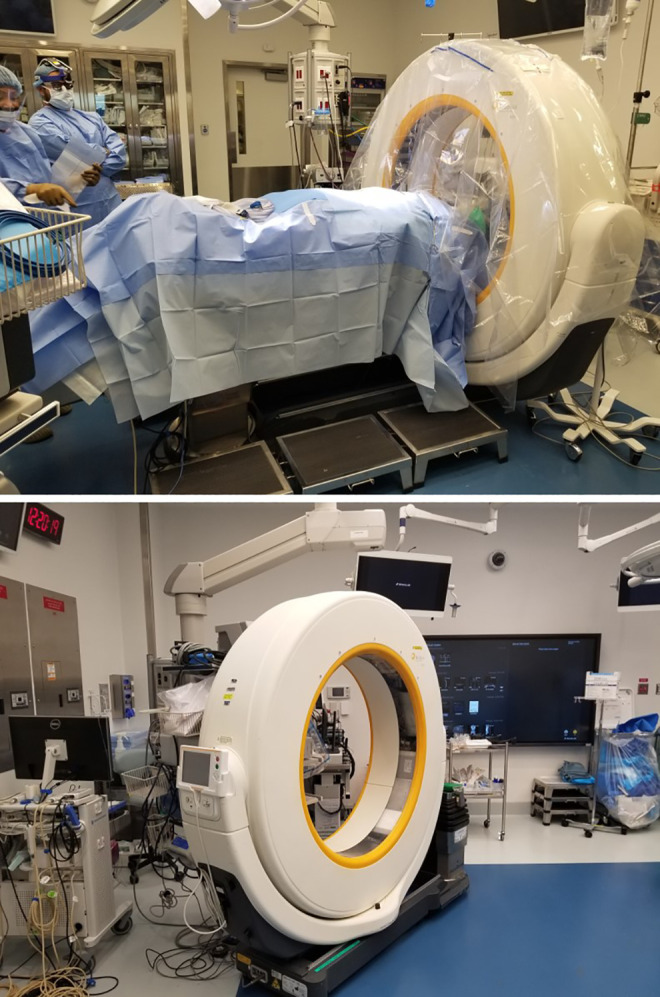
Operating room setup of intraoperative computed tomography (CT) image-guidance navigation system for transforaminal lateral interbody fusions (TLIFs) at our institution.
Data Collection and Outcome Measures
Chart review was performed for demographic data (age, gender, body mass index [BMI], and Charlson comorbidity index [CCI]), preoperative and intraoperative radiation dose and perioperative outcomes. Preoperative CT scan utilization and radiation dose, intraoperative radiation dose and time (via use of fluoroscopy and/or CT) and total-procedure radiation dose (sum of preoperative CT and intraoperative radiation doses) were assessed. Total-procedure radiation dose per level involved during surgery was also calculated by dividing total-procedure radiation dose by the number of levels involved in surgery. All radiation doses were determined from the DICOM Radiation Dose Structured Reports (RDSR) and quantified in milliGrays (mGy). Surgical data assessed included levels fused, estimated blood loss (EBL), length of stay (LOS), operative time, and general perioperative complications (including cardiac, urinary and neurologic complications, along with ileus and surgical site infection).
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences, version 23 (IBM Corp, Armonk, NY). Univariate analysis and multivariate logistic regression analysis for categorical variables and 1-way analysis of variance for continuous variables were utilized. Significance was set at P < .05.
Results
Patient Sample and Demographics
A total of 165 patients underwent 1- or 2-level TLIF during the study period (51.83% female, 48.17% male). Mean age was 59.13 ± 13.18 years, BMI was 29.43 ± 6.72 kg/m2, and CCI 1.20 ± 1.56. Twelve cases utilized intraoperative CT IGN for pedicle screw placement and 62 used robotic assistance. In all, 56 cases were open and 35 were fluoroscopically guided MIS. The open (38.18% female) and fluoro-MIS (45.71% female) patient groups had a lower proportion of females compared with the IGN (66.67% female) and robotic (64.52% female) patient groups (P = .021, Table 2). Patients were also younger in the MIS group (50.63 years) compared with the other groups (63.42 years in IGN, 61.74 years in robotic, 60.63 years in open; P < .001). There were no differences in BMI (P = .313) among any of the groups, though there was a trend toward higher CCI in IGN (1.83) and open (1.54) patients compared with robotic (0.98) and MIS patients (0.83, P = .051).
Table 2.
Differences in Demographics Among Different TLIF Methods.
| Total (n = 165) | IGN (n = 12) | Robotic (n = 62) | Open (n = 56) | MIS (n = 35) | P | |
|---|---|---|---|---|---|---|
| Gender (female), % | 51.83 | 66.67 | 64.52 | 38.18 | 45.71 | .021 |
| Age, y | 59.13 ± 13.18 | 63.42 ± 8.56 | 61.74 ± 11.78 | 60.63 ± 13.9 | 50.63 ± 12.48 | <.001 |
| BMI, kg/m2 | 29.43 ± 6.72 | 27.1 ± 3.36 | 28.79 ± 6.97 | 29.82 ± 6.67 | 30.78 ± 7.1 | .313 |
| CCI | 1.20 ± 1.56 | 1.83 ± 1.34 | 0.98 ± 1.19 | 1.54 ± 1.94 | 0.83 ± 1.4 | .051 |
Abbreviations: TLIF, transforaminal lateral interbody fusion; IGN, intraoperative computed tomography image-guided navigation; robotic, robotic assistance; MIS, minimally invasive surgery with fluoroscopy; BMI, body mass index; CCI, Charlson Comorbidity Index.
Perioperative Outcomes
The fluoro-MIS group had the lowest mean posterior levels fused (1.06 levels in MIS vs 1.42 in IGN, 1.27 in robotic, and 1.32 in open; P = .015, Table 3). EBL was significantly lower in the MIS group compared with all others (162.14 vs 441.67 mL in IGN, 380.24 mL in robotic, and 355.36 mL in open; P = .002) and this was confirmed with post hoc Tukey test. LOS was also lowest in the MIS group (2.83 vs 4.75 days in IGN, 3.89 days in robotic, and 3.89 days in open; P = .039), but post hoc Tukey test showed that this was not a significant difference in paired comparisons. Operative time was highest for IGN patients (303.5 vs 264.85 minutes robotic, 229.91 minutes open, and 213.43 minutes MIS; P < .001). Post hoc Tukey test showed that there was a significant difference in operative time comparing IGN patients with MIS and open patients but not with robotics patients. There were no significant differences in postoperative complications among any of the groups (P = .644).
Table 3.
Differences in Perioperative Outcomes Among Different TLIF Methods.
| Total (n = 165) | IGN (n = 12) | Robotic (n = 62) | Open (n = 56) | MIS (n = 35) | P | |
|---|---|---|---|---|---|---|
| Posterior levels fused | 1.25 ± 0.44 | 1.42 ± 0.51 | 1.27 ± 0.45 | 1.32 ± 0.47 | 1.06 ± 0.24 | .015 |
| EBL, mL | 330 ± 308.14 | 441.67 ± 180.7 | 380.24 ± 349.04 | 355.36 ± 301.01 | 162.14 ± 207.93 | .002 |
| LOS, d | 3.73 ± 2.31 | 4.75 ± 1.14 | 3.89 ± 2.64 | 3.89 ± 1.74 | 2.83 ± 2.55 | .039 |
| Operating time, min | 244.9 ± 70.38 | 303.5 ± 67.53 | 264.85 ± 73.16 | 229.91 ± 60.03 | 213.43 ± 60.46 | <.001 |
| Postoperative complication, % | 15.15 | 8.33 | 19.35 | 14.29 | 11.43 | .644 |
Abbreviations: TLIF, transforaminal lateral interbody fusion; IGN, intraoperative computed tomography image-guided navigation; robotic, robotic assistance; MIS, minimally invasive surgery with fluoroscopy; EBL, estimated blood loss; LOS, length of stay.
Radiation Exposure
Total-procedure radiation was highest in the fluoro-MIS group, followed by the robotics, IGN and then open groups (P < .001, Table 4). Total-procedure radiation per level fused was also highest in the MIS group, following the same hierarchy (P < .001). This was also the case for intraoperative radiation (P < .001). A higher proportion of robotic and lower proportion of MIS patients underwent preoperative CT (25% in IGN, 82.26% in robotic, 37.5% in open, 8.57% in MIS; P < .001). There was no difference in preoperative radiation dose among patients who underwent preoperative CT (P = .931).
Table 4.
Differences in Radiation Exposure Among Different TLIF Methods.
| Total (n = 165) | IGN (n = 12) | Robotic (n = 62) | Open (n = 56) | MIS (n = 35) | P | |
|---|---|---|---|---|---|---|
| Total-procedure radiation, mGy | 51.19 ± 42.02 | 50.21 ± 22.84 | 59.84 ± 32.48 | 22.56 ± 22.62 | 82.02 ± 56.24 | <.001 |
| Total-procedure radiation per level, mGy | 45.52 ± 40.97 | 41.88 ± 27.70 | 51.18 ± 32.05 | 18.56 ± 19.53 | 79.41 ± 54.25 | <.001 |
| Intraoperative radiation, mGy | 42.16 ± 41.81 | 44.69 ± 13.08 | 44.85 ± 34.61 | 14.81 ± 15.54 | 80.28 ± 55.6 | <.001 |
| Preoperative CT, % | 47.27 | 25.00 | 82.26 | 37.50 | 8.57 | <.001 |
| Preoperative CT radiation, mGy | 19.88 ± 9.48 | 22.09 ± 19.67 | 19.36 ± 7.32 | 20.67 ± 12.28 | 20.28 ± 12.16 | .931 |
Abbreviations: TLIF, transforaminal lateral interbody fusion; IGN, intraoperative computed tomography image-guided navigation; robotic, robotic assistance; MIS, minimally invasive surgery with fluoroscopy.
Fluoroscopy Time
Mean intraoperative fluoroscopy time by surgeon by type of guidance used for pedicle screw insertion was assessed to see if there were differences in radiation usage time among different surgeons. Surgeons were grouped by those who performed majority MIS cases, majority open cases and majority robotically assisted cases. Surgeons 5 and 12, who performed majority IGN cases, were excluded due to minimal fluoroscopy usage during IGN cases. Surgeons 1, 4, 6, 8, 10, and 16 were excluded from comparison due to having 2 or fewer total patients. There was no significant difference in fluoroscopy time among the surgeons who performed majority open cases and majority robotic cases (P = .782 and .785, respectively, Table 5). There was a greater variation in fluoroscopy time for surgeons performing the majority MIS cases, with Surgeons 11 and 15 in particular having higher mean fluoroscopy times (P = .074, Table 5).
Table 5.
Mean Intraoperative Fluoroscopy Time by Surgeon by Type of Guidance Used for Pedicle Screw Insertion.a
| Surgeon | Fluoroscopy Time, s | P | |
|---|---|---|---|
| Majority MIS cases | 2 | 49.60 | .074 |
| 3 | 37.45 | ||
| 11 | 106.42 | ||
| 15 | 101.58 | ||
| 19 | 67.61 | ||
| Majority open cases | 9 | 24.24 | .782 |
| 13 | 12.27 | ||
| 17 | 21.75 | ||
| Majority robotic cases | 7 | 48.12 | .785 |
| 14 | 44.40 | ||
| 18 | 43.47 |
Abbreviation: MIS, minimally invasive surgery with fluoroscopy.
a Surgeons 5 and 12, who had majority cases using intraoperative computed tomography image-guided navigation, were excluded due to minimal fluoroscopy usage during those cases. Surgeons 1, 4, 6, 8,10, and 16 were excluded due to having 2 or fewer total patients.
Discussion
Advancements in imaging technology have diversified the options of image guidance techniques in the surgical procedures of various disciplines. Robotic navigational guidance and intraoperative CT IGN have been increasingly used in spine surgeries in an attempt to improve patient safety and the precision of pedicle screw placement. In addition to assessing cost-effectiveness and accuracy of screw positioning in these operations, an important topic of discussion pertaining to novel methods of navigational assistance is radiation exposure. While several studies have explored the risks of radiation exposure for the patient, surgeon, and operating room staff when using fluoroscopic guidance, there is a lack information comparing it with robotic assistance and IGN.6,22-25 Because of this, our study aimed to assess the relationship between types of guidance used for pedicle screw placement and patient radiation exposure, along with perioperative outcomes.
In this study, we compared 4 types of guidance for pedicle screw placement in TLIF surgeries: IGN, robotic assistance, open surgery, and fluoroscopic-MIS. Overall, the fluoro-MIS cohort had younger patients and less levels fused and also trended toward a lower mean CCI. Unsurprisingly, EBL was also lowest in the MIS cohort in comparison with the IGN, robotic and open groups, which were comparable. Though analysis of variance showed a significant difference in LOS between groups (P = .039), with the MIS group having the lowest mean, post hoc analysis demonstrated that there were no significant differences between groups. We also found a higher proportion of females in the open and fluoro-MIS cohorts compared with the IGN and robotic groups (P = .021), though it is questionable how clinically relevant this is, especially given some of the smaller cohort sizes. There were no significant differences in postoperative complications among any of the groups.
Regarding operative time, surgeries using IGN had the longest procedures on average (303.5 minutes), followed by robotically assisted surgeries (264.85 minutes), both of which were longer than open (229.91 minutes) and MIS (213.43 minutes) procedures with conventional fluoroscopy use (P < .001). While IGN procedures were significantly longer than open and MIS procedures, post hoc analysis showed no difference compared with robotic surgeries. This could possibly stem from additional time required for setting up the IGN and robotic systems, which has been reported to prolong operating time.3 However, other studies have reported that total-procedure time is not significantly extended, despite a longer setup time, because navigation allows for quicker placement of pedicle screws.30,31 In addition, a patient-level cost analysis conducted by Dea et al32 in 2016 reported a cost-effective reduction in revision surgery for symptomatic postoperative screw malposition using CT-based navigation. Taking all of this into account, future comparison studies would be beneficial and could incorporate cost data for a more complete picture of cost-effectiveness.
Perhaps of more concern in this study is our findings on radiation dosage. Because open procedures generally used minimal intraoperative fluoroscopy, the radiation doses for these patients were predictably the lowest. More strikingly, radiation dosage was highest for intraoperative, total-procedure and total-procedure per level measurements in the fluoro-MIS patient group. This is not necessarily a surprising finding, as past studies have reported higher radiation exposure in less invasive spine approaches.23 However, the contrast in mean dosage between the MIS group and both the IGN and robotic groups is starker than one may expect. Mean total-procedure radiation for the MIS cohort was 82.02 mGy compared with 50.21 mGy and 59.84 mGy in the IGN and robotic groups, respectively (P < .001). The MIS patients also had the fewest number of levels fused on average, so the difference was more apparent when calculating total-procedure radiation per level (79.41 mGy for MIS versus 41.88 mGy for IGN and 51.18 mGy for robotic; P > .001). Despite the MIS patients having by far the lowest rate of preoperative CT, which adds approximately 20 mGy of radiation to the total-procedure sum, they were still exposed to substantially more radiation overall.
In assessing for differences in intraoperative fluoroscopy time by different surgeons, we found no significant difference between surgeons who primarily performed open and robotic cases. There was, however, a greater variation in fluoroscopy time among surgeons who performed majority MIS cases, with 2 surgeons in particular averaging over 100 seconds of fluoroscopy use per case while the others averaged in the 40- to 70-second range (P = .074). Because of the images being taken by radiation technicians who were used by all surgeons, we believe that this reflects some surgeons acquiring a larger number of images, rather than higher radiation dose per image. Furthermore, the lower doses of radiation used by Surgeons 2 and 3 suggests that surgeon technique may help reduce radiation doses in the MIS group.
While the aforementioned radiation measurements are for the patient, because this radiation dose total stemmed primarily from intraoperative fluoroscopy in MIS cases, it raises concerns about the proportion of total radiation dose applied to the surgeon and operating staff, who are performing these surgeries on a regular basis. Moreover, the surgeons are positioned in closest proximity to intraoperative radiation scatter, whereas for intraoperative CT or preoperative CT, they are not in close proximity to the radiation source.
Radiation exposure in medicine is by no means benign. It can lead to illness and injury in patients and surgeons alike and is estimated to cause 0.6% to 3% of cancer cases worldwide.33 There are currently no specific guidelines regarding radiation exposure limits to surgeons and other operative room staff, though there are general recommendations to minimize radiation through various methods, such as the use of barriers and other protective equipment.34 Regardless of the system used for guidance when placing pedicle screws, there should be an attempt to maximize the configuration of imaging equipment to achieve ALARA (as low as reasonably achievable) patient radiation dose.35 IGN and robotic assistance are still less utilized at many institutions, with high costs and an involved training process likely being primary prohibitive factors. Nonetheless, these methods would likely expose both the patient and the operative staff to significantly less radiation in nonopen cases, compared with conventional fluoroscopic guidance, without compromising perioperative outcomes. For this reason, the judicious usage of IGN and robotic assistance could prove to be advantageous, and this study suggests that further exploration into their utilization is warranted.
Our study has some limitations. Because this was a retrospective cohort study, it has all of the accompanying limitations of such a study. We also had a limited sample size, particularly in the IGN group. Differences in intraoperative radiation exposure may also be affected by the fact that procedures in different groups were not all performed by the same surgical team. Future studies could benefit from having a larger sample size as more procedures are performed using these methods. A multi-institutional study would also increase the sample size and may reduce potential for surgeon technique and selection bias in testing IGN and robotic guidance systems as well. Additionally, further exploration into assessing occupational radiation exposure with different methods of guidance would benefit this conversation, as few studies have directly investigated radiation exposure to surgeons and the rest of the operating room staff themselves. In particular, further research involving data collected from surgeon-worn dosimeters would better quantify radiation dosage received in relation to fluoroscopy time. Nevertheless, this study is, to our knowledge, the first to compare patient radiation exposure between IGN, robotic assistance, and conventional intraoperative fluoroscopic guidance in both MIS and open TLIF surgeries. While there were no significant differences in perioperative outcomes among groups, with the exception of the expected lower EBL and LOS in MIS procedures, further discussion about the costs and benefits of IGN and robotic guidance systems are warranted, especially with regard to radiation safety in comparison with conventional fluoroscopy use.
Conclusion
Intraoperative CT IGN and robotic assistance in posterior lumbar fusion were associated with higher intraoperative and total-procedure radiation exposure than open cases without IGN or robotics; however, fluoroscopic-MIS has the highest total procedural radiation dose. While fluoroscopic-MIS procedures reported highest radiation exposure to the patient, an additional significant concern is that the proportion of total radiation dose also applied to the surgeon and operating room staff in the fluoroscopic-MIS group is much higher than that in IGN, robotics, and open groups. More comprehensive or consistent training for surgeons about fluoroscopy use may be beneficial to reduce unnecessary intraoperative radiation exposure, particularly in MIS cases.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Themistocles S. Protopsaltis, MD  https://orcid.org/0000-0002-4978-2600
https://orcid.org/0000-0002-4978-2600
Aaron J. Buckland, MBBS, FRACS  https://orcid.org/0000-0002-9424-0843
https://orcid.org/0000-0002-9424-0843
Ethical Approval: Each institution obtained approval from their local Institutional Review Board to analyze or review patients in the retrospective database. Waiver of consent was obtained from each patient.
References
- 1. Farah K, Coudert P, Graillon T, et al. Prospective comparative study in spine surgery between O-arm and Airo systems: efficacy and radiation exposure. World Neurosurg. 2018;118:e175–e184. doi:10.1016/j.wneu.2018.06.148 [DOI] [PubMed] [Google Scholar]
- 2. Gautschi OP, Schatlo B, Schaller K, Tessitore E. Clinically relevant complications related to pedicle screw placement in thoracolumbar surgery and their management: a literature review of 35 630 pedicle screws. Neurosurg Focus. 2011;31:E8. doi:10.3171/2011.7.FOCUS11168 [DOI] [PubMed] [Google Scholar]
- 3. Waschke A, Walter J, Duenisch P, Reichart R, Kalff R, Ewald C. CT-navigation versus fluoroscopy-guided placement of pedicle screws at the thoracolumbar spine: Single center experience of 4500 screws. Eur Spine J. 2013;22:654–660. doi:10.1007/s00586-012-2509-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meng XT, Guan XF, Zhang HL, He SS. Computer navigation versus fluoroscopy-guided navigation for thoracic pedicle screw placement: a meta-analysis. Neurosurg Rev. 2016;39:385–391. doi:10.1007/s10143-015-0679-2 [DOI] [PubMed] [Google Scholar]
- 5. Tian NF, Xu HZ. Image-guided pedicle screw insertion accuracy: a meta-analysis. Int Orthop. 2009;33:895–903. doi:10.1007/s00264-009-0792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mulconrey DS. Fluoroscopic radiation exposure in spinal surgery: in vivo evaluation for operating room personnel. Clin Spine Surg. 2013;29:E331–E335. doi:10.1097/bsd.0b013e31828673c1 [DOI] [PubMed] [Google Scholar]
- 7. Hart RA, Hansen BL, Shea M, Hsu F, Anderson GJ. Pedicle screw placement in the thoracic spine. Spine (Phila Pa 1976). 2005;30:E326–E331. doi:10.1097/01.brs.0000166621.98354.1d [DOI] [PubMed] [Google Scholar]
- 8. Ravi B, Zahrai A, Rampersaud R. Clinical accuracy of computer-assisted two-dimensional fluoroscopy for the percutaneous placement of lumbosacral pedicle screws. Spine (Phila Pa 1976). 2011;36:84–91. doi:10.1097/BRS.0b013e3181cbfd09 [DOI] [PubMed] [Google Scholar]
- 9. Bransford R, Bellabarba C, Thompson JH, Henley MB, Mirza SK, Chapman JR. The safety of fluoroscopically-assisted thoracic pedicle screw instrumentation for spine trauma. J Trauma. 2006;60:1047–1052. doi:10.1097/01.ta.0000215949.95089.18 [DOI] [PubMed] [Google Scholar]
- 10. Mirza SK, Wiggins GC, Kuntz C, 4th, et al. Accuracy of thoracic vertebral body screw placement using standard fluoroscopy, fluoroscopic image guidance, and computed tomographic image guidance: a cadaver study. Spine (Phila Pa 1976). 2003;28:402–413. doi:10.1097/01.BRS.0000048461.51308.CD [DOI] [PubMed] [Google Scholar]
- 11. Amiot LP, Lang K, Putzier M, Zippel H, Labelle H. Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine (Phila Pa 1976). 2000;25:606–614. doi:10.1097/00007632-200003010-00012 [DOI] [PubMed] [Google Scholar]
- 12. Guzey FK, Emel E, Seyithanoglu MS, et al. Accuracy of pedicle screw placement for upper and middle thoracic pathologies without coronal plane spinal deformity using conventional methods. J Spinal Disord Tech. 2006;19:436–441. doi:10.1097/00024720-200608000-00011 [DOI] [PubMed] [Google Scholar]
- 13. Assaker R, Reyns N, Vinchon M, Demondion X, Louis E. Transpedicular screw placement: image-guided versus lateral-view fluoroscopy: in vitro simulation. Spine (Phila Pa 1976). 2001;26:2160–2164. doi:10.1097/00007632-200110010-00024 [DOI] [PubMed] [Google Scholar]
- 14. Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine (Phila Pa 1976). 2007;32:E111–E120. doi:10.1097/01.brs.0000254048.79024.8b [DOI] [PubMed] [Google Scholar]
- 15. Costa F, Cardia A, Ortolina A, Fabio G, Zerbi A, Fornari M. Spinal navigation: Standard preoperative versus intraoperative computed tomography data set acquisition for computer-guidance system: radiological and clinical study in 100 consecutive patients. Spine (Phila Pa 1976). 2011;36:2094–2098. doi:10.1097/BRS.0b013e318201129d [DOI] [PubMed] [Google Scholar]
- 16. Holly LT, Foley KT. Image guidance in spine surgery. Orthop Clin North Am. 2007;38:451–461. doi:10.1016/j.ocl.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 17. Mason A, Paulsen R, Babuska JM, et al. The accuracy of pedicle screw placement using intraoperative image guidance systems. J Neurosurg Spine. 2014;20:196–203. doi:10.3171/2013.11.SPINE13413 [DOI] [PubMed] [Google Scholar]
- 18. Shin BJ, James AR, Njoku IU, Härtl R. Pedicle screw navigation: a systematic review and meta-analysis of perforation risk for computer-navigated versus freehand insertion. J Neurosurg Spine. 2012;17:113–122. doi:10.3171/2012.5.spine11399 [DOI] [PubMed] [Google Scholar]
- 19. Tang J, Zhu Z, Sui T, Kong D, Cao X. Position and complications of pedicle screw insertion with or without image-navigation techniques in the thoracolumbar spine: a meta-analysis of comparative studies. J Biomed Res. 2014;28:228–329. doi:10.7555/jbr.28.20130159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tian NF, Huang QS, Zhou P, et al. Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J. 2011;20:846–859. doi:10.1007/s00586-010-1577-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsalafoutas IA, Tsapaki V, Kaliakmanis A, et al. Estimation of radiation doses to patients and surgeons from various fluoroscopically guided orthopaedic surgeries. Radiat Prot Dosimetry. 2008;128:112–119. doi:10.1093/rpd/ncm234 [DOI] [PubMed] [Google Scholar]
- 22. Theocharopoulos N, Perisinakis K, Damilakis J, Papadokostakis G, Hadjipavlou A, Gourtsoyiannis N. Occupational exposure from common fluoroscopic projections used in orthopaedic surgery. J Bone Joint Surg Am. 2003;85:1698–1703. doi:10.2106/00004623-200309000-00007 [DOI] [PubMed] [Google Scholar]
- 23. Yu E, Khan SN. Does less invasive spine surgery result in increased radiation exposure? A systematic review. Clin Orthop Relat Res. 2014;472:1738–1748. doi:10.1007/s11999-014-3503-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rampersaud YR, Foley KT, Shen AC, Williams S, Solomito M. Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine (Phila Pa 1976). 2000;25:2637–2645. doi:10.1097/00007632-200010150-00016 [DOI] [PubMed] [Google Scholar]
- 25. Bandela JR, Jacob RP, Arreola M, Griglock TM, Bova F, Yang M. Use of CT-based intraoperative spinal navigation: management of radiation exposure to operator, staff, and patients. World Neurosurg. 2013;79:390–394. doi:10.1016/j.wneu.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 26. Nachabe R, Strauss K, Schueler B, Bydon M. Radiation dose and image quality comparison during spine surgery with two different, intraoperative 3D imaging navigation systems. J Appl Clin Med Phys. 2019;20:136–145. doi:10.1002/acm2.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mendelsohn D, Strelzow J, Dea N, et al. Patient and surgeon radiation exposure during spinal instrumentation using intraoperative computed tomography-based navigation. Spine J. 2016;16:343–354. doi:10.1016/j.spinee.2015.11.020 [DOI] [PubMed] [Google Scholar]
- 28. Bindal RK, Glaze S, Ognoskie M, Tunner V, Malone R, Ghosh S. Surgeon and patient radiation exposure in minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Spine. 2008;9:570–573. doi:10.3171/SPI.2008.4.08182 [DOI] [PubMed] [Google Scholar]
- 29. Ul Haque M, Shufflebarger HL, O’Brien M, Macagno A. Radiation exposure during pedicle screw placement in adolescent idiopathic scoliosis: is fluoroscopy safe? Spine (Phila Pa 1976). 2006;31:2516–2520. doi:10.1097/01.brs.0000238675.91612.2f [DOI] [PubMed] [Google Scholar]
- 30. Tabaraee E, Gibson AG, Karahalios DG, Potts EA, Mobasser JP, Burch S. Intraoperative cone beam–computed tomography with navigation (O-ARM) versus conventional fluoroscopy (C-ARM): a cadaveric study comparing accuracy, efficiency, and safety for spinal instrumentation. Spine (Phila Pa 1976). 2013;38:1953–1958. doi:10.1097/BRS.0b013e3182a51d1e [DOI] [PubMed] [Google Scholar]
- 31. Lian X, Navarro-Ramirez R, Berlin C, et al. Total 3D Airo® navigation for minimally invasive transforaminal lumbar interbody fusion. Biomed Res Int. 2016;2016:5027340. doi:10.1155/2016/5027340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dea N, Fisher CG, Batke J, et al. Economic evaluation comparing intraoperative cone beam CT-based navigation and conventional fluoroscopy for the placement of spinal pedicle screws: a patient-level data cost-effectiveness analysis. Spine J. 2016;16:23–31. doi:10.1016/j.spinee.2015.09.062 [DOI] [PubMed] [Google Scholar]
- 33. De González AB, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363:345–351. doi:10.1016/S0140-6736(04)15433-0 [DOI] [PubMed] [Google Scholar]
- 34. Giordano BD, Grauer JN, Miller CP, Morgan TL, Rechtine GR, 2nd. Radiation exposure issues in orthopaedics. J Bone Joint Surg Am. 2011;93:e69(1-10). doi:10.2106/JBJS.J.01328 [DOI] [PubMed] [Google Scholar]
- 35. International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP. 2007;37:1–332. doi:10.1016/j.icrp.2004.12.002 [DOI] [PubMed] [Google Scholar]