Abstract
Study Design:
This was a retrospective cohort study.
Objectives:
When anterior cervical osteophytes become large enough, they may cause dysphagia. There is a paucity of work examining outcomes and complications of anterior cervical osteophyte resection for dysphagia.
Methods:
Retrospective review identified 19 patients who underwent anterior cervical osteophyte resection for a diagnosis of dysphagia. The mean age was 71 years and follow-up, 4.7 years. The most common level operated on was C3-C4 (13, 69%).
Results:
Following anterior cervical osteophyte resection, 79% of patients had improvement in dysphagia. Five patients underwent cervical fusion; there were no episodes of delayed or iatrogenic instability requiring fusion. Fusion patients were younger (64 vs 71 years, P = .05) and had longer operative times (315 vs 121 minutes, P = .01). Age of 75 years or less trended toward improvement in dysphagia (P = .09; OR = 18.8; 95% CI 0.7-478.0), whereas severe dysphagia trended toward increased complications (P = .07; OR = 11.3; 95% CI = 0.8-158.5). Body mass index, use of an exposure surgeon, diffuse idiopathic skeletal hyperostosis diagnosis, surgery at 3 or more levels, prior neck surgery, and fusion were not predictive of improvement or complication.
Conclusions:
Anterior cervical osteophyte resection improves swallowing function in the majority of patients with symptomatic osteophytes. Spinal fusion can be added to address stenosis and other underlying cervical disease and help prevent osteophyte recurrence, whereas intraoperative navigation can be used to ensure complete osteophyte resection without breaching the cortex or entering the disc space. Because of the relatively high complication rate, patients should undergo thorough multidisciplinary workup with swallow evaluation to confirm that anterior cervical osteophytes are the primary cause of dysphagia prior to surgery.
Keywords: cervical spine, osteophyte, syndesmophyte, DISH, dysphagia, cheilectomy
Introduction
Anterior vertebral osteophytes and syndesmophytes are common radiological findings seen in the elderly adult population; yet they are rarely symptomatic.1-3 These may be caused by spinal degeneration, previous trauma, prior surgery, or pathological conditions such as diffuse idiopathic skeletal hyperostosis (DISH).4-6 When these bony growths become large enough, they can lead to dysphagia, dysphonia, dyspnea, and obstructive sleep apnea.3,6-8 Previous studies have shown that less than 1% of cervical osteophytes lead to dysphagia, and only 1.7% of cases of dysphagia are caused by cervical osteophytes.3,5,9 Osteophytes may cause dysphagia via multiple mechanisms, including mechanical compression of the esophagus, interference with normal epiglottis movement, compression of the Auerbach’s myenteric plexus, and the induction of inflammation and edema about the esophagus, which can lead to fibrosis and adhesions, preventing normal motility and causing cricopharyngeal spasm.10,11 Most cases can be treated nonsurgically with diet modification, muscle relaxants, anti-inflammatories, postural changes during eating, phonophoresis, and swallowing rehabilitation programs.12,13 When conservative treatment fails, surgical osteophyte resection can improve hyoid movement, leading to enhanced upper esophageal sphincter opening11 and symptoms of dysphagia.3,6,7,11,14-24
Given the relative rarity of symptomatic anterior cervical osteophytes being treated with surgical resection, the current literature consists of case reports and case series with relatively few patients.3,6,7,9,11,14-25 The purpose of this study was to review demographics, clinical characteristics, preoperative assessment, swallowing outcome, need for cervical fusion, delayed cervical instability, and osteophyte regrowth following primary resection of anterior cervical osteophytes as a treatment for dysphagia at our tertiary referral center. We also report our current preferred surgical workup and operative technique using intraoperative navigation.
Materials and Methods
Following institutional review board approval, we identified all patients who underwent anterior cervical osteophyte resection for a diagnosis of dysphagia over an 18-year period (1999-2017). This cohort was reviewed to determine patient demographics, clinical presentation, preoperative assessment, medical history, prior neck surgery, preoperative spinal alignment and size of osteophytes/syndesmophytes, swallowing function, and outcomes, including intraoperative and postoperative complications, concomitant cervical fusion, osteophyte regrowth, need for revision osteophyte removal, and improvement in swallowing function.
Preoperative swallowing function was measured using the dysphagia severity scale described by Miyamoto et al16 and the Functional Outcome Swallowing Scale (FOSS).16,26 Briefly, the dysphagia severity scale is graded as mild, moderate, or severe based on swallowing symptoms. Mild dysphagia was defined by abnormal sensation or painful swallowing, moderate dysphagia as difficulty swallowing solid boluses, and severe as unable to swallow small solid boluses or experiencing aspiration and coughing during swallowing.16 The FOSS staging system defines stage 0 as normal function; stage I, episodic or daily dysphagia; stage II, significant dietary modifications or prolonged mealtime; stage III, decompensated function, with weight loss of 10% or less of body weight; stage IV, severely decompensated, with weight loss of more than 10% body weight or severe aspiration with bronchopulmonary complications; and stage V, requiring nonoral feeding.
Patient Group
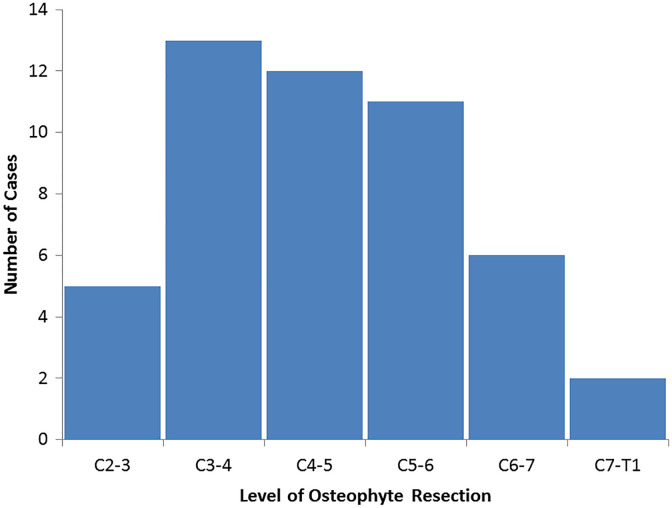
Over the study period, 19 patients underwent anterior cervical osteophyte resection for a diagnosis of dysphagia. There were 17 (89%) men, with a mean age of 71 years and mean body mass index (BMI) of 26.4 kg/m2. There were 3 (16%) current tobacco users and 10 (53%) former tobacco users. A preoperative diagnosis of diabetes mellitus was present in 11% (2) of patients. The mean length of preoperative dysphagia symptoms was 6.6 years. Mean clinical follow-up was 4.7 years (range, 2-10 years; Table 1). A total of 16 patients had final follow-up cervical spine imaging, with a mean of 2.4 years (range, 2.5 months to 6.5 years), after surgery. Also, 16 patients underwent swallow evaluation after surgery prior to starting an oral diet. The most common spinal level operated on was C3-4 (Figure 1), and the average number of levels operated on was 2.6 ± 1.7 levels (range 1-6 levels). Seven patients (37%) had a history of prior cervical spine or anterior neck surgery, 12 patients (63%) had a diagnosis of diffuse idiopathic skeletal hyperostosis (DISH), 1 patient (5%) had a diagnosis of ankylosing spondylitis, and 1 patient (5%) had rheumatoid arthritis.
Table 1.
Demographics of Patients Undergoing Anterior Cervical Osteophyte Resection for Dysphagia.
| Demographic | |
|---|---|
| Male patients | 17 (89%) |
| Female patients | 2 (11%) |
| Mean age (±SD) years | 70.6 ± 7.7 |
| Mean BMI (±SD) kg/m2 | 26.4 ± 4.8 |
| Mean follow-up (±SD) | 4.5 ± 2.0 |
Abbreviations: BMI, body mass index.
Figure 1.
Levels of anterior osteophyte resection in 19 patients undergoing surgery for a diagnosis of dysphagia.
Preoperative Assessment
Preoperative workup included video fluoroscopic swallowing exam and evaluation by an otolaryngologist (100%), computed tomography (CT) scan (74%), magnetic resonance imaging (MRI; 73%,) cervical spine X-rays (37%), electromyograph (5%), and evaluation by gastroenterology (47%), physical medicine and rehabilitation/speech (37%), and neurology (11%).
Surgical Treatment
Surgeries were performed by either a spine fellowship–trained orthopedic surgeon or neurosurgeon. An exposure surgeon trained in otolaryngology was utilized in 12 of the 19 cases (63%). All patients were positioned supine on a flat-top table and underwent general anesthesia. A Smith-Robinson approach with either a transverse or vertical oblique skin incision was utilized in all cases.6,27 In all joint cases, the otolaryngologist performed the exposure and the orthopedic spine or neurosurgery team performed the cheilectomy with or without fusion. Osteophyte resection was completed using a combination of osteotomes, punches, rongeurs, and high-speed drill with a diamond or matchstick bur. Fluoroscopic imaging or surgical navigation was used intraoperatively to assist with removal of the osteophyte complex.
Intraoperative Surgical Navigation
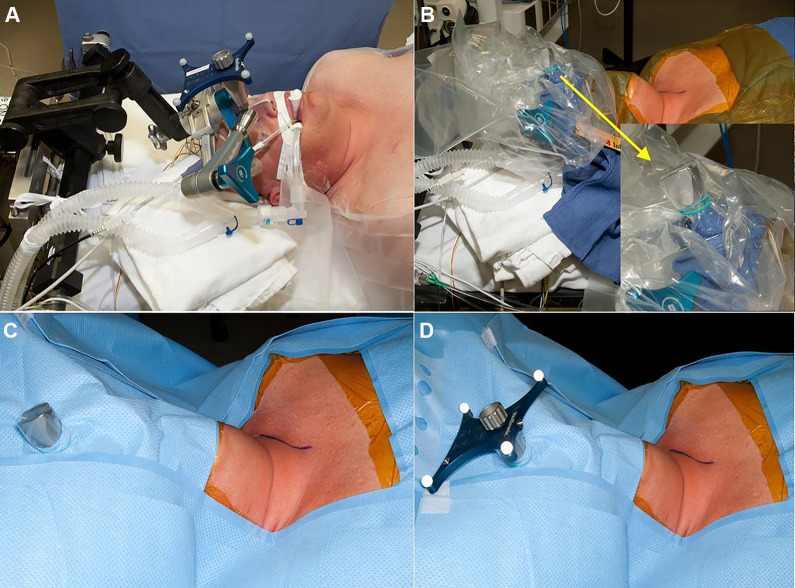
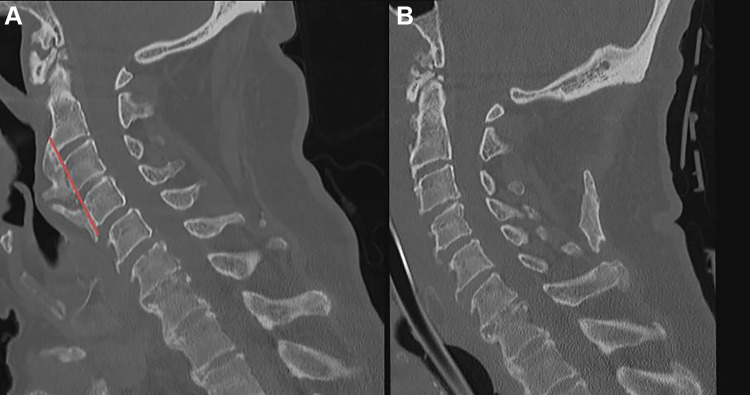
The StealthStation Surgical Navigation System (Stealth) and O-Arm Surgical Imaging System (Medtronic, Dublin, Ireland) was used for intraoperative surgical navigation. A Mayfield cranial stabilization system (Integra LifeSciences, Plainsboro, NJ) is used to hold the skull and cervical spine in place during the operation and allow for placement of the reference frame attachment (Figure 2). Exposure and provisional osteophyte removal are completed prior to O-Arm imaging. This allows intraoperative 3-D imaging to determine which portions and how much of the osteophyte(s) remain after provisional resection. Navigated probes and burs are then used for final osteophyte resection to the native anterior vertebral body cortex without breaching the cortex or entering the disc space (Figure 3).
Figure 2.
Example of draping for stereotactic navigation setup for cervical spine surgery. A Mayfield cranial stabilization system (Integra LifeSciences, Plainsboro, NJ) is used to hold the skull and cervical spine in place during the operation. The reference frame attachment for the stereotactic navigation setup is connected directly to the Mayfield (A). A sterile drape is then placed over the attachment and rubber banded in place (B). The patient is then draped in the usual sterile fashion, and a hole is cut in the drape to allow the attachment to come into the surgical field (C). The sterile reference frame is then placed on the attachment. This setup allows for sterility and excellent working space between the navigational tools and the reference frame.
Figure 3.
Intraoperative stereotactic navigation can be used to determine the junction between the pathological osteophyte and the native anterior cortex and disc space, thereby ensuring complete but not overresection. Either of these 2 extremes is easily obtained without some measure of image guidance to ensure that the osteophyte is completely resected, whereas the native spinal column elements are preserved for stability. Image (A) demonstrates patients with anterior cervical syndesmophytes with significant compression on the esophagus at C3-4. The red line is the planned resection of osteophytes to remove esophageal compression without entering the disc space and destabilizing the cervical spine. Image (B) demonstrates resection of syndesmophytes after surgical resection using stereotactic navigation intraoperatively.
Statistical Analysis
Continuous variables between groups were analyzed using the Student t-test or Wilcoxon test; categorical variables were compared using the Fisher exact test; and odds ratios were calculated when feasible. Multivariate nominal logistic regression analysis of surgical and patient characteristics, including age >75 years, BMI >27.5 kg/m2, and preoperative dysphagia severity, was completed to determine impact on improvement in dysphagia and complications after surgery. A P value <.05 was considered significant for all statistical analyses.
Results
The average size of resected osteophytes was 14.2 mm (4-23.9 mm); 8 patients (42%) had the maximal size of the osteophyte at the C3-4 level, 8 (42%) at the C4-5 level, and 3 (16%) at the C5-6 level. Cheilectomy was performed at a single level in 7 patients, 2 levels in 5 patients, and 3 or more levels in 7 patients. Patients with a diagnosis of DISH underwent surgery at more levels than those patients without a DISH diagnosis (3.1 ± 2.0 vs 1.7 ± 0.8 levels; P = .05).
Prior to surgery, 7 patients (37%) had severe dysphagia, 11 (58%), moderate dysphagia, and 1 (5%), mild dysphagia (Table 2). There was no correlation between dysphagia severity and osteophyte size (P = .5) or number of levels operated on (P = .3). When dysphagia was measured using FOSS, 6 patients (32%) had stages II, III, and IV, each, whereas 1 patient (5%) had a stage of V. There was no correlation between FOSS and osteophyte size (P = .44) or number of levels operated on (P = .94). More than half of the patients (53%) reported significant weight loss prior to surgery (mean 11.8 lb; 0-40 lb).
Table 2.
Patient Characteristics, Surgical Characteristics, Dysphagia Improvement, and Complications of Those Undergoing Primary Anterior Cervical Osteophyte Resection for Dysphagia.a
| Patient | Age and Sex | Clinical Follow-up (Radiographic Follow-up, in Years) | Previous Surgery | Dysphagia Severity and FOSS | Operative Levels | Fusion | Improvement in Dysphagia | Osteophyte Regrowth | Complication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 76, M | 2.7 (2.7) | Tracheostomy and anterior neck skin grafting | Severe, IV | C3-C4 | No | No | Left-sided vocal cord paralysis (chronic) and right-side vocal cord paresis (acute), PEG | |
| 2 | 67, M | 5.0 (5.0) | Moderate, II | C3-C5 | No | Yes | C3-4 and C4-5 (11 mm at 5 years) | ||
| 3 | 62, M | 9.8 (1.8) | Severe, IV | C3-C7 | C3-7 PSF | Yes | Pseudoarthrosis at C6-7 | ||
| 4 | 64, F | 4.7 (4.0) | Thyroidectomy | Moderate, III | C2-T1 | C5-6 ACDF | Yes | Esophageal injury leading to deep infection, diskitis/osteomyelitis, and pseudoarthrosis | |
| 5 | 77, M | 3.3 (2.2) | C4-5 ACDF | Severe, IV | C5-C6 | No | Some | C5-6 (12 mm at 2 years) | Aspiration pneumonia |
| 6 | 76, M | 5.9 (0.9) | Thyroid surgery | Moderate, IV | C3-C7 | No | Yes | ||
| 7 | 71, M | 6.1 (0.3) | Severe, V | C2-C3 | No | Yes | |||
| 8 | 70, M | 6.2 (3.5) | Moderate, II | C4-C5 | No | Yes | C4-5 (7 mm at 3.5 years) | ||
| 9 | 66, M | 7.2 | Moderate, II | C4-C7 | No | Yes | |||
| 10 | 79, M | 5.7 (5.0) | Moderate, III | C3-C4 | C3-4 ACDF | Yes | C2-3 (6 mm at 5 years) | ||
| 11 | 65, M | 2.0 (0.3) | C5-7 ACDF | Moderate, II | C5-C6 | No | Yes | ||
| 12 | 63, M | 2.4 (1.3) | Moderate, II | C4-C6 | C4-5 ACDF | Yes | PEG | ||
| 13 | 88, M | 2.1 (0.3) | Severe, IV | C2-C7 | No | Some | PEG | ||
| 14 | 75, M | 2.1 (0.3) | Moderate, III | C2-T1 | No | Yes | |||
| 15 | 67, F | 7.3 (6.5) | C5-6 ACDF | Moderate, III | C3-5 | No | Some | C2-3 and C3-4 (13 mm at 6.5 years) | |
| 16 | 54, M | 5.5 (5.0) | Mild, II | C2-6 | C2-6 ACF | Yes, and improved breathing | |||
| 17 | 75, M | 5.1 (0.2) | C4-5 ACDF | Severe, III | C3-4 and C5-6 | No | Yes | Aspiration pneumonia | |
| 18 | 75, M | 3.7 | Severe, IV | C3-4 | No | Yes | |||
| 19 | 72, M | 2.6 | Moderate, III | C3-5 | No | Yes | Superior laryngeal nerve injury with dysphonia |
Abbreviations: FOSS, Functional Outcome Swallowing Scale; M, male; F, female; PEG, percutaneous endoscopic gastrostomy; PSF, posterior spinal fusion; ACDF, anterior cervical discectomy and fusion; ACF, anterior cervical fusion.
a Bold text in the Osteophyte Regrowth column indicates asymptomatic regrowth of osteophytes.
Following anterior cervical osteophyte resection for a diagnosis of dysphagia, 15 of the 19 patients (79%) had a significant improvement in their dysphagia, 3 had some improvement (16%), and 1 had no improvement (5%; Figure 4). Mean time to improvement in dysphagia was 36 days (range 1-244 days). The average increase in BMI after surgery was 2.1 kg/m2. Patients who underwent cheilectomy at C5-6 and below showed a 50% rate of improvement compared with a rate of improvement of 82% in patients who had osteophytes removed from the C4-5 level or above (P = .39). Patients ≤75 years old saw improvement in their dysphagia 93% of the time compared with only 40% in patients >75 years old. This was significant on univariate analysis (P = .04; OR = 19.5; 95% CI = 1.3-292.8) and trended toward significance on multivariate nominal logistic regression analysis (P = .09; OR = 18.8; 95% CI = 0.7-478.0). Use of an exposure surgeon, surgery at 3 or more levels, prior neck surgery, fusion at the time of cheilectomy, osteophyte regrowth, and DISH diagnosis were not found to be predictive of improvement on univariate analysis (Table 3). BMI >27.5 kg/m2 and severe preoperative dysphagia were not independent prognostic factors on univariate or multivariate analysis (Table 4).
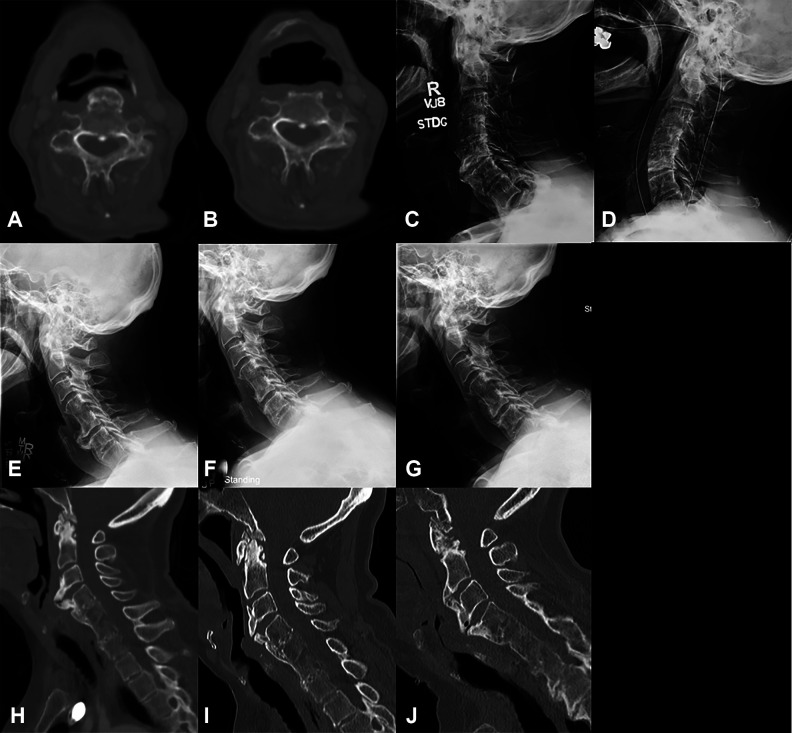
Figure 4.
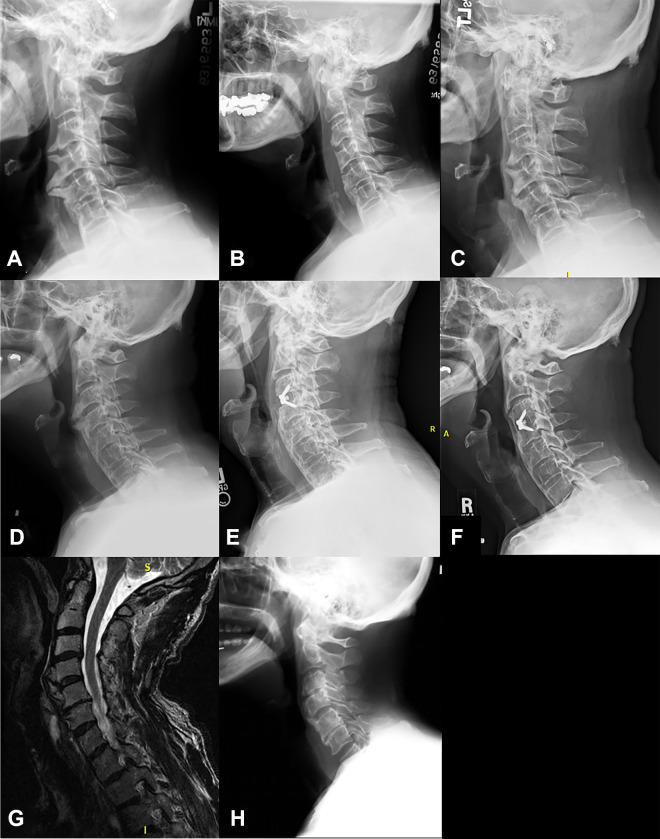
Imaging of patients who did not see significant improvement in their dysphagia after anterior cervical osteophyte resection. Axial computed tomography (CT) scan through the C4 vertebral body preoperatively (A) and 2 months postoperatively of patient 1 showing minimal residual anterior osteophyte and decreased esophageal compression. Lateral X-rays of patient 13, preoperatively (C) and 1 month postoperatively (D) showing full resection of osteophytes. Radiographic imaging of patient 5 who had a 16-mm osteophyte at C5-6 preoperatively (E), 6 mm postoperatively (F), and osteophyte regrowth to 11.5 mm 2 years postoperatively (G). CT imaging of patient 15 who had a 16.5 mm osteophyte at C3-4 preoperatively (H), 8 mm 14 months postoperatively (I), and regrowth to 13 mm at 6.5 years after osteophyte resection (J).
Table 3.
Factors Predictive of Improvement of Dysphagia and Complication Following Cheilectomy Using Univariate Analysis.
| Improvement | P | OR | CI, Lower 95% | CI, Upper 95% |
|---|---|---|---|---|
| Age 75 years or less | .04a | 19.50 | 1.30 | 292.75 |
| Exposure surgeon | .60 | 2.00 | 0.21 | 18.69 |
| BMI > 27.5 kg/m2 | .58 | 3.43 | 0.29 | 40.95 |
| Surgery at 3 or more levels | 1.00 | 2.00 | 0.17 | 24.07 |
| Severe dysphagia | .18 | 0.12 | 0.01 | 1.53 |
| Prior neck surgery | .60 | 0.50 | 0.05 | 4.67 |
| Fusion at time of cheilectomy | .53 | NAb | NAb | NAb |
| DISH | .60 | 2.00 | 0.21 | 18.69 |
| Osteophyte regrowth | .18 | 0.15 | 0.01 | 1.80 |
| Complication | P | OR | CI, Lower 95% | CI, Upper 95% |
| Age 75 years or less | .60 | 0.37 | 0.05 | 3.01 |
| Exposure surgeon | .38 | 0.38 | 0.06 | 2.55 |
| BMI > 27.5 kg/m2 | .17 | 0.19 | 0.03 | 1.43 |
| Surgery at 3 or more levels | 1.00 | 1.05 | 0.16 | 6.92 |
| Severe dysphagia | .07 | 7.50 | 0.92 | 61.05 |
| Prior neck surgery | 1.00 | 1.05 | 0.16 | 6.92 |
| Fusion at the time of cheilectomy | .60 | 2.70 | 0.33 | 21.98 |
| DISH | 1.00 | 0.95 | 0.14 | 6.28 |
| Osteophyte regrowth | .60 | 0.38 | 0.03 | 4.55 |
Abbreviations: BMI, body mass index; OR, odds ratio; DISH, diffuse idiopathic skeletal hyperostosis.
b Unable to calculate OR because all patients with fusion showed improvement.
Table 4.
Factors Predictive of Improvement of Dysphagia and Complication Following Cheilectomy Using Nominal Regression Multivariate Analysis.
| Factors Predictive of Improvement | P Value | OR | CI, Lower 95% | CI, Upper 95% |
|---|---|---|---|---|
| Age 75 years or less | .10 | 15.91 | 0.62 | 409.21 |
| BMI above 27.5 kg/m2 | .98 | 1.04 | 0.04 | 27.57 |
| Severe dysphagia | .23 | 0.16 | 0.01 | 3.13 |
| Factors Predictive of Complication | P Value | OR | CI, Lower 95% | CI, Upper 95% |
| Age 75 years or less | .83 | 1.39 | 0.06 | 29.88 |
| BMI above 27.5 kg/m2 | .13 | 0.12 | 0.01 | 1.85 |
| Severe dysphagia | .07 | 11.27 | 0.80 | 158.46 |
Abbreviations: BMI, body mass index; OR, odds ratio.
Five patients (26%) underwent cervical fusion in conjunction with the osteophyte resection (Figure 5). Four of these patients had a planned fusion for concomitant spinal stenosis with radiculopathy (25%), myelopathy (25%), or significant stenosis on MRI (50%). The fifth patient underwent fusion to prevent osteophyte recurrence, given the hyperlordotic alignment and multiple levels of fused segments below the construct. No patient underwent anterior spinal fusion for concerns of iatrogenic instability related to the osteophyte resection. The average preoperative cervical lordosis was 26.3° (−7° to 52.3°; Table 5). There was no significant difference in lordosis between those who underwent fusion and those who did not (36.7° vs 21.9°, P = .08). There was 1 patient with a C2-3 anterolisthesis who did not undergo fusion. All other patients did not have a cervical spondylolisthesis. The average motion of the cervical segment with the largest osteophyte that underwent resection was 3.1° ± 2.2°. There was no difference between motion of the cervical spine in those who underwent fusion and those who did not (3.2° vs 3.1°, P = 1). There was no difference in the osteophyte size, length of dysphagia, DISH diagnosis, or BMI between those undergoing fusion and those who did not; however, the fusion group was significantly younger (64 vs 73 years, P = .05). The use of a cervical collar after surgery was significantly increased in the fusion group (80% vs 14%; P = .01; OR = 24; 95% CI = 1.7-341.0).
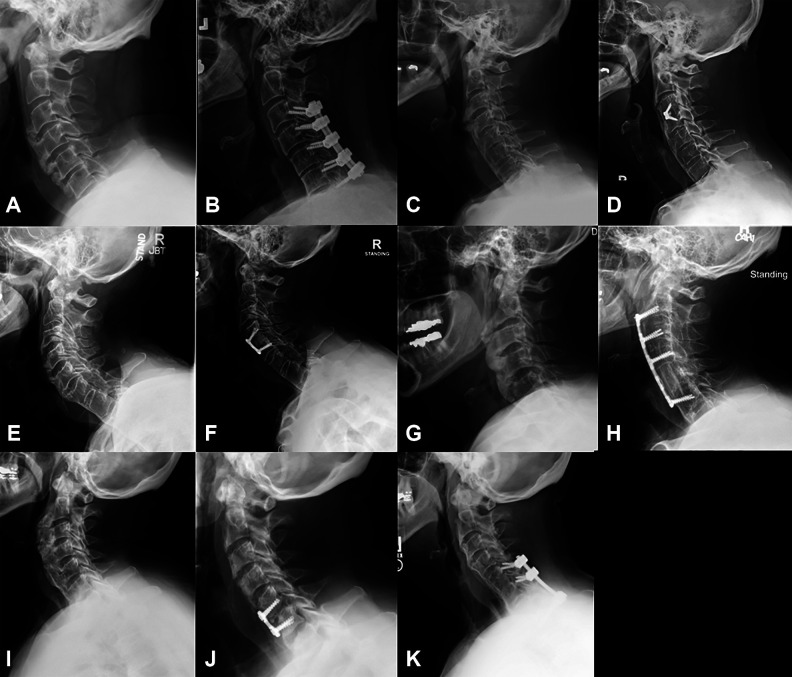
Figure 5.
Five patients underwent concurrent cervical fusion surgery along with osteophyte resection. Lateral preoperative (A) and 2-year postoperative (B) X-rays for patient 3 who underwent C3-7 osteophyte resection and posterior decompression and fusion for concurrent myelopathy. Preoperative (C) and 5-year postoperative (D) lateral X-rays of patient 10 who underwent C3-4 anterior cervical discectomy and fusion (ACDF) for critical cervical stenosis at the time of osteophyte removal. Preoperative (E) and 2-year postoperative (F) X-rays of patient 12, who underwent prophylactic C4-5 ACDF to help prevent recurrent osteophyte regrowth. Lateral preoperative (G) and 5-years postoperative (H) X-rays of patient 17 who underwent C5 corpectomy with C2-6 anterior cervical fusion for cervical stenosis from OPLL along with osteophyte removal of dysphagia and sleep apnea. Preoperative (I) and postoperative (J) X-rays of patient 4 who underwent C5-6 ACDF for C6 radiculopathy along with C2-T1 cheilectomy and cricopharyngeal myotomy for dysphagia. The patient developed a postoperative infection with osteomyelitis, requiring a 2-stage operation with anterior debridement, with partial C5 and C6 corpectomy, revision C5-6 ACDF, and posterior C5-T1 spinal fusion, which showed good alignment 4 years postoperatively (K).
Table 5.
Characteristics of Patients Undergoing Anterior Osteophyte Resection for Dysphagia With Comparison of Those Undergoing Fusion and Those Not Undergoing Fusion.
| Combined | Fusion | No Fusion | P Value | |
|---|---|---|---|---|
| Osteophyte size (mm) | 14.2 ± 4.8 | 15.6 ± 3.7 | 13.6 ± 5.2 | .43 |
| Length of dysphagia (years) | 3.6 ± 3.7 | 3.0 ± 2.0 | 3.8 ± 4.2 | 1.00 |
| Cervical lordosis (degrees) | 26.3 ± 13.8 | 36.7 ± 12.7 | 21.9 ± 12.2 | .07 |
| Motion at osteophyte segment (degrees) | 3.2 ± 2.3 | 3.2 ± 2.3 | 3.3 ± 2.5 | 1.00 |
| Age (years) | 70.6 ± 7.7 | 64.4 ± 9.1 | 72.9 ± 6.0 | .05a |
| BMI (kg/m2) | 26.4 ± 4.8 | 28.8 ± 3.6 | 25.5 ± 5.0 | .21 |
| Cervical collar use | 6 (31.6%) | 4 (80.0%) | 2 (14.3%) | .01a |
| DISH diagnosis | 12 (63.2%) | 4 (80.0%) | 8 (57.1%) | .36 |
| Operative time (minutes) | 178 ± 137 | 315 ± 174 | 121 ± 67 | .01a |
| EBL (cc) | 162 ± 156 | 245 ± 233 | 117 ± 75 | .63 |
| Length of stay (days) | 3.3 ± 2.3 | 4.2 ± 2.0 | 3.0 ± 3.6 | .07 |
Abbreviations: BMI, body mass index; DISH, diffuse idiopathic skeletal hyperostosis; EBL, estimated blood loss.
The overall complication rate for this cohort was 42%. There were no episodes of delayed instability requiring fusion; however, there was 1 pseudoarthrosis that was lost to follow-up after 2 years. This patient underwent a C3-7 posterior spinal fusion for coexisting myelopathy and developed a pseudoarthrosis at the bottom of the construct at C6-7. There was also a case of pseudoarthrosis of a C5-6 anterior cervical discectomy and fusion (ACDF) with deep infection with diskitis and vertebral osteomyelitis, where the patient underwent a 2-stage anterior-posterior fusion with irrigation, debridement, and revision C5-6 ACDF with iliac crest bone graft and posterior cervical fusion from C5-T1. Of note, this patient also underwent myotomy of the esophagus at the time of cheilectomy, leading to esophageal injury. This patient ultimately went on to clear the infection and develop a stable fusion after reoperation. There was 1 case of bilateral vocal cord dysfunction. This patient had a history of anterior neck and chest burns requiring tracheostomy and skin grafting 40 years prior. They had chronic left vocal cord paralysis and acquired a right vocal cord paresis after osteophyte resection (via a right-sided approach). This patient required percutaneous endoscopic gastrostomy (PEG) placement 9 days postoperatively. His right vocal cord paresis improved, and he was weaned from PEG feeds by 8 months. One patient had a PEG tube in place before surgery, and one other required PEG tube placement during their recovery. All patients had their PEG tube removed within 9 months of surgery. One patient had a tracheostomy prior to surgery, which too was removed after cheilectomy. No patient in this cohort required tracheostomy after cheilectomy. Other complications included 1 superior laryngeal nerve injury and 2 cases of aspiration pneumonia. There were no patients who underwent a revision anterior osteophyte resection. Severe dysphagia prior to surgical intervention trended toward an increased risk of complications on univariate analysis (71% vs 25%; P = .07; OR = 7.5; 95% CI = 0.9-61.1), whereas a BMI >27.5 kg/m2, age of 75 years or less, use of an exposure surgeon, surgery at 3 or more levels, prior neck surgery, fusion at the time of cheilectomy, osteophyte regrowth, or DISH diagnosis did not appear to relate to complications (Table 3). On nominal regression multivariate analysis, severe dysphagia again trended toward increased risk of complications (OR = 11.3; 95% CI = 0.8-158.5), whereas a BMI >27.5 kg/m2 and an age >75 years were less predictive of complication (Table 4).
There were 5 (26%) cases of osteophyte regrowth, one of which underwent fusion. Mean regrowth was 2.0 ± 0.5 mm per year in these patients. Three patients had asymptomatic osteophytes measuring 11 mm at 5 years, 6 mm at 5 years (proximal level to osteophyte resection and ACDF), and 7 mm at 3.5 years after surgery (Figure 6). Another patient had osteophyte regrowth seen 14 months after surgery measuring 8 mm (this patient did not have postoperative X-rays, so some of this bony osteophyte may have been from incomplete resection) and 13 mm at 6.5 years postoperatively (Figures 4H-4J). This patient had some improvement after surgery but continued to complain of progressive dysphagia over the next few years and ultimately underwent an esophageal dilation 8 years after her osteophyte resection. The final patient with regrowth had 6 mm of residual osteophyte postoperatively, which grew to 11.5 mm 2 years postoperatively (Figures 4E-4G). This patient did not have full resolution of their dysphagia, which was thought to be multifactorial as a result of tongue and pharyngeal weakness along with the anterior cervical osteophytes.
Figure 6.
Preoperative (A) radiograph of patient 2, who underwent C3-5 anterior osteophytectomy (21 mm) with postoperative radiograph (B) demonstrating complete excision of the C3-4 and C4-5 osteophyte and subsequent asymptomatic regrowth of osteophytes to 11 mm (C) at the 5-year follow-up. Preoperative (D) radiographs of patient 10 who underwent C3-4 anterior cervical discectomy and fusion and cheilectomy (E) who had regrowth of osteophytes (6 mm) proximally at the C2-3 level at the 5-year follow-up. Preoperative T2 magnetic resonance image (G) of patient 8 who underwent C4-5 osteophyte resection (13 mm) and had asymptomatic regrowth of osteophytes at C4-5 of 7 mm at the 3.5-year follow-up (H).
Mean operative time was 178 minutes (33-561 minutes), with mean estimated blood loss of 162 cc (50-500 cc), and mean length of stay was 3.3 (1-13 days) days after surgery. Patients who underwent fusion had longer operative times (315 ± 174 vs 121 ± 67 minutes, P = .01) and trended toward longer hospital stays (4.2 ± 2.0 vs 3.0 ± 3.6 days, P = .07). Estimated blood loss was not statistically different between those who underwent fusion and those who did not (245 ± 233 vs 117 ± 75 cc, P = .63).
Discussion
Anterior osteophytes occur commonly along the length of the spine; however, when they occur in the tight confines of the neck, they can produce symptomatic mass effect on the adjacent structures, leading to dysphagia. The purpose of this study was to review our experience with primary anterior osteophyte resection and report our current preferred surgical workup and operative technique.
Here, we report that the majority (79%) of patients had significant improvement in dysphagia after surgical resection of anterior cervical osteophytes. This is similar to previous studies, which have shown improvement in 70% to 100% of patients.6,14,16,20,22,28 Patients 75 years or older trended toward less improvement, which may be a result of increased frailty and decreased functional reserve in these patients.29 Additionally, those who failed to improve after surgery had multifactorial dysphagia, including vocal cord dysfunction, esophageal dysmotility and weakness, altered peristalsis, and esophageal strictures, along with anterior cervical osteophytes. This highlights the importance of a thorough preoperative evaluation because it is important to rule out other causes of dysphagia, which should be treated prior to osteophyte resection. Additionally, if patients have other factors contributing to dysphagia, preoperative counseling is key to managing expectations regarding dysphagia improvement.
Previous studies have described failure resulting from incomplete resection of osteophytes and osteophyte regrowth.16,23 In this cohort, there were 5 patients (2 symptomatic and 3 asymptomatic) who showed osteophyte regrowth after osteophyte resection. However, previous studies have shown that it can take 10 or more years to become symptomatic from osteophyte regrowth.16
Some studies have advocated for prophylactic cervical fusion in patients <70 years of age to prevent regrowth of osteophytes.16 We found regrowth to be 2 mm per year in those who had osteophyte regrowth, which is higher than that previously reported (1 mm per year).16 In this cohort, coexisting spinal stenosis and spinal cord or nerve root impingement was the most common reason for fusion, whereas 1 patient underwent fusion to prevent regrowth. Previous studies have suggested the use of nonsteroidal anti-inflammatory drugs to prevent osteophyte recurrence; however, the value of this has not been elucidated.16,22,30 Likewise, application of bone wax to the exposed cut bone helps with hemostasis and may reduce the rate of bone reaccumulation.31,32
This is the first study to describe the use of intraoperative navigation for the resection of anterior cervical osteophytes, which has multiple advantages. Following provisional resection, intraoperative 3-D scanning can determine the amount and location of remaining osteophytes, whereas navigated probes and burs allow for real-time guidance during final bony resection. Navigation also allows the surgeon to “visualize” the native disc space and remove bridging osteophytes anterior to the disc space without entering it or damaging the annulus fibrosis. This prevents iatrogenic destabilization requiring fusion or late instability, which would require subsequent surgery and fusion. Surgical navigation does, however, come at the expense of increased cost and surgical time for intraoperative imaging and the learning curve inherent to surgical navigation.33-36
In this cohort, the most symptomatic level of cervical osteophytes was C3-4 followed by C4-5 and C5-6. All osteophytes resected at the C6-7 and C7-T1 level were in conjunction with osteophyte complexes higher in the cervical spine. Osteophytes at C3-4 and C4-5 have been shown to restrict laryngeal closure by the epiglottis and at C5-6 and C6-7 lead to the retention of solid food in the pharynx, both of which can result in aspiration.16,18 Additionally, osteophytes at the C5-6 level and below have more room for growth without impingement on the esophagus because the soft-tissue space between the anterior spine and the esophagus measures approximately 6 mm at C2 and 22 mm at C6.37 For these reasons, osteophytes below the C4-5 levels are less likely to cause dysphagia, and improvement after resection is less predictable (82% vs 50% improvement in this series).
Our preferred management of these patients includes a multidisciplinary approach involving otolaryngology and speech pathology preoperatively to evaluate for other causes of dysphagia. All patients should have preoperative flexion and extension radiographs to evaluate for instability, spondylolisthesis, osteophyte size, and location. CT and MRI are adjunct studies used to help with preoperative planning and evaluate for spinal stenosis or nerve root impingement in those with symptoms of radiculopathy or myelopathy on exam because this should be addressed at the same time as osteophyte resection. Surgical exposure is typically completed by our otolaryngology colleagues given the more challenging exposure in the upper cervical level with the dramatic changes in anatomy caused by the large osteophytes. Likewise, this allows the otolaryngologist to have direct intraoperative anatomical knowledge of the esophagus and perform laryngoscopy/esophagoscopy if there is question regarding perforation or thinning of the posterior esophageal wall. Intraoperative navigation is used to ensure complete osteophyte resection and to avoid entering the disc spaces at levels where fusion is not planned. We believe that fusion should be considered in those patients with concomitant spinal cord/nerve root compression or spondylolisthesis or young patients with significant mobility at the cervical levels in question. Older patients with nonmobile segments can forgo fusion because this may increase operative times and complications for an already vulnerable population. Postoperative care should involve close monitoring of dysphagia and airway compromise. If there are airway concerns, delayed extubation and intensive care unit monitoring is warranted. All patients remain NPO (nothing by mouth) until after video swallow study to ensure safe swallowing prior to advancing the patient’s diet. It is not uncommon to use a temporary nasogastric feeding tube in patients with severe and prolonged preoperative dysphagia. The need for short-term and possibly long-term tube feeding must be discussed during preoperative counseling, along with the risk of esophageal injury and superior and recurrent laryngeal nerve palsy. Follow-up should include postoperative X-rays and follow-up X-rays every 3 to 5 years or if new dysphagia symptoms arise.
Limitations of this study include biases inherent to a retrospective review of a rare condition and the relatively low number of patients; however, this is the largest series to date. Additionally, not all patients had long-term follow-up cervical imaging to evaluate for osteophyte regrowth. Similarly, not all patients had flexion-extension radiographs to evaluate postoperative instability, although no patient has subsequently returned for cervical fusion to address instability or stenosis symptoms. Although our duration of radiographic follow-up (2.4 years) limits our ability to empirically comment on potential for recurrent anterior osteophytosis, symptomatic recurrence has not been our anecdotal experience. This may be a result of the fact that the many patients presenting with dysphagia (such as patient 13; Figures 4C-4D) have developed large anterior osteophytes in the context of generalized advanced spondylosis and resultant ankyloses, in which disc collapse as well as uncovertebral and facet arthroses are created. Resecting anterior osteophytes does not undo this generalized stiffening, and no patient required revision surgery for recurrent dysphagia. Finally, there was no postoperative scoring system or patient-reported outcome (PRO) utilized for these patients after surgery to evaluate quantitative change after osteophyte resection. Future studies should incorporate both preoperative and postoperative PRO measures such as the Swallow Quality of Life Questionnaire, Sydney Swallow Questionnaire, or Swallowing Quality of Care, which are high-quality PRO measures for mechanical and neuromyogenic oropharyngeal dysphagia.38
In conclusion, anterior cervical osteophyte resection improves swallowing function in the majority of patients with dysphagia caused by esophageal compression. Prior to surgery, patients should undergo thorough swallow evaluation to ensure that the anterior cervical osteophytes are the primary cause of dysphagia, whereas the use of intraoperative navigation confirms complete resection. Additionally, there is a relatively high complication rate, which highlights the need for a multidisciplinary approach to the workup and treatment of these patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Joshua M. Kolz, MD, MS  https://orcid.org/0000-0001-8085-3633
https://orcid.org/0000-0001-8085-3633
Brett A. Freedman, MD  https://orcid.org/0000-0002-3408-0163
https://orcid.org/0000-0002-3408-0163
References
- 1. Parker MD. Dysphagia due to cervical osteophytes: a controversial entity revisited. Dysphagia. 1989;3:157–160. [DOI] [PubMed] [Google Scholar]
- 2. Klaassen Z, Tubbs RS, Apaydin N, Hage R, Jordan R, Loukas M. Vertebral spinal osteophytes. Anat Sci Int. 2011;86:1–9. doi:10.1007/s12565-010-0080-8 [DOI] [PubMed] [Google Scholar]
- 3. Vodicar M, Kosak R, Vengust R. Long-term results of surgical treatment for symptomatic anterior cervical osteophytes: a case series with review of the literature. Clin Spine Surg. 2016;29:E482–E487. doi:10.1097/BSD.0b013e31829046af [DOI] [PubMed] [Google Scholar]
- 4. Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology. 1976;119:559–568. doi:10.1148/119.3.559 [DOI] [PubMed] [Google Scholar]
- 5. Strasser G, Schima W, Schober E, Pokieser P, Kaider A, Denk DM. Cervical osteophytes impinging on the pharynx: importance of size and concurrent disorders for development of aspiration. AJR Am J Roentgenol. 2000;174:449–453. doi:10.2214/ajr.174.2.1740449 [DOI] [PubMed] [Google Scholar]
- 6. Carlson ML, Archibald DJ, Graner DE, Kasperbauer JL. Surgical management of dysphagia and airway obstruction in patients with prominent ventral cervical osteophytes. Dysphagia. 2011;26:34–40. doi:10.1007/s00455-009-9264-6 [DOI] [PubMed] [Google Scholar]
- 7. Giger R, Dulguerov P, Payer M. Anterior cervical osteophytes causing dysphagia and dyspnea: an uncommon entity revisited. Dysphagia. 2006;21:259–263. doi:10.1007/s00455-006-9049-0 [DOI] [PubMed] [Google Scholar]
- 8. Kmucha ST, Cravens RB, Jr. DISH syndrome and its role in dysphagia. Otolaryngol Head Neck Surg. 1994;110:431–436. doi:10.1177/019459989411000414 [DOI] [PubMed] [Google Scholar]
- 9. Akbal A, Kurtaran A, Selcuk B, Gurcan A, Ersoz M, Akyuz M. The development of dysphagia and dysphonia due to anterior cervical osteophytes. Rheumatol Int. 2009;29:331–334. doi:10.1007/s00296-008-0669-6 [DOI] [PubMed] [Google Scholar]
- 10. Angelos C, Dimitra A. Dysphagia due to anterior cervical osteophytes complicated with hypopharynx abscess. BMJ Case Rep. 2011;2011:bcr1120103551. doi:10.1136/bcr.11.2010.3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeong H, Seo HG, Han TR, Chung CK, Oh BM. Kinematic changes in swallowing after surgical removal of anterior cervical osteophyte causing dysphagia: a case series. Ann Rehabil Med. 2014;38:865–870. doi:10.5535/arm.2014.38.6.865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Unlu Z, Orguc S, Eskiizmir G, Aslan A, Tasci S. The role of phonophoresis in dyshpagia due to cervical osteophytes. Int J Gen Med. 2008;1:11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi HE, Jo GY, Kim WJ, Do HK, Kwon JK, Park SH. Characteristics and clinical course of dysphagia caused by anterior cervical osteophyte. Ann Rehabil Med. 2019;43:27–37. doi:10.5535/arm.2019.43.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ozgursoy OB, Salassa JR, Reimer R, Wharen RE, Deen HG. Anterior cervical osteophyte dysphagia: manofluorographic and functional outcomes after surgery. Head Neck. 2010;32:588–593. doi:10.1002/hed.21226 [DOI] [PubMed] [Google Scholar]
- 15. Vengust R, Mihalic R, Turel M. Two different causes of acute respiratory failure in a patient with diffuse idiopathic skeletal hyperostosis and ankylosed cervical spine. Eur Spine J. 2010;19(suppl 2):S130–S134. doi:10.1007/s00586-009-1159-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyamoto K, Sugiyama S, Hosoe H, Iinuma N, Suzuki Y, Shimizu K. Postsurgical recurrence of osteophytes causing dysphagia in patients with diffuse idiopathic skeletal hyperostosis. Eur Spine J. 2009;18:1652–1658. doi:10.1007/s00586-009-1133-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Constantoyannis C, Papadas T, Konstantinou D. Diffuse idiopathic skeletal hyperostosis as a cause of progressive dysphagia: a case report. Cases J. 2008;1:416. doi:10.1186/1757-1626-1-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seidler TO, Alvarez JCP, Wonneberger K, Hacki T. Dysphagia caused by ventral osteophytes of the cervical spine: clinical and radiographic findings. Eur Arch Otorhinolaryngol. 2009;266:285–291. doi:10.1007/s00405-008-0735-4 [DOI] [PubMed] [Google Scholar]
- 19. De Jesus-Monge WE, Cruz-Cuevas EI. Dysphagia and lung aspiration secondary to anterior cervical osteophytes: a case report and review of the literature. Ethn Dis. 2008;18(2, suppl 2):S2–137 -140. [PMC free article] [PubMed] [Google Scholar]
- 20. Oppenlander ME, Orringer DA, La Marca F, et al. Dysphagia due to anterior cervical hyperosteophytosis. Surg Neurol. 2009;72:266–271. doi:10.1016/j.surneu.2008.08.081 [DOI] [PubMed] [Google Scholar]
- 21. Solaroglu I, Okutan O, Karakus M, Saygili B, Beşkonakli E. Dysphagia due to diffuse idiopathic skeletal hyperostosis of the cervical spine. Turk Neurosurg. 2008;18:409–411. [PubMed] [Google Scholar]
- 22. Urrutia J, Bono CM. Long-term results of surgical treatment of dysphagia secondary to cervical diffuse idiopathic skeletal hyperostosis. Spine J. 2009;9:e13–e17. doi:10.1016/j.spinee.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 23. Scholz C, Naseri Y, Hohenhaus M, Hubbe U, Klingler JH. Long-term results after surgical treatment of diffuse idiopathic skeletal hyperostosis (DISH) causing dysphagia. J Clin Neurosci. 2019;67:151–155. doi:10.1016/j.jocn.2019.05.057 [DOI] [PubMed] [Google Scholar]
- 24. Egerter AC, Kim ES, Lee DJ, et al. Dysphagia secondary to anterior osteophytes of the cervical spine. Global Spine J. 2015;5:e78–e83. doi:10.1055/s-0035-1546954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin HW, Quesnel AM, Holman AS, Curry WT, Jr, Rho MB. Hypertrophic anterior cervical osteophytes causing dysphagia and airway obstruction. Ann Otol Rhinol Laryngol. 2009;118:703–707. doi:10.1177/000348940911801004 [DOI] [PubMed] [Google Scholar]
- 26. Aronson N, Filtzer DL, Bagan M. Anterior cervical fusion by the Smith-Robinson approach. J Neurosurg. 1968;29:396–404. [PubMed] [Google Scholar]
- 27. Salassa JR. A Functional Outcome Swallowing Scale for staging oropharyngeal dysphagia. Dig Dis. 1999;17:230–234. doi:10.1159/000016941 [DOI] [PubMed] [Google Scholar]
- 28. Verlaan JJ, Boswijk PF, de Ru JA, Dhert WJ, Oner FC. Diffuse idiopathic skeletal hyperostosis of the cervical spine: an underestimated cause of dysphagia and airway obstruction. Spine J. 2011;11:1058–1067. doi:10.1016/j.spinee.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 29. Deiner S, Silverstein JH. Long-term outcomes in elderly surgical patients. Mt Sinai J Med. 2012;79:95–106. doi:10.1002/msj.21288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tu TH, Wu JC, Huang WC, et al. Postoperative nonsteroidal antiinflammatory drugs and the prevention of heterotopic ossification after cervical arthroplasty: analysis using CT and a minimum 2-year follow-up. J Neurosurg Spine. 2015;22:447–453. doi:10.3171/2014.10.Spine14333 [DOI] [PubMed] [Google Scholar]
- 31. Alberius P, Klinge B, Sjogren S. Effects of bone wax on rabbit cranial bone lesions. J Craniomaxillofac Surg. 1987;15:63–67. [DOI] [PubMed] [Google Scholar]
- 32. Vestergaard RF, Jensen H, Vind-Kezunovic S, Jakobsen T, Søballe K, Hasenkam JM. Bone healing after median sternotomy: a comparison of two hemostatic devices. J Cardiothorac Surg. 2010;5:117. doi:10.1186/1749-8090-5-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bai YS, Zhang Y, Chen ZQ, et al. Learning curve of computer-assisted navigation system in spine surgery. Chin Med J (Engl). 2010;123:2989–2994. [PubMed] [Google Scholar]
- 34. Ryang YM, Villard J, Obermuller T, et al. Learning curve of 3D fluoroscopy image-guided pedicle screw placement in the thoracolumbar spine. Spine J. 2015;15:467–476. doi:10.1016/j.spoinee.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 35. Watkins RG, Gupta A, Watkins RG. Cost-effectiveness of image-guided spine surgery. Open Orthop J. 2010;4:228–233. doi:10.2174/1874325001004010228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al-Khouja L, Shweikeh F, Pashman R, Johnson JP, Kim TT, Drazin D. Economics of image guidance and navigation in spine surgery. Surg Neurol Int. 2015;6(suppl 10):S323–S326. doi:10.4103/2152-7806.159381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeBehnke DJ, Havel CJ. Utility of prevertebral soft tissue measurements in identifying patients with cervical spine fractures. Ann Emerg Med. 1994;24:1119–1124. doi:10.1016/s0196-0644(94)70242-x [DOI] [PubMed] [Google Scholar]
- 38. Patel DA, Sharda R, Hovis KL, et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1–23. doi:10.1093/dote/dow028 [DOI] [PMC free article] [PubMed] [Google Scholar]