Abstract
The role of nitrogen (N) fixation in determining the frequency, magnitude, and extent of harmful algal blooms (HABs) has not been well studied. Dolichospermum is a common HAB species that is diazotrophic (capable of N fixation) and thus growth is often considered never to be limited by low combined N sources. However, N fixation is energetically expensive and its cost during bloom formation has not been quantified. Additionally, it is unknown how acclimation to differing nutrient ratios affects growth and cellular carbon (C):N stoichiometry. Here, we test the hypotheses that diazotrophic cyanobacteria are homeostatic for N because of their ability to fix atmospheric N2 and that previous acclimation to low N environments will result in more fixed N and lower C:N stoichiometry. Briefly, cultures that varied in resource N:phosphorus (P) ranging from 0.01–100 (atom), were seeded with Dolichospermum which were previously acclimated to low and high N:P conditions and then sampled temporally for growth and C:N stoichiometry. We found that Dolichospermum was not homeostatic for N and displayed classic signs of N limitation and elevated C:N stoichiometry, highlighting the necessary growth trade-off within cells when expending energy to fix N. Acclimation to N limited conditions caused differences in both C:N and fixed N at various time points in the experiment. These results highlight the importance of environmentally available N to a diazotrophic bloom, as well as how previous growth conditions can influence population growth during blooms experiencing variable N:P.
Keywords: Ecological Stoichiometry, Harmful Algal Blooms, Acclimation, Homeostasis
Introduction
Anthropogenic climate change has caused stronger stratification of freshwater systems due to increased temperature, and when coupled with eutrophication has led to a surge in cyanobacterial harmful algal blooms (HABs) worldwide (Paerl, 2014; Paerl, 2017). These HAB events are largely unpredictable due to the multifaceted nature of bloom formation (Brooks et al., 2018), but the effect of HABs can be wide ranging, and can include increased turbidity, large diurnal dissolved oxygen swings, and the presence of cyanotoxins (Brooks et al., 2017). Cyanotoxins pose many risks to birds and mammals, which include a suite of health issues and harmful effects to humans (Buratti et al., 2017). Some harmful cyanobacteria species (diazotrophs) have the capacity to fix atmospheric nitrogen (N), giving them a competitive advantage over other phytoplankton during periods of N deficiency or starvation (Carey et al., 2012). But, the relative importance of N fixation in regulating toxic cyanobacterial blooms remains understudied (Gobler et al., 2016; Grover et al., 2020). Thus, there is a growing need for understanding how a changing environment effects the role of diazotrophic cyanobacteria in bloom formation (Lu et al., 2019).
A well-suited framework to examine mechanisms of bloom formation is ecological stoichiometry (ES), which explains how elements move from the abiotic to the biotic environment and how the supply of elements can alter food webs and ecosystem dynamics (Sterner and Elser, 2002). One central premise of ES is that autotrophic producers (phytoplankton) are plastic in their internal nutrient composition while consumers are more invariable (Sterner and Elser, 2002). Initially, heterotrophic bacteria were thought to be homeostatic, meaning that variability in their internal nutrient content was extremely low across highly variable nutrient supply ratios (Makino et al., 2003). However, more recent evidence suggests that the internal nutrient composition can be substantially variable under different environmental conditions (Scott et al., 2012; Godwin and Cotner, 2015a). Surprisingly, the stoichiometry of diazotrophic cyanobacteria remains empirically understudied (Grover et al., 2020) even though these species are ideal model organisms to test the potential for stoichiometric homeostasis in an autotroph with the unique capacity to access a N pool unavailable to most organisms.
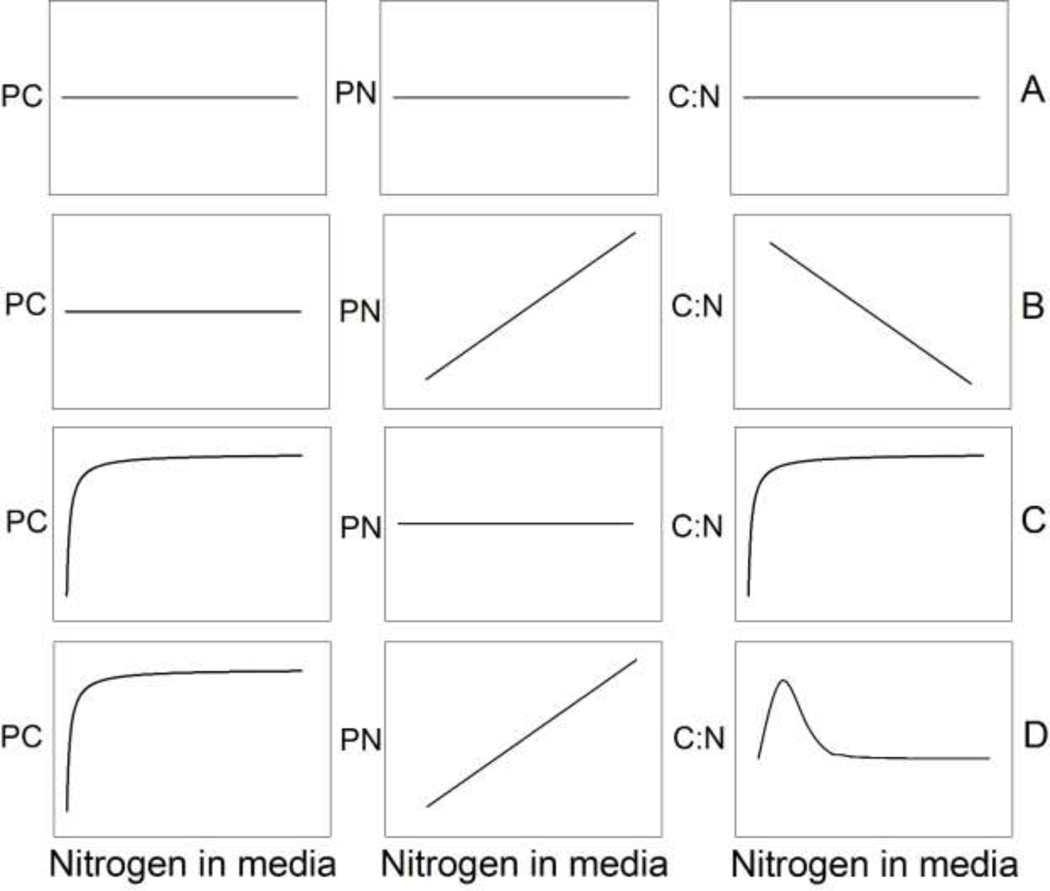
Stoichiometric theory suggests that under variable resource N concentrations, diazotrophic cyanobacteria should be homeostatic because they have the unique capacity to access unlimited N through atmospheric N2. However, N fixation is energetically expensive (Flores and Herrero, 2005), which decreases carbon (C) fixation efficiency when diazotrophs rely predominantly on fixed N2 (Boatman et al., 2018; Grover et al., 2020). To illustrate predictions using stoichiometric theory, we constructed four hypothetically possible growth trade-off scenarios regarding the relationship between diazotrophic cyanobacterial bloom biomass production (as particulate carbon; PC), biomass N content (as particulate N; PN), and the biomass C:N ratio relative to an increasing resource N supply, but no other changes, in the environment (Fig. 1). These hypothetical scenarios represent one time-point in a diazotroph blooms growth, while fixing N. Scenario A shows the expected pattern of complete stoichiometric homeostasis with little or no growth reduction due to active N fixation. These organisms may use dissolved inorganic N (DIN) when it is available, but also may use atmospheric N2 to grow just as efficiently. Scenario B shows a similar invariable growth response (biomass as C) as scenario A, but a variation in diazotroph N content in response to N availability. This scenario is possible because diazotrophs are known to store excess N in the form of cyanophycin (Kromkamp, 1987) when grown under N replete conditions. Thus, even though there may be no growth tradeoff to using N2 when needed, diazotroph N content increases as N availability increases, which causes the C:N ratio of diazotrophs to decline with increasing N availability. In scenario C, diazotroph biomass increases with increasing N availability, but bloom N content is invariable because N fixation offsets the N supply at low water column concentrations. As the combined resource N concentration increases, the need to acquire N through N fixation decreases, and the growth penalty is eliminated. This scenario describes a growth tradeoff that is caused by the energy requirement for maintaining a minimum cellular N through N fixation (Fay, 1992). In this scenario, the diazotroph C:N ratio increases with increasing N availability due to increasingly efficient growth (biomass production) with increasing water column N concentration. Scenario D portrays a growth tradeoff similar to scenario C, however this scenario also assumes that diazotroph N content varies with N availability, similar to scenario B. As a result, the diazotroph C:N exhibits a „hump-shaped‟ curve across N availability. Diazotrophs growing strictly on atmospheric N2 or DIN have the lowest C:N, but C:N increases at moderate N availability as diazotrophs rely on both N sources.
Fig. 1:
Hypothetical graphs showing the possible growth trade-offs from diazotrophic cyanobacteria dependent upon how much available nitrogen (N) is in the environment. Four different scenarios (A, B, C, D) exist to show this in terms of particulate carbon (PC), particulate nitrogen (PN), and the carbon to nitrogen (C:N) response.
To test these hypothetical relationships, we asked how does Dolichospermum flos-aquae regulate C:N under differentially supplied nutrients in culture conditions, and does population acclimation to differing nutrient ratio conditions have an effect on N fixation? Briefly, D. flos-aquae acclimated to different initial nutrient conditions was grown under different N concentrations, which were standardized to a single phosphorus (P) concentration to generate a wide range of N:P levels that are representative of natural lakes and reservoirs (Scott et al., 2019). We hypothesize our hypothetical scenario C (Fig. 1), because D. flos-aquae is generally assumed to be homeostatic for N, but also likely to exhibit a growth tradeoff caused by the increased energetic demand to fix N2 (Cobb and Myers, 1964). It was also hypothesized that D. flos-aquae acclimated to low resource N:P conditions would have lower C:N in low N environments compared to populations acclimated at high N:P due to the N limited acclimated cells being primed for N fixation.
2. Methods
2.1. Maintenance culture conditions
Dolichospermum flos-aquae was purchased from the University of Texas Culture Collection (UTEX #1444) and maintained in lab for over 18 months. Maintenance batch cultures were grown at 26ºC, with a light:dark cycle of 14h:10h, light intensity of 100 μmol m2 s−1 with one-half strength (0.5x) BG-11 media (Sigma), containing 123.2 mg L−1 N and 3.41 mg L−1 P, with added 1.35 μg L−1 vitamin B12 (VB12). Batch cultures were renewed every four weeks by transferring 1% of the cells into freshly prepared and sterilized 0.5x BG-11 media with 1.35 μg L−1 VB12. In June 2017 the stock culture was split into two additional cultures to begin acclimation to different resource N supplies. In these new cultures, 1% aliquots of the existing D. flos-aquae were transferred into 5% or 20x diluted N-free BG-11 amended with 1.35 μg L−1 VB12, which were then amended with sodium nitrate (N-NaNO3) to achieve N:P resource of 2 and 20 by atom. These cultures (referred to as low N acclimation and high N acclimation, respectively) were then maintained continuously by renewing them every four weeks as described above over a period of four months.
2.2. Homeostasis Experimental Design
A 12-day experiment was used to test for homeostasis of D. flos-aquae. We used 5% or 20x diluted N-free BG-11 media that contains 0.357 mg L−1 of P (K2HPO4) and manipulated N as NaNO3 to alter the resource N:P. These N additions generated a range of N:P (by atom) from 1–100 representing a gradient of severe N limitation to N sufficiency in the media (Supplementary Table 1A). Quadruplicate cultures were initiated using 0.85 L of 5% BG-11-N amended with VB12 (1.35 μg L−1) and inoculated to a final concentration of 0.01 mg L−1 of D. flos-aquae C from the maintenance culture stock source (see 2.1). Experimental units were then randomly placed in two identical incubators set at 26⁰C with a light intensity of 100 μmol m−2s−1 and a light:dark cycle of 14h:10h for 12 days. Each day cultures were shaken to prevent cells from settling. Each experimental unit was subsampled for PC and PN after 5–8, 10, and 12 days of growth by using vacuum filtration onto precombusted glass fiber filters (0.7μm GF/F Whatman) and stored at −20 ºC until analyzed.
2.3. Acclimation Experimental Design
For the acclimation experiment, 0.75 L cultures were created ranging in N:P ratios from 0.01–50 (atom) to test for acclimation responses in D. flos-aquae. Three P concentrations were generated by using 2.5%, 5%, or 10% N-free BG-11 that resulted in P concentrations (as K2HPO4) of 0.178, 0.357, and 0.714 mg L−1 (or 133.5, 267.8, and 535.5 μg P, respectively), and media was amended with different amounts of N (as NaNO3) to achieve the various N:P conditions (Supplementary Table 1B). Vitamin B12 was amended to these cultures as previously described (1.35 μg L−1). Experimental cultures were inoculated to a final concentration of approximately 0.1 mg L−1 C D. flos-aquae which had been previously acclimated to N:P 2 or N:P 20 (by atom) media for five months (~68 generations). Microscopic analysis was used to verify that the low N:P acclimated population were actively generating heterocyst and the high N:P acclimated population were not. Experimental cultures with acclimated populations were placed in two incubators at 26⁰C under a 14h:10h light:dark cycle at 100 μmol m−2 s−1 light intensity for four weeks. Cultures were shaken and rotated within and between incubators every other day. To adjust for evaporation, the volume of water within each experimental unit was measured just prior to and after sampling. On the day of sampling, deionized water was added to replace the amount of water lost caused by evaporation, shaken and then sampled weekly for four weeks, which allowed for cultures below N:P 50 to reach stationary phase by the end of the four weeks, for PC and PN onto precombusted 0.7μm GF/F Whatman filters. Filters were immediately frozen at −20⁰C until analyzed. Dissolved nutrients were sampled by collecting the filtrate from a 0.45μm filter and stored at −20⁰C until analyzed.
2.4. Nutrient analysis
Filters analyzed for PN and PC were first dried at 60⁰C for 24h and analyzed on a Thermo Finnigan Flash EA 1112 elemental analyzer (Thermo Fisher Scientific, Waltham, Massachusetts). Mass of C and N was determined by producing an aspartic acid standard curve and fitting the area given to the produced equation. Analysis for dissolved nutrients was performed on a Lachat 8500 flow-injection auto-analyzer with an ASX-520 autosampler (Hach Co., Loveland, Colorado). Dissolved nutrients (ammonia and nitrate/nitrite) were analyzed using EPA QA/QC standards and APHA/CRASR protocols (APHA, 2005; Center for Reservoir and Aquatic Systems Research, Waco, Texas, USA).
2.5. Statistical analysis
2.5.1. Homeostasis Experiment
A linear mixed-model analysis was performed for both day and N:P effect on cellular stoichiometry using the lme4 package (Bates et al., 2015) in R v 3.5.1 (R Core Team, 2019). The best model was selected according to the AIC value, and an analysis of variance (ANOVA) was done on the selected model to determine the Chi-squared (χ2) values and p-values within the model. Changes in cellular stoichiometry were analyzed between nutrient supply ratios within each day among N:P treatment and between days within a N:P treatment through a one-way ANOVA using the car package in R (Fox and Weisberg, 2011) with a Tukey post-hoc test done to determine the differences among either N:P treatments or days.
2.5.2. Acclimation Experiment
To assess differences between acclimation populations and P levels, treatments were standardized by using a N imbalance equation (Scott et al., 2019). This equation is a simple mass balance of the concentration of available N and P within the system that is available for growth compared to the optimum N:P (by mass) of the organism. The resulting imbalance, if positive, indicates that P was limiting the growth, while a negative imbalance indicates N was limiting the growth. The resulting answer also indicates the degree of imbalance, with larger positive or negative numbers indicating a larger imbalance and numbers closer to zero representing small imbalances or optimal nutrient conditions in the case of zero imbalance.
The N imbalance for each treatment was calculated using equation 1 (Scott et al., 2019):
| (1) |
where NI is the N imbalance relative to P of the culture, Nx and Px are the experimental concentrations of N and P added to the cultures in μg L−1, and N:Popt is the stoichiometric optimum of the N:P ratio for D. flos-aquae, which was determined to be 17.7 by atom (Klausmeier et al., 2004) or ~8 by mass.
2.5.3. C:N Stoichiometry and Biomass
Differences between the C:N of the low and high N acclimated populations for each day were compared using a paired two-tailed t-test in R (R Core Team, 2019). Statistically significant differences in these values indicated that the C:N stoichiometry differed between the different populations acclimated to a variable range of N:P. To assess differences in biomass (PC) of treatments, 95% prediction intervals were calculated using the lme function in R v 3.5.1 (R Core Team, 2019). These prediction intervals were graphed between low and high N acclimated populations, and if the intervals overlapped between the acclimations, it was determined for that N:P treatment there was no significant difference between the acclimation populations.
2.5.4. Dissolved Inorganic Nitrogen
To test for differences in dissolved inorganic N (DIN) uptake between low and high N acclimations, the lsmeans package in R v. 3.5.1 (Lenth, 2016; R Core Team, 2019) was used to compare the slopes between linear regressions and exponential decay models produced between DIN and days 0–14 of each N:P treatments for each of the acclimations. These days were chosen as this was when the most dynamic change in DIN uptake occurred for all treatments before biomass saturation, and thus would then prevent the regression line to be driven by an extreme high and low value. Linear and exponential decay models were then produced for the DIN uptake of each treatment (Sigma version 14) over all sampling days. The highest r2 value between the linear and exponential decay models was used to determine which model produced the best fit, which overall were exponential decay models.
2.5.5. Nitrogen Fixation Estimates
To examine N fixation, we first found the residuals between PN and initial culture DIN. The difference between the measured PN concentrations from all experiments from their expected values assuming no N fixation (i.e. initial DIN concentration in media) was computed per equation 2:
| (2) |
Positive values indicated the concentration of the DIN surplus and negative values indicated the concentration of fixed N in each experimental bloom. These DIN-PN residuals were calculated for all measured PN samples and then graphed against the N imbalance of the cultures by week. The slopes were compared using SMATR (Falster et al. 2006; Warton et al. 2006) to determine if the ordinary least squares regression showed significantly different slopes between populations with different acclimations. A significant difference in slopes between the high and low N acclimated populations demonstrated that N fixation was dependent on the acclimation conditions of D. flos-aquae. Slopes were also compared through time within an acclimation treatment. Differences of slopes through time indicated the rate of N fixation is time dependent.
3. Results
3.1. Homeostasis Experiment
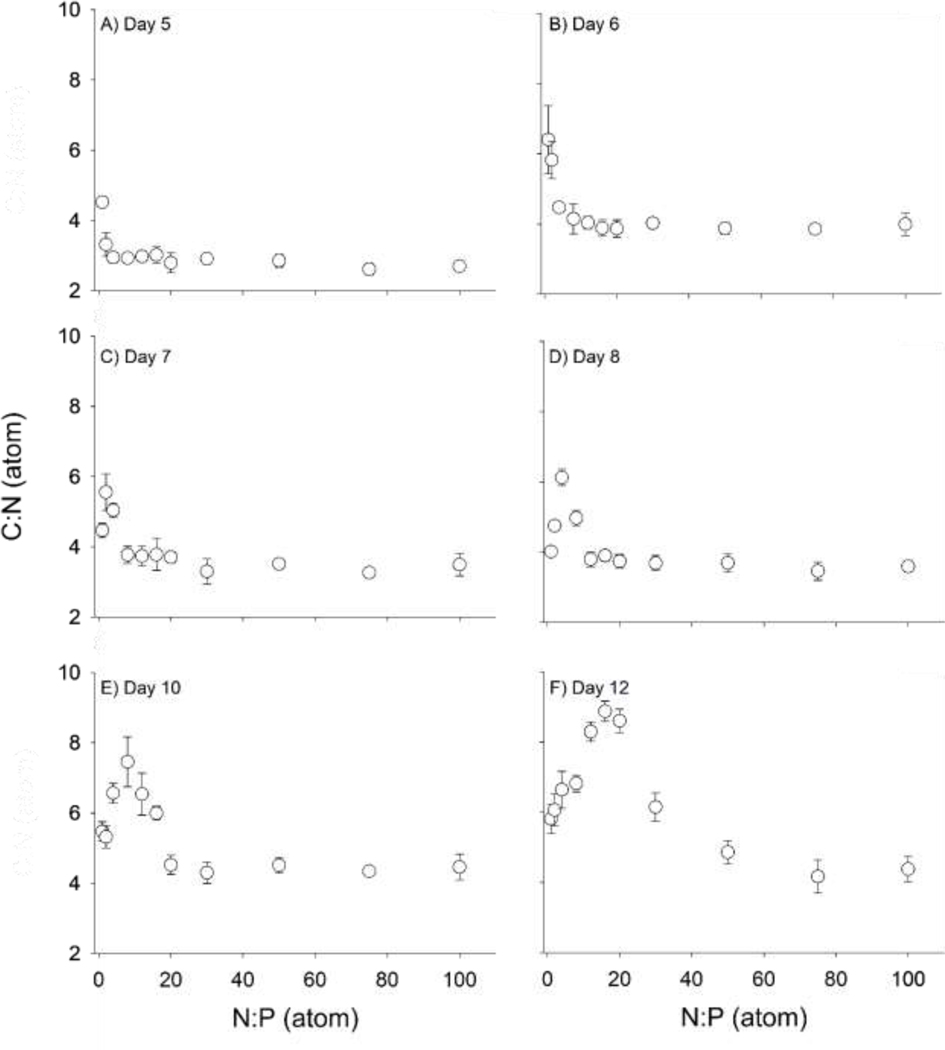
Dolichospermum flos-aquae elemental composition varied among N:P supply and by sampling day of the cultures (Table 1, Fig. 2). The C:N for day 0, which was from the maintenance culture used to inoculate the cultures, was 4.42 ± 0.133. A wave pattern occurred temporally across the N:P gradient, starting with N:P 1 having the highest C:N on day 5, then N:P 2 on day 6 (Table 1, Fig. 2). After each D. flos-aquae N:P treatment displayed a peak C:N, the biomass C:N decreased to just above the baseline C:N of 4–5 thus completing the wave pattern (Fig. 2). After 12 days of growth, this wave pattern resulted in the highest C:N of 9 in the N:P 16 treatment, with decreasing C:N in N:P treatments lower than N:P 16 and increasing C:N in N:P 20 and 30 treatments (Table 1, Fig. 2). C:N of the treatments did increase through time within a treatment.
Table 1:
Linear model results showing Chi-squared (χ2), degrees of freedom (DF), and p-values. Tukey’s post-hoc test results between nitrogen:phosphorus (N:P) treatments within day for the homeostasis experiment. For post-hoc analysis of the means note that D>C>B>A.
| Variable | χ2 | DF | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N:P | 518.5 | 10 | <0.001 | ||||||||
| Day | 3311 | 5 | <0.001 | ||||||||
| N:P(Day) | 1046 | 50 | <0.001 | ||||||||
| Post hoc Comparisons | |||||||||||
| Day | |||||||||||
| Treatment | 5 | 6 | 7 | 8 | 10 | 12 | |||||
| (N:P) | |||||||||||
| 1 | C | A | BC | A | BD | B | |||||
| 2 | B | A | D | B | CD | BC | |||||
| 4 | AB | B | CD | C | EF | BC | |||||
| 8 | AB | B | AB | B | F | C | |||||
| 12 | AB | B | AB | A | EF | D | |||||
| 16 | AB | B | AB | A | DE | D | |||||
| 20 | A | B | A | A | AC | D | |||||
| 30 | AB | B | A | A | A | BC | |||||
| 50 | AB | B | A | A | AC | A | |||||
| 75 | A | B | A | A | ABC | A | |||||
| 100 | A | B | A | A | AC | A | |||||
| Post hoc Comparisons | |||||||||||
| N:P treatment | |||||||||||
| Day | 1 | 2 | 4 | 8 | 12 | 16 | 20 | 30 | 50 | 75 | 100 |
| 5 | AC | A | A | A | A | A | A | A | A | A | A |
| 6 | D | C | B | BC | B | B | B | C | B | BD | BC |
| 7 | AB | BC | B | AB | B | B | B | AB | B | AB | B |
| 8 | A | B | C | C | B | B | B | BC | B | BC | B |
| 10 | BCD | BC | C | D | C | C | C | C | C | D | C |
| 12 | CD | C | C | D | D | D | D | D | C | CD | C |
Fig. 2:
The carbon to nitrogen (C:N) ratio by atom among N to phosphorus (N:P) supply for the homeostasis experiment through time (A) Day 5, (B) Day 6, (C) Day 7, (D) Day 8, (E) Day 10, (F) Day 12. Tukey post-hoc test results for the homeostasis experiment shown in Table 1.
3.2. Acclimation Experiment
3.2.1. C:N Stoichiometry and Biomass
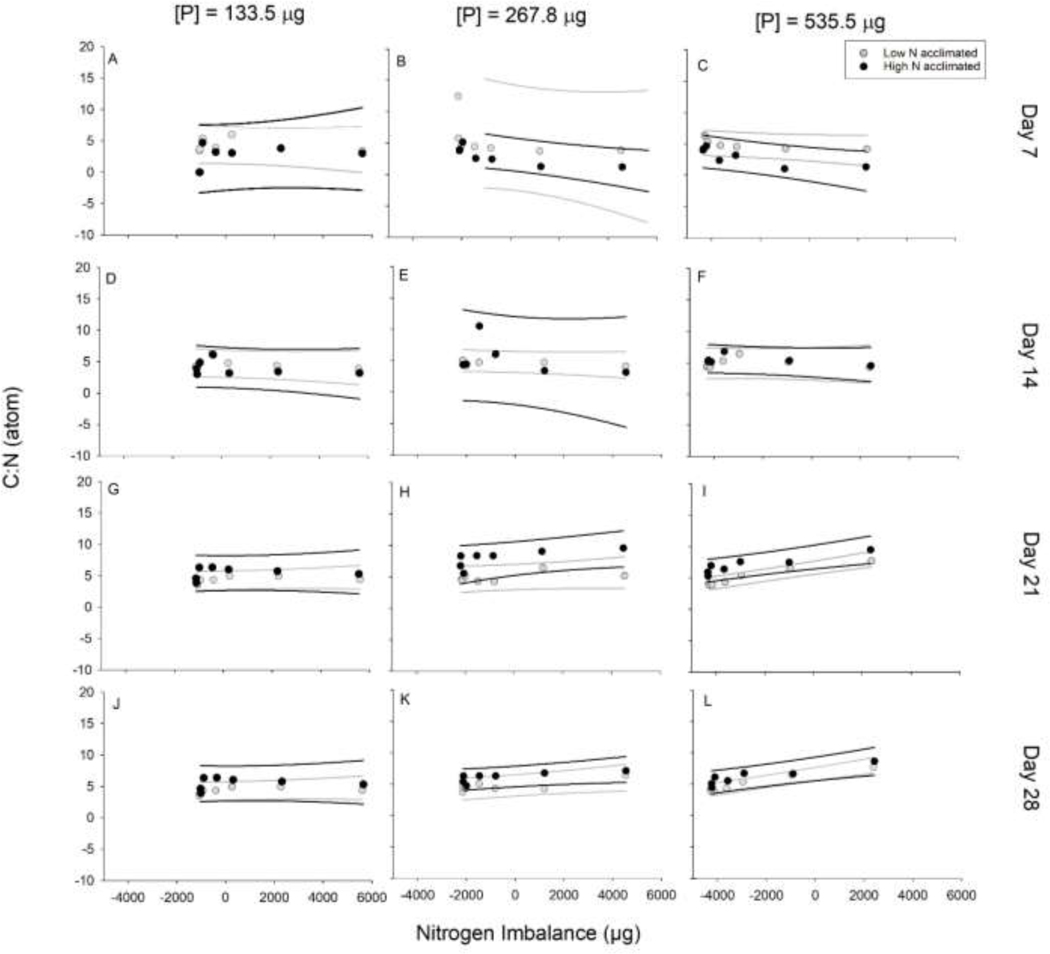
The C:N was significantly different between the low and high N acclimations across N:P treatments for days 7, 21, and 28 for all three P levels (Table 2; Fig. 3). On day 7, the low N acclimated populations had a higher C:N ranging between 3.4–12.5 than the high N acclimated populations ranging between 1.0–4.9, regardless of P treatment (Table 2; Fig. 3A-C). On day 14, the C:N of the acclimations were not significantly different with ranges between 3.1–10.5 (Table 2; Fig. 3D-F). On day 21 significant differences between the low and high N acclimation were identified with the high N acclimated populations having higher C:N values, ranging from 4.6–8.8 for all P levels, while the low N acclimated populations C:N ranged over 3.7–6.4 (Table 2; Fig. 3G-I). Day 28 had the same significant pattern with the high N acclimated populations having a higher C:N, that ranged between 4.6–8.8 compared to the low N acclimation populations that ranged between 3.5–7.7 for all P levels (Table 2; Fig. 3J-L).
Table 2:
Carbon:nitrogen (C:N) ranges, encompassing all N:phosphorus (P) conditions, and previous N acclimation for each day for the acclimation experiment. Test statistic and p-value from paired t-test are shown.
| Day | PO4−P (μg) | Nitrogen acclimation | C:N (atom) range over all N:P | Test statistic (t) | p-value |
|---|---|---|---|---|---|
| Low | 3.4–6.0 | 2.81 | 0.031 | ||
| 133.5 | High | 3.0–4.7 | |||
| 7 | Low | 3.7–12.5 | 2.71 | 0.035 | |
| 267.8 | High | 1.0–4.9 | |||
| Low | 4.2–6.3 | 4.00 | 0.007 | ||
| 535.5 | High | 1.6–4.9 | |||
| Low | 3.9–6.3 | 1.90 | 0.10 | ||
| 133.5 | High | 3.1–6.3 | |||
| Low | 4.1–5.9 | −0.36 | 0.73 | ||
| 14 | 267.8 | High | 3.1–10.5 | ||
| Low | 4.3–6.4 | −1.39 | 0.22 | ||
| 535.5 | High | 4.0–5.0 | |||
| Low | 3.7–4.9 | −4.79 | 0.003 | ||
| 133.5 | High | 4.3–6.3 | |||
| 21 | Low | 4.4–6.4 | −3.49 | 0.013 | |
| 267.8 | High | 4.8–7.2 | |||
| Low | 3.9–6.5 | −5.16 | 0.002 | ||
| 535.5 | High | 4.6–8.8 | |||
| Low | 3.5–4.9 | −4.79 | 0.003 | ||
| 133.5 | High | 4.6–6.3 | |||
| 28 | Low | 4.2–6.4 | −3.49 | 0.013 | |
| 267.8 | High | 4.8–7.2 | |||
| Low | 3.9–7.7 | −5.16 | 0.002 | ||
| 535.5 | High | 4.6–8.8 | |||
Fig. 3:
The carbon:nitrogen (C:N) by atom for each acclimation treatment over the four sampling events and for each of the phosphorus (P) levels. Lines represent 95% prediction intervals. (A) Day 7 for P of 133.5 μg, (B) Day 7 for P of 267.8 μg, (C) Day 7 for P of 535.5 μg, (D) Day 14 for P of 133.5 μg, (E) Day 14 for P of 267.8 μg, (F) Day 14 for P of 535.5 μg, (G) Day 21 for P of 133.5 μg, (H) Day 21 for P of 267.8 μg, (I) Day 21 for P of 535.5 μg, (J) Day 28 for P of 133.5 μg, (K) Day 28 for P of 267.8 μg, (L) Day 28 for P of 535.5 μg.
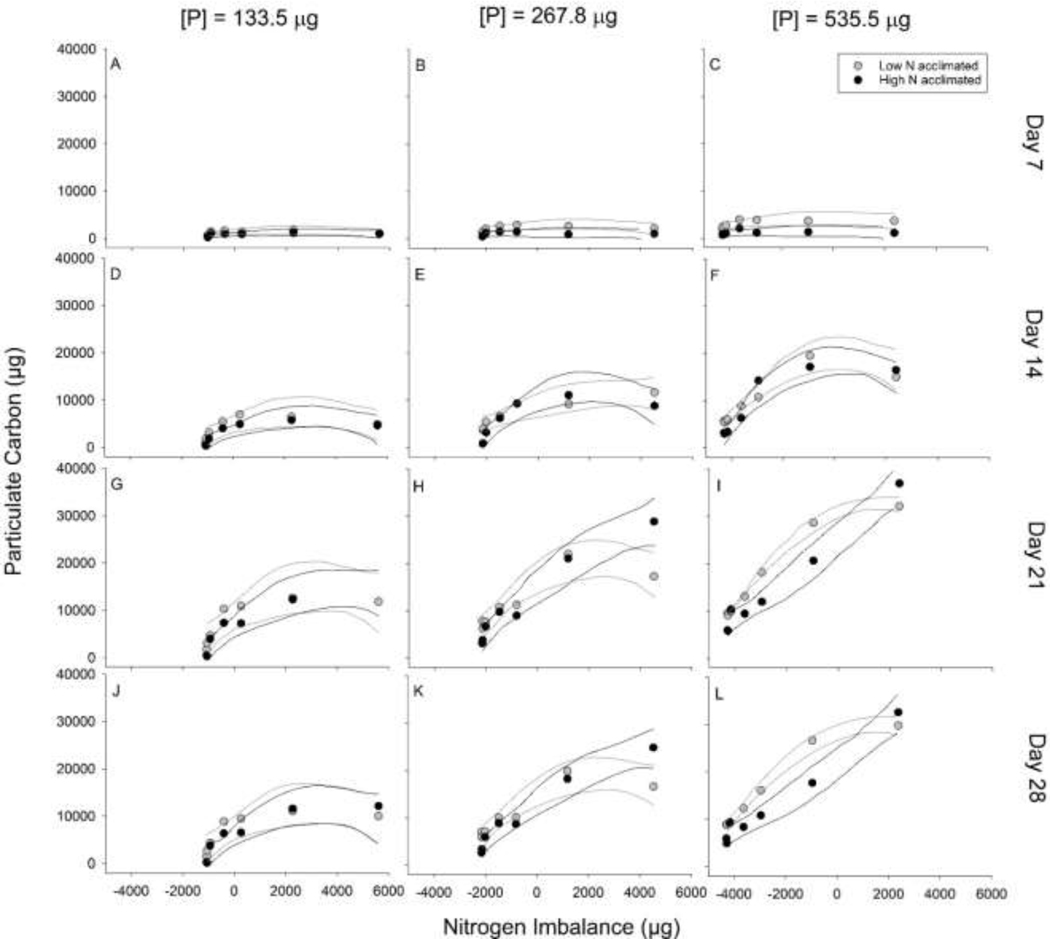
After 7 days of growth, acclimation conditions resulted in no significant differences in growth, as PC, regardless of N imbalance in the low and medium P treatments (Fig. 4A, 4B). However, in the high P treatment the low N acclimated population produced more biomass on day 7 than the high N acclimated population, regardless of N imbalance (Fig. 4C). No consistent significant differences occurred between high or low N acclimated populations irrespective of N imbalance or P level for most of the duration of the experiment (Fig. 4). However, there were areas of segregated errors on day 21 and 28 in the high P level where the low N acclimated populations had significantly increased PC at both intermediate and high N imbalances (Fig. 4I, 4L). The relative shape of the data varied through time, however, with both the low and high N acclimations showing saturating curves on day 7 regardless of P treatment. By day 21, the high N acclimated strain in the intermediate and high P cultures showed a near-linear response to increasing N imbalance relative to P.
Fig. 4:
The particulate carbon (PC) mass for each acclimation treatment over the four sampling events and for each of the phosphorus (P) levels. Lines represent 95% prediction intervals. (A) Day 7 for P of 133.5 μg, (B) Day 7 for P of 267.8 μg, (C) Day 7 for P of 535.5 μg, (D) Day 14 for P of 133.5 μg, (E) Day 14 for P of 267.8 μg, (F) Day 14 for P of 535.5 μg, (G) Day 21 for P of 133.5 μg, (H) Day 21 for P of 267.8 μg, (I) Day 21 for P of 535.5 μg, (J) Day 28 for P of 133.5 μg, (K) Day 28 for P of 267.8 μg, (L) Day 28 for P of 535.5 μg.
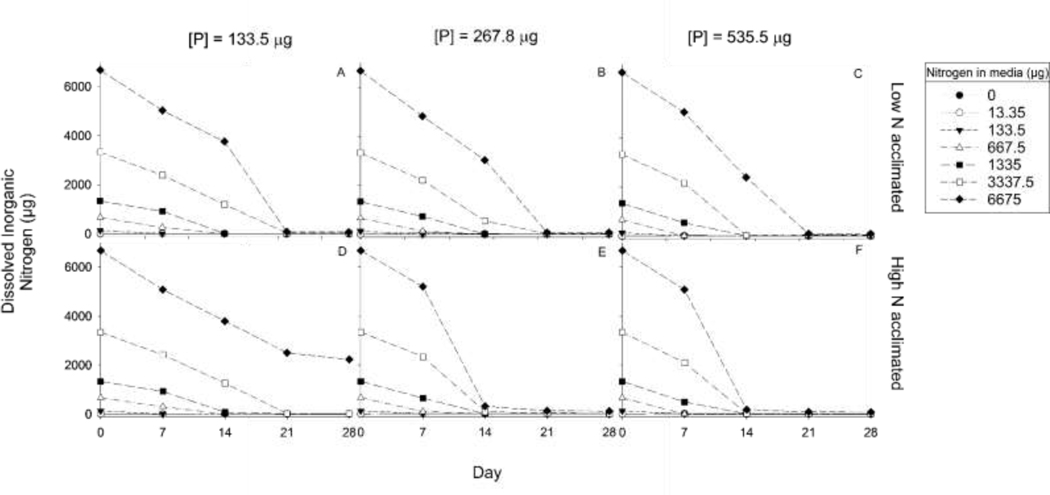
3.2.2. Dissolved Inorganic Nitrogen
Differences in DIN uptake slopes between high and low acclimated populations within the same N:P conditions revealed no significant differences (p>0.05) for any N:P regardless of P treatment (Fig. 5). Over the 28 day experiment, exponential decay models overall explained DIN uptake rates better than linear regressions, and from these models the 95% confidence intervals confirmed there were no differences between decay rates (Supplementary Table 2). However, a few uptake differences between acclimations are notable within the high N populations. The high N acclimated populations high N treatments (6675 μg) in the mid and high P levels utilized up to 90% of the DIN in media by Day 14 (Fig. 5E; 5F). The low N acclimated populations did not reach this amount of DIN uptake in these specific treatment conditions until Day 21 (Fig. 5B; 5C). However in the high N treatment and low P level, the high N acclimated population did not uptake all available DIN by the end of the 28 days, stopping at ~75% DIN uptake from the beginning media conditions (Fig. 5D), and this was not seen in the low N acclimated populations, which utilized ~99% of the available DIN by day 28 (Fig. 5A).
Fig. 5:
Total dissolved inorganic nitrogen (μg; N) for each of the acclimation populations for all four sampling events. (A) Low N acclimated populations with an initial phosphorus (P) of 133.5 μg, (B) Low N acclimated populations with an initial P of 267.8 μg, (C) Low N acclimated populations with an initial P of 535.5 μg, (D) High N acclimated populations with an initial P of 133.5 μg, (E) High N acclimated populations with an initial P of 267.8 μg, (F) High N acclimated populations with an initial P of 535.5 μg.
3.2.3. Nitrogen Fixation
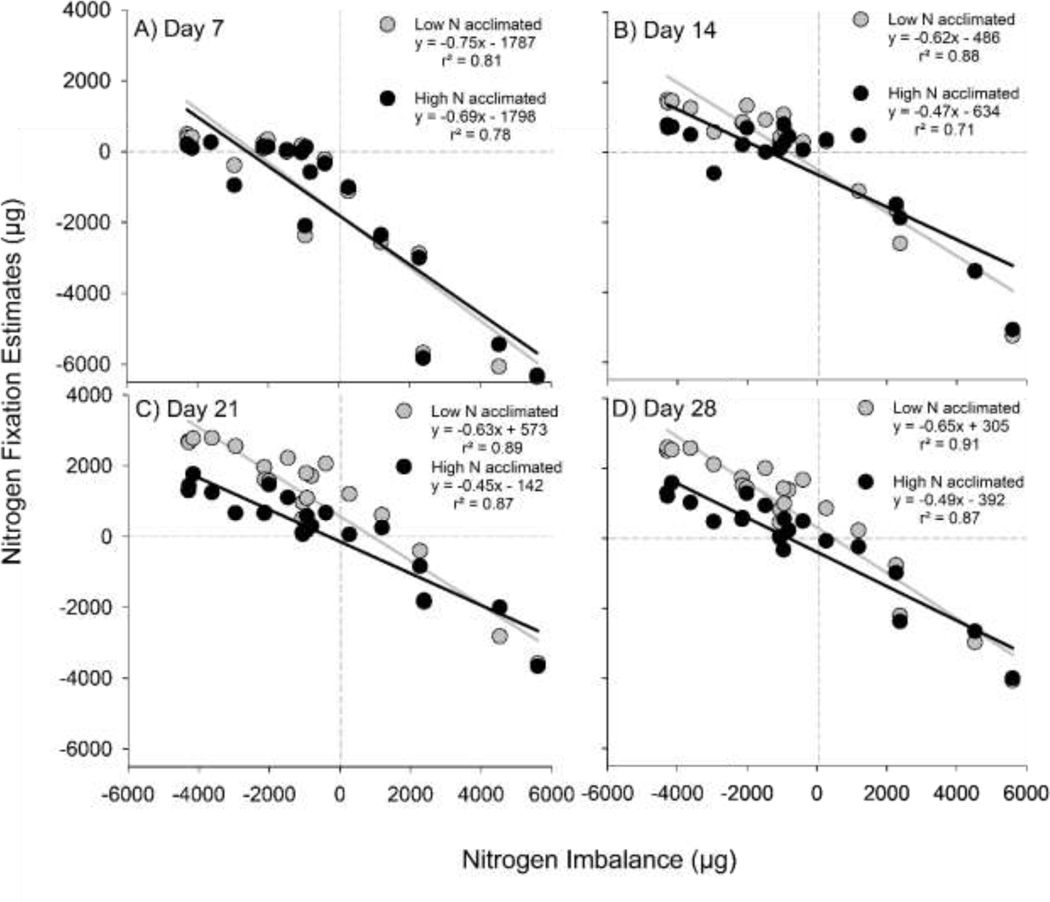
The mass of N fixed varied with the N imbalance of media but there was no difference in the slopes between N fixation and N imbalance for low and high N acclimated populations after 7 and 14 days of growth (Fig. 6A; t=0.187, p=0.644, Fig. 6B; t=2.97, p=0.081). However, after 21 and 28 days of growth, low N acclimated populations exhibited statistically greater N fixation rates that increased with increasing N deficit (i.e. negative N imbalance; Fig. 6C; t=6.41, p=0.012, Fig. 6D; t=5.61, p=0.018). Nitrogen fixation estimates significantly differed over time for the high N acclimated populations (t=13.5, p=0.006) with the slopes for day 21 and 28 being shallower than the slope from day 7 (Fig. 6), and the remaining temporal comparisons having the same slope. This was not the case for the low N acclimated populations, with a constant slope identified temporally (t=4.95, p=0.183).
Fig. 6:
Particulate nitrogen (N) residuals from the N added to the cultures plotted against the N imbalance of the media over the four sampling events for each acclimation (A) Day 7, (B) Day 14, (C) Day 21, (D) Day 28.
4. Discussion
4.1. Overview of C:N Stoichiometry
These results support the hypothetical scenario D from the conceptual figure (Fig. 1), with the C:N displaying a hump-shaped curve with increasing resource N concentration. Currently, the literature is sparse on cyanobacteria, specifically diazotroph, stoichiometry studies, with the focus typically on cellular N:P responses rather than C:N. For example, in a study examining the optimum N:P of phytoplankton, only 17% of examined genera were diazotrophic (Klausmeier et al., 2004). However, quantifying C:N stoichiometry may be particularly important for predicting the potential toxicity of diazotrophic cyanobacterial blooms (van de Waal, 2014). Cellular C:N stoichiometry in D. flos-aquae ranged between 1–12.5, with most values ranging from 4–9, which was dependent on the initial N:P supply, P treatment, and the duration of growth. These results indicate that diazotrophic cyanobacteria are plastic in their C:N stoichiometry and there is a trade-off between growth and the energy required to fix N.
Sampling through time allowed for identifying when this C:N „hump‟ occurred, which could have been missed in a longer temporal study with a more coarse sampling regime. Additionally, the hypothesis that acclimation would affect the C:N stoichiometry of D. flos-aquae populations was found to be supported under certain P treatments and over different times. Acclimating populations to either low or high N resulted in C:N differences throughout the initial and late periods of culture growth. These ranges in C:N were related to the timing of populations becoming N limited, as the low N acclimated populations initially had higher C:N over all N:P treatments, even though this pattern switched after 21 days. These results highlight that antecedent environmental conditions affect the cellular stoichiometry and biomass production on timescales typical of a HAB. Overall, the results suggest that not only nutrient supply, but acclimation affect the size and stoichiometry of a diazotrophic cyanobacterial bloom. This study lends to the growing literature on diazotrophic cyanobacteria stoichiometry.
4.2. Homeostasis
The findings in this study add more evidence indicating bacteria can be non-homeostatic with regard to nutrient stoichiometry (Sterner and Elser, 2002). Much of the evidence to support strict homeostasis in bacteria stemmed from an E. coli laboratory strain which exhibited minimal plasticity in C:P and N:P and no plasticity in the C:N when grown on C:N:P nutrient supply ratios that varied by an order of magnitude (Makino et al., 2003), and a meta-analysis that indicated bacteria species are largely homeostatic for C:P, C:N and N:P (Persson et al., 2010). Since then, both weak and non-homeostatic stoichiometry has been found in more recently isolated strains across environmentally relevant nutrient gradients (Scott et al., 2012; Godwin and Cotner, 2015a; Godwin and Cotner, 2015b). Less is known about C:N homeostasis in bacteria and cyanobacteria. Cyanobacteria, including Dolichospermum, were thought to have a C:N of 5.6 (atom) that was homeostatic (Wolk, 1973), and deviations in homeostasis were caused by temporally decoupling of C and N acquisition (Forchhammer and Selim, 2020). However, non-diazotrophic cyanobacteria populations can have C:N stoichiometry that ranges between 4 and 20 (Wagner et al., 2019) that is likely a result of increased glycogen storage in biomass once dissolved N is exhausted (Forchhammer and Selim, 2020). These results show that diazotrophic C:N stoichiometry is less variable and constrained ranging between 4–9. There was a slight increase in the baseline C:N through time (Figure 2). This may have been due to another factor becoming limiting in the cultures, such as light, or possibly P, that would then contribute to this decoupling of C and N. Regardless, these data support the hypothesis that diazotrophs ability to fix atmospheric N2 results in a greater degree of homeostasis compared to non-diazotrophs, but not strict C:N homeostasis as typically defined in ecological stoichiometry.
If variable nutrient supply rates maintained a dynamic demand for oscillating N fixation within a population, these data predict non-homeostasis for active N fixers. The temporal plasticity of cellular C:N in diazotrophs is likely caused by the molecular changes resulting from acquiring N from DIN to N fixation. DIN scarcity initiates an increase in the cellular C:N ratio in D. flos-aquae and C and N metabolism begins to decouple, causing increases of tricarboxylic acid cycle intermediate metabolite, 2-oxoglutarate (2-OG) (Torres-Sánchez et al., 2015). High concentrations of 2-OG causes a N sensing promoter, NtcA, to begin the cascading gene signaling process for heterocysts differentiation that is needed for nitrogenase synthesis and protection (Herrero et al., 2004). Consequently, the energetic requirements for this multi-step physiological response causes a decrease in growth (Fay, 1992). The growth decrease and the increase in cellular N caused by reliance on atmospheric N2 permits D. flos-aquae to increase N cell quota causing the C:N to decrease. Thus, stoichiometric homeostasis appears temporally dependent on extracellular DIN availability in diazotrophic organisms. Furthermore, if a population is driven to complete dependence on DIN or atmospheric N2, then this period of non-homeostasis appears temporary.
4.3. Diazotroph growth trade-off
Diazotrophs grown solely on atmospheric N2 maintained slightly elevated C:N stoichiometry compared to populations grown exclusively on DIN. However, this near homeostatic response comes at a substantial growth penalty. This growth penalty is well documented (Fogg, 1964), and is caused by the increased energetic requirements to assimilate N from atmospheric N2 (Flores and Herrero, 2005) and possibly by replacing up to 10% of the vegetative cells used for growth to heterocysts used to fix N (Herrero et al., 2016). Diazotrophic species have a selective advantage in low DIN waters because of the ability to fix N (Wood et al., 2010). However, under N limited conditions other phytoplankton groups with lower optimal N:P may be able to effectively compete with diazotrophic cyanobacteria. The optimum phytoplankton N:P has shown to vary between genera (Klausmeier et al., 2004), as differences in nutrient requirements are based on physiological conditions. These differences in N:P optima contribute to the community structure seen in natural systems, as different nutrient requirements would allow for multiple genera to co-exist (Hutchinson, 1961). Comparable between divisions, optimum N:P has been shown to be lower in diatoms and higher in cyanobacteria (Hillebrand et al., 2013). Static and non-static responses to environmental nutrient changes effects which genera can be found in different environments. Maintaining a fairly constant C:N when exclusively grown on atmospheric N2 generates a fitness advantage in N limited systems. This N limitation, while being represented here as N:P values, is also related to the concentration of nutrients used to create the ratio (Morel, 1987). It has been shown that the half saturation constant of N is not only related to ambient N, but also cell size, showing a physiological trade-off of growth and nutrient uptake (Smith et al., 2014). This study clearly shows that this fitness advantage comes at high cost of growth for Dolichospermum.
4.4. Acclimation
The importance of acclimation in phytoplankton populations has recently been explored with a focus on conditions that effect growth and nutrient uptake, as these are correlated to acclimations to their environments (Litchman et al., 2007). In diatoms, Chaetoceros simplex chronically exposed to N limitation was unable to acclimate to grow under high temperatures compared to strains that were grown in N-replete conditions (Aranguren‐Gassis et al., 2019). Temporal differences between P transporter genes under differing P additions was found in batch diatom monocultures, highlighting that a temporal sequence of molecular responses occurs in phytoplankton nutrient uptake acclimation (Cáceres et al., 2019). Phenotypic plasticity has been observed in Microcystis, with upwards of a 5-fold increase in maximum CO2 uptake rates which were not previously acclimated to high CO2, suggesting blooms acclimated to high CO2 will intensify with rising atmospheric CO2 (Ji et al., 2020). Microcystis acclimated to both elevated N and temperature showed a significant increase in growth rates, but exhibited no significant changes in microcystin production (Rouco et al., 2011). In N-fixers, phycobiliproteins in Dolichospermum (reported as Anabeana) exhibited chromatic acclimation with differing light qualities (Ojit et al., 2015).
Acclimating diazotrophs to low or high N conditions varied C:N stoichiometry and biomass production between populations. The C:N plasticity between low and high acclimated populations varied depending on the duration of the experiment, possibly due to differences in intracellular N storage pools and the breakdown and consumption of these storage pools when populations became N limited (Allen et al., 1980). Additionally, low N acclimated populations produced more biomass in the high P treatments when the N imbalance was low during most of the experiment. Acclimation to temperature and resource limitation in Chlamydomonas populations resulted in similar responses to which the acclimated treatment out competes the non-acclimated populations when exposed to similar conditions (Kremer et al., 2018; Bernhardt et al. 2020). For example, when Chlamydomonas acclimated to temperatures of both 14°C and 33°C were then exposed to temperatures ranging from 14–33°C, it was found that the populations with 14°C acclimation displayed higher growth rates overall compared to the 33°C acclimated populations (Kremer et al., 2018). Additionally, Chlamydomonas acclimated to light, N, or P limitation resulted in higher growth rates under limited conditions compared to non-acclimated populations, which could be described by the difference in the Monod curve allowing for higher growth and decreasing the half saturation constant (Bernhardt et al. 2020).
4.5. Nitrogen fixation
Although DIN uptake was similar between the low and high N acclimated populations, the low N acclimated populations were able to fix more N in less time (Fig. 6). This could be explained by low N acclimated populations having a greater density of heterocysts that enabled a rapid N fixation response. Dolichospermum only produces heterocysts to fix N when environmental N is limited (Yema et al., 2016). Heterocysts typically form within 24 hours after N limitation occurs (Kumar et al., 2010) and the N-fixing (nif) genes are expressed late during this developmental period (Elhai and Wolk, 1990; Golden et al., 1991). As the low N acclimated populations were previously primed for N fixation, these populations may have had the morphological and epigenetic machinery in place to shorten the response time to N deficiency. Although increased DIN availability inhibits N fixation, regaining nitrogenase activity in existing heterocysts may provide a functionally competitive mechanism for D. flos-aquae to respond to short-term N deficiency. While studies have shown growth differences for diazotrophs grown under differing N conditions both in lab and in field studies (Zulkefli and Hwang, 2020; Thomas and Litchman, 2016; Stockner and Shortreed, 1988), growth is usually measured as chlorophyll-a and C:N is not typically published or considered in the discussion of the findings. Further, the literature on ES of N fixation is strongly focused on N:P ratios. Of diazotrophs where C:N has been studied, the C:N of Trichodesmium while fixing N has been found to range from 4.1–6.5 (Holl and Montoya, 2008 and references within).
Relative to in situ N:P conditions, a 20-year study over three lakes of differing trophic status showed ranges in N:P from 15 – 1219 (by atom; Adamovich et al., 2019), which were relevant to our studies N:P culture conditions, showing that Dolichospermum very realistically could grow under such conditions and would expect C:N to respond as shown in Fig. 2. While eutrophic lakes have typically been of concern for cyanobacterial blooms, Dolichospermum blooms in oligotrophic lakes have been noted. Dolichospermum blooms in an oligotrophic lake during summer months had a C:N of 8.7 and 4.7 (by atom) in 2010 and 2011 (Callieri et al., 2014), a range supported by results from this study (Fig. 2). However without confirmed heterocyst counts from the Callieri et al. (2014) study, the high C:N merely infers N fixation. It is notable that Dolichospermum blooms have been found to occur in lakes that do not have a typically low N:P ratio (Sterner et al., 2020), suggesting that this genus utilizes other methods, such as akinetes (Carey et al., 2009) to outperform other genera. Rivers may also be driving these Dolichospermum blooms, as the lower N:P riverine and cooler environment would prime these filaments, and then once reaching the lake body where higher temperatures and light levels allowed for growth a bloom becomes possible (Reinl et al., 2020). How the C:N of Dolichospermum would respond if blooming in an oligotrophic lake is still comparable to our study, as the production of heterocyst is dependent on N availability. If the bloom utilizes the available N, heterocyst will form and the „hump‟ shaped curve signifying cell N limitation should be observable through time, even in lakes with low productivity.
Resource competition and competitive exclusion theories provide a predictive framework on interspecific competition and states that organisms that have a lower nutrient requirement would outcompete organisms with a higher nutrient requirement (Hutchinson, 1961; Tilman, 1977). For example, species with lower half saturation constants for nitrate will be able to obtain limiting resources and outcompete species with higher half saturation constants (Reynolds, 2006). Seasonal N drawdown typically occurs over the course of the late summer months (Salk et al., 2018) in many lakes until fall turnover increases available N. During the months when N is limiting, Dolichospermum can begin to acclimate and increase the amount of N fixed leaving them primed to respond to a pulse of N availability during seasonal mixing. Our results indicate the potential importance of this phenomena, but targeted field studies are needed to confirm that N fixation may simply position a diazotrophic species for seasonal blooms rather than fuel them.
5. Conclusion
Diazotrophs are unique in terms of their ability to fix atmospheric N2, which confer a unique advantage to some HAB species. Here ES theory was used to evaluate the potential for elemental homeostasis of N in a common diazotrophic phytoplankton, D. flos-aquae. This diazotroph responded dynamically to changing nutrient supply and this response depended on the conditions in which a blooming population was acclimated. This study shows the potential for C:N homeostasis within diazotrophs, but only with obvious growth/homeostasis tradeoffs. Short term studies on bloom forming cyanobacteria may miss this critical dynamic. These results span across a variable productivity gradient from eutrophic to hypereutrophic and provide evidence for the importance of controlling for both N and P as the main macronutrients for cyanobacteria of emerging concern (Glibert and Burford, 2017). Without readily available N, growth rates of diazotrophs cannot reach the maximum growth rate because N fixation induces a strict homeostasis growth tradeoff.
Supplementary Material
Highlights.
Dolichospermum flos-aquae was acclimated to low and high N:P conditions.
Populations were tested for temporal biomass and C:N stoichiometric changes.
D. flos-aquae (UTEX 1444) does not exhibit strict stoichiometric homeostasis for N.
Acclimated populations of D. flos-aquae exhibited differences in fixed N and C:N.
Acknowledgements and Funding Source
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under award number 1P01ES028942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would also like to thank Dr. Jeff Back, as well as the Center for Reservoir and Aquatic Systems Research at Baylor University, for running the dissolved nutrient samples and for use of their facility.
Footnotes
Declaration of Competing Interest
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- APHA. 2005. Standard methods for the examination of water and wastewater (22nd edn), American Water Works Association, Washington, DC. [Google Scholar]
- Adamovich BV, Medvinsky AB, Nikitina LV, Radchikova NP, Mikheyeva TM, Kovalevskaya RZ, Veres Yu. K., Chakraborty A, Rusakov AV, Nurieva NI, Zhukova TV 2019. Relations between variations in the lake bacterioplankton abundance and the lake trophic state: Evidence from the 20-year monitoring. Ecol. Indic 97, 120–129. 10.1016/j.ecolind.2018.09.049 [DOI] [Google Scholar]
- Allen MM, Hutchison F, Weathers PJ 1980. Cyanophycin granule polypeptide formation and degradation in the cyanobacterium Aphanocapsa 6308. J. Bacteriol 141 (2), 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranguren‐Gassis M, Kremer CT, Klausmeier CA, Litchman E 2019. Nitrogen limitation inhibits marine diatom adaptation to high temperatures. Ecol. Lett 22 (11), 1860–1869. 10.1111/ele.13378 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S 2015. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw 67 (1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bernhardt JR, Kratina P, Pereira AL, Tamminen M, Thomas MK, Narwani A 2020. The Evolution of Competitive Ability for Essential Resources. Phil. Trans. R. Soc. B 375. 10.1098/rstb.2019.0247 [DOI] [PMC free article] [PubMed]
- Boatman TG, Davey PA, Lawson T, Geider RJ 2018. The physiological cost of diazotrophy for Trichodesmium erythraeum IMS101. PLoS One. 13 (4). 10.1371/journal.pone.0195638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BW, Lazorchak JM, Howard MDA, Johnson M-VV, Morton SL, Perkins DAK, Reavie ED, Scott GI, Smith SA, Steevens JA 2017. In some places, in some cases, and at some times, harmful algal blooms are the greatest threat to inland water quality: Inland HABs: Transformative threats to water quality? Environ. Toxicol. Chem 36 (5), 1125–1127. 10.1002/etc.3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BW, Lazorchak JM, Howard MDA, Johnson M-VV, Morton SL, Perkins DAK, Reavie ED, Scott GI, Smith SA, Steevens JA 2018. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem 35 (1), 6–13. 10.1002/etc.3220@10.1002/(ISSN)1552-8618.FOCUS [DOI] [PubMed] [Google Scholar]
- Buratti FM, Manganelli M, Vichi S, Stefanelli M, Scardala S, Testai E, Funari E 2017. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol 91 (3), 1049–1130. 10.1007/s00204-016-1913-6 [DOI] [PubMed] [Google Scholar]
- Cáceres C, Spatharis S, Kaiserli E, Smeti E, Flowers H, Bonachela JA 2019. Temporal phosphate gradients reveal diverse acclimation responses in phytoplankton phosphate uptake. ISME J. 13. 2834–2845. 10.1038/s41396-019-0473-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callieri C, Bertoni R, Contesini M, Bertoni F 2014. Lake Level Fluctuations Boost Toxic Cyanobacterial “Oligotrophic Blooms.” PLoS One. 9 (10). 10.1371/journal.pone.010952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CC, Weathers KC, Cottingham KL 2009. Increases in phosphorus at the sediment-water interface may influence the initiation of cyanobacterial blooms in an oligotrophic lake. SIL Proc. 1922–2010, 30 (8), 1185–1188. 10.1080/03680770.2009.11923908 [DOI] [Google Scholar]
- Carey CC, Ibelings BW, Hoffmann EP, Hamilton DP, Brookes JD 2012. Ecophysiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 46 (5), 1394–1407. 10.1016/j.watres.2011.12.016 [DOI] [PubMed] [Google Scholar]
- Cobb HD, Myers J 1964. Comparative Studies of Nitrogen Fixation and Photosynthesis in Anabaena Cylindrica. Am. J. Bot 51 (7), 753–762. 10.1002/j.1537-2197.1964.tb06697.x [DOI] [Google Scholar]
- Elhai J, Wolk CP 1990. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 9 (10), 3379–3388. 10.1002/j.1460-2075.1990.tb07539.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falster D, Warton D, Wright I 2006. Standardized Major Axis Tests and Routines, Ver 2.0.
- Fay P 1992. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol. Rev 56 (2), 340–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores E, Herrero A 2005. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans 33 (1), 164–167. 10.1042/BST0330164 [DOI] [PubMed] [Google Scholar]
- Forchhammer K Selim K 2020. Carbon/Nitrogen Homeostasis Control in Cyanobacteria. FEMS Microbiol. Rev 44 (1), 33–53. 10.1093/femsre/fuz025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg GE 1964. Environmental Conditions and the Pattern of Metabolism in Algae. In: Jackson DF (eds) Algae and Man. Springer, Boston, MA. 77–85. 10.1007/978-1-4684-1719-7_5 [DOI] [Google Scholar]
- Fox J, Weisberg S 2011. Multivariate Linear Models in R. 31. [Google Scholar]
- Glibert PM, Burford MA 2017. Globally Changing Nutrient Loads and Harmful Algal Blooms: Recent Advances, New Paradigms, and Continuing Challenges. Oceanogr. 30 (1), 58–69. [Google Scholar]
- Gobler CJ, Burkholder JM, Davis TW, Harke MJ, Johengen T, Stow CA, Van de Waal DB 2016. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae. 54 (Supplement C), 87–97. 10.1016/j.hal.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Godwin CM, Cotner JB 2015a. Aquatic heterotrophic bacteria have highly flexible phosphorus content and biomass stoichiometry. ISME J. 9 (10), 2324–2327. 10.1038/ismej.2015.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin CM, Cotner JB 2015b. Stoichiometric flexibility in diverse aquatic heterotrophic bacteria is coupled to differences in cellular phosphorus quotas. Front. Microbiol 6 (159), 1–15. 10.3389/fmicb.2015.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JW, Whorff LL, Wiest DR 1991. Independent regulation of nifHDK operon transcription and DNA rearrangement during heterocyst differentiation in the cyanobacterium Anabaena sp. Strain PCC 7120. J. Bacteriol 173 (22), 7098–7105. 10.1128/jb.173.22.7098-7105.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover JP, Scott JT, Roelke DL, Brooks BW 2020. Dynamics of nitrogen-fixing cyanobacteria with heterocysts: A stoichiometric model. Mar. Freshw. Res 71 (5), 644–658. 10.1071/MF18361 [DOI] [Google Scholar]
- Herrero A, Muro-Pastor AM, Valladares A, Flores E 2004. Cellular differentiation and the NtcA transcription factor in filamentous cyanobacteria. FEMS Microbiol. Rev 28 (4), 469–487. 10.1016/j.femsre.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Herrero A, Stavans J, Flores E 2016. The Multicellular Nature of Filamentous Heterocyst-Forming Cyanobacteria. FEMS Microbiol. Rev 40 (6), 831–854. 10.1093/femsre/fuw029 [DOI] [PubMed] [Google Scholar]
- Hillebrand H, Steinert G, Boersma M, Malzahn A, Meunier CL, Plum C, Ptacnik R 2013. Goldman revisited: Faster-growing phytoplankton has lower N:P and lower stoichiometric flexibility. Limnol. Oceanogr 58 (6), 2076–2088. doi: 10.4319/lo.2013.58.6.2076 [DOI] [Google Scholar]
- Holl CM, Montoya JP 2008. Diazotrophic Growth of the Marine Cyanobacterium Trichodesmium Ims101 in Continuous Culture: Effects of Growth Rate on N2-Fixation Rate, Biomass, and C:n:p Stoichiometry1. J. Phycol 44 (4), 929–937. 10.1111/j.1529-8817.2008.00534.x [DOI] [PubMed] [Google Scholar]
- Hutchinson GE 1961. The Paradox of the Plankton. Am. Nat 95, 137–145. [Google Scholar]
- Ji X, Verspagen JMH, de Waal DBV, Rost B, Huisman J 2020. Phenotypic plasticity of carbon fixation stimulates cyanobacterial blooms at elevated CO2. Sci. Adv 6 (8), 1–9. 10.1126/sciadv.aax2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausmeier CA, Litchman E, Daufresne T, Levin SA 2004. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature. 429 (6988), 171–174. 10.1038/nature02454 [DOI] [PubMed] [Google Scholar]
- Kremer CT, Fey SB, Arellano AA, Vasseur DA 2018. Gradual plasticity alters population dynamics in variable environments: thermal acclimation in the green alga Chlamydomonas reinhartdii. Proc. R. Soc. B 285 (1870). 10.1098/rspb.2017.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromkamp J 1987. Formation and functional significance of storage products in cyanobacteria. N.Z. J. Mar. Freshw. Res 21 (3), 457–465. 10.1080/00288330.1987.9516241 [DOI] [Google Scholar]
- Kumar K, Mella-Herrera RA, Golden JW 2010. Cyanobacterial Heterocysts. Cold Spring Harb. Perspect. Biol 2 (4). 10.1101/cshperspect.a000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R 2016. Least-Squares Means. R Package „lsmeans‟. (https://cran.r-project.org/web/packages/lsmeans/lsmeans.pdf)
- Litchman E, Klausmeier CA, Schofield OM, Falkowski PG 2007. The role of functional traits and trade-offs in structuring phytoplankton communities: Scaling from cellular to ecosystem level. Ecol. Lett 10 (12), 1170–1181. 10.1111/j.1461-0248.2007.01117.x [DOI] [PubMed] [Google Scholar]
- Lu J, Zhu B, Struewing I, Xu N, Duan S 2019. Nitrogen–phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by meta-transcriptomics. Sci. Rep 9 (1), 1–11. 10.1038/s41598-019-38481-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino W, Cotner JB, Sterner RW, Elser JJ 2003. Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C : N : P stoichiometry. Funct. Ecol 17 (1), 121–130. 10.1046/j.1365-2435.2003.00712.x [DOI] [Google Scholar]
- Morel FMM 1987. Kinetics of Nutrient Uptake and Growth in Phytoplankton1. J. Phycol 23 (1), 137–150. 10.1111/j.0022-3646.1987.00137.x [DOI] [Google Scholar]
- Ojit SK, Indrama T, Gunapati O, Avijeet SO, Subhalaxmi SA, Silvia C, Indira DW, Romi K, Minerva S, Thadoi DA, Tiwari ON, Sharma GD 2015. The response of phycobiliproteins to light qualities in Anabaena circinalis. J. Appl. Biol. Biotechnol 3 (3), 1–6. 10.7324/JABB.2015.3301 [DOI] [Google Scholar]
- Paerl HW 2014. Mitigating Harmful Cyanobacterial Blooms in a Human- and Climatically-Impacted World. Life. 4 (4), 988–1012. 10.3390/life4040988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl HW 2017. Controlling harmful cyanobacterial blooms in a climatically more extreme world: Management options and research needs. J. Plankton Res 39 (5), 763–771. 10.1093/plankt/fbx042 [DOI] [Google Scholar]
- Perrson J, Fink P, Goto A, Hood JA, Jonas J, Kato S 2010. To Be or Not To Be What You Eat: Regulation of Stoichiometric Homeostasis Among Autotrophs and Heterotrophs. Oikos. 119, 741–751. 10.1111/j.1600-0706.2009.18545.x. [DOI] [Google Scholar]
- R Core Team. 2019. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. [Google Scholar]
- Reinl KL, Sterner RW, Lafrancois BM, Brovold S 2020. Fluvial seeding of cyanobacterial blooms in oligotrophic Lake Superior. Harmful Algae. 100. 10.1016/j.hal.2020.101941 [DOI] [PubMed] [Google Scholar]
- Reynolds CS 2006. The Ecology of Phytoplankton. Cambridge University Press. 10.1017/CBO9780511542145 [DOI] [Google Scholar]
- Rouco M, López-Rodas V, Flores-Moya A, Costas E 2011. Evolutionary Changes in Growth Rate and Toxin Production in the Cyanobacterium Microcystis aeruginosa Under a Scenario of Eutrophication and Temperature Increase. Microb. Ecol 62 (2), 265–273. 10.1007/s00248-011-9804-0 [DOI] [PubMed] [Google Scholar]
- Salk KR, Bullerjahn GS, McKay RML, Chaffin JD, Ostrom NE 2018. Nitrogen cycling in Sandusky Bay, Lake Erie: oscillations between strong and weak export and implications for harmful algal blooms. Biogeosciences. 15, 2891–2907. 10.5194/bg-15-2891-2018 [DOI] [Google Scholar]
- Scott T, Cotner J, LaPara T 2012. Variable Stoichiometry and Homeostatic Regulation of Bacterial Biomass Elemental Composition. Front. Microb 3 (42), 1–8. 10.3389/fmicb.2012.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JT, McCarthy MJ, Paerl HW 2019. Nitrogen transformations differentially affect nutrient-limited primary production in lakes of varying trophic state. Limnol. Oceanogr. Lett 4 (4), 96–104. 10.1002/lol2.10109 [DOI] [Google Scholar]
- Smith SL, Merico A, Hohn S, Brandt G 2014. Sizing-up nutrient uptake kinetics: Combining a physiological trade-off with size-scaling of phytoplankton traits. Mar. Ecol. Prog. Ser 511, 33–39. 10.3354/meps10903 [DOI] [Google Scholar]
- Sterner RW, Elser JJ 2002. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press. [Google Scholar]
- Sterner RW, Reinl KL, Lafrancois BM, Brovold S, Miller TR 2020. A first assessment of cyanobacterial blooms in oligotrophic Lake Superior. Limnol, Oceanogr. 65 (12), 2984–2998. [Google Scholar]
- Stockner JG, Shortreed KS 1988. Response of Anabaena and Synechococcus to manipulation of nitrogen: Phosphorus ratios in a lake fertilization experiment. Limnol, Oceanogr. 33 (6), 1348–1361. 10.4319/lo.1988.33.6.1348 [DOI] [Google Scholar]
- Thomas MK, Litchman E 2016. Effects of temperature and nitrogen availability on the growth of invasive and native cyanobacteria. Hydrobiologia. 763 (1), 357–369. 10.1007/s10750-015-2390-2 [DOI] [Google Scholar]
- Tilman D, 1977. Resource competition between planktonic algae: an experimental and theoretical approach. Ecology 58, 338–348. [Google Scholar]
- Torres-Sánchez A, Gómez-Gardeñes J, Falo F 2015. An Integrative Approach for Modeling and Simulation of Heterocyst Pattern Formation in Cyanobacteria Filaments. PLoS Comput. Biol 11 (3). 10.1371/journal.pcbi.1004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Waal DB, Smith VH, Declerck SAJ, Stam ECM, Elser JJ 2014. Stoichiometric regulation of phytoplankton toxins. Ecol. Lett 17 (6), 736–742. 10.1111/ele.12280 [DOI] [PubMed] [Google Scholar]
- Wagner ND, Osburn FS, Wang J, Taylor RB, Boedecker AR, Chambliss CK, Brooks BW, Scott JT 2019. Biological Stoichiometry Regulates Toxin Production in Microcystis aeruginosa (UTEX 2385). Toxins. 11 (10), 601. 10.3390/toxins11100601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warton D, Wright I, Falster D, Westoby M 2006. Bivariate line-fitting methods for allometry. Biol. Rev. 81, 259–291. [DOI] [PubMed] [Google Scholar]
- Wolk PC 1973. Physiology and Cytological Chemistry of Blue-Green Algae. Bacteriol. Rev 37 (1), 32–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SA, Prentice MJ, Smith K, Hamilton DP 2010. Low dissolved inorganic nitrogen and increased heterocyte frequency: precursors to Anabaena planktonica blooms in a temperate, eutrophic reservoir. J. Plankton Res. 32 (9), 1315–1325. 10.1093/plankt/fbq048 [DOI] [Google Scholar]
- Yema L, Litchman E, de Tezanos Pinto P 2016. The role of heterocytes in the physiology and ecology of bloom-forming harmful cyanobacteria. Harmful Algae. 60, 131–138. 10.1016/j.hal.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Zulkefli NS, Hwang SJ 2020. Heterocyst Development and Diazotrophic Growth of Anabaena variabilis under Different Nitrogen Availability. Life. 10 (11), 279. 10.3390/life10110279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.