Abstract
Modeling human behavior within mathematical models of infectious diseases is a key component to understand and control disease spread. We present a mathematical compartmental model of Susceptible–Infectious–Removed to compare the infected curves given by four different functional forms describing the transmission rate. These depend on the distance that individuals keep on average to others in their daily lives. We assume that this distance varies according to the balance between two opposite thrives: the self-protecting reaction of individuals upon the presence of disease to increase social distancing and their necessity to return to a culturally dependent natural social distance that occurs in the absence of disease. We present simulations to compare results for different society types on point prevalence, the peak size of a first epidemic outbreak and the time of occurrence of that peak, for four different transmission rate functional forms and parameters of interest related to distancing behavior, such as: the reaction velocity of a society to change social distance during an epidemic. We observe the vulnerability to disease spread of close contact societies, and also show that certain social distancing behavior may provoke a small peak of a first epidemic outbreak, but at the expense of it occurring early after the epidemic onset, observing differences in this regard between society types. We also discuss the appearance of temporal oscillations of the four different transmission rates, their differences, and how this oscillatory behavior is impacted through social distancing; breaking the unimodality of the actives-curve produced by the classical SIR-model.
Subject terms: Applied mathematics, Epidemiology
Introduction
Epidemics and pandemics are a thread for public health. More pandemic situations such as the current pandemic caused by the viral disease COVID-19 may come in the future. Such a pandemic can cause a devastating public health, social and economic impact across the world. In a pandemic situation governments may be forced to impose and promote restrictive measures to control disease spread. The approach different societies take may vary according to cultural, political and economic realities of each country1. Restrictive measures may eventually have to be relaxed due to the economic and social impact that these can provoke, especially in poorer societies2, while finding a balance between health and economic factors, and trusting on companies and individuals to implement and maintain protective measures3. Social distancing is one of the main recommended individual protective measures during pandemic situations caused by directly transmitted diseases4. Social distancing has been shown to be an effective measure for controlling disease burden for instance during the SARS epidemic of 2003 in Hong Kong1, or during the current COVID-19 pandemic5, 6. Epidemic situations force individuals to develop a change in their social behavior. For instance, there is evidence that the appearance of new behaviors could be conditioned by fears, worries and anxiety among individuals, which recently has been measured by the use of The Fear of COVID-19 Scale7. Hence, in such situations, societies are forced to make cultural changes that strengthen the awareness for public health. These cultural changes if maintained, could help prevent the dissemination of infectious diseases and future epidemics8.
We focus on studying through a mathematical model the epidemiological effects of keeping a certain social distance when encounters are not to be avoided during an epidemic outbreak of a directly transmitted disease. Mathematical modeling of human behavior is an essential tool to guide control strategies, impulsed for instance to prevent infection in risk groups8, 9. To incorporate in mathematical epidemiological models variables or parameters that describe social behavior is an important challenge10. With our model, we seek to: first, compare disease dynamics for different types of societies under different distance dependent transmission rate functional forms; second, understand and describe how the dynamics of the social distance—depending on the observed point prevalence—affects the transmission rates and disease dynamics; third, identify epidemiological and social/cultural factors relevant for disease mitigation.
Methodological aspects
There exists an extensive number of mathematical models that explain, characterize and project the evolution of different infectious diseases that affect humans11–15. In addition to present a compartmental model that classically describes disease dynamics, we incorporate social distancing as a dependent variable following a dynamic law based on point prevalence and socio-behavioral factors. Theories of human behavior state that there exist environmental factors (e.g., climate, demographic growth, location) and psycho-social aspects (e.g., degree of aggregation, economic prosperity, culture) that influence the distance that individuals maintain from each other in their daily lives8, 16–18. Additionally, human groups define cultural norms that can be classified into the following types19: (i) Contact cultures, which relate through close personal distance emphasizing physical contact; (ii) non-contact cultures, in which individuals keep further distance from each other, avoiding physical contact. For instance, contact cultures are found in Southern Europe, Latin America and the Arab countries, while non-contact cultures are found in North America, North of Europe and Asia.
When modeling a disease, it would be best to have a clear understanding about how interactions between people occur, for then recognize social patterns. There are social studies that provide information on social distancing, in particular on the average distance between susceptible and infectious individuals, which is very useful to understand disease spread20. There are methods based on statistics that determine the distance distribution using the number of infectious events associated to all possible susceptible-infectious cases20. Other studies state that the probability of infection between susceptible and infectious decreases with distance according to a formulation of the Power Law21. In general, we assume that an average behavior– connected to social and cultural characteristics of a population– offers, up to a certain level useful information to answer questions at population level, in terms of ecological and epidemiological nature22. There are also studies in the literature related to social distancing, which incorporate the effect on disease dynamics of frequency-duration of physical contact and distances that exist between households in social settings23, 24. In the aforementioned study24, the recorded data are social distances of 1,821 individuals living in Southern China, aggregated in different environments, such as: age and rural or urban conditions. As a result, the study reveals that distance is inversely proportional to the probability of infection. In addition, it was shown that social contacts and their duration decrease with chronological age. Those results provide contact patterns that are consistent with similar research studies conducted in European countries19. For technical simplicity and lack of more accurate information, in this study we assume a uniformly distributed distancing behavior, in populations aggregated by culture. In other words, we assume that all individuals with the same cultural background follow the same social distancing behavior; as we describe in the next section.
There exist several articles that study the spread of infectious diseases related to human behavior through mathematical models. One of the first generalizations of the Kermack-McKendrick deterministic epidemic model25 in that respect was given by Capasso and Serio in 197826, where they present the force of infection in an SIR (Susceptible–Infected–Removed) compartmental type model of differential equations as a function g(I), which saturates for large levels of infectives in the population, changing the transmission rate of the classical SIR model from constant to non-constant. After a study that these authors conducted about the cholera epidemic in Bari, Italy, they wanted to reflect—with the saturation of the force of infection—the psychological effect in the population that leads to adopt more self-protective measures when the number of infected individuals is high. Also, in27, a non-linear force of infection including a saturation function represents the influence of human behavioral change in a cholera model due to health education, hygiene and sanitation practices. In28, an SIR model with exponential saturation of the force of infection of the form is presented, with the intent to capture disease dynamics as an outbreak progresses and behaviors change, where for instance the parameter a is reduced by mask wearing. There are several other articles incorporating similar non-linear force of infection terms (see28–33 and references therein). Additionally, the article by D’Onofrio et al.34 incorporates a non-constant transmission rate that depends not only on the current number of infectives but also on M, representing an information index that summarizes the current and past history of disease prevalence. Their results show that social behavioral change may trigger oscillations in the infectious population. On the other hand, Pedro et al.35 extend an SIR type model incorporating the effect of social support for school and workplace closure on disease dynamics, and study socio-economic conditions for a second COVID-19 wave. In the aforementioned study, the authors define a transmission rate that captures the impact of closure through a function of time. This function is governed by a dynamic law explained by Imitation Dynamics36 to describe population-level support for closure. The article in9 also uses Imitation Dynamics to present the competing dynamics between a resident pathogenic strain and a mutant strain with higher virulence. That article studies a population in which individuals learn and develop a behavior to protect each other. Other dynamic mathematical models have included behavior by dividing the population into different risk groups, and this way studying the epidemiological effects based on social distancing while including individuals’ risk perception, awareness, fear, cooperation or activity level37–46. Specifically, a model that quantifies the epidemiological impact of the size of groups of individuals who do or do not follow responsible behavior can be seen in43. The study shows how the Basic Reproduction Number (an epidemiological threshold that generally determines disease dynamics47) and disease prevalence changes according to each responsible individual. It also discusses the necessity of quantifying the effect that distance between individuals has on disease transmission. On the other hand, the article in40 studies media induced social distancing in an SIR type model including an extra social distancing compartment, whose influx rate is influenced by media; and the authors in39 present a compartmental model that stratifies the population not only by disease status but also by disease awareness status.
Also, there exists evidence for the changing temporal behavior of disease transmission in epidemic or pandemic settings28, 48–55, which justifies extending an SIR type model by incorporating a non-constant transmission rate. In particular, there are articles—some as a result of the high demand in understanding COVID-19—that fit mathematical models in order to represent the decrease in the transmission rate27, 28, 31, 49. For instance, the article in49 includes a time varying exponential decay log function for the transmission rate, to capture this way the early decreasing shape of the transmission rate of COVID-19 thought to be due to enforced lockdowns and disease mitigation interventions.
We consider a deterministic mathematical model based on ordinary differential equations that divide the human population into Susceptible–Infectious–Removed (SIR)56, and extend it including a non-constant transmission rate. The transmission rate of a disease depends on the effective contact rate of individuals, which depends on individual distancing behavior, and determines the occurrence of infection10. The novelty of the model we present is to assume that the transmission rate is represented by different functional forms that depend inversely on a dynamic distance that individuals keep from each other. The dynamics of this distance depends on the point prevalence of the disease and the resistance to change, which comes from the necessity people feel to return to their natural social distance. We make two assumptions regarding the average distance between individuals: (a) in the absence of disease, individuals tend to maintain a certain average distance from each other, which we will call natural-distance, and (b) in the presence of disease, individuals respond by increasing their social distance according to the appreciation of point prevalence levels, and hence the natural-distance becomes a dynamic distance that we will call interaction-distance. We first compare epidemic curves, and the size and timing of the first appearing epidemic peak, for society types that differ according to social distancing behavior related to assumptions (a) and (b). We study the disease dynamics of these societies for different transmission rate functional forms that are interaction-distance dependent, and for different parameter values appearing in these transmission rates. Then, we discuss the temporal dynamics of the four transmission rate functions, how their shape is explained through social distancing behavior and their added practical significance to the classical SIR model when modeling the propagation of infectious diseases.
Cultural distance as risk factor for disease transmission
In the field of semiotic, the discipline that studies the organization of space in terms of linguistic communication is called Proxemic18. In the present work, we will take few elements of this area, in particular related to the types of space that surround the human body—their limits and use–, which could help us characterize distancing behavior in different cultural settings, essential for disease transmission.
Generally speaking, a person defines his or her distance range or degree of physical contact according to the social interconnection she/he experiences with the counterpart (e.g., family, friends, colleagues or strange). Some studies also point out that personal differences such as: personality, age, gender, social conditions, etc., are crucial for a person to decide his or her personal distance boundaries8, 17, 18, 57. Nevertheless, the main factor that determines the distance that individuals keep from each other is cultural related18, which is associated with the geographical region the population is located. As mentioned before, we consider an average distancing behavior assumed equal for all individuals within the same cultural background. Thereby, different average distancing behaviors might affect differently the transmission rate of the disease, leading to cultural changes in disease dynamics.
The term Proxemic is conceptualized by the notion of personal space when referred to the form by which human beings physically interact with each other, either with peers or objects18. In this respect, physical distance is correlated with the social closeness that individuals keep from each other, being characterized in the following way: (i) intimate, (ii) personal, (iii) social and public. Specifically, to each social relationship type corresponds a personal space, which is configured by concentric bubbles, of radius: (i) from 0 to 0.45 (m) for intimate distance ; (ii) from 0.45 to 1.2 (m) for personal distance ; (iii) more than 3.5 (m) for social distance. Latin communities for instance tend to interact socially keeping less distance compared to Anglo-American societies. Indeed, the work in58, titled Proxemics and Tactility in Latin America states that there exist different ways of proxemic communication between individuals belonging to different Latin American countries and even between gender encounters (man-man; man-woman; or woman-woman). The aforementioned study revealed that the encounter between gender, together with the country of origin are determinant factors that affect the average distance individuals keep from each other. It was performed through a multivariate analysis of variance to determine if gender and culture have an effect on human-distances with pairs using groups of individuals from Costa Rica, Panama and Colombia respectively. As a result, it was proven that Costa Rica interacts significantly closer than the rest of the countries located on the south and the mean distance for female pairs is significantly smaller than other gender's encounters.
A global study in the field of Cross Cultural Psychology revealed a comparative interpersonal distancing world wide, using a large data set of 8,943 participants from 42 countries19. According to those authors, Southern European, Latin American and Arabian countries are considered closer cultures with notable physical contact behaviors; whilst North America, Northern Europe and Asian countries prefer more distant encounters and non contact behaviors. As a result, a list of global comparative social distances19, comprised by countries with small, medium and large social distancing allowed among peers is shown in Table 1. It shows the average natural social distance given by the culture of each country. In the Americas, the frequency of physical contacts and their distances decrease progressively as we move from North America to South America. Therefore, it is impossible to determine a common universal contact index for all cultures18. We include this cultural difference using a specific base parameter that is interpreted as the distance that individuals would keep to each other culturally in the absence of the disease. This is the parameter we refer to as natural-distance.
Table 1.
Average social natural-distances measured in meters (m) for different countries of origin.
| Type of social natural-distance | Distance interval (m) | Country of origin |
|---|---|---|
| Small | [0; 1) | Italy–Argentina–Bulgaria–Greece |
| Ukraine–Russia–Slovakia–Austria | ||
| Serbia–Peru–Spain | ||
| Medium | [1; 1.2] | USA–Germany–Indonesia–Estonia |
| England–Poland–Canada–Norway | ||
| China–Brazil–Nigeria–South Korea | ||
| India–Switzerland–Kenya–Portugal | ||
| Czech Republic–Malaysia–Iran | ||
| Pakistan–Croatia–Mexico–Ghana | ||
| Hong Kong | ||
| Large | Uganda–Hungary–Saudi Arabia | |
| Romania–Turkey |
(Information was obtained from the article by Sorokowska et al.19).
Distance-contagion model
We consider a Susceptible–Infectious–Removed (SIR) type model with recovery and transmission rates given respectively by and . The latter, is assumed to be dependent on the average dynamic distance that individuals usually keep from each other, which we denote D and call interaction-distance. The functional form that takes will be introduced in the next section. We assume in our model, that the dynamic for D depends on the level of infectious and that in the absence of disease the interaction -distance returns to its natural equilibrium , which represents the natural-distance of the society. We also suppose no demographic change, no immigration, and a constant total population size . The system of differential equations that determines the dynamic is:
| 1 |
with positive initial conditions , , , . The rate [1/time] measures the rate of resistance, per distant unit, to change distancing behavior. It measures how fast individuals return to their natural-distance , or in other words, the rate at which individuals return to natural distance habits, given by their culture. [distance/time] determines the reaction-velocity by which change occurs according to how people perceive point prevalence levels. Observe that if there is no resistance and D increases () proportional to point prevalence: D increases steeply if people react fast (large ) and increases slightly if they react slow (small ); on the contrary, if individuals tend to return to their natural-distance fast, so there is a large resistance to change their natural way of living. Also, if , the population does not react to point prevalence levels and hence the distance decreases and tends to the equilibrium , as long as ; if on the contrary , the population is very perceptive and reacts quickly to change, even when point prevalence levels may be low.
Observe that, when solving the last equation from the system in Eq. (1), we obtain
| 2 |
which is a function such that if (i.e. society follows its natural distancing behavior when the first infectious person appears), then .
Moreover, its asymptotic behavior () is as follows
| 3 |
Since in Eq. (1), the flow is uni-directional, we have . Hence, given , there exists such that for . Therefore, in Eq. (3) is bounded by and by making we conclude that exponentially, see Fig. 6b, such that the interaction-distance converges to the constant natural-distance of the society. Notice that once expressing I/N in terms of D, we obtain for S that , which when integrating over [0, t] provides the following expression
| 4 |
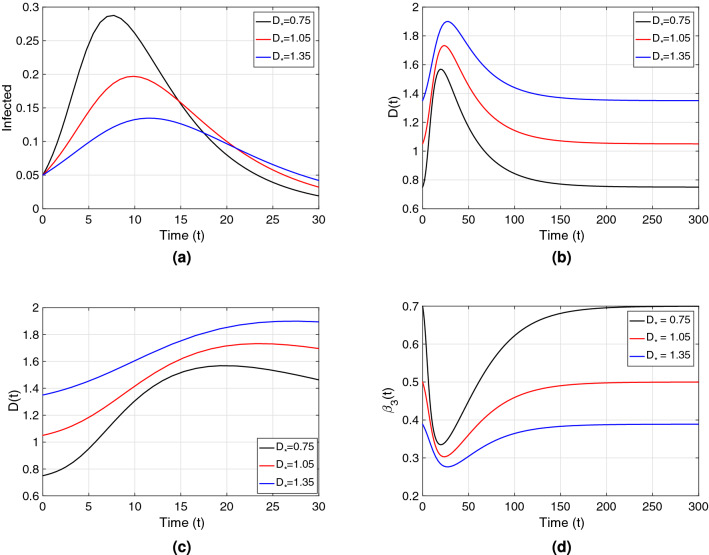
Figure 6.
(a) Point prevalence, I(t)/N, from the system in Eq. (1) with respect to time. (b) Distance D(t) kept by individuals through the epidemic. (c) Zoomed version of (b). (d) Transmission rate from Eq. (8). All plots in the figure consider as the transmission rate. Black, red and blue curves correspond to , , and respectively. These correspond to societies of small ([0, 100)), medium ([100, 120]) and large () natural-distance types respectively (see Table 1). The other parameter values were taken to be ; , , , . The value for each society type is: (black), (red), (blue). The initial condition was used for each type of society.
We denote the value of S for infinite time, and obtain
| 5 |
where . As is to be expected, the epidemics will end with more susceptibles if: the reaction velocity () is large, the resistance () and/or the natural-distance is small. Additionally, if is given by Eq. (8) (shown in the next section), with , we have that , with , an increasing function.
Many factors determine change in behavior, and in particular the dynamics of the interaction-distance between individuals. It may be difficult to quantify parameters related to those changes, such as the rate of resistance to change (), or the reaction-velocity to change (). But, assumptions could be made on how on average the population thinks. In general, there are different types of behavioral changes, as described in16, such as: definite or momentary; local or global; uni-causal or multi-causal; group influenced or individual; superficial or profound. When a change in habits occurs, it is generally difficult to maintain over time and according to the authors in16, maintaining it depends on cultural re-education initiatives.
Distance dependent transmission rate and the basic reproduction number ()
In this section, we will describe how the transmission rate varies with social distance D. We assume a base line transmission rate and a scaling distance , such that . In what follows, we will present four functional forms.
| 6 |
, was inspired on below, and found in59. On one hand, it similarly decreases in a convex form, but keeping its own structural geometry and qualitative differences.
| 7 |
, was introduced in59 using a Maximum Likelihood estimation for the Blue Tongue virus serotype 8 epidemic with a data set from Netherlands and Germany and described in Eqn. (8) was obtained through a parameter estimation of the transmission rate with data from Belgium based on distances between farms. In both cases D, represents the inter farm distance; the initial rate of transmission or base line transmission, and a scaling distance59. Meanwhile is a parameter that measures the decrease in the infectious rate with distance at farm level60.
| 8 |
| 9 |
The form for was introduced in57 in the context of social mixing patterns in rural and urban areas of Southern China aimed to quantify the human interactions targeted for better understanding on the transmission of respiratory infectious diseases. In this study, the contact duration was assigned as an integer number multiplied by the number of individuals following an exponential distribution to each contact event.
Notice that the functional forms given in Eqs. (6)–(9), are decreasing, convex functions such that for ; but for small values of D they differ in the following way: , , and .
In what follows of this article, we are going to consider that the scaling distance is an average of the distance types D_* from Table 1 and hence is fixed at . This way, we can observe: for societies that are experiencing medium interaction-distance , the transmission rate is the base line transmission rate ; for societies with interaction-distance the transmission rate is larger than ; and, for societies with interaction-distance , the transmission rate is smaller than .
Figure 1 shows the transmission curves of the four functional forms from Eqs. (6)–(9): (black), (red), (blue) and (purple), with scaling distance . It can be seen that for , for , which is the base line constant transmission rate. This means that once the interaction-distance D is close to , all transmission rates are similar and close to . On the contrary, if the interaction-distance reaches the type small, medium or large (see Table 1), the transmission rates differ from each other accordingly. Observe that before , the order of the transmission rates is and afterwards it changes to .
Figure 1.
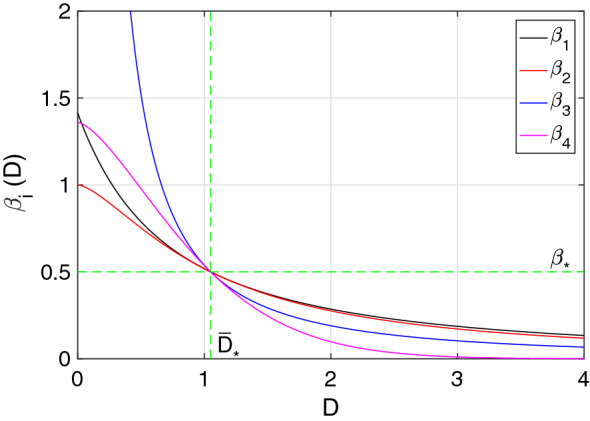
The different transmission rate functional forms , are pictured. The scaling distance was taken to be for all transmission rates, which was considered to be an average of the distance types D_* from Table 1. The other parameters were chosen to be and .
Figure 2 shows for the scaling distance and for different values of . From the picture it can be appreciated that for societies experiencing an interaction-distance D less than , the transmission rate increases with increasing , and, on the contrary, that for societies with interaction-distance D greater than , the transmission rate decreases with increasing .
Figure 2.
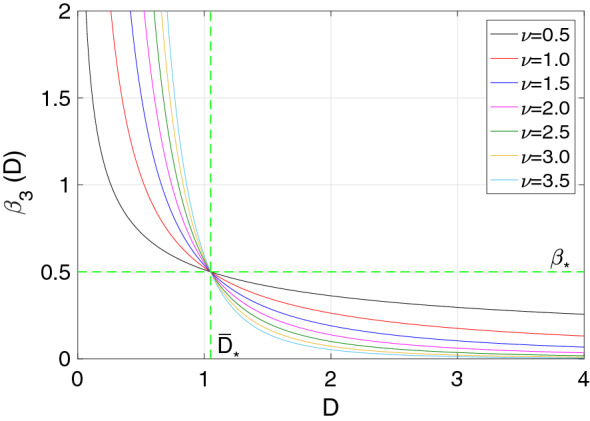
The transmission rate functional form for is pictured. The scaling distance was taken to be for all transmission rates, which was considered to be an average of the distance types D_* from Table 1. The base line transmission rate was chosen to be .
The basic reproduction number, , is an important threshold quantity that generally determines the course of an epidemic and the corresponding dynamics of the system describing it, such that usually an epidemic peak occurs if , and on the other hand, the disease is not able to invade the population if 47. Linearizing the system in Eq. (1) around the disease free state and considering D_0=D_*, reduces to , , and using a similar approach as in61 we obtain the known form for for an SIR model without demography—whose value depends on the natural-distance for each society type from Table 1–, which is
| 10 |
Numerical results
We present numerical simulations to study disease dynamics for different societies under distinct transmission rate functional forms and distance-related parameters. We also show the practical significance of these four functional forms according to their dynamics in time and dependency on interaction-distance. The software MATLAB62 was used to create all figures in this section, as well as Figs. 1 and 2 from the previous section.
Disease transmission and dynamics under different natural-distance () assumptions
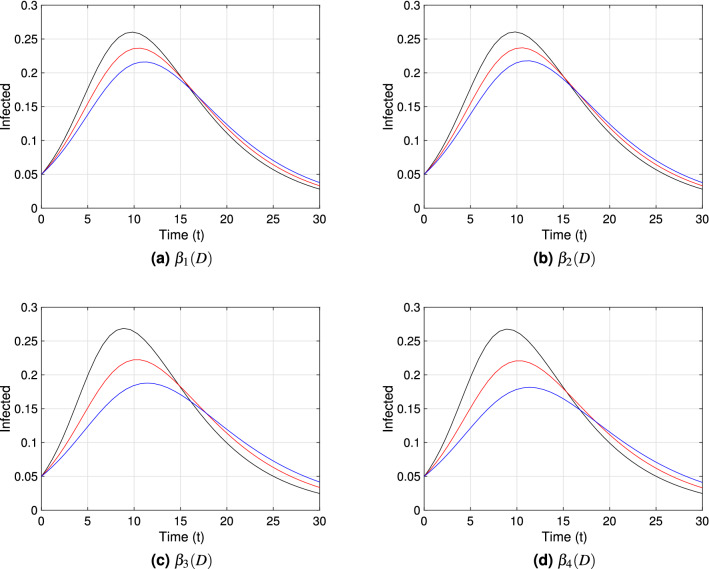
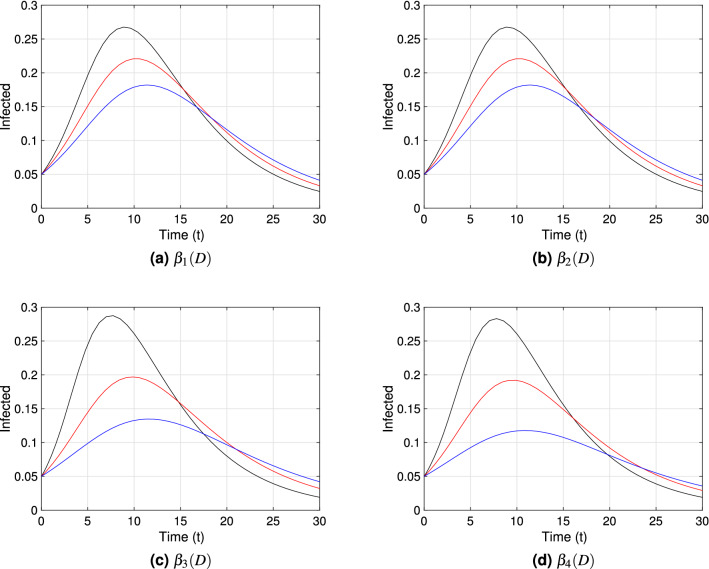
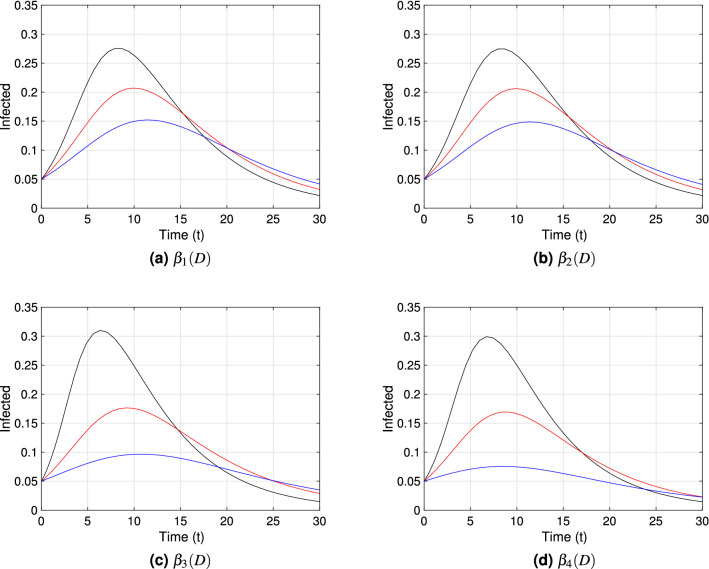
Each graph within Figs. 3, 4 and 5 shows the point prevalence curve (I(t)/N) from the system in Eq. (1) with respect to time, for the different transmission rate forms as in Eqs. (6)–(9): , , and , with scaling distance . The colors correspond to the natural-distances value in the system given in Eq. (1), for three different types of societies: (i) small social natural-distance (black, ), within the range [0, 1) [m]; (ii) medium social natural-distance (red, ), within the range [1, 1.2] [m]; and (iii) large social natural-distance (blue, ), within the range . We consider , and in Figs. 3, 4 and 5 respectively, to account for the effect of the shape of the transmission functions on disease dynamics. To study the first impact of an epidemic, the time frame chosen for the mentioned figures shows the peak of a first epidemic outbreak, considering that our model may allow for further smaller peaks (see Fig. 9 in the next subsection).
Figure 3.
Point prevalence, I(t)/N, from the system in Eq. (1) with respect to time for each transmission functional form and . Black, red and blue curves correspond to , , and respectively. These correspond to societies of small ([0, 100)), medium ([100, 120]) and large () natural-distance types respectively (see Table 1). The other parameter values are fixed at ; , , , . The value for each society are for (a) and (b): (black), (red), (blue); (c) and (d): (black), (red), (blue). The initial condition was used for each type of society.
Figure 4.
Point prevalence, I(t)/N, from the system in Eq. (1) with respect to time for each transmission functional form and . Black, red and blue curves correspond to , , and respectively. These correspond to societies of small ([0, 100)), medium ([100, 120]) and large () natural-distance types respectively (see Table 1). The other parameter values were taken to be ; , , , . The value for each society are for (a) and (b): (black), (red), (blue); (c): (black), (red), (blue); (d): (black), (red), (blue). The initial condition was used for each type of society.
Figure 5.
Point prevalence, I(t)/N, from the system in Eq. (1) with respect to time for different transmission functional forms and . Black, red and blue curves correspond to , , and respectively. These correspond to societies of small ([0, 100)), medium ([100, 120]) and large () natural-distance types respectively (see Table 1). The other parameter values were taken to be ; , , , . The value for each society are for (a) and (b): (black), (red), (blue); (c): (black), (red), (blue); (d): (black), (red), (blue). The initial condition was used for each type of society.
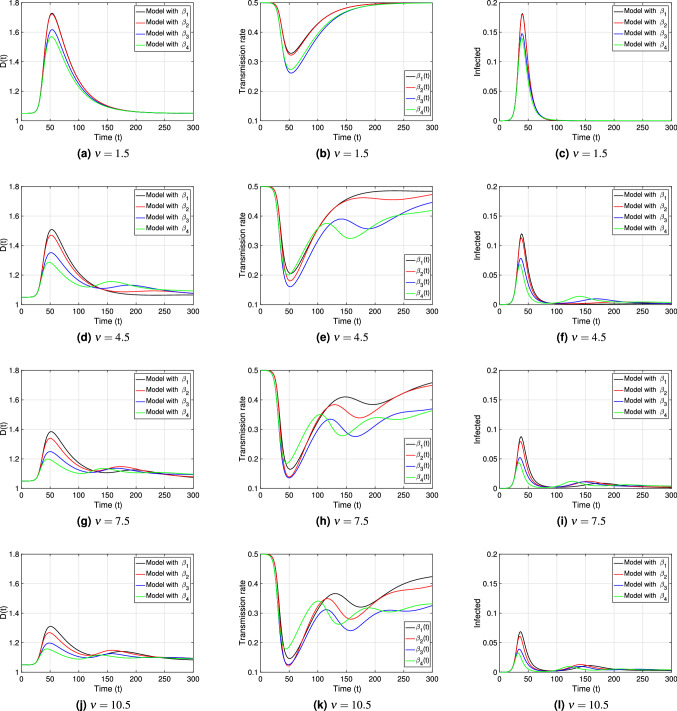
Figure 9.
Temporal evolution of the interaction-distance D(t) (fist column); the transmission rates , , from Eqs. (6)–(9) (second column); and the point prevalence I(t)/N (third column); for . The remaining parameter values were taken to be ; , , and , with . The initial conditions used are , , and .
Comparing Figs. 3, 4 and 5, we can observe especially for and societies of small natural-distance type (black) that, the higher is the larger is the size of the peak and the sooner does the peak occur (compare subfigure (c) in Figs. 3, 4, 5). Also, significant differences in point prevalence levels can be observed between societies of different natural-distance types (small, medium or large), especially for and large values (see Figs. 4c, 5c). Figures 4c and 5c show clearly that societies of small natural-distance type (black) show the greatest increase in peak size but also the largest shift in the occurrence of the peak when compared to others. In general, the smaller the natural-distance type of the society is, the sooner does the peak occur. These culturally driven differences are less if we observe the dynamics for and , especially for small values, and are most noticeable for and for large values.
For each type of society, Fig. 6a describes the dynamics of infected individuals (I(t)/N); Fig. 6b,c shows the evolution of the interaction-distance D(t) from the system in Eq. (1), kept by individuals though the course of the epidemic for a certain distance-related parameter set; and Fig. 6d describes the corresponding temporal dynamics of the transmission rate from Eq. (8). The interaction-distance D(t) reaches a peak, which occurs after the epidemic peak (compare Fig. 6a,c). After attaining the peak, the interaction-distance curves converge to their respective culturally determined natural-distance (see Fig. 6b). Figure 6a shows that the peak of the infected curve, as discussed earlier, shifts according to the natural-distance type of the society (determined by the value of ), as do the peaks of the distance curves (see Fig. 6b), in reaction to the disease peak. One can also observe from Fig. 6b that the absolute change in interaction-distance is largest for societies of small natural-distance type (black curve), compared to other types. The transmission rate behaves as expected, inversely proportional to interaction-distance, being the societies of large natural-distance types the ones with the smallest transmission rate as well as the smallest absolute change in transmission (see Fig. 6d).
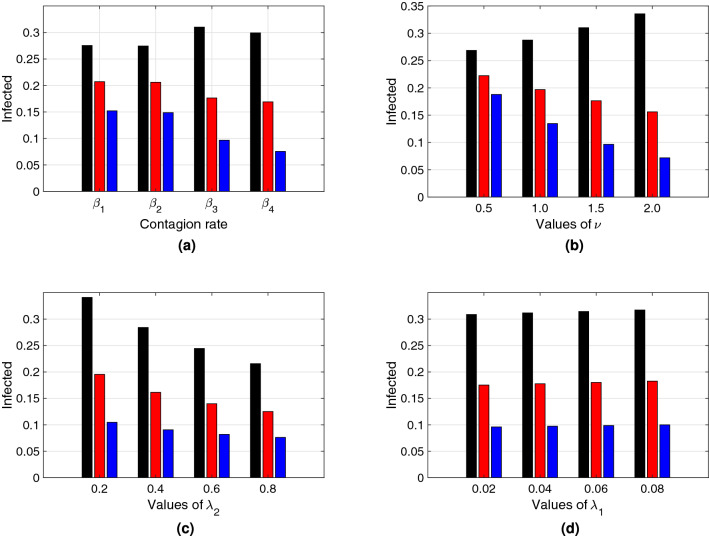
Figures 7 and 8 depict bar plots that illustrate, respectively, the height of the peak of a first epidemic outbreak and its time of occurrence for: (a) each transmission rate , ; (b) different values (); (c) different values (); (d) different values (); each for the three different types of social natural-distance (small, medium, large) represented by colors (black, red, blue). Figure 7a for instance, shows that returns the highest peak compared to the other transmission rate functional forms, for small social natural-distance societies (black), and also that the difference in peak size between different societies is biggest for and . Figure 7b shows that for societies of small natural-distance type, the higher the value, the higher is the epidemic peak, and that the contrary is true for societies of large natural-distance type. Figure 7c depicts that the higher the reaction velocity to change () is, the lower is the infection peak, especially noticeable for societies of small natural-distance types, and Fig. 7d illustrates that for the parameter range chosen, there is little effect on peak size of the rate () at which individuals return to their natural-distance . In general, all four subplots show that the smaller the natural-distance type of a society is, the larger is the epidemic peak size.
Figure 7.
Size of the peak of a first epidemic outbreak from the system in Eq. (1) with respect to (a) contagion rates , , (b) measure of decrease of transmission rate with distance , (c) reaction velocity to change , (d) rate of resistance to change , for different society types: Black, red and blue bars correspond to , , and respectively. These correspond to societies of small ([0, 100)), medium ([100, 120]) and large () natural-distance types respectively (see Table 1). The height of each bar represents the size of the peak of the epidemic curve. The other parameter values were taken to be and . For (a) , , . For (b) we used as the transmission rate, and . For (c) we also used as the transmission rate, and . For (d) , and as the transmission rate. The initial condition was used for each type of society.
Figure 8.
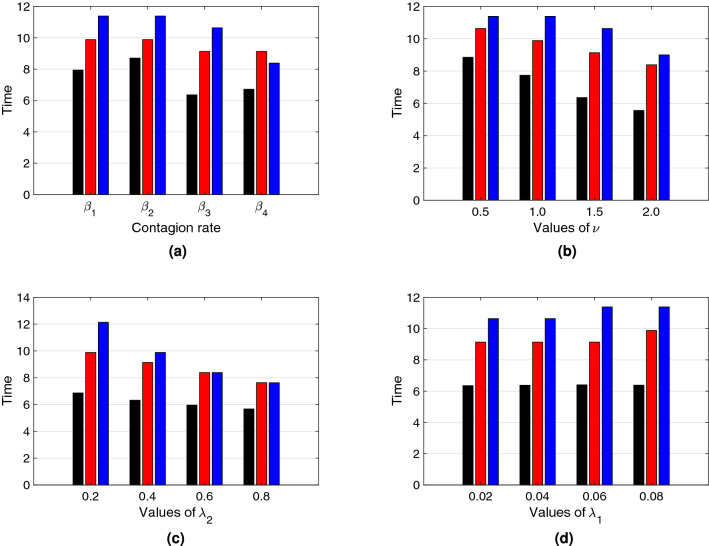
Time of occurrence of the peak of a first epidemic outbreak from the system in Eq. (1) with respect to, (a) contagion rates , , (b) measure of decrease of transmission rate with distance , (c) reaction velocity to change , (d) rate of resistance to change , for different society types: Black, red and blue bars correspond to , , and respectively. These correspond to societies of small ([0, 100)), medium ([100, 120]) and large () natural-distance types respectively (see Table 1). The height of each bar represents the time of occurrence of the peak of the epidemic curve. The other parameter values were taken to be and . For (a) , . For (b) we used as the transmission rate, and . For (c) we also used as the transmission rate, and . For (d) , and as the transmission rate. The initial condition was used for each type of society.
Additionally, Fig. 8 shows in general that, the smaller the natural-distance type of a society is, the sooner occurs the peak. In particular, Fig. 8a depicts that for and for societies of small natural-distance type, the peak occurs the earliest. On the other hand, in Fig. 8b we observe that, the larger the value for is, the sooner is the timing of the peak. Figure 8c depicts that the time of the peak does not experience such a great change according to compared to the effect on peak size, especially for societies of small natural-distance type; but, for societies of large natural-distance type, increasing may have an effect on earlier peak occurrence. So, comparing Fig. 7c with Fig. 8c, especially for societies of medium and large natural-distance type (red, blue), one can observe that, the larger , the smaller is the peak but, at the same time, the sooner it occurs. Hence, there is a trade off between reduced peak size and early occurrence of the peak. Finally, Fig. 8d illustrates that the greater , the later the peak may occur, mainly for societies of medium or large natural-distance types.
Temporal dynamics of the transmission rates impacted by interaction-distance (D(t))
Figure 9 shows the evolution in time of the interaction-distance D(t), the four transmission rates from Eqs. (6)–(9), and the point prevalence I(t)/N for different values. Since larger values account for important differences among societies during the initial period of disease propagation (see Figs. 3, 4, and 5) we choose in the larger range. We describe in Fig. 9 the practical significance of the different transmission rate functional forms, their correlation with interaction-distance, and their impact on the curves of infected; in the setting of an average society with natural-distance . We first describe general temporal features common to all four transmission rate functions and then point out specific characteristics that make them differ in their practical significance for disease modeling.
Upon the arrival of an infectious disease with high morbidity and/or mortality, a decrease of the transmission rate during the initial period of disease expansion can be observed28, 48, 49. We can observe in Fig. 9, that our model describes that behavior for the transmission rates , . Additionally, one of the novelties of our model is that it explains the decreasing behavior of the transmission rates by a behavioral change in the population, represented by social distancing; i.e., it shows that the initial decrease in the transmission rates may be due to an increment of the interaction-distance D(t), whose dynamic depends on the increase in active cases and some correlated behavioral factor (see the equation for D(t) in Eq. (1)). In fact, just as we have observed previously in Fig. 6 for , in Fig. 9 we see that for all four transmission rates, during the first 50 days of disease propagation, the interaction-distance D(t) increases (see Fig. 9a,d,g,j), which produces a reduction in the transmission rate during the same time period (see Fig. 9b,e,h,k) and a first epidemic peak in that time frame (see Fig. 9c,f,i,l).
During the course of a pandemic, the change in social distancing behavior affects the rate of efficient contacts for disease transmission and, therefore, the transmission rate. The rising or falling of the transmission rate is one of the reasons that explains the change in the effective reproduction number—a dynamic measure of the average number of secondary cases per infected case in a population composted by susceptible and non-susceptible individuals—that has been observed during epidemic outbreaks, since this measure is a function of the efficient contacts, among others50–55. Our model, with its different transmission rate functions correlated to distancing behavior, gives a range of practical scenarios for the evolution of a changing transmission rate responsible for disease propagation. This evolution is characterized by the transmission rate functions given in Eqs. (6)–(9) that are defined by and their dependency on D(t).
First, we observe how the parameter affects the characterization of disease transmission in general. We see from Fig. 9 that after the first minimum value of each transmission rate, the rates start increasing, tending to return to their initial state . We observe that the convergence to their initial value happens in a shorter time-frame for small values than for large ones. Indeed, we can see clearly from Fig. 9b,e,h,k, that for instance , , for , and . We also observe that oscillations appear for larger values during the recovery phase of the transmission rates, which we will discuss in more detail below.
It is important to add to the discussion how the dependency on the interaction-distance D(t) of the different transmission rates affect their timely evolution. Observe that the efficiency of D in lowering each transmission rate differs for different values: the larger is, the more efficient is an absolute increase/reduction in interaction-distance in reducing/increasing each transmission rate; e.g., only a small increment in D(t) from to is necessary to achieve a significant reduction in each transmission rate during that time period. As a consequence, observe that for large values, only a small initial increase in the interaction-distance produces a low first epidemic peak. That efficiency of D in reducing each transmission rate is at a cost: a low first epidemic peak in exchange for breaking the unimodality of the active-infected-curve produced by the classical SIR model with constant transmission rate (one bell-shaped infection curve due to the epidemic growth being limited by the proportion of susceptible individuals)63, 64, and hence our model may produce several further epidemic peaks (see Fig. 9c,f,i,l). This is a direct consequence of the oscillatory recovery of the transmission rate mentioned before, produced by the oscillatory behavior of D(t) in combination with its efficiency in reducing transmission.
Next, we will discuss some specific characteristics of the transmission rate functions. We observe from Fig. 9e,h,k differences between the four transmission rates in their oscillations that describe disease dynamics: oscillations of (in green) for any value are ahead of the oscillations of any of the other three transmission rates, producing earlier epidemic peaks; on the contrary, (in black) produces oscillations the latest, producing later epidemic peaks; the transmission rate function pairs and , and and generate similar dynamic behavior for small values, but their behavior drifts apart for increasing . Also, we can see in Fig. 9f,i,l that peak sizes and time-spans between peaks change according to different transmission rate functions and their oscillatory shape.
Discussion and conclusions
To control the spread of a disease causing an epidemic or pandemic, the only effective measure may be to reduce the effective contact rate by social distancing. In fact, there is scientific evidence that suggests that the transmission of pathogenic agents occurs with sensitivity to human behavior, in particular to the distance between individuals24, 65. The importance of social distancing—to keep infectious diseases from spreading and mitigate their morbidity and mortality—was revealed in a historic article that studied the data of Pneumonic Plague in Manchurian in north-eastern Asia during the years 1910-11 and 1920-2165. That study evidenced an epidemiological risk for pneumonia for distances between 5 cm to 2 m. This makes it clear that incorporating into mathematical models the factor of social distance is important if more precision is needed to sustain and guide measures of sanitary intervention24. Our simple model supports these findings.
The model results describe how the distance that individuals keep from each other varies in time and with respect to point prevalence (see Fig. 6). In particular, the simulations illustrate that the first peak in distancing after the onset of an epidemic (the moment when people keep the largest distance from each other) occurs—as a reactive reaction—after the first peak of infections happens, varying the time of occurrence according to the type of society. We could also observe that societies where people keep a small natural-distance from each other, have to change their distancing behavior the most to counteract disease spread (see Fig. 6b).
Our results in Figs. 7 and 8 confirm the importance of social distancing, and show differences in peak size and peak time of a first epidemic outbreak for different cultural settings. In particular, our results show clearly the vulnerability of societies of small social natural-distance type—in which individuals maintain a distance of less than one meter from each other. Such societies could experience a mayor epidemic peak that occurs early after the onset of the epidemic. On the other hand, societies in which individuals maintain a distance from each other of more than one meter, experience a lower peak that occurs later after the beginning of the epidemic, as compared to peak size and time for other types of societies.
Our simulations also show differences in peak size and time for different epidemiological and social distancing related parameters for each society type, during a first epidemic outbreak. For instance, the form of the transmission rate—which is distance dependent—affects greatly size and time of the epidemic peak. Also, parameters related to how fast individuals change behavior according to point prevalence levels () and how resistant () individuals are to change their natural distance (), may be key for disease dynamics. For instance, in general, populations that react quickly to the observed point prevalence experience smaller peaks, which is specially pronounced for societies of small natural-distance type (see Fig. 7c); but, for small peak sizes, there is a trade off: and the peak may occur sooner, especially for societies of large natural-distance type (see Fig. 8c). Hence, a society of large natural-distance type that reacts fast to change when there is disease present, may experience a small but early first epidemic peak.
The shape of the infected curve beyond the first epidemic outbreak in a pandemic situation changes from country to country, as has been observed for instance during the current COVID-19 pandemic66. In particular, how close or how high possible further epidemic peaks are varies. Our numerical results show that the transmission rate functional forms used—since they are able to produce oscillations (see Fig. 9)—give us a range of possibilities that may help to describe the qualitative behavior of different infection curve scenarios. Additionally, we can explain a possible cause for the changing transmission rates in terms of a tangible variable: interaction-distance D(t); that describes the distancing behavior of individuals in time. We can also describe how efficient social distancing is in changing disease transmission (using the parameter ), which may vary for different populations. This efficiency determines the form of the oscillatory behavior of the transmission rates and hence, the appearance of several further peaks; this way breaking the unimodality of the active-infected-curve produced by the classical SIR model.
In order to obtain better guidelines from the model, we plan in future work to extend the model including more epidemiological classes and an additional structure that further describes human behavior in an epidemic situation. For instance, for modeling COVID-19, additional classes for pre-symptomatic, asymptomatic and hospitalized individuals may be necessary. We also would like to conduct some sensitivity analysis. For instance, to compute the Partial Rank Correlation Coefficient (PRCC) for each parameter and parameter ranges could give insight into which parameters affect epidemic peak and peak time the most. We also would like to address, which of the four transmission rate functional forms would best fit for instance the COVID-19 epidemic data for different types of societies, as well as consider age-group differences, among other factors.
Our model and its results are a first approach for analyzing the effect of initiatives for pandemic preparedness under different epidemiological and cultural settings, determined by: (1) the transmission rate of a particular disease, which is inversely proportional to distance; (2) the velocity of the population to react to the presence of the disease (); (3) the resistance that individuals experience to change their natural distance (). Indeed, if the goal would be to reduce peak size during a first epidemic outbreak and postpone its timing (for instance to gain time to implement proper healthcare conditions to treat infected individuals) and the society affected is of small social natural-distance type (less than one meter), then, measures that change the society type—by increasing the natural distance given by the culture () to more than one meter—would lower the peak and postpone it. Such measures in the short term could be for instance quarantine, and in the long term cultural re-education initiatives that change the distancing behavior of the population. Changing the natural-distance that people keep from each other- in other words, to change society type- may be more effective for lowering the peak of a first epidemic outbreak than not changing society type and instead, finding measures that increase public health awareness and improve the velocity of reaction to change () of the society. Indeed, for instance for societies of large natural-distance type, to increase the reaction velocity may anticipate the peak, which may not be desired.
Control measures such as quarantine, indeed aim to stop social activities as a way to obtain large social distancing, and this way increase the natural-distance given by the culture. Without those measures, it is extremely difficult to control that our personal space is respected by others, especially in cultures of small natural-distance type. Government imposed control measures are not sustainable in the long term, and hence cultural re-education initiatives are necessary to get individuals accustomed to change their social behavior. A cultural change is necessary. As our results show, in general, societies that show during a first outbreak the smallest peak size, occurring late after the onset of the epidemics, are societies where the natural-distance given culturally is large (individuals upon encounter maintain a distance larger than 1.2 meters from each other), almost independent of the transmission rate form (see Figs. 7a,b and 8a,b).
Even though it is not easy to change habits acquired throughout the years, it is our obligation to make the change. We have to insist that public health authorities and their technical advisors, as well as individuals in the population, impulse initiatives for cultural re-education to confront epidemics to come. As stated in16, while learning from history, now may be our opportunity to make progress in this direction.
Acknowledgements
This work was partially funded by Proyecto Ingeniería 2030 14ENI2-26865 from the Facultad de Ingeniería y Ciencias at Universidad Adolfo Ibáñez, a proyect financed by the Chilean economic development agency CORFO.
Author contributions
All authors developed the mathematical framework. J.G. and K.V. developed simulations. All authors analyzed results, developed the introduction, methodological background and discussion. K.V. edited the final draft. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Maritza Cabrera, Fernando Córdova-Lepe, Juan Pablo Gutiérrez-Jara and Katia Vogt-Geisse.
Contributor Information
Juan Pablo Gutiérrez-Jara, Email: jpgutierrezjara@gmail.com.
Katia Vogt-Geisse, Email: katia.vogt@uai.cl.
References
- 1.Li H-L, Jecker NS, Chung RY-N. Reopening economies during the covid-19 pandemic: reasoning about value tradeoffs. Am. J. Bioeth. 2020;20:136–138. doi: 10.1080/15265161.2020.1779406. [DOI] [PubMed] [Google Scholar]
- 2.Khoo EJ, Lantos JD. Lessons learned from the covid-19 pandemic. Acta Paediatrica. 2020;109:1323–1325. doi: 10.1111/apa.15307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pronk NP, Kassler WJ. Balancing health and economic factors when reopening business in the age of covid-19. J. Occup. Environ. Med. 2020;62:e540–e541. doi: 10.1097/JOM.0000000000001955. [DOI] [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention (CDC)- COVID-19- Social Distancing, (accessed 22 October 2020) https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html (2020).
- 5.Courtemanche C, Garuccio J, Le A, Pinkston J, Yelowitz A. Strong social distancing measures in the united states reduced the covid-19 growth rate: study evaluates the impact of social distancing measures on the growth rate of confirmed covid-19 cases across the united states. Health Aff. 2020;39:10–1377. doi: 10.1377/hlthaff.2020.00608. [DOI] [PubMed] [Google Scholar]
- 6.Andersen, M. Early evidence on social distancing in response to covid-19 in the united states. Available at SSRN 3569368, (2020).
- 7.Martínez-Lorca, M. et al. The fear of covid-19 scale: validation in spanish university students. Psychiatry Res. 113350, (2020). [DOI] [PMC free article] [PubMed]
- 8.González Pérez U. El modo de vida en la comunidad y la conducta cotidiana de las personas. Revista Cubana de Salud Pública. 2005;31:0. [Google Scholar]
- 9.Pharaon, J. & Bauch, C. The influence of social behavior on competition between virulent pathogen strains. bioRxiv 293936 (2018). [DOI] [PMC free article] [PubMed]
- 10.Funk S, et al. Nine challenges in incorporating the dynamics of behaviour in infectious diseases models. Epidemics. 2015;10:21–25. doi: 10.1016/j.epidem.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Allen, L. J., Brauer, F., Van den Driessche, P. & Wu, J. Mathematical Epidemiology Vol. 1945 (Springer, 2008).
- 12.Brauer, F. & Castillo-Chavez, C. Mathematical Models in Population Biology and Epidemiology Vol. 2 (Springer, 2012).
- 13.Diekmann, O. & Heesterbeek, J. A. P. Mathematical Epidemiology of Infectious Diseases: Model Building, Analysis and Interpretation Vol. 5 (John Wiley & Sons, 2000).
- 14.Chowell G, Sattenspiel L, Bansal S, Viboud C. Mathematical models to characterize early epidemic growth: a review. Phys. Life Rev. 2016;18:66–97. doi: 10.1016/j.plrev.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutiérrez-Jara JP, Córdova-Lepe F, Muñoz-Quezada MT. Dynamics between infectious diseases with two susceptibility conditions: a mathematical model. Math. Biosci. 2019;309:66–77. doi: 10.1016/j.mbs.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Acosta, C. A. Cuatro preguntas para iniciarse en cambio organizacional. Revista colombiana de psicología 9–24, (2002).
- 17.Amaoka, T., Laga, H., Saito, S. & Nakajima, M. Personal space modeling for human–computer interaction. In International Conference on Entertainment Computing, 60–72 (Springer, 2009).
- 18.Hall ET, et al. Proxemics [and comments and replies] Curr. Anthropol. 1968;9:83–108. doi: 10.1086/200975. [DOI] [Google Scholar]
- 19.Sorokowska A, et al. Preferred interpersonal distances: a global comparison. J. Cross-Cult. Psychol. 2017;48:577–592. doi: 10.1177/0022022117698039. [DOI] [Google Scholar]
- 20.Salje H, Cummings DA, Lessler J. Estimating infectious disease transmission distances using the overall distribution of cases. Epidemics. 2016;17:10–18. doi: 10.1016/j.epidem.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ster IC, Ferguson NM. Transmission parameters of the 2001 foot and mouth epidemic in great britain. PloS one. 2007;2:e502. doi: 10.1371/journal.pone.0000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley S, Eames K, Isham V, Mollison D, Trapman P. Five challenges for spatial epidemic models. Epidemics. 2015;10:68–71. doi: 10.1016/j.epidem.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Read, J. M., Bridgen, J. R., Cummings, D. A., Ho, A. & Jewell, C. P. Novel coronavirus 2019-ncov: early estimation of epidemiological parameters and epidemic predictions. MedRxiv (2020). [DOI] [PMC free article] [PubMed]
- 24.Read JM, et al. Social mixing patterns in rural and urban areas of southern china. Proc. R. Soc. Lond. B Biol. Sci. 2014;281:20140268. doi: 10.1098/rspb.2014.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kermack WO, McKendrick AG. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Char. 1927;115:700–721. [Google Scholar]
- 26.Capasso V, Serio G. A generalization of the kermack-mckendrick deterministic epidemic model. Math. Biosci. 1978;42:43–61. doi: 10.1016/0025-5564(78)90006-8. [DOI] [Google Scholar]
- 27.Wang X, Gao D, Wang J. Influence of human behavior on cholera dynamics. Math. Biosci. 2015;267:41–52. doi: 10.1016/j.mbs.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolokolnikov T, Iron D. Law of mass action and saturation in sir model with application to coronavirus modelling. Infect. Dis. Model. 2021;6:91–97. doi: 10.1016/j.idm.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Driessche P, Watmough J. A simple sis epidemic model with a backward bifurcation. J. Math. Biol. 2000;40:525–540. doi: 10.1007/s002850000032. [DOI] [PubMed] [Google Scholar]
- 30.Kochańczyk M, Grabowski F, Lipniacki T. Dynamics of covid-19 pandemic at constant and time-dependent contact rates. Math. Model. Natl. Phenomena. 2020;15:28. doi: 10.1051/mmnp/2020011. [DOI] [Google Scholar]
- 31.Taghvaei A, Georgiou T, Norton L, Tannenbaum A. Fractional sir epidemiological models. Sci. Rep. 2020;10:20882. doi: 10.1038/s41598-020-77849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan S, Wang W. Dynamical behavior of an epidemic model with a nonlinear incidence rate. J. Differ. Equ. 2003;188:135–163. doi: 10.1016/S0022-0396(02)00089-X. [DOI] [Google Scholar]
- 33.Reluga TC. Game theory of social distancing in response to an epidemic. PLOS Comput. Biol. 2010;5:e1000793. doi: 10.1371/journal.pcbi.1000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.d’Onofrio A, Manfredi P. Information-related changes in contact patterns may trigger oscillations in the endemic prevalence of infectious diseases. J. Theor. Biol. 2009;256:473–478. doi: 10.1016/j.jtbi.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Pedro, S. A. et al. Conditions for a second wave of covid-19 due to interactions between disease dynamics and social processes. medRxiv (2020).
- 36.Bauch CT. Imitation dynamics predict vaccinating behaviour. Proc. R. Soc. B Biol. Sci. 2005;272:1669–1675. doi: 10.1098/rspb.2005.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenhalgh D, Rana S, Samanta S, et al. Awareness programs control infectious disease: multiple delay induced mathematical model. Appl. Math. Comput. 2015;251:539–563. [Google Scholar]
- 38.Poletti P, Ajelli M, Stefano M. The effect of risk perception on the 2009 h1n1 pandemic influenza dynamics. PLOS ONE. 2011;6:e16460. doi: 10.1371/journal.pone.0016460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teslya A, et al. Impact of self-imposed prevention measures and short-term government-imposed social distancing on mitigating and delaying a covid-19 epidemic: A modelling study. PLoS Med. 2020;17:e1003166. doi: 10.1371/journal.pmed.1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mummert A, Weiss H. Get the news out loudly and quickly: the influence of the media on limiting emerging infectious disease outbreaks. PloS one. 2013;8:e71692. doi: 10.1371/journal.pone.0071692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agaba G, Kyrychko Y, Blyuss K. Mathematical model for the impact of awareness on the dynamics of infectious diseases. Math. Biosci. 2017;286:22–30. doi: 10.1016/j.mbs.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Zhao, S. et al. Imitation dynamics in the mitigation of the novel coronavirus disease (covid-19) outbreak in wuhan, china from 2019 to 2020. Ann. Transl. Med.8, (2020). [DOI] [PMC free article] [PubMed]
- 43.Córdova-Lepe, F., Cabrera Hernández, M. & Gutiérrez-Jara, J. P. Modeling the epidemiological impact of a preventive behavioral group. Medwave18 (2018). [DOI] [PubMed]
- 44.Karlsson C-J, Rowlett J. Decisions and disease: a mechanism for the evolution of cooperation. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-69546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Epstein JM, Parker J, Cummings D, Hammond RA. Coupled contagion dynamics of fear and disease: mathematical and computational explorations. PLoS One. 2008;3:e3955. doi: 10.1371/journal.pone.0003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Valle S, Hethcote H, Hyman JM, Castillo-Chavez C. Effects of behavioral changes in a smallpox attack model. Math. Biosci. 2005;195:228–251. doi: 10.1016/j.mbs.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002;180:29–48. doi: 10.1016/S0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 48.Ke, R., Sanche, S., Romero-Severson, E. & Hengartner, N. Fast spread of covid-19 in europe and the us suggests the necessity of early, strong and comprehensive interventions. medRxiv (2020).
- 49.Law K, Peariasamy K, Gill B, et al. Tracking the early depleting transmission dynamics of covid-19 with a time varying sir model. Sci. Rep. 2020;10:21721. doi: 10.1038/s41598-020-78739-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Towers, S., Patterson-Lomba, O. & Castillo-Chavez, C. Temporal variations in the effective reproduction number of the 2014 west africa ebola outbreak. PLoS Curr.6, (2014). [DOI] [PMC free article] [PubMed]
- 51.Cowling BJ, et al. The effective reproduction number of pandemic influenza: prospective estimation. Epidemiology. 2010;21:842. doi: 10.1097/EDE.0b013e3181f20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tariq A, et al. Transmission dynamics and control of covid-19 in chile, march–october, 2020. PLoS Negl. Trop. Dis. 2021;15:e0009070. doi: 10.1371/journal.pntd.0009070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santamaría L, Hortal J. Covid-19 effective reproduction number dropped during spain’s nationwide dropdown, then spiked at lower-incidence regions. Sci. Total Environ. 2021;751:142257. doi: 10.1016/j.scitotenv.2020.142257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang, J., Park, H., Jung, J., Kim, S.-H. & Kim, N. Basic and effective reproduction numbers of covid-19 cases in south korea excluding sincheonji cases. Medrxiv (2020).
- 55.Tang B, et al. An updated estimation of the risk of transmission of the novel coronavirus (2019-ncov) Infect. Dis. Model. 2020;5:248–255. doi: 10.1016/j.idm.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hethcote HW. The mathematics of infectious diseases. SIAM Rev. 2000;42:599–653. doi: 10.1137/S0036144500371907. [DOI] [Google Scholar]
- 57.Read JM, et al. Social mixing patterns in rural and urban areas of southern china. Proc. R. Soc. B Biol. Sci. 2014;281:20140268. doi: 10.1098/rspb.2014.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shuter R. Proxemics and tactility in latin america. J. Commun. 1976;26:46–52. doi: 10.1111/j.1460-2466.1976.tb01902.x. [DOI] [PubMed] [Google Scholar]
- 59.de Koeijer AA, et al. Quantitative analysis of transmission parameters for bluetongue virus serotype 8 in western europe in 2006. Vet. Res. 2011;42:53. doi: 10.1186/1297-9716-42-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boender, G. J. et al. Risk maps for the spread of highly pathogenic avian influenza in poultry. PLoS Comput. Biol.3, (2007). [DOI] [PMC free article] [PubMed]
- 61.Vogt-Geisse K, Ngonghala CN, Feng Z. The impact of vaccination on malaria prevalence: a vaccine-age-structured modeling approach. J. Biol. Syst. 2020;28:475–513. doi: 10.1142/S0218339020400094. [DOI] [Google Scholar]
- 62.The Mathworks, Inc., Natick, Massachusetts. MATLAB version 9.1 (R2016b) (2016).
- 63.Anderson, R. M. & May, R. M. Infectious Diseases of Humans: Dynamics and Control (Oxford University Press, 1992).
- 64.Keeling, M. & Danon, L. Mathematical modelling of infectious diseases. Br. Med. Bull.92, (2009). [DOI] [PubMed]
- 65.Kool JL, Weinstein RA. Risk of person-to-person transmission of pneumonic plague. Clin. Infect. Dis. 2005;40:1166–1172. doi: 10.1086/428617. [DOI] [PubMed] [Google Scholar]
- 66.CSSE. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU), (accessed 02 2021). https://coronavirus.jhu.edu/data/new-cases.