Abstract
Background:
Insulin degludec/liraglutide (IDegLira) results in glycated hemoglobin (HbA1c) levels comparable with basal-bolus (BB) therapy. Here, we assessed the effect of once-daily IDegLira compared with BB (once-daily insulin glargine 100 U/mL and insulin aspart ≤4 times/day) across subgroups with varying characteristics.
Materials and Methods:
DUAL VII trial participants (type 2 diabetes [T2D], HbA1c 53-86 mmol/mol [7.0%-10.0%]) were subgrouped post hoc based on the following baseline characteristics: HbA1c (≤58.5, >58.5 to ≤69.4, and >69.4 mmol/mol; ≤7.5%, >7.5 to ≤8.5%, and >8.5%), body mass index (<30, ≥30 to <35, and ≥35 kg/m2), age (18 to <65 and ≥65 years), duration of diabetes (≥0 to 10 and ≥10 years), total pretrial daily basal insulin dose (20 to <30, ≥30 to <40, and ≥40 to ≤50 U), and fasting plasma glucose (<7.2 mmol/L/<130 mg/dL and ≥7.2 mmol/L/≥130 mg/dL).
Results:
Compared with BB, and in all subgroups, IDegLira treatment consistently gave similar HbA1c reductions, less severe or blood glucose-confirmed hypoglycemia, lower end-of-trial (EOT) total daily insulin dose, and weight loss. In all subgroups, mean EOT HbA1c was ≤53 mmol/mol (≤7.0%). The greatest HbA1c reduction occurred in the highest baseline HbA1c subgroup. Overall, mean EOT daily insulin dose was 0.43 to 0.52 U/kg with IDegLira and 0.74 to 1.07 U/kg with BB. More participants achieved the triple composite endpoint (HbA1c <53 mmol/mol [<7.0%] without weight gain or hypoglycemia) with IDegLira vs BB across the baseline HbA1c subgroups (≤58.5 mmol/mol [44.6% vs 7.0%], >58.5 to ≤69.4 mmol/mol [41.1% vs 8.3%], and >69.4 mmol/mol [23.8% vs 3.4%]).
Conclusion:
These results support initiating IDegLira in patients with varying baseline characteristics and uncontrolled T2D on basal insulin.
ClinicalTrials.gov registration:
Keywords: GLP-1 analog, insulin therapy, liraglutide, randomized trial, type 2 diabetes
Introduction
As the number of type 2 diabetes (T2D) therapies available has expanded, clinicians have more opportunity to employ a personalized approach to the treatment of their patients. The American Diabetes Association/European Association for the Study of Diabetes consensus report states that intensification strategies should be individualized to unique patient factors such as glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) levels, and whether patients are overweight, have a history of recurrent hypoglycemia, or have risk factors for comorbid conditions.1
The use of basal insulins in combination with glucagon-like peptide-1 receptor agonists (GLP-1RA) is an effective treatment option for people with T2D requiring intensification beyond insulin therapy, and is an option that is now available in the form of fixed-ratio combinations such as insulin degludec/liraglutide (IDegLira).1 The safety and efficacy of IDegLira has been demonstrated in the Dual Action of Liraglutide and Insulin Degludec in Type 2 Diabetes (DUAL) clinical trial program2-9 Importantly, results from the DUAL VII trial showed that a once-daily injection of IDegLira was noninferior to multiple injections of basal-bolus (BB) insulin therapy (insulin glargine 100 units [U]/mL [IGlar U100] + insulin aspart [IAsp] ≤4 times daily) for reduction in HbA1c, and was associated with a significantly lower rate of hypoglycemia.8 In addition to providing a simple, less burdensome injectable therapy, IDegLira was associated with weight loss in DUAL VII, whereas weight gain was observed with BB insulin therapy.8
The way in which patients with varying baseline characteristics respond to different treatment regimens is important when tailoring therapy to the patient’s needs. In order to investigate whether the benefits of IDegLira over BB insulin therapy in the overall trial population were preserved across subsets of the participants, we performed a post hoc analysis to examine whether participants’ baseline characteristics in DUAL VII influenced their responses to these diabetes interventions. To do this, DUAL VII trial participants were divided into subgroups based on six baseline parameters: (1) HbA1c, (2) body mass index (BMI), (3) age, (4) duration of diabetes, (5) total pretrial daily basal insulin dose, and (6) FPG.
Methods
DUAL VII (trial registration: NCT02420262; www.clinicaltrials.gov) was a phase 3b, multinational, open-label, two-arm parallel, randomized, controlled trial in participants with T2D from 12 countries.8 The study design, methodology, and primary results have been described previously; the primary endpoint was change in HbA1c from baseline to week 26 of treatment.8 The study was conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice10 and the Declaration of Helsinki.11 Briefly, participants were adults with T2D diagnosed clinically ≥6 months prior to screening, HbA1c 53 to 86 mmol/mol (7.0%-10.0%), BMI ≤40 kg/m2, receiving stable daily doses of 20 to 50 U of IGlar U100 and metformin ≥1500 mg (or maximum tolerated dose) for >90 days prior to screening.8
A total of 506 participants were randomized to either once-daily IDegLira or once-daily IGlar U100 and IAsp up to four times daily. The doses of IDegLira or IGlar U100 were titrated twice weekly, based on the mean of three prebreakfast self-measured blood glucose levels. Metformin was continued at pretrial doses. For the purposes of this analysis, participants were stratified into subgroups according to six baseline parameters: (1) HbA1c (≤58.5 mmol/mol/≤7.5%, >58.5 to ≤69.4 mmol/mol/>7.5 to ≤8.5%, and >69.4 mmol/mol/>8.5%); (2) BMI (<30, ≥30 to <35, and ≥35 kg/m2); (3) age (18 to <65 and ≥65 years); (4) duration of diabetes (≥0-10 and ≥10 years); (5) total pretrial daily basal insulin dose (20 to <30, ≥30 to <40, and ≥40 to ≤50 U); and (6) FPG (<7.2 mmol/L/<130 mg/dL and ≥7.2 mmol/L/≥130 mg/dL).
The primary endpoint, change in HbA1c, was assessed in the subgroup analysis. The following secondary endpoints were also assessed: (1) change in body weight; (2) number of treatment-emergent severe (requiring third-party assistance) or blood glucose-confirmed (<3.1 mmol/L; 56 mg/dL) symptomatic hypoglycemic episodes; (3) end-of-trial (EOT) daily insulin dose (total and basal [U/kg]); and (4) the triple composite endpoint of achieving HbA1c <53 mmol/mol (<7.0%) with no weight gain and without hypoglycemia (“achieving HbA1c <53 mmol/mol (<7.0%) with no weight gain” was measured at week 26; “without hypoglycemia” refers to hypoglycemic events occurring during the last 12 weeks of treatment), presented for baseline HbA1c, baseline BMI, and duration of diabetes groups.
Statistical Methods
The sample size calculation was previously described.8 Because of the post hoc nature of this study, no power calculation was performed. All postbaseline HbA1c and body weight measurements obtained at planned visits before discontinuation from randomized treatment were analyzed using a linear mixed normal model using an unstructured residual covariance matrix for corresponding measurements within the same participant. The model included subgroup, treatment, visit, and region (Europe/North America/South America) as fixed factors and baseline response as covariate. The interactions between visit and region, visit, and covariate, and between visit, subgroup, and treatment were included in the analysis model. Total and basal daily insulin doses were analyzed using a mixed model of repeat measurement with compound symmetric covariance structure. The model included subgroup, treatment, visit, and region as fixed factors and baseline HbA1c and basal insulin dose at screening as covariates. The interactions between subgroup, treatment, and visit, and between visit and all other covariates/factors were included in the model. The number of treatment-emergent severe or blood glucose-confirmed symptomatic hypoglycemic episodes was analyzed using a negative binomial regression model with a log link and the logarithm of the time period in which a hypoglycemic episode was considered treatment-emergent as offset. The model included subgroup, treatment, and region as fixed factors. The interaction between subgroup and treatment was included in the model. Hypoglycemic episodes were defined as treatment-emergent if the onset of the episode occurred on or after the first day of trial product administration, and no later than seven calendar days after the last day on-trial product. The triple responder endpoint was analyzed using a logistic regression model with treatment and region as fixed factors and baseline HbA1c and body weight values as covariates. Testing for the treatment by subgroup interaction was performed for all of the above.
Results
Tables 1-3 present, respectively, the change in HbA1c, change in weight, and EOT total insulin dose (U/kg) according to the six baseline clinical subgroups based on (1) HbA1c, (2) BMI, (3) age, (4) duration of diabetes, (5) total pretrial daily basal insulin dose, and (6) FPG. Supplemental Table S1 shows the EOT basal insulin dose (U/kg) and Figure 1 shows the number of treatment-emergent severe or blood glucose-confirmed symptomatic hypoglycemic episodes, both according to these six baseline characteristics.
Table 1.
Change in Glycated Hemoglobin by Baseline Characteristic.
| Baseline characteristics | Week 26, N |
HbA1c, change from baseline,
mmol/mol |
ETD [95% CI] | HbA1c, change from baseline,
% |
ETD [95% CI] | Subgroup
interaction P-value |
|||
|---|---|---|---|---|---|---|---|---|---|
| IDegLira | Basal-bolus | IDegLira | Basal-bolus | IDegLira | Basal-bolus | ||||
| HbA1c | |||||||||
| ≤58.5 mmol/mol | 54 | 54 | −10.40 (7.75) | −8.78 (5.80) | −1.11 [−4.32; 2.09] | −0.95 (0.71) | −0.80 (0.53) | −0.10 [−0.40; 0.19] | .4703 |
| >58.5 to ≤69.4 mmol/mol | 107 | 101 | −14.61 (9.30) | −14.35 (7.55) | −0.79 [−3.10; 1.51] | −1.34 (0.85) | −1.31 (0.69) | −0.07 [−0.28; 0.14] | |
| >69.4 mmol/mol | 77 | 78 | −22.77 (9.35) | −23.54 (10.05) | 1.11 [−1.54; 3.75] | −2.08 (0.86) | −2.15 (0.92) | 0.10 [−0.14; 0.34] | |
| BMI | |||||||||
| <30 kg/m2 | 92 | 89 | −15.69 (10.27) | −15.66 (9.29) | −0.62 [−3.08; 1.85] | −1.44 (0.94) | −1.43 (0.85) | −0.06 [−0.28; 0.17] | .6505 |
| ≥30 to <35 kg/m2 | 94 | 81 | −16.65 (9.58) | −16.13 (10.01) | −0.57 [−3.07; 1.94] | −1.52 (0.88) | −1.48 (0.92) | −0.05 [−0.28; 0.18] | |
| ≥35 kg/m2 | 52 | 63 | −16.71 (11.04) | −16.83 (10.70) | 1.09 [−2.01; 4.20] | −1.53 (1.01) | −1.54 (0.98) | 0.10 [−0.18; 0.38] | |
| Age | |||||||||
| <65 years | 170 | 185 | −16.18 (10.47) | −15.93 (10.09) | −0.25 [−2.01; 1.51] | −1.48 (0.96) | −1.46 (0.92) | −0.02 [−0.18; 0.14] | .8439 |
| ≥65 years | 68 | 48 | −16.57 (9.37) | −16.94 (9.21) | 0.11 [−3.02; 3.24] | −1.52 (0.86) | −1.55 (0.84) | 0.01 [−0.28; 0.30] | |
| Diabetes duration | |||||||||
| <10 years | 90 | 83 | −15.74 (10.41) | −16.21 (11.05) | −0.07 [−2.59; 2.45] | −1.44 (0.95) | −1.48 (1.01) | −0.01 [−0.24; 0.22] | .8872 |
| ≥10 years | 148 | 150 | −16.63 (10.00) | −16.10 (9.25) | −0.29 [−2.22; 1.63] | −1.52 (0.92) | −1.47 (0.85) | −0.03 [−0.20; 0.15] | |
| Total pretrial daily basal insulin dose | |||||||||
| 20 to <30 U | 91 | 96 | −16.37 (9.22) | −15.65 (9.73) | −0.16 [−2.58; 2.26] | −1.50 (0.84) | −1.43 (0.89) | −0.01 [−0.24; 0.21] | .8628 |
| ≥30 to <40 U | 56 | 61 | −17.21 (9.60) | −16.41 (11.15) | −1.04 [−4.11; 2.02] | −1.58 (0.88) | −1.50 (1.02) | −0.10 [−0.38; 0.19] | |
| ≥40 to ≤50 U | 91 | 76 | −15.65 (11.35) | −16.52 (9.15) | 0.01 [−2.55; 2.57] | −1.43 (1.04) | −1.51 (0.84) | 0.00 [−0.23; 0.24] | |
| FPG | |||||||||
| <7.2 mmol/L | 86 | 82 | −15.24 (10.75) | −13.34 (8.20) | −1.17 [−3.74; 1.39] | −1.39 (0.98) | −1.22 (0.75) | −0.11 [−0.34; 0.13] | .3599 |
| ≥7.2 mmol/L | 151 | 151 | −16.84 (9.79) | −17.65 (10.43) | 0.32 [−1.58; 2.22] | −1.54 (0.90) | −1.62 (0.95) | 0.03 [−0.15; 0.20] | |
Data are mean (SD) unless otherwise stated. Changes from baseline are absolute changes using descriptive statistics based on the FAS. All postbaseline HbA1c measurements obtained at planned visits before discontinuation from randomized treatment were analyzed using a linear mixed normal model using an unstructured residual covariance matrix for HbA1c measurements within the same participant. The model included subgroup, treatment, visit, and region as fixed factors and baseline HbA1c as covariate. The interactions between subgroup*treatment*visit, region*visit, and baseline HbA1c*visit were included in the model.
Basal-bolus, insulin glargine 100 U/mL + insulin aspart; BMI, body mass index; ETD, estimated treatment difference; FAS, full analysis set; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; IDegLira, insulin degludec/liraglutide; N, number of participants; SD, standard deviation; U, units.
Table 2.
Change in Body Weight by Baseline Characteristic.
| Baseline characteristics | Week 26, N |
Body weight, change from baseline,
kg |
ETD [95% CI] | Subgroup
interaction P-value |
||
|---|---|---|---|---|---|---|
| IDegLira | Basal-bolus | IDegLira | Basal-bolus | |||
| HbA1c | ||||||
| ≤58.5 mmol/mol | 54 | 54 | −1.03 (3.16) | 2.15 (3.21) | −3.12 [−4.42; −1.83] | .2472 |
| >58.5 to ≤69.4 mmol/mol | 107 | 101 | −1.12 (3.27) | 2.05 (3.58) | −3.23 [−4.16; −2.30] | |
| >69.4 mmol/mol | 77 | 78 | −0.54 (3.23) | 3.75 (4.01) | −4.31 [−5.38; −3.23] | |
| BMI | ||||||
| <30 kg/m2 | 92 | 89 | −1.09 (2.68) | 2.30 (3.70) | −3.36 [−4.37; −2.36] | .7399 |
| 30 to <35 kg/m2 | 94 | 81 | −0.97 (3.13) | 2.39 (3.39) | −3.47 [−4.50; −2.45] | |
| ≥35 kg/m2 | 52 | 63 | −0.52 (4.20) | 3.44 (4.07) | −3.98 [−5.25; −2.72] | |
| Age | ||||||
| <65 years | 170 | 185 | −0.83 (3.28) | 2.63 (3.75) | −3.57 [−4.29; −2.86] | .9536 |
| ≥65 years | 68 | 48 | −1.13 (3.12) | 2.67 (3.65) | −3.53 [−4.81; −2.25] | |
| Diabetes duration | ||||||
| <10 years | 90 | 83 | −0.51 (3.50) | 2.84 (3.70) | −3.40 [−4.43; −2.38] | .6724 |
| ≥10 years | 148 | 150 | −1.16 (3.04) | 2.53 (3.73) | −3.68 [−4.46; −2.90] | |
| Total pretrial daily basal insulin dose | ||||||
| 20to <30 U | 91 | 96 | −0.06 (3.22) | 2.84 (3.90) | −2.80 [−3.77; −1.83] | .0326 |
| 30 to <40 U | 56 | 61 | −0.42 (2.70) | 2.60 (2.96) | −3.03 [−4.26; −1.80] | |
| 40 to ≤50 U | 91 | 76 | −2.07 (3.23) | 2.42 (4.06) | −4.59 [−5.62; −3.56] | |
| FPG | ||||||
| <7.2 mmol/L | 86 | 82 | −1.15 (3.22) | 1.95 (3.57) | −3.06 [−4.10; −2.03] | .2089 |
| ≥7.2 mmol/L | 151 | 151 | −0.84 (3.19) | 3.01 (3.76) | −3.89 [−4.66; −3.12] | |
Data are mean (SD) unless otherwise stated. Changes from baseline are absolute changes using descriptive statistics based on the FAS. All postbaseline body weight measurements obtained at planned visits before discontinuation from randomized treatment were analyzed using a linear mixed normal model using an unstructured residual covariance matrix for body weight measurements within the same participant. The model included subgroup, treatment, visit, and region as fixed factors and baseline body weight as covariate. The interactions between subgroup*treatment*visit, region*visit, and baseline HbA1c*visit were included in the model.
Basal-bolus, insulin glargine 100 U/mL + insulin aspart; BMI, body mass index; ETD, estimated treatment difference; FAS, full analysis set; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; IDegLira, insulin degludec/liraglutide; N, number of participants; SD, standard deviation; U, units.
Table 3.
End-of-Trial Total Daily Insulin Dose (U/kg) by Baseline Characteristic.
| Baseline characteristics | Week 26, N |
End-of-trial insulin dose,
U/kg |
ETD [95% CI] | Subgroup
interaction P-value |
||
|---|---|---|---|---|---|---|
| IDegLira | Basal-bolus | IDegLira | Basal-bolus | |||
| HbA1c | ||||||
| ≤58.5 mmol/mol | 54 | 54 | 0.46 (0.13) | 0.74 (0.30) | −0.31 [−0.39; −0.23] | <.0001 |
| >58.5 to ≤69.4 mmol/mol | 107 | 100 | 0.47 (0.12) | 0.92 (0.41) | −0.44 [−0.50; −0.38] | |
| >69.4 mmol/mol | 77 | 78 | 0.49 (0.14) | 1.05 (0.53) | −0.56 [−0.63; −0.49] | |
| BMI | ||||||
| <30 kg/m2 | 92 | 88 | 0.50 (0.15) | 0.90 (0.51) | −0.42 [−0.48; −0.35] | .1643 |
| 30 to <35 kg/m2 | 94 | 81 | 0.48 (0.12) | 0.94 (0.43) | −0.45 [−0.51; −0.38] | |
| ≥35 kg/m2 | 52 | 63 | 0.43 (0.08) | 0.92 (0.39) | −0.52 [−0.60; −0.44] | |
| Age | ||||||
| <65 years | 170 | 184 | 0.48 (0.13) | 0.92 (0.46) | −0.46 [−0.51; −0.41] | .5562 |
| ≥65 years | 68 | 48 | 0.48 (0.13) | 0.91 (0.42) | −0.43 [−0.51; −0.35] | |
| Diabetes duration | ||||||
| <10 years | 90 | 83 | 0.49 (0.12) | 0.96 (0.51) | −0.47 [−0.53; −0.40] | .5553 |
| ≥10 years | 148 | 149 | 0.47 (0.13) | 0.90 (0.42) | −0.44 [−0.49; −0.39] | |
| Total pretrial daily basal insulin dose | ||||||
| 20 to <30 U | 91 | 96 | 0.43 (0.14) | 0.79 (0.41) | −0.35 [−0.42; −0.29] | .0003 |
| 30 to <40 U | 56 | 60 | 0.49 (0.13) | 0.94 (0.48) | −0.47 [−0.55; −0.39] | |
| 40 to ≤50 U | 91 | 76 | 0.52 (0.10) | 1.07 (0.43) | −0.54 [−0.61; −0.48] | |
| FPG | ||||||
| <7.2 mmol/L | 86 | 81 | 0.45 (0.13) | 0.80 (0.38) | −0.36 [−0.43; −0.30] | .0012 |
| ≥7.2 mmol/L | 151 | 151 | 0.50 (0.12) | 0.99 (0.47) | −0.50 [−0.55; −0.45] | |
Data are mean (SD) unless otherwise stated. EOT dose analyzed are absolute changes using descriptive statistics based on the FAS. Total daily insulin dose (U/kg) was analyzed using a mixed model of repeated measurement with a compound symmetric covariance structure. The model included treatment, visit, and region as fixed factors and baseline HbA1c and basal insulin dose at screening as covariates. The interaction between subgroup*treatment*visit was included in the model.
Basal-bolus, insulin glargine 100 U/mL + insulin aspart; BMI, body mass index; EOT, end-of-trial; ETD, estimated treatment difference; FAS, full analysis set; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; IDegLira, insulin degludec/liraglutide; N, number of participants; SD, standard deviation; U, units.
Figure 1.
Rate of treatment-emergent hypoglycemic episodes (severe or blood glucose-confirmed symptomatic) per 100 participant-years by (a) glycated hemoglobin, (b) body mass index, (c) age, (d) diabetes duration, (e) total pretrial daily basal insulin dose, and (f) fasting plasma glucose at baseline. Data are rates of treatment-emergent hypoglycemic episodes per 100 PYE. A number of treatment-emergent (severe or blood glucose-confirmed symptomatic) hypoglycemic episodes were analyzed using a negative binomial regression model with a log link and the logarithm of the exposure time as offset. The model included subgroup, treatment, and region as fixed factors. The interaction between subgroup and treatment was included in the model.
Basal-bolus, insulin glargine 100 U/mL + insulin aspart; BMI, body mass index; CI, confidence interval; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; IDegLira, insulin degludec/liraglutide; PYE, participant-years of exposure.
Change in HbA1c
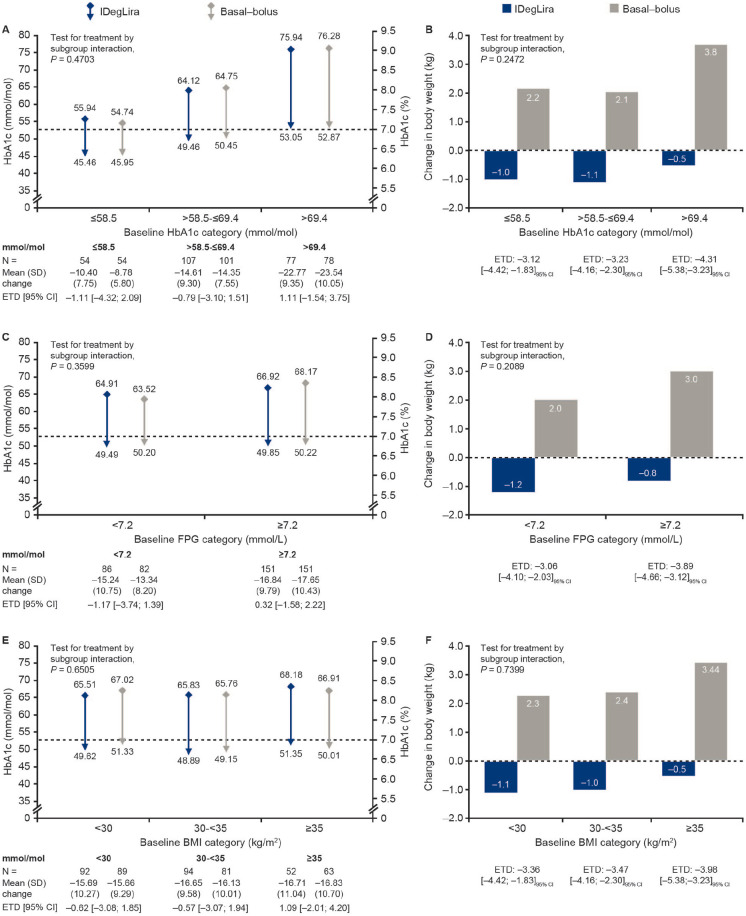
No significant differences were observed for change in HbA1c from baseline to week 26 between IDegLira and BB insulin therapy, regardless of baseline subgroup (Table 1; Figure 2). Across both treatment arms, the smallest change in HbA1c was seen in the lowest baseline HbA1c subgroup (−10.40 mmol/mol [−0.95%] for IDegLira and −8.78 mmol/mol [−0.80%] for BB insulin) compared with the greatest change seen in the highest HbA1c subgroup (−22.77 mmol/mol [−2.08%] for IDegLira and −23.54 mmol/mol [−2.15%] for BB insulin). Likewise, in both treatment arms, change in HbA1c from baseline to week 26 increased with increasing baseline HbA1c, BMI, age, and FPG. Change in HbA1c was greater with increasing baseline diabetes duration in the IDegLira treatment arm only, while change in HbA1c was greater with increasing baseline pretrial daily basal insulin dose in the BB insulin arm only. No significant interaction was observed between treatment and any of the subgroups for change in HbA1c. Across all six baseline clinical subgroups, mean EOT HbA1c was ≤53 mmol/mol (≤7.0%) regardless of treatment arm (data shown for HbA1c, FPG, and BMI subgroups only; Figure 2).
Figure 2.
Change in glycated hemoglobin and body weight from baseline to week 26 by baseline glycated hemoglobin group (a, b), fasting plasma glucose (c, d), and body mass index (e, f). Data are mean (SD) unless otherwise stated. Changes from baseline are absolute changes using descriptive statistics based on the FAS. All postbaseline HbA1c measurements obtained at planned visits before discontinuation from randomized treatment were analyzed using a linear mixed normal model using an unstructured residual covariance matrix for HbA1c measurements within the same participant. For (a), (c), and (e), the dotted line represents the American Diabetes Association HbA1c target <53 mmol/mol (<7.0%). The model included subgroup, treatment, visit, and region as fixed factors and baseline HbA1c as covariate. The interactions between subgroup*treatment*visit, region*visit, baseline HbA1c*visit were included in the model. * indicates an interaction.
Basal-bolus, insulin glargine 100 U/mL + insulin aspart; BMI, body mass index; CI, confidence interval; ETD, estimated treatment difference; FAS, full analysis set; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; IDegLira, insulin degludec/liraglutide; N, number of participants; SD, standard deviation; U, unit.
Change in Body Weight
IDegLira was associated with a mean reduction in body weight across all six clinical subgroups assessed, compared with a weight gain with BB insulin therapy. In the IDegLira arm, mean weight loss was greater with increasing baseline age, diabetes duration, and pretrial daily basal insulin dose, but decreased with increasing baseline BMI and FPG. Treatment differences between IDegLira and BB insulin therapy for change in body weight were significant across all subgroups (Figure 2; Table 2). Only total basal insulin dose had a positive interaction with treatment for the endpoint change in body weight (P = .0326).
Number of Treatment-Emergent Severe or Blood Glucose-Confirmed Symptomatic Hypoglycemic Episodes
Rates of blood glucose-confirmed hypoglycemia were lower for IDegLira (30.1-222.0 events per 100 participant-year of exposure [PYE]) compared with BB insulin therapy (656.5-946.6 events per 100 PYE; Figure 1) across all subgroups assessed. A significant interaction was observed between treatment and the subgroups based on HbA1c (P = .0004), BMI (P < .0001), and pretrial daily basal insulin dose (P = .0003). No significant interaction was observed between treatment and either age, diabetes duration, or FPG at baseline.
End-of-Trial Daily Insulin Dose (Total and Basal)
The mean EOT total daily insulin dose for IDegLira ranged from 0.43 U/kg (absolute dose: 36.0 U degludec and 1.3 mg liraglutide) to 0.52 U/kg (absolute dose: 44.9 U degludec and 1.6 mg liraglutide) across the subgroups assessed and was significantly lower than the doses used with BB insulin therapy (0.74-1.07 U/kg; Table 3). The greatest differences in EOT total daily insulin dose in favor of IDegLira vs BB were observed in patients with a BMI ≥35 kg/m2, baseline HbA1c >69.4 mmol/mol, and a pretrial daily basal insulin dose ≥40 to ≤50 U (Table 3). In both treatment arms, the mean total daily EOT insulin dose was greater with increasing baseline HbA1c, total pretrial daily basal insulin dose, and FPG (Table 3). Significant interaction was observed between treatment and HbA1c, total pretrial daily basal insulin dose, and FPG (P < 0.0001, P = 0.0003 and P = 0.0012, respectively). No significant interaction was observed between treatment and BMI, age, or diabetes duration at baseline.
End-of-trial daily basal insulin dose was lower for all subgroups in the IDegLira-treated arm compared with the BB-treated arm, and this difference was greatest in the subgroups with the highest HbA1c, BMI, and total pretrial basal insulin dose at baseline (supplemental Table S1). No significant interactions were observed between treatment and HbA1c, age, or diabetes duration, but significant interactions were observed with BMI (P = .0376), total pretrial insulin dose (P < .0001), and FPG (P = .0121).
Triple Composite Endpoint of Achieving HbA1c <53 mmol/mol (<7.0%) With No Weight Gain and Without Hypoglycemia
The odds of participants achieving the triple composite endpoint of HbA1c <53 mmol/mol (<7.0%) with no weight gain and without hypoglycemia were significantly greater with IDegLira than BB insulin therapy across all HbA1c, BMI, and duration of diabetes subgroups (supplemental Figure S1).
The percentages of participants achieving the triple composite endpoint with IDegLira vs BB insulin therapy were as follows: in the HbA1c subgroups of ≤58.5 mmol/mol (≤7.5%), 44.6% vs 7.0%, (estimated odds ratios [EOR]: 13.43 [4.23; 42.62]95% CI); in the >58.5 to ≤69.4 mmol/mol (>7.5% to ≤8.5%) cohort, 41.1% vs 8.3% (EOR: 7.80 [3.56; 17.08]95% CI); and in the >69.4 mmol/mol (>8.5%) cohort, 23.8% vs 3.4% (EOR: 15.81 [3.57; 70.05]95% CI) (supplemental Figure S1A). No significant interaction was observed between treatment and baseline HbA1c subgroup (P = .6072).
Greater percentages of participants treated with IDegLira in all BMI subgroups achieved the triple composite endpoint: in the BMI <30 kg/m2 cohort, 34.4% vs 4.1% (EOR: 13.65 [4.56; 40.81]95% CI); in the BMI ≥30 to <35/m2 cohort, 39.4% vs 7.8% (EOR: 10.53 [4.15; 26.76]95% CI); and in the BMI ≥35 kg/m2 cohort, 33.3% vs 7.5% (EOR: 7.75 [2.62; 22.87]95% CI). There was no significant interaction between treatment and BMI subgroup (P = .7697) (supplemental Figure S1B).
Similarly, of participants with diabetes duration <10 years, 36.7% of IDegLira-treated participants vs 5.6% of BB-treated participants achieved the triple composite endpoint (EOR: 12.08 [4.43; 32.94]95% CI), and 35.7% and 6.7% (EOR: 9.52 [4.59; 19.73]95% CI), respectively, of those with diabetes duration ≥10 years (supplemental Figure S1C). No significant treatment effect was observed by duration of diabetes (P = .7057).
Discussion
This post hoc analysis of DUAL VII aimed to assess whether the benefit of IDegLira vs BB insulin therapy was applicable to a broad participant population or only defined segments of the population. Previous studies have investigated treatment effect across various baseline characteristics and many have reported greater reductions in HbA1c in participants with higher baseline HbA1c values compared with those with lower baseline values.12-17 This approach includes a post hoc analysis of the DUAL V trial, which demonstrated that IDegLira treatment (compared with continued IGlar U100) resulted in greater HbA1c reductions, a greater percentage of participants achieving glycemic targets, weight loss (vs weight gain), and lower hypoglycemia rates across baseline HbA1c, FPG, and BMI subgroups.12 Consistent with the results of this study, the DUAL V post hoc analysis also showed that with an increasing baseline HbA1c, there was a decrease in the proportion of participants achieving the triple composite endpoint. In addition, and similar to the results of our study, in DUAL V, EOT insulin dose was relatively stable across baseline HbA1c groups with IDegLira (40-42 U), whereas it increased (from a mean dose of 60 to 73 U) with increasing baseline HbA1c in the IGlar U100 treatment arm.12 However, it is important to note that while the insulin dose in the IDegLira arm was capped at 50 U (due to the maximum dose of the liraglutide component of the fixed-ratio combination), the insulin dose in the comparator arms of both DUAL V and VII was not capped.
The current study demonstrated that the benefit of IDegLira compared with BB insulin therapy in DUAL VII was consistent across six different baseline characteristics. Furthermore, a greater percentage of participants with IDegLira vs BB insulin therapy achieved the triple composite endpoint of HbA1c <53 mmol/mol (<7.0%) without hypoglycemia and with no weight gain regardless of baseline HbA1c, BMI, or duration of diabetes.
These benefits are considered attributable to the complementary mechanisms of action of basal insulin and GLP-1RA therapy and the resulting insulin dose-sparing properties of IDegLira. Similar levels of glycemic control can be achieved with IDegLira at a lower insulin dose compared with basal insulin or BB insulin therapy because of the additive effect of the basal insulin and GLP-1RA components of IDegLira on glycemic control.18 In turn, the side effects of insulin therapy—namely hypoglycemia and weight gain—are reduced.19,20 Therefore, it is not surprising that the magnitude of weight loss and hypoglycemia rate reduction with IDegLira was greatest in participants switching from the highest pretrial insulin dose to the relatively low starting dose of 16 U IDegLira as these patients benefitted from the greatest relative reduction in overall insulin exposure.
High doses of insulin are often required to improve HbA1c with BB insulin therapy and this inevitably comes with an increased risk of hypoglycemia and weight gain.21 With increasing baseline HbA1c and increasing pretrial daily basal insulin dose, a need for gradually higher insulin doses was evident in the BB treatment arm; however, the insulin dose was relatively consistent across these categories with IDegLira treatment. The greatest differences in insulin dose between treatments were observed in those patients with the highest baseline HbA1c (>69.4 mmol/mol) and highest pretrial basal insulin dose (40 to ≤50 U). DUAL VII demonstrated weight benefit and lower rates of hypoglycemia with IDegLira compared with BB therapy across all patient subgroups. The findings suggest that the greatest weight benefit of IDegLira treatment may be seen in patients with poor glycemic control and in patients that are on high doses of insulin. Further, the composite endpoint findings suggest that IDegLira offers a spectrum of clinical benefits over BB insulin therapy, regardless of a patient’s duration of diabetes, baseline HbA1c, or baseline BMI at treatment initiation.
There was a significant interaction between treatment and baseline BMI subgroup for the number of hypoglycemic episodes; the reason that rates were higher in the <30 kg/m2 baseline BMI subgroup for both treatment arms is unclear and warrants further investigation. Similarly, it should be noted that the trend of decreasing weight loss with increasing baseline BMI subgroup in the IDegLira treatment arm was in contrast to results reported from the similar post hoc analysis of DUAL V12; the reason for this disparity is unclear and it is possible that it could be due to chance.
The widely reported phenomenon of significantly greater reductions in HbA1c with increasing baseline HbA1c16,17 was observed in this study and as steeper improvements in glycemic control are likely to be associated with higher hypoglycemia rates—particularly with insulin therapy—this may partly explain the contrast in terms of hypoglycemia rates in the highest baseline HbA1c subgroup.
The findings from this analysis cannot necessarily be generalized to clinical practice without further corroboration because this is a post hoc analysis and therefore designed to generate hypotheses rather than conclusions. However, the analysis was conducted using data from a large randomized clinical trial allowing for meaningful clinical analysis. Further limitations include the exclusion of participants with HbA1c >86 mmol/mol (10.0%) or BMI >40 kg/m2 from the overall trial, use of more than 50 U/day of IGlar U100, use of any medication indicated for diabetes or obesity other than those stated in the inclusion criteria 90 days before screening, and the lack of correction for multiple testing in this post hoc analysis. The hypotheses generated from this study could be tested for generalizability in a pragmatic evidence study or a randomized controlled trial comparing outcomes in users of IDegLira and BB insulin therapy.22
Conclusion
The present post hoc analysis demonstrates that the benefits of IDegLira vs BB insulin therapy—namely that good glycemic control can be achieved with lower insulin requirements, lower hypoglycemia rates, and weight loss—are consistent across a range of different baseline characteristics and degrees of disease progression. Altogether, these results support the initiation of IDegLira in a broad general population of patients with poor glycemic control on 20 to 50 U of basal insulin. The convenience of once-daily IDegLira, which reduces the number of injections compared with BB therapy, provides a less burdensome injectable treatment alternative with the potential to improve patient adherence.
Supplemental Material
Supplemental material, 1128008_Efficacy_by_Baseline_Characteristics_manuscript_Fig_S1_1 for The Benefit of Insulin Degludec/Liraglutide (IDegLira) Compared With Basal-Bolus Insulin Therapy is Consistent Across Participant Subgroups With Type 2 Diabetes in the DUAL VII Randomized Trial by Liana K. Billings, Bue F. Ross Agner, Yuksel Altuntas, Randi Grøn, Natalie Halladin, David C. Klonoff, Nikolaos Tentolouris and Esteban Jódar in Journal of Diabetes Science and Technology
Acknowledgments
The authors are grateful to the people who participated in this study. The study was sponsored by Novo Nordisk. Medical writing and editorial support was provided by Victoria Atess and Catherine Jones of Watermeadow Medical, UK, an Ashfield company, part of UDG Healthcare, funded by Novo Nordisk, in accordance with Good Publication Practice (GPP3) guidelines.
Footnotes
Author Contributions: All authors confirm that they meet the International Committee of Medical Journal Editors (ICJME) uniform requirements for authorship. All authors had full access to all data, were responsible for data interpretation and manuscript preparation, and had final responsibility for the decision to submit for publication.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Bue Ross Agner, Natalie Halladin, and Randi Grøn are all employees and shareholders in Novo Nordisk. Nikolaos Tentolouris has participated in advisory panels for Merck Sharp Dohme (MSD), AstraZeneca, Sanofi, Novo Nordisk, ELPEN, Eli Lilly, Servier, Boehringer Ingelheim, and Novartis, and has received research support from MSD, Eli Lilly, Novo Nordisk, Sanofi, Pfizer, AstraZeneca, Janssen, Cilag, GlaxoSmithKline, and Novartis. Liana K. Billings has received consulting fees from Novo Nordisk, Sanofi, Dexcom, and Lily; research support from Novo Nordisk, Sanofi, Dexcom, and Lilly; and participates in the speakers’ bureau for Novo Nordisk. David Klonoff is a consultant for Ascensia, Eoflow, Lifecare, Novo, Roche, and Voluntis. Esteban Jódar has appeared on advisory panels for Amgen, AstraZeneca, Fresenius, Janssen, Lilly, MSD, Novo Nordisk, Shire, and UCB; has received research support from AstraZeneca, Janssen, Lilly, MSD, Novo Nordisk, Pfizer, and Sanofi; and is on the speakers’ bureau for Amgen, AstraZeneca, Boehringer Ingelheim, FAES, Janssen, Lilly, MSD, Novartis, and Novo Nordisk.
Data Availability: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Novo Nordisk.
ORCID iD: Liana K. Billings  https://orcid.org/0000-0001-7991-3010
https://orcid.org/0000-0001-7991-3010
Previous Presentation: Parts of this study were presented as a poster presentation at the American Diabetes Association, 78th Annual Scientific Sessions, June 22-26, 2018, Orlando, Florida. The primary results from DUAL VII have been published.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893. [DOI] [PubMed] [Google Scholar]
- 3. Buse JB, Vilsboll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933. [DOI] [PubMed] [Google Scholar]
- 4. Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther. 2017;8(1):101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodbard HW, Bode BW, Harris SB, et al. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naive people with Type 2 diabetes: the DUAL IV trial. Diabet Med. 2017;34(2):189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lingvay I, Manghi FP, Garcia-Hernandez P, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315(319):898-907. [DOI] [PubMed] [Google Scholar]
- 7. Harris SB, Kocsis G, Prager R, et al. Safety and efficacy of IDegLira titrated once weekly versus twice weekly in patients with type 2 diabetes uncontrolled on oral antidiabetic drugs: DUAL VI randomized clinical trial. Diabetes Obes Metab. 2017;19(6):858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41(5):1009-1016. [DOI] [PubMed] [Google Scholar]
- 9. Philis-Tsimikas A, Billings L, Busch RS, et al. Superior efficacy of insulin degludec/liraglutide (IDegLira) vs. insulin glargine (IGlar U100) as add-on to sodium-glucose cotransporter-2 inhibitor (SGLT2i) ± oral antidiabetic drug (OAD) therapy in patients with type 2 diabetes (T2D)—DUAL IX trial. Diabetes. 2018;67(suppl 1):127. [Google Scholar]
- 10. International Conference of Harmonisation. ICH Harmonised Tripartite Guideline: Good Clinical Practice. 1996. http://apps.who.int/medicinedocs/en/m/abstract/Js22154en/. Accessed December 10, 2018.
- 11. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 12. Lingvay I, Harris S, Jaeckel E, Chandarana K, Ranthe MF, Jodar E. Insulin degludec/liraglutide (IDegLira) was effective across a range of dysglycaemia and body mass index categories in the DUAL V randomized trial. Diabetes Obes Metab. 2018;20(1):200-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilding JP, Blonde L, Leiter LA, et al. Efficacy and safety of canagliflozin by baseline HbA1c and known duration of type 2 diabetes mellitus. J Diabetes Complications. 2015;29(3):438-444. [DOI] [PubMed] [Google Scholar]
- 14. Gallwitz B, Dagogo-Jack S, Thieu V, et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20(2):409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeFronzo RA, Ferrannini E, Schernthaner G, et al. Slope of change in HbA1c from baseline with empagliflozin compared with sitagliptin or glimepiride in patients with type 2 diabetes. Endocrinol Diabetes Metab. 2018;1(2):e00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose-lowering therapies: a meta-analysis of randomized clinical trials. Diabet Med. 2010;27(3):309-317. [DOI] [PubMed] [Google Scholar]
- 17. Giugliano D, Maiorino M, Bellastella G, Chiodini P, Esposito K. Relationship of baseline HbA1c, HbA1c change and HbA1c target of <7% with insulin analogues in type 2 diabetes: a meta-analysis of randomised controlled trials. Int J Clin Pract. 2011;65(5):602-612. [DOI] [PubMed] [Google Scholar]
- 18. Owens DR, Monnier L, Bolli GB. Differential effects of GLP-1 receptor agonists on components of dysglycaemia in individuals with type 2 diabetes mellitus. Diabetes Metab. 2013;39(6):485-496. [DOI] [PubMed] [Google Scholar]
- 19. Inman TR, Plyushko E, Austin NP, Johnson JL. The role of basal insulin and GLP-1 receptor agonist combination products in the management of type 2 diabetes. Ther Adv Endocrinol Metab. 2018;9(5):151-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moreira RO, Cobas R, Lopes Assis Coelho RC. Combination of basal insulin and GLP-1 receptor agonist: is this the end of basal insulin alone in the treatment of type 2 diabetes? Diabetol Metab Syndr. 2018;10(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20(3):488-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klonoff DC. The expanding role of real-world evidence trials in health care decision making. J Diabetes Sci Technol. 2020;14(1):174-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 1128008_Efficacy_by_Baseline_Characteristics_manuscript_Fig_S1_1 for The Benefit of Insulin Degludec/Liraglutide (IDegLira) Compared With Basal-Bolus Insulin Therapy is Consistent Across Participant Subgroups With Type 2 Diabetes in the DUAL VII Randomized Trial by Liana K. Billings, Bue F. Ross Agner, Yuksel Altuntas, Randi Grøn, Natalie Halladin, David C. Klonoff, Nikolaos Tentolouris and Esteban Jódar in Journal of Diabetes Science and Technology