Abstract
Objectives:
Gastrointestinal bleeding is a morbid complication of dual antiplatelet therapy (DAPT). We evaluated the extent to which contemporary trials of DAPT included steps to ensure appropriate use of proton pump inhibitor (PPI) gastroprotection and reported rates of PPI use.
Methods:
A methodological review of randomized trials comparing varying durations of DAPT after percutaneous coronary intervention.
Results:
Among 21 trials, none incorporated protocol procedures or guidance for prescribing PPIs. Five reported rates of PPI use (range 25.6-69.1%).
Discussion:
PPI gastroprotection is overlooked in major trials of DAPT. Appropriate use of PPI gastroprotection represents an important opportunity to improve patient safety.
Keywords: Dual Antiplatelet Therapy, Clinical Trial, Proton Pump Inhibitor, Gastroprotection
Introduction
The gastrointestinal (GI) tract is the most common site of clinically significant hemorrhage in patients using dual antiplatelet therapy (DAPT) (1). In such patients, proton pump inhibitors (PPIs) reduce the odds of overt or occult upper GI bleeding by approximately two-thirds (2). Accordingly, professional societies in the United States recommend PPIs for bleeding prevention in high-risk patients using DAPT, especially those with a history of upper GI bleeding (3,4), and in Europe recommend PPIs in all patients using DAPT (5). However, in clinical practice only a minority of these patients are treated with PPIs (6).
In this study, we sought to determine the extent to which contemporary clinical trials of DAPT included steps in the protocol to ensure use of PPIs to prevent upper GI bleeding.
Methods
We performed a methodological review to determine the extent to which clinical trials that evaluated the safety and efficacy of varying durations of DAPT included procedures in the study protocol or other guidance for prescribing PPIs, as well as to identify exclusion criteria related to GI bleeding risk and reported rates of PPI use. Trials of this type were selected for review because they represent a contemporary group of DAPT trials conducted during a period when high-quality evidence was available to support the use of PPI gastroprotection.
To identify relevant trials, we used a previous systematic review that investigated outcomes of varying durations of DAPT after percutaneous coronary intervention (PCI) with publication dates between June 1983 and April 2018 (7). To identify more recent trials, we repeated the identical search but limited the results to studies published between January 2018 and October 2019. The search strategy included Medline, Embase, Cochrane Library for Clinical Trials, PubMed, Web of Science, ClinicalTrials.gov, and Clinicaltrialsregister.eu. The full search strategy can be found in Supplement 1. Eligible studies were randomized controlled trials with adult participants who received DAPT following PCI with a drug-eluting stent, that compared two of three possible durations of DAPT (short-term [<6 months), standard term [12 months], and long term [>12 months]), with outcomes including death, myocardial infarction, stroke, and bleeding. Studies that evaluated ≤1 month of DAPT or included patients using oral anticoagulants were excluded.
Two authors independently reviewed all titles and abstracts to select studies for full text review (KP and ANK) and performed data abstraction (ANK and DH). A third author (JEK) adjudicated any discrepancies. For each included trial, we reviewed all available online supplementary materials, clinical trial registries, and other publications linked to the trial NCT (clinicaltrials.gov identifier) number to identify relevant data. The review protocol was published in OSF (8).
Results
Twenty-one trials were included (Figure 1). In addition to the primary publications, study information was identified from separate trial rationale and design publications (n=7), online supplementary materials (n=13), supplementary protocols (n=4), and other publications of trial results (n=4; see Supplementary Table 2). The trials were published between 2010 and 2019 (Table 1). Sixteen trials began enrollment of patients after the 2008 publication of relevant multispecialty guidelines on PPI gastroprotection (9), and 9 trials began enrollment after the 2010 publication of the COGENT trial, which demonstrated a reduction in GI events in patients using omeprazole with clopidogrel, with no increase in cardiovascular events (10).
Figure 1.
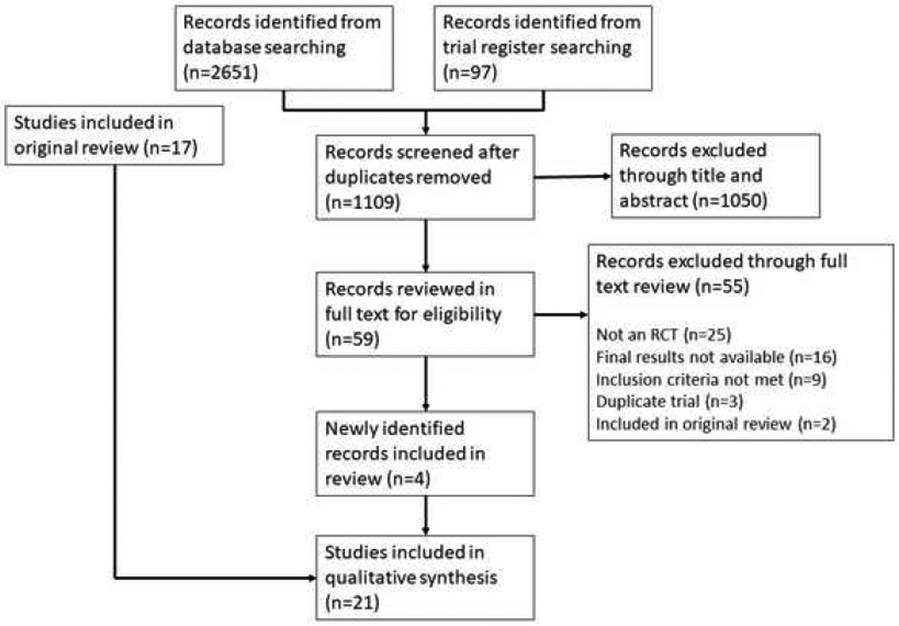
PRISMA flow diagram
Table 1.
Characteristics of Included Studies
| Trial Name & NCT Number |
DAPT duration (months) |
N | Year Published |
Date of First Enrollment |
Location | Study procedures regarding PPI prescribing |
|---|---|---|---|---|---|---|
| ARCTIC-Interruption NCT00827411 | 12 / 18-30 | 1259 | 2014 | January 2011 | 38 sites in France | None |
| DAPT-STEMI NCT01459627 | 6 / 12 | 870 | 2018 | December 2011 | 17 sites across 4 countries | None |
| DAPT Study NCT00977938 | 12 / 30 | 9961 | 2014 | August 2009 | 452 sites in 11 countries | None |
| DES LATE NCT01186146 | 12 / 36 | 5045 | 2014 | July 2007 | 24 sites in Korea | Stated that physicians may prescribe additional medications based on standard of care, did not specifically mention PPIs. |
| DETECT-OCT NCT01752894 | 3 / 12 | 779 | 2018 | January 2013 | 7 sites in Korea | None |
| EXCELLENT NCT00698607 | 6 / 12 | 1443 | 2012 | June 2008 | 19 sites in Korea | None |
| I-LOVE-IT 2 NCT01681381 | 6 / 12 | 1829 | 2016 | September 2012 | 32 sites in China | None |
| ISAR-SAFE NCT00661206 | 6 / 12 | 4000 | 2015 | October 2008 | 40 sites worldwide | Stated that non-study medications were prescribed at the discretion of the treating physician but did not specifically mention PPIs. |
| ITALIC NCT00780156, NCT01476020 | 6 / 24 | 1987 | 2017 | November 2008 | 70 sites in Europe and the Middle East | None |
| IVUS-XPL NCT01308281 | 6 / 12 | 1400 | 2016 | October 2010 | 20 sites in Korea | None |
| NIPPON NCT01514227 | 6 / 18 | 3307 | 2017 | December 2011 | 130 sites in Japan | None |
| OPTIDUAL NCT00822536 | 12 / 48 | 1385 | 2015 | January 2009 | 58 sites in France | None |
| OPTIMA-C NCT03056118 | 6 / 12 | 1367 | 2017 | April 2011 | 10 sites in South Korea | None |
| OPTIMIZE NCT01113372 | 3 / 12 | 3119 | 2013 | April 2010 | 33 sites in Brazil | None |
| PRODIGY NCT00611286 | 6 / 24 | 1970 | 2012 | December 2006 | 3 sites in Italy | Stated that decision to start PPI left to physician’s discretion. |
| REAL ZEST-LATE NCT00484926, NCT00590174 | 12 / 36 | 2701 | 2010 | July 2007 | 22 sites in South Korea | None |
| RESET NCT01145079 | 3 / 12 | 2117 | 2012 | April 2009 | 26 sites in Korea | None |
| SECURITY NCT00944333 | 6 / 12 | 1399 | 2014 | July 2009 | Multisite International Trial | None |
| SMART-CHOICE NCT02079194 | 3 / 12 | 2993 | 2019 | March 2014 | 33 sites in Korea | None |
| SMART-DATE NCT01701453 | 6 / 12 | 2712 | 2018 | September 2012 | 31 sites in South Korea | None |
| TWILIGHT NCT02270242 | 3 / 15 | 7119 | 2019 | July 2015 | 187 sites in 11 countries | None |
DAPT = Dual antiplatelet therapy; NCT Number = ClinicalTrials.gov identifier number; PPI = Proton pump inhibitor.
None of the trials included procedures in the protocol or any guidance for prescribing PPIs. One study protocol noted that PPI use was left to the discretion of the treating physician. Two study protocols specified that treating physicians could prescribe additional, non-study mandated medications but did not specifically mention PPIs. Five studies reported rates of PPI use at the time of randomization or shortly thereafter, which ranged from 25.6-69.1% (Table 2).
Table 2.
Prevalence of proton pump inhibitor use by assigned duration of dual antiplatelet therapy in the five studies that reported such data
| Trial | Prevalence of PPI use, by assigned duration of DAPT |
|---|---|
| ARCTIC-Interruption (2014) | 12 months: 29% 18-30 months: 33% |
| ISAR-SAFE (2015) | 6 months: 26.5% 12 months: 25.6% |
| NIPPON (2017) | 6 months: 69.1% 18 months: 67.6% |
| OPTIDUAL (2015) | 12 months: 46.8% 48 months: 49.8% |
| PRODIGY (2012) | 6 months: 36.9% 24 months: 38% |
All studies recorded medication use prior to or at time of randomization except PRODIGY, which measured PPI use at multiple time points. The data shown are from the 30-day time point. DAPT = Dual antiplatelet therapy. PPI= Proton pump inhibitor.
Trials’ exclusion criteria related to bleeding are shown in Supplement 3. Nine of the trials excluded patients who had had GI bleeding or other bleeding events (without reference to an anatomic site) that occurred during some previous interval; however, none of the trials explicitly excluded patients who had such events more than one year ago.
Discussion
DAPT quadruples the risk of upper GI bleeding (11). PPI gastroprotection is recommended in DAPT patients at high risk but is underused in clinical practice (3,5,6,9). In 21 contemporary clinical trials of DAPT, a highly controlled setting in which evidence-based care is expected, none of the studies included procedures in the protocol or other guidance for prescribing PPIs to prevent upper GI bleeding, and few reported rates of PPI use. These findings highlight an important opportunity, and arguably an obligation, to improve patient safety. Systematic use of PPI gastroprotection in these trials could also lead to a better understanding of the risk-benefit profile of DAPT in the context of best practices for upper GI bleeding prevention.
It is possible that study leaders had concerns about PPIs’ reducing the anti-platelet effect of thienopyridines, an interaction that has been hypothesized. However, a clinically significant interaction has not been borne out in meta-analysis (12), and professional guidance statements still recommend PPIs in high-risk DAPT patients (3). None of the trials excluded all patients at high risk for upper GI bleeding. Another possibility is unawareness of the substantial body of evidence supporting the role of PPIs for upper GI bleeding prevention. Yet, 16 of the 21 trials commenced after publication of relevant multi-society guidelines in 2008 (9), and nearly half after publication of the COGENT trial in 2010 (9,10).
As a limitation, the results of this study should not be generalized to all trials of antithrombotic drugs. In addition, it is possible that details of study procedures related to PPIs were not captured using our search strategy, but unlikely since we reviewed not only primary publications but also clinical trial registries and all available supplementary materials.
In conclusion, contemporary clinical trials of DAPT do not incorporate guideline-recommended steps to prevent upper GI bleeding, presenting an opportunity to improve patient safety, and to emphasize the importance of PPI gastroprotection to clinicians in practice. Future trials should ensure PPI gastroprotection is incorporated into study protocols.
Supplementary Material
Acknowledgments
Sources of Funding
Kurlander: VA Health System and NIDDK K23 DK118179-01A1. The views represented in this manuscript do not represent the views of either the VA Health System or the NIH.
Footnotes
Disclosures
Geoffrey Barnes discloses grant funding from Pfizer/Bristol-Myers Squibb and consulting fees from Pfizer/Bristol-Myers Squibb, Janssen, Portola, and AMAG Pharmaceuticals. Devraj Sukul receives salary support from Blue Cross Blue Shield of Michigan (BCBSM). However, the opinions, beliefs and viewpoints expressed by the authors do not necessarily reflect those of BCBSM or any of its employees. Hitinder Gurm receives research support from Blue Cross and Blue Shield of Michigan, the National Institutes of Health Center for Accelerated Innovations, and Michigan Translational Research and Commercialization for Life Sciences Innovation Hub and is a consultant for Osprey Medical. None of the other authors report any conflicts of interest.
References
- 1.Berger Peter B, Bhatt Deepak L, Valentin Fuster, et al. Bleeding Complications With Dual Antiplatelet Therapy Among Patients With Stable Vascular Disease or Risk Factors for Vascular Disease. Circulation 2010;121:2575–2583. [DOI] [PubMed] [Google Scholar]
- 2.Khan M, Siddiqui W, Alvarez C, et al. Reduction in postpercutaneous coronary intervention angina in addition to gastrointestinal events in patients on combined proton pump inhibitors and dual antiplatelet therapy: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol 2018;30:847–853. [DOI] [PubMed] [Google Scholar]
- 3.Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;122:2619–2633. [DOI] [PubMed] [Google Scholar]
- 4.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol 2016;68:1082–1115. [DOI] [PubMed] [Google Scholar]
- 5.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTSThe Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 6.Hartman J, Nauka PC, Priyanka S, et al. Proton Pump Inhibitors Are Underprescribed in Patients Discharged on Dual Antiplatelet Therapy After Acute Coronary Syndrome. Gastroenterology 2018;155:e37. [Google Scholar]
- 7.Yin S-H-L, Xu P, Wang B, et al. Duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stent: systematic review and network meta-analysis. BMJ 2019;365:l2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurlander JE, Helminski D, Platt K, et al. Specifications for PPI Use in Study Protocols for Randomized Controlled Trials of Dual Antiplatelet Therapy (DAPT): A Systematic Review. [Internet]. 2020;Available from: https://osf.io/a34n7 [Google Scholar]
- 9.Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risks of Antiplatelet Therapy and NSAID Use A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008;118:1894–1909. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without Omeprazole in Coronary Artery Disease. N. Engl. J. Med 2010;363:1909–1917. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez LAG, Lin KJ, Hernández-Díaz S, et al. Risk of Upper Gastrointestinal Bleeding With Low-Dose Acetylsalicylic Acid Alone and in Combination With Clopidogrel and Other MedicationsClinical Perspective. Circulation 2011;123:1108–1115. [DOI] [PubMed] [Google Scholar]
- 12.Demcsák A, Lantos T, Bálint ER, et al. PPIs Are Not Responsible for Elevating Cardiovascular Risk in Patients on Clopidogrel—A Systematic Review and Meta-Analysis [Internet]. Front. Physiol 2018;9[cited 2020 Feb 25] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6252380/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


