Abstract
Study Design:
Narrative review.
Objectives:
Artificial intelligence (AI) and machine learning (ML) have emerged as disruptive technologies with the potential to drastically affect clinical decision making in spine surgery. AI can enhance the delivery of spine care in several arenas: (1) preoperative patient workup, patient selection, and outcome prediction; (2) quality and reproducibility of spine research; (3) perioperative surgical assistance and data tracking optimization; and (4) intraoperative surgical performance. The purpose of this narrative review is to concisely assemble, analyze, and discuss current trends and applications of AI and ML in conventional and robotic-assisted spine surgery.
Methods:
We conducted a comprehensive PubMed search of peer-reviewed articles that were published between 2006 and 2019 examining AI, ML, and robotics in spine surgery. Key findings were then compiled and summarized in this review.
Results:
The majority of the published AI literature in spine surgery has focused on predictive analytics and supervised image recognition for radiographic diagnosis. Several investigators have studied the use of AI/ML in the perioperative setting in small patient cohorts; pivotal trials are still pending.
Conclusions:
Artificial intelligence has tremendous potential in revolutionizing comprehensive spine care. Evidence-based, predictive analytics can help surgeons improve preoperative patient selection, surgical indications, and individualized postoperative care. Robotic-assisted surgery, while still in early stages of development, has the potential to reduce surgeon fatigue and improve technical precision.
Keywords: artificial intelligence, machine learning, spine surgery, robotic spine surgery, radiology, review
Introduction
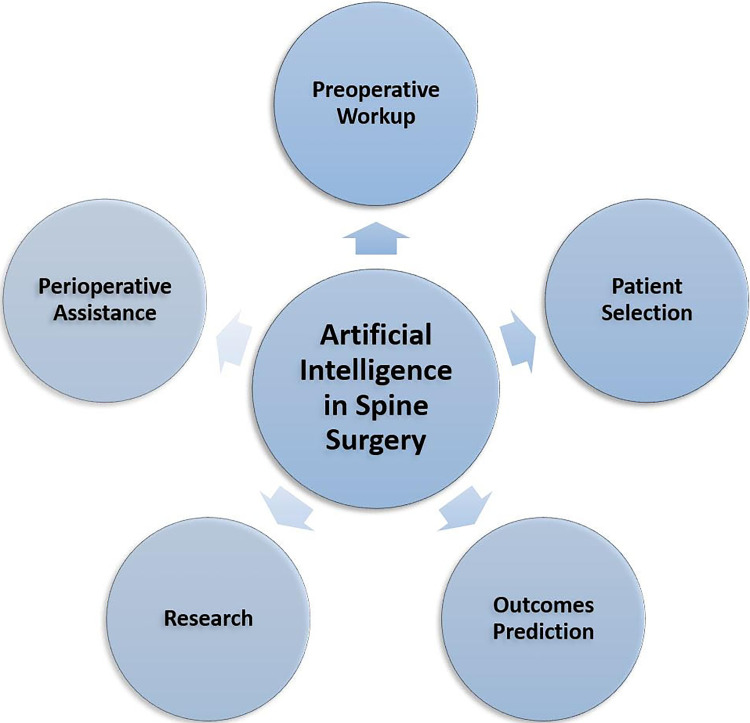
Artificial intelligence (AI) has emerged as a disruptive technology that could guide clinical decision making in spine surgery.1-5 While AI applications to fields such as radiology and dermatology have been transformative, it has yet to be widely adopted or well understood by most spine surgeons.1,3,6 AI could potentially enhance the delivery of spine care in 4 primary ways: (1) preoperative workup, patient selection, outcome prediction; (2) enhancing the quality and reproducibility of spine research; (3) perioperative surgical assistance and data tracking; and (4) intraoperative surgical performance (Figure 1). The purpose of this narrative review is to concisely compile, analyze, and discuss current trends and applications of AI in spine surgery.
Figure 1.
Artificial Intelligence will disrupt the spine service line by improving the quality and delivery of care. This is accomplished through AI’s ability to enhance (1) preoperative patient workup, patient selection, and outcome prediction; (2) quality and reproducibility of spine research; (3) perioperative surgical assistance and data tracking optimization; and (4) intraoperative surgical performance.
Background
Economics of Spine Surgery: Rising Costs and Mitigating Downstream Financial Risks
Spine care is expensive: annual expenditures in the United States currently average $110 billion in direct costs.7,8 By 2025, total health expenditures are expected to average $5.3 trillion, or close to 20% of the gross domestic product of the United States.8,9 Furthermore, the expenses are progressively increasing; spinal fusions performed today cost approximately 12 times more than fusions performed 30 years ago.10 In 2014, spinal fusions had the highest aggregate cost for an entire hospital stay in the United States, beating costs of both total hip and knee arthroplasty.11 It is tempting to assume this increase in spending is fueled driven by supply and demand economics, or in other words, that current trends in spine care spending are unsustainable. However, what we have observed has been quite the opposite.
The increasing cost of health care is quickly spiraling out of control, and Medicare reimbursements are facing billions of dollars in planned cuts over the next several years.12 Furthermore, health care reform has developed into an environment of decreasing reimbursements, increasing payer denials, and profiling hospitals and physicians on the utilization and quality of care.8 Without innovation, or significant downsizing, the current trajectory of spine surgery expenditures is not sustainable.7,8,10
Heterogeneity in the Delivery of Care and Research
Spine surgery is fraught with a myriad of different surgical perspectives for a given condition, often informed by practice and experience.13 A 2014 survey of neurosurgical and orthopedic spine surgeons in the United States demonstrated disagreement on the surgical treatment of a recurrent lumbar disc herniations in 69% of the surgeons.14 In 2016, another survey demonstrated a 75% rate of disagreement among spine surgeons on various treatments for chronic low back pain patients.15 This heterogeneity of care leads to variability in treatment and cost. This was demonstrated by a cross-sectional study by Alvin and colleagues, who demonstrated a direct correlation between treatment costs and patient volume along with years of experience for recurrent lumbar disc herniations.13 In addition, private practice spine surgeons were shown to be more cost-conscious compared with their counterparts in academic practice.13
To further complicate matters, heterogeneity also exists in patient-reported outcome instruments (PROIs), which are used to evaluate surgeon performance and judge the benefit of a particular surgery. While they are not perfect measures of outcome, they are nonetheless necessary to address possible discrepancies in care. Still, the limitations of patient reports are important to note. For instance, a recent cross-sectional study examined the use of PROIs in spine surgery and found approximately 206 unique PROIs with minimal expert consensus regarding measurements of outcome.16 With these factors in mind, there exist numerous areas where AI algorithms (eg, machine learning [ML], deep learning) can be used to improve care and develop consensus among surgeons.
Artificial Intelligence in Spine Surgery
There has been increasing attention and interest in the system-based benefits of AI and its applications to spine surgery.1,3,6,17 AI can help clinicians and hospital centers define the quality and cost of care, improve outcomes, and mitigate downrange financial exposures to institution and to payers.1-3 While there has also been controversy surrounding AI, if implemented appropriately, it has the potential to revolutionize the standard of care in spine surgery, reduce cost and waste, and improve the efficiency and patient care. In addition, AI could enhance individualized care to patients and reduce heterogeneity in both clinical practice and research.1,3,18
Preoperative Patient Care and Outcome Prediction
As an illustrative example, a patient with medically refractory mechanical back pain and radiculopathy secondary to a single-level, degenerative lumbar spondylolisthesis could potentially undergo a lumbar laminectomy, laminoforaminotomy, laminectomy with in situ posterolateral fusion, laminectomy with instrumented posterolateral fusion, transforaminal interbody fusion, anterior lumbar interbody fusion, lateral interbody fusion, or some combination of the above.14,15 While there is evidence to support certain surgical treatments over others, a surgeon’s choice in treatment is often dictated by training, experience, and personal performance.14 Furthermore, there are many patient-specific variables that influence cost and outcomes such as body mass index, the presence and severity of comorbidities, tobacco use, and psychosocial factors, to name a few. It is difficult, if not impossible, for the clinician to reconcile and weight all of the discrete data points and his/her personal performance when indicating such a patient for surgery. AI can assist with such decision making. While most published literature are level III evidence or expert-based guidelines, most cannot guide decision making for complex spine surgery or when there is clinical equipoise.
In this setting, AI could assist surgeons in identifying optimal surgical candidates, advise the surgeon on operative approaches, and predict the likelihood of success, cost, and/or payments of various treatment pathways.1,5,17,19 Ghogawala and colleagues examined this AI-driven approach in the setting of degenerative lumbar spondylolisthesis, specifically in utilizing expert-reviewed imaging data from the upcoming SLIP II study to create a supervised ML model.3 They also raise the issue of unsupervised or semisupervised modeling projects as well. While the SLIP II study will not suffer from this issue, these models require standardized methodologies of reporting radiology images, preoperative workup, surgical data, and PROIs, along with the high expense associated with obtaining expert opinions (often called data labeling) on the images. These innovative approaches could allow for a stronger guarantee of optimized patient outcomes in certified surgical candidates through ensuring proper surgical selection.
In addition, Ames and colleagues examined AI-driven risk prediction models in a retrospective multicenter study that examined preoperative decision making with the largest adult spinal deformity patient cohort to date.1 Their model predicted 2-year outcomes by constructing a visual risk-benefit grid; furthermore, their model also provides the surgeon insight into which surgical intervention would yield the highest probability of success with the lowest risk. Given that adult spinal deformity is a heterogeneous patient cohort, hierarchical clustering was used to create 3 distinct demographic/pathological and 4 distinct surgical clusters combined to produce 12 separate groups of patients for whom extensive patient-reported outcomes were collected. These groups were then compared on the basis of complication rates and postoperative disability and functional outcome scores, allowing surgeons to assess patients’ placement into these categories and subsequently predict the outcomes of their surgeries. In essence, these models successfully converted surgeons’ gestalt about a patient’s probability of surgical success into an accurate, reproducible, and homogenous clinical decision-making tool in a population of patients at high risk of poor outcome. Ideally, these tools will be developed for a variety of patient populations in the future.
While these innovative approaches to ML in spine surgery have not yet been scaled to encompass the whole field, models that help surgeons determine the probability of an adverse event following spine surgery have emerged in recent years. Arvind and Kim both developed AI models to predict surgical complications in patients after anterior cervical discectomy and fusion and posterior lumbar fusion, respectively.19,20 Both studies utilized a variety of different methodologies, including artificial neural networks, support vector machine, logistic regression, and random forest, all compared to the predictive power of the American Society of Anesthesiologists physical status classification for predicting postoperative complications. While the authors hypothesized that the receiver-operating characteristics of their model would improve as more patient information was added to the database over time, very few of their models performed with an area under the receiver operating characteristic curve (AUC) greater than 0.7. Furthermore, their predictive values for models utilizing American Society of Anesthesiologists physical status classification were often worse than chance (AUCs 0.35 to 0.57), which calls into question the validity of these models as well. Efforts by Stopa and colleagues using ML algorithms to predict patients at risk of nonroutine discharge after elective lumbar spine surgery have fared better, as their external validation of a previously developed model for nonroutine discharge netted an AUC of 0.89.5 Regardless, ML models that help surgeons predict complications preoperatively will inevitably improve with the availability of more data, hopefully to the point of effective clinical implementation.
Research
At its core, AI is fundamentally a research tool that could be both powerful and disruptive to the current body of spine surgery literature. As ML applications improve, this may ultimately lead to a paradigm shift in the way evidence-based guidelines are used and interpreted. Most expert-based, societal guidelines are developed through modified Delphi approaches with expert panels.21 The evidence used to create the guidelines that ultimately dictate the standard practice of spine surgery is mostly derived through large, multicenter, retrospective database studies. The results produced from these studies are wholly dependent on the data that is utilized to generate these hypotheses.22
AI-based research enables clinical data to speak for itself. Rather than utilizing a data mining approach, which drives much spine research, AI has the ability to revolutionize the field. To best appreciate the potential breakthroughs that may result from exploiting AI in spine research, it is useful to survey the advances that have already been made in this arena. In particular, a recent review by Galbusera and colleagues identified several key areas of spine research that have benefited from AI and ML, namely, diagnostic imaging, outcome prediction, clinical decision support, and biomechanics.2
Diagnostic Imaging
The classification of degenerative discs via feature-extraction from MRI (magnetic resonance imaging) imaging exemplifies the critical contributions that AI has already made to the field of spine surgery.2 By extracting salient features of discs, such as the shape and intensity, and utilizing convoluted neural networks, which are intrinsically adept at processing visual data, this AI-based algorithm was able to achieve a 70.1% concordance with human observations, which is extremely comparable to the documented rate of agreement between individual expert radiologists (70.4%).2,23,24 With further training, the accuracy of this classification scheme improved to 97%.2 This Pfirrman classification system, named after the radiologist who first described it, is commonly utilized in clinical practice and allows standardization of this diagnostic classification.2,23 Other areas of spine research to profit from AI include evaluation of severity in adolescent idiopathic scoliosis by utilizing surface topography and support vector machines and classification of spinal deformities through automated Cobb angle analyses.2 In doing so, it complements surgeon expertise with standardized recommendations derived from large collections of patient data, and thereby decreases the subjectivity associated with these tasks.
Advances in technology are facilitating the transformation of image analysis, from qualitative, subjective assessment to acquisition of quantitative, reproducible data. Many computer vision ML applications are focused on lesion detection on conventional images. But the truly transformative innovations involve analysis of tissues at a pixel level with MR fingerprinting and texture analysis and acquisition and analysis of tissue properties with synthetic MRI.25 In contrast to subjective visual analysis of signal intensity differences in a conventional MR image, synthetic MRI produces objective numeric values that can be used with or without production of actual images. Applications for spine surgery could include preoperative assessment of osteoporosis, and postoperative assessment of changes in disc composition, marrow composition, or spinal cord or nerve root tissue characterization.26 In the future, quantitative data acquisition with AI analysis will allow direct transfer of information, with characterization and interpretation of patient pathology, from imaging equipment to the surgeon, without the need for radiology interpretation and reports.
The adoption of AI tools by other members of the larger health care team will also affect surgical evaluation and workflow. Algorithms that provide image augmentation with deep learning have recently been cleared by the Federal Drug Administration (FDA).27 This technology facilitates MR image acquisition in a fraction of the normal study time, by collecting less data and using the algorithm to interpolate the missing information based on training from large, high-quality data sets. These time savings benefit the surgeon and patient: exams are substantially shorter, reducing motion artifact and enhancing image quality; patient satisfaction is improved with less time in the MR scanner; and the time from initial order to delivered report is substantially decreased. Algorithms that automate evaluation of spinal hardware location and integrity on routine longitudinal radiographic follow-up are currently being developed. These AI tools can analyze multiple prior studies in a fraction of the time and cost of a human interpretation.
Outcome Prediction
Predictive models using ML techniques such as decision tree and random forest enable surgeons to anticipate everything from the best course of care—surgical intervention versus conservative, for example—to operative complications, such as those following spinal deformity surgery. The complexity of these tasks render them poor candidates for traditional statistical methods such as logistic regression and more promising candidates for ML, which is far more proficient in processing nonlinear data and identifying relationships not superficially apparent or readily found using statistical models alone. Indeed, studies have shown that statistical predictive models of complications in spine surgery—surgical site infection, for example—often perform poorly on external validation, indicating that the corresponding results are representative only of a specific cohort and cannot be generalized to the patient population.17 On the contrary, ML techniques have shown remarkable promise in tasks of similar complexity. For instance, a large retrospective study recently described a random forest approach to predicting intraoperative and perioperative complications following spinal deformity surgery that reached an accuracy of 87.6%.2,18
Translational Research
Integration of AI into biomechanical investigations represents yet another frontier of spine surgery research. While the usage of AI in this field is still in its infancy, AI has promising applications in this arena. Analysis of gait and motion patterns, along with identification of abnormal gait in spinal disorders, represents one area that can benefit from AI usage.28-30 While this field has traditionally relied on integrating discrete variables such as velocity and angle of joint rotation to quantitatively describe motion, ML allows identification and classification of abnormal gait patterns that cannot be detected with traditional methods alone.28-30 Additionally, ML can also be utilized to estimate biomechanical variables such as stress and load on specific joints and predict how bone and tissue would respond to such forces. If such an algorithm were optimized, it would allow the surgeon to tailor the treatment to the individual patient and the specific biomechanical properties of their spine.
Perioperative Assistance and Robotic Surgery
Over the past several years, technical advancements in surgical simulation, augmented reality, and robotic-assisted spine surgery have led to fundamental changes in spine surgery practice. While perioperative AI platforms are currently in development and still experimental at the time of this writing, the 2 most prominent technological advances in the modern era of spine surgery, namely, neuronavigation and surgical robotics, have the potential to incorporate AI and are especially well suited to AI.4,31 For one, robotic-assisted spine surgery is often associated with minimally invasive procedures that feature small incisions and exposures. As such, perioperative surgical planning and intraoperative navigation become exceedingly important to facilitate optimal outcomes and minimize iatrogenic injury. Since image processing is a key strength of AI programs, neuronavigation stands to particularly benefit from evolving AI techniques. Furthermore, AI can facilitate the individualization of management and surgical planning to each patient. By accounting for anatomical variations among patients, the image processing prowess of AI allows exact reconstruction of relevant spinal anatomy during surgical planning.
The advent of advanced navigation technologies allows surgeons construct a 3-dimensional rendering of the spine that provides real-time positional feedback during the operation, thereby allowing visualization of deeper structures. Thus, intraoperatively, the operating team can also use AI-powered image guidance to direct anatomical positioning of constructs and avoid iatrogenic injury. Indeed, computer-assisted navigation (CAN) platforms are widely utilized throughout operating rooms in the United States in operations ranging from resection of spinal tumors to spinal deformity surgery. Multiple studies have established that CAN usage not only improves accuracy in operative tasks like pedicle screw placement but also improves the efficiency of operations, thereby reducing the duration of generalized anesthesia and decreasing complications associated with screw misplacement.4,31 Furthermore, usage of CAN platforms can also reduce the need for fluoroscopic guidance during procedures and thereby decrease exposure to harmful radiation.4,31
In addition to CAN, surgical robotics have the potential to transform the field of spine surgery by increasing precision, thereby lowering complications from human error, and efficiency (Table 1).32,33 One of the biggest challenges in spine surgery is maintaining precision and accuracy of motion during lengthy operations. It is not only human to error, but also human to fatigue, and even the best trained spine surgeon is not an exception to this human attribute. Robotics address these drawbacks by providing a precision and indefatigability impossible to consistently reproduce in a surgeon. To appreciate the contributions of surgical robots to spine surgery, it is useful to discuss 4 of the most widely used and well-studied robots: SpineAssist (MAZOR Robotics Inc, Caesarea, Israel), ROSA (Medtech, SA, Montpellier, France), the Excelsius GPS Robot (Globus Medical, Inc, Audubon, PA), and the Da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA).4,31,33-37
Table 1.
Review of Published Literature Examining the Precision and Accuracy of Robotic Pedicle Screw Placement.
| Author, Year | Robotic System | Study Design | Number of Patients, Pedicle Screws | Findings |
|---|---|---|---|---|
| Elswick et al, 202057 | Excelsius GPS | Retrospective review | 28 patients, 127 screws | 97.6% accuracy with robot |
| Zygourakis et al, 201858 | Excelsius GPS | Case report | 1 patient, 8 screws | Successful revision decompression and fusion from L3 to S1 |
| Chenin et al, 201732 | ROSA | Retrospective review | 25 patients, 110 screws | 96.3% accuracy with robot |
| Lonjon et al, 201659 | ROSA | Retrospective review | 10 patients, 40 screws | 97.3% accuracy with robot |
| Hyun et al, 201736 | SpineAssist | Prospective randomized trial | 30 patients, 130 screws | 100% accuracy with robot |
| Keric et al, 201739 | SpineAssist | Retrospective review | 66 patients, 341 screws | 90.0% accuracy with robot |
| Kim et al, 201747 | SpineAssist | Prospective randomized trial | 37 patients, 158 screws | 99.4% accuracy with robot |
| Kuo et al, 201648 | SpineAssist | Retrospective review | 64 patients, 317 screws | 98.7% accuracy with robot; K-wire deviation is usually caudal and lateral |
| Macke et al, 201649 | SpineAssist | Retrospective review | 50 patients, 662 screws | 92.7% accuracy with robot in patients with adolescent idiopathic scoliosis |
| Bederman et al, 201644 | SpineAssist | Retrospective review | 14 patients, 31 screws | 100% accuracy with robot |
| Sensakovic et al, 201652 | SpineAssist | Retrospective review | 34 patients | Radiation dose reduced with robotic pediatric spine surgery |
| Tsai et al, 201653 | SpineAssist | Retrospective review | 35 patients, 176 screws | 98.9% accuracy with robot; robotic grading classification system assesses K-wire placement accuracy |
| van Dijk et al, 201554 | SpineAssist | Retrospective review | 112 patients, 494 screws | 97.9% accuracy with robot |
| Hu et al, 201541 | SpineAssist | Retrospective review | 9 patients | Robotic spinal tumor surgery appears safe |
| Schatlo et al, 201542 | SpineAssist | Retrospective review | 258 patients, 1265 screws | 96.2% accuracy; peak in screw inaccuracy between cases 10 and 20 |
| Onen et al, 201437 | SpineAssist | Retrospective review | 27 patients, 136 screws | 98.6% accuracy with robot; reduced radiation exposure |
| Schatlo et al, 201443 | SpineAssist | Retrospective review | 55 patients, 244 screws | 91.4% accuracy with robot |
| Hu and Lieberman, 201435 | SpineAssist | Retrospective review | 162 patients | Accuracy increases with surgeon experience |
| Dreval et al, 201445 | SpineAssist | Retrospective review | 77 patients | Robotic surgery can be used for GO-LIF and spine tumor surgery |
| Hu et al., 201338 | SpineAssist | Retrospective review | 102 patients, 1085 screws | 98.9% accuracy with robot |
| Roser et al, 201355 | SpineAssist | Prospective randomized trial | 18 patients, 72 screws | 99% accuracy with robot |
| Ringel et al, 201256 | SpineAssist | Prospective randomized trial | 30 patients, 146 screws | 85% accuracy with robot (lower than freehand) |
| Schizas et al, 201251 | SpineAssist | Retrospective review | 11 patients, 64 screws | 95% accuracy with robot |
| Kantelhardt et al, 201146 | SpineAssist | Retrospective review | 55 patients, 250 screws | 95% accuracy with robot |
| Devito et al, 201033 | SpineAssist | Retrospective review | 635 patients, 3271 screws | 98.3% accuracy with robot |
| Pechlivanis et al, 200950 | SpineAssist | Prospective case series | 31 patients, 133 screws | 99.2% accuracy with robot |
| Sukovich et al, 200640 | SpineAssist | Retrospective review | 14 patients, 98 screws | 96.0% accuracy with robot |
| Barzilay et al, 200634 | SpineAssist | Prospective case series | 15 patients | Described technical challenges |
The SpineAssist robot mounts directly onto bony landmarks, such as a spinous process, during the operation.32,33,38-40 With 6 degrees of freedom, this device allows for precise positioning of surgical instruments and interface with a CAN, ultimately identifying the most accurate location for pedicle placement based on the location of the designated entry point and screw trajectory.4,31,41-43 Several studies have documented the accuracy of the SpineAssist robot.44-54 For instance, Roser et al found an accuracy rate of 99% in pedicle placement with SpineAssist, compared with an accuracy of 92% with navigation only.55 Interestingly, a randomized control trial by Ringel et al was the only study to find a significantly decreased accuracy associated with screw placement using SpineAssist robots (85% vs 93% for fluoroscopy-guidance, P = .019), though the authors note that this result could be in part due to placement of only one K-wire as recommended by the manufacturer.56 Furthermore, the authors note that the pedicle screw entry point usually slopes down laterally, which can lead to slipping of the cannula and subsequent lateral breach during pedicle screw placement. All of these concerns came without a corresponding decrease in intraoperative radiation exposure, and surgeons should take these into account when utilizing the SpineAssist system.
The Excelsius Robot System, which gained FDA approval in 2017, has been the subject of an operative technique case report and a case series since its approval.57,58 Its rigid arm and freestanding nature allow it to be utilized without rigid fixation to the spine.58 While elementary compared to conventional CAN systems, the Excelsius system allows for navigational capacity, which could allow for minimally invasive technique utilization and the minimization of intraoperative radiation exposure to the surgical team. A case series of the Excelsius system suggests that up to 97.6% of pedicle screws can be placed with a GRS A or B rating, with all of the placement errors in the reported case series being on patient’s left side and most likely to happen at the L5 level.57 However, unlike the SpineAssist, randomized evidence is currently lacking, and further studies will be needed to elucidate specific outcomes for this device in comparison to freehand pedicle screw placement.
The ROSA robot, though initially designed for intracranial operations, can also be adopted in spine surgery and may even address some shortcomings associated with SpineAssist.4,31,32 A freestanding robotic assistant, the ROSA does not require fixation to any part of the spine, thereby alleviating errors associated with incorrect fixation, as described by Ringel et al. As the robotic arm moves with the patient via a camera that monitors movement of the patient through several percutaneously placed pins on bony landmarks, there is a further risk reduction for disconnect between the system and the patient during surgery. While this technology has not yet been validated for spine surgery, preliminary results are promising and indicate that the ROSA has an accuracy of 97.3% in pedicle screw instrumentation, compared with 92% in the fluoroscopy-guided group, although this difference was not statistically significant.4,31 Furthermore, it was associated with more than 70 extra minutes added to the operative time and increased, rather than decreased, radiation exposure for the surgical team.59 The authors attribute these difficulties to the learning curve of using the new device and small number of patients in the study. These results, however, highlight the vital importance of further study on this system before widespread adoption in spine surgery.60-62
Last, the Da Vinci Surgical System, perhaps the most well-known robotic suite was approved by the FDA in 2000 for laparoscopic procedures such as hysterectomies.4,31 The Da Vinci exemplifies the telesurgical model, in which the surgeon operates the robot from a remote booth that is equipped with technology allowing the surgeon to control the robotic arms and override their actions.4,31 This model lends itself well to training and education, as it strikes a balance between autonomy and oversight. Despite the abandonment of the laparoscopic approach to the anterior lumbar interbody fusion, the Da Vinci holds promise in the area of a minimally invasive approach to anterior lumbar interbody fusion, and a number of case reports and small studies have begun to examine this topic in animals and humans. While this system is not yet FDA approved for placement of instrumentation, it carries promising potential in this arena, and further studies of its accuracy in instrumentation placement will be needed to assess its long-term value as a disruptive innovation in spine surgery.
It is important to note that while surgical robotics are clearly advancing spine surgery, they do not supplement the surgeon. Even in the most technically challenging cases, which would benefit from the increased precision of robotics, it is still the surgeon that makes operative decisions and guides the robots in their function. Like any instrument, surgical robots are tools—albeit particularly powerful ones—in the spine surgeon’s armamentarium. Furthermore, there is a large cost burden to overcome with surgical robotic devices, which may prove to be a barrier to widespread implementation of these devices in the future. Future research is necessary to explore the cost-effectiveness of these technologies and to expand the repertoire of operations for robots like the ones described in this review. While AI-driven technologies for perioperative surgical assistance are still in their infancy, the potential for predictive analytics to guide the robotic-guided fusions is certainly on the horizon.
Conclusion
Artificial intelligence has tremendous potential in revolutionizing comprehensive spine care. AI’s evidence-based, predictive analytics can help surgeons improve preoperative patient selection, surgical indications, and improve individualized postoperative care. In the realm of research, AI computing capacity can be used to collect, process, and analyze volumes of patient information to extract valuable clinical information for studies. Robotic-assisted surgery, while still new and improving, has potential to help reduce surgeon fatigue and improve technical precision. Ultimately, in the ever-evolving landscape of spine surgery, one thing is certain: artificial intelligence technologies have arrived—and they are here to stay.
Footnotes
Authors’ Note: All authors contributed to the creation of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jonathan J. Rasouli, MD  https://orcid.org/0000-0002-5085-8422
https://orcid.org/0000-0002-5085-8422
References
- 1. Ames CP, Smith JS, Pellise F, et al. Artificial intelligence based hierarchical clustering of patient types and intervention categories in adult spinal deformity surgery: towards a new classification scheme that predicts quality and value. Spine (Phila Pa 1976). 2019;44:915–926. [DOI] [PubMed] [Google Scholar]
- 2. Galbusera F, Casaroli G, Bassani T. Artificial intelligence and machine learning in spine research. JOR Spine. 2019;2:e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghogawala Z, Dunbar M, Essa I. Artificial intelligence for the treatment of lumbar spondylolisthesis. Neurosurg Clin N Am. 2019;30:383–389. [DOI] [PubMed] [Google Scholar]
- 4. Kochanski RB, Lombardi JM, Laratta JL, Lehman RA, O’Toole JE. Image-guided navigation and robotics in spine surgery. Neurosurgery. 2019;84:1179–1189. [DOI] [PubMed] [Google Scholar]
- 5. Stopa BM, Robertson F, Karhade AV, et al. Predicting nonroutine discharge after elective spine surgery: external validation of machine learning algorithms. J Neurosurg Spine. 2019;26:1–6. [DOI] [PubMed] [Google Scholar]
- 6. Buchlak QD, Esmaili N, Leveque JC, et al. Machine learning applications to clinical decision support in neurosurgery: an artificial intelligence augmented systematic review [published online August 17, 2019]. Neurosurg Rev. doi:10.1007/s10143-019-01163-8 [DOI] [PubMed] [Google Scholar]
- 7. Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parker SL, Chotai S, Devin CJ, et al. Bending the cost curve-establishing value in spine surgery. Neurosurgery. 2017;80(3S):S61–S69. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Medicare and Medicaid Services. NHE fact sheet. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet . Accessed March 11, 2020.
- 10. Waterman BR, Belmont PJ, Jr, Schoenfeld AJ. Low back pain in the United States: incidence and risk factors for presentation in the emergency setting. Spine J. 2012;12:63–70. [DOI] [PubMed] [Google Scholar]
- 11. McDermott KW, Freeman WJ, Elixhauser A. Statistical Brief #233: Overview of Operating Room Procedures During Inpatient Stays in U.S. Hospitals, 2014. Rockville, MD: Healthcare Cost and Utilization Project; 2006. [PubMed] [Google Scholar]
- 12. Larkin DJ, Swanson RC, Fuller S, Cortese DA. The Affordable Care Act: a case study for understanding and applying complexity concepts to health care reform. J Eval Clin Pract. 2016;22:133–140. [DOI] [PubMed] [Google Scholar]
- 13. Alvin MD, Lubelski D, Alam R, et al. Spine surgeon treatment variability: the impact on costs. Global Spine J. 2018;8:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mroz TE, Lubelski D, Williams SK, et al. Differences in the surgical treatment of recurrent lumbar disc herniation among spine surgeons in the United States. Spine J. 2014;14:2334–2343. [DOI] [PubMed] [Google Scholar]
- 15. Lubelski D, Williams SK, O’Rourke C, et al. Differences in the surgical treatment of lower back pain among spine surgeons in the united states. Spine (Phila Pa 1976). 2016;41:978–986. [DOI] [PubMed] [Google Scholar]
- 16. Guzman JZ, Cutler HS, Connolly J, et al. Patient-reported outcome instruments in spine surgery. Spine (Phila Pa 1976). 2016;41:429–437. [DOI] [PubMed] [Google Scholar]
- 17. Janssen DMC, van Kuijk SMJ, d’Aumerie BB, Willems PC. External validation of a prediction model for surgical site infection after thoracolumbar spine surgery in a Western European cohort. J Orthop Surg Res. 2018;13:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Durand WM, DePasse JM, Daniels AH. Predictive modeling for blood transfusion after adult spinal deformity surgery: a tree-based machine learning approach. Spine (Phila Pa 1976). 2018;43:1058–1066. [DOI] [PubMed] [Google Scholar]
- 19. Kim JS, Merrill RK, Arvind V, et al. Examining the ability of artificial neural networks machine learning models to accurately predict complications following posterior lumbar spine fusion. Spine (Phila Pa 1976). 2018;43:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arvind V, Kim JS, Oermann EK, Kaji D, Cho SK. Predicting surgical complications in adult patients undergoing anterior cervical discectomy and fusion using machine learning. Neurospine. 2018;15:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6:e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kilkenny MF, Robinson KM. Data quality: “garbage in—garbage out”. Health Inf Manag. 2018;47:103–105. [DOI] [PubMed] [Google Scholar]
- 23. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26:1873–1878. [DOI] [PubMed] [Google Scholar]
- 24. Ruiz-Espana S, Arana E, Moratal D. Semiautomatic computer-aided classification of degenerative lumbar spine disease in magnetic resonance imaging. Comput Biol Med. 2015;62:196–205. [DOI] [PubMed] [Google Scholar]
- 25. Vargas MI, Drake-Perez M, Delattre BMA, Boto J, Lovblad KO, Boudabous S. Feasibility of a synthetic MR imaging sequence for spine imaging. AJNR Am J Neuroradiol. 2018;39:1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nam KH, Seo I, Kim DH, Lee JI, Choi BK, Han IH. Machine learning model to predict osteoporotic spine with Hounsfield units on lumbar computed tomography. J Korean Neurosurg Soc. 2019;62:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaharchuk G, Gong E, Wintermark M, Rubin D, Langlotz CP. Deep learning in neuroradiology. AJNR Am J Neuroradiol. 2018;39:1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fukuchi RK, Eskofier BM, Duarte M, Ferber R. Support vector machines for detecting age-related changes in running kinematics. J Biomech. 2011;44:540–542. [DOI] [PubMed] [Google Scholar]
- 29. Leardini A, Biagi F, Merlo A, Belvedere C, Benedetti MG: Multi-segment trunk kinematics during locomotion and elementary exercises. Clin Biomech (Bristol, Avon). 2011;26:562–571. [DOI] [PubMed] [Google Scholar]
- 30. Zhang J, Lockhart TE, Soangra R. Classifying lower extremity muscle fatigue during walking using machine learning and inertial sensors. Ann Biomed Eng. 2014;42:600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Overley SC, Cho SK, Mehta AI, Arnold PM. Navigation and robotics in spinal surgery: where are we now? Neurosurgery. 2017;80(3 suppl):S86–S99. [DOI] [PubMed] [Google Scholar]
- 32. Chenin L, Capel C, Fichten A, Peltier J, Lefranc M. Evaluation of screw placement accuracy in circumferential lumbar arthrodesis using robotic assistance and intraoperative flat-panel computed tomography. World Neurosurg. 2017;105:86–94. [DOI] [PubMed] [Google Scholar]
- 33. Devito DP, Kaplan L, Dietl R, et al. Clinical acceptance and accuracy assessment of spinal implants guided with SpineAssist surgical robot: retrospective study. Spine (Phila Pa 1976). 2010;35:2109–2115. [DOI] [PubMed] [Google Scholar]
- 34. Barzilay Y, Liebergall M, Fridlander A, Knoller N. Miniature robotic guidance for spine surgery—introduction of a novel system and analysis of challenges encountered during the clinical development phase at two spine centres. Int J Med Robot. 2006;2:146–153. [DOI] [PubMed] [Google Scholar]
- 35. Hu X, Lieberman IH. What is the learning curve for robotic-assisted pedicle screw placement in spine surgery? Clin Orthop Relat Res. 2014;472:1839–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Minimally invasive robotic versus open fluoroscopic-guided spinal instrumented fusions: a randomized controlled trial. Spine (Phila Pa 1976). 2017;42:353–358. [DOI] [PubMed] [Google Scholar]
- 37. Onen MR, Simsek M, Naderi S. Robotic spine surgery: a preliminary report. Turk Neurosurg. 2014;24:512–518. [DOI] [PubMed] [Google Scholar]
- 38. Hu X, Ohnmeiss DD, Lieberman IH. Robotic-assisted pedicle screw placement: lessons learned from the first 102 patients. Eur Spine J. 2013;22:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keric N, Eum DJ, Afghanyar F, et al. Evaluation of surgical strategy of conventional vs. percutaneous robot-assisted spinal trans-pedicular instrumentation in spondylodiscitis. J Robot Surg. 2017;11:17–25. [DOI] [PubMed] [Google Scholar]
- 40. Sukovich W, Brink-Danan S, Hardenbrook M. Miniature robotic guidance for pedicle screw placement in posterior spinal fusion: early clinical experience with the SpineAssist. Int J Med Robot. 2006;2:114–122. [DOI] [PubMed] [Google Scholar]
- 41. Hu X, Scharschmidt TJ, Ohnmeiss DD, Lieberman IH. Robotic assisted surgeries for the treatment of spine tumors. Int J Spine Surg. 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schatlo B, Martinez R, Alaid A, et al. Unskilled unawareness and the learning curve in robotic spine surgery. Acta Neurochir (Wien). 2015;157:1819–1823. [DOI] [PubMed] [Google Scholar]
- 43. Schatlo B, Molliqaj G, Cuvinciuc V, Kotowski M, Schaller K, Tessitore E. Safety and accuracy of robot-assisted versus fluoroscopy-guided pedicle screw insertion for degenerative diseases of the lumbar spine: a matched cohort comparison. J Neurosurg Spine. 2014;20:636–643. [DOI] [PubMed] [Google Scholar]
- 44. Bederman SS, Hahn P, Colin V, Kiester PD, Bhatia NN. Robotic guidance for S2-alar-iliac screws in spinal deformity correction. Clin Spine Surg. 2017;30:E49–E53. [DOI] [PubMed] [Google Scholar]
- 45. Dreval ON, Rynkov IP, Kasparova KA, Bruskin A, Aleksandrovskii V, Zil’bernshtein V. Results of using Spine Assist Mazor in surgical treatment of spine disorders. Zh Vopr Neirokhir Im N N Burdenko. 2014;78:14–20. [PubMed] [Google Scholar]
- 46. Kantelhardt SR, Martinez R, Baerwinkel S, Burger R, Giese A, Rohde V. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur Spine J. 2011;20:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim HJ, Jung WI, Chang BS, Lee CK, Kang KT, Yeom JS. A prospective, randomized, controlled trial of robot-assisted vs freehand pedicle screw fixation in spine surgery. Int J Med Robot. 2017;13. doi:10.1002/rcs.1779 [DOI] [PubMed] [Google Scholar]
- 48. Kuo KL, Su YF, Wu CH, et al. Assessing the intraoperative accuracy of pedicle screw placement by using a bone-mounted miniature robot system through secondary registration. PLoS One. 2016;11:e0153235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Macke JJ, Woo R, Varich L. Accuracy of robot-assisted pedicle screw placement for adolescent idiopathic scoliosis in the pediatric population. J Robot Surg. 2016;10:145–150. [DOI] [PubMed] [Google Scholar]
- 50. Pechlivanis I, Kiriyanthan G, Engelhardt M, et al. Percutaneous placement of pedicle screws in the lumbar spine using a bone mounted miniature robotic system: first experiences and accuracy of screw placement. Spine (Phila Pa 1976). 2009;34:392–398. [DOI] [PubMed] [Google Scholar]
- 51. Schizas C, Thein E, Kwiatkowski B, Kulik G. Pedicle screw insertion: robotic assistance versus conventional C-arm fluoroscopy. Acta Orthop Belg. 2012;78:240–245. [PubMed] [Google Scholar]
- 52. Sensakovic WF, O’Dell MC, Agha A, Woo R, Varich L. CT radiation dose reduction in robot-assisted pediatric spinal surgery. Spine (Phila Pa 1976). 2017;42:E417–E424. [DOI] [PubMed] [Google Scholar]
- 53. Tsai TH, Wu DS, Su YF, Wu CH, Lin CL. A retrospective study to validate an intraoperative robotic classification system for assessing the accuracy of Kirschner wire (K-wire) placements with postoperative computed tomography classification system for assessing the accuracy of pedicle screw placements. Medicine (Baltimore). 2016;95:e4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Dijk JD, van den Ende RP, Stramigioli S, Kochling M, Hoss N. Clinical pedicle screw accuracy and deviation from planning in robot-guided spine surgery: robot-guided pedicle screw accuracy. Spine (Phila Pa 1976). 2015;40:E986–E991. [DOI] [PubMed] [Google Scholar]
- 55. Roser F, Tatagiba M, Maier G. Spinal robotics: current applications and future perspectives. Neurosurgery. 2013;72(suppl 1):12–18. [DOI] [PubMed] [Google Scholar]
- 56. Ringel F, Stuer C, Reinke A, et al. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: a prospective randomized comparison to conventional freehand screw implantation. Spine (Phila Pa 1976). 2012;37:E496–E501. [DOI] [PubMed] [Google Scholar]
- 57. Elswick CM, Strong MJ, Joseph JR, Saadeh Y, Oppenlander M, Park P. Robotic-assisted spinal surgery: current generation instrumentation and new applications. Neurosurg Clin N Am. 2020;31:103–110. [DOI] [PubMed] [Google Scholar]
- 58. Zygourakis CC, Ahmed AK, Kalb S, et al. Technique: open lumbar decompression and fusion with the Excelsius GPS robot. Neurosurg Focus. 2018;45(video suppl 1):V6. [DOI] [PubMed] [Google Scholar]
- 59. Lonjon N, Chan-Seng E, Costalat V, Bonnafoux B, Vassal M, Boetto J. Robot-assisted spine surgery: feasibility study through a prospective case-matched analysis. Eur Spine J. 2016;25:947–955. [DOI] [PubMed] [Google Scholar]
- 60. Beutler WJ, Peppelman WC, Jr, DiMarco LA. The da Vinci robotic surgical assisted anterior lumbar interbody fusion: technical development and case report. Spine (Phila Pa 1976). 2013;38:356–363. [DOI] [PubMed] [Google Scholar]
- 61. Lee JY, Bhowmick DA, Eun DD, Welch WC. Minimally invasive, robot-assisted, anterior lumbar interbody fusion: a technical note. J Neurol Surg A Cent Eur Neurosurg. 2013;74:258–261. [DOI] [PubMed] [Google Scholar]
- 62. Yang MS, Yoon DH, Kim KN, et al. Robot-assisted anterior lumbar interbody fusion in a Swine model in vivo test of the da Vinci surgical-assisted spinal surgery system. Spine (Phila Pa 1976). 2011;36:E139–E143. [DOI] [PubMed] [Google Scholar]