Abstract
The concept of implantable glucose sensors has been promulgated for more than 40 years. It is now accepted that continuous glucose monitoring (CGM) increases quality of life by allowing informed diabetes management decisions as a result of more optimized glucose control. The focus of this article is to provide a brief overview of the CGM market history, emerging technologies, and the foreseeable challenges for the next CGM generations as well as proposing possible solutions in an effort to advance the next generation of implantable sensor.
Keywords: continuous glucose monitoring, diabetes, foreign body reaction, inflammation
Introduction
The domestic prevalence of diabetes mellitus (DM) has increased from 11 million in 2000 to approximately 30 million in less than two decades.1 Approximately 90% to 95% of the diabetic population is classified as type 2 diabetes in which an individual produces an insufficient amount of insulin to meet their metabolic requirements.2 This number is predicted to rise to nearly 55 million people by 2030 representing an estimated increase of 54%.3 The World Health Organization estimates that approximately 422 million adults or 8.5% of the world’s population are currently affected, which represent a fourfold increase in less than 40 years. This exponential rise in DM implies an incipient global pandemic.
Traditionally, the principal method of glucose monitoring for people with diabetes has been through self-monitoring of blood glucose (SMBG) without a clear consensus on sampling frequency that varied between four and ten times per day, for insulin-dependent patient with diabetes.4 This variation reflects differences in activity levels, lifestyle, insulin injection regimens, and agreements brokered between the clinician and the patient. Nevertheless, few patients adhere to the rigid SMBG regimen necessary to delay the onset and slow the progression of diabetic complications including retinopathy, nephropathy, and neuropathy that can result in limb amputations.4 The concept of implantable glucose sensors has been promulgated for more than 40 years.5 Extensive research and development resulted in the first marketable transdermal implantable glucose sensors in 1999 and early 2000. These first sensors had limited clinical utility as they experienced significant drift in sensitivity over the initial FDA-approved three-day implantation period.6,7 Given the superior in vitro performance, this in vivo sensor output drift was unexpected such that enthusiasm waned even within the scientific community in these early continuous glucose monitoring (CGM) days.8-10 Further technological advances in CGM revolutionized diabetes care. It is now accepted that CGM increases quality of life by allowing informed diabetes management decisions as a result of more optimized glucose control.11 This leads to a better health and a reduction in diabetic complications.12 Although CGM requires a higher initial investment, the trust is that long-term health benefits of CGM are cost-effective when compared to daily use of test strips.12-15 Nevertheless, a consensus on CGM and cost-effectiveness has not yet been reached.16,17 Notably, the hybrid closed loop insulin delivery system, which is linked to a glucose sensor, was named as the most disruptive medical technology in healthcare at an industry summit in 2018.
The next technological wave will focus on advancing closed-loop artificial pancreas device (APD) systems that incorporate a long-term functional implantable sensor device directed at normalizing blood glucose levels. As innovation in diabetes management progresses, an overarching factor in the development of any successful APD system is the advancement of a highly accurate and long-term functional glucose sensor. Insulin infusion systems, which are a vital part of the APD system, have their own challenges as they are currently FDA approved for three days of consecutive use. Although tangentially important, they are not addressed in this overview of CGM technology innovations. Rather, the focus of this article is to provide an overview of the CGM market history, emerging technologies, and the foreseeable challenges for the next CGM generations as well as proposing possible solutions in an effort to advance the next generation of APD systems.
Overview of the Current CGM Technology
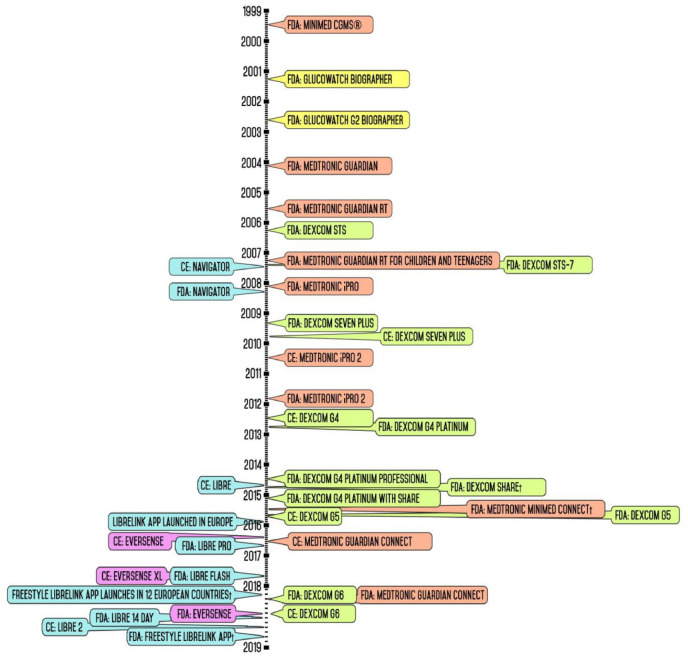
Figure 1 depicts the release dates of CGM devices in American and European markets beginning with its introduction nearly two decades ago to the present day. This timeline also includes secondary display accessories and downloadable applications as a means of emphasizing the industry’s shift to establish mobile device compatibility in continuous glucose monitors. Otherwise, these timeline events focus primarily on the release of standalone CGM; a designation indicating that these devices can be employed to monitor a patient’s interstitial glucose levels either independently or as part of an insulin infusion system. Given that a CGM device is typically understood to be composed of a sensor, a transmitter, and a receiver or monitor, and sold as a unit with no interchangeable components, the timeline does not depict major technological developments in these individual components. Thus, specific product releases such as the launch of the Enlite glucose sensor or Guardian 3 glucose sensor—glucose sensors, which are sold as stand-alone products and are compatible with more than one type of insulin pump or CGM system—are not included in Figure 1. Given the specific scope of this review, readers who are interested in the history and development of CGMs are advised to read other previous reviews in addition to the background literature.5,18-20
Figure 1.
Timeline of continuous glucose monitoring market release.
In 1999, Minimed marketed the first CGM system that enabled recording of a patient’s glucose values over a span of three days, which relied on repetitive sensor calibration with a finger stick glucose sample every 6 to 12 hours.21 The sensor and its receiver were physically connected through a cable such that it functioned similar to the currently available wireless CGM. However, the data were not available to the patient in real time as these data had to be downloaded to a healthcare provider for post hoc analysis. Thus, this device was designed to supplement rather than replace traditional blood glucose monitoring.21 In 2004, the Medtronic Guardian introduced wireless transmitting from sensor to receiver as well as programmable high and low glucose alerts; features that became an industry standard. Subsequently, the advent of the Medtronic Guardian RT and the Dexcom STS—launched in 2005 and 2006, respectively—allowed the user to view their calculated glucose levels in real time for up to three days.20
In 2007, Dexcom introduced the STS-7, which was the first device to allow patients to continuously monitor their glucose levels for seven days as opposed to three.20 Prior to the STS-7, Dexcom focused primarily on the development of a totally implantable glucose sensor device to function for a month to a year at a time.22,23 In 2014, Abbott obtained the CE mark for FreeStyle Libre. In 2018, Abbott launched the FreeStyle Libre in the United States, the first flash glucose monitoring system that had several transformative innovations. Notably, this device permitted the user to scan the receiver over the sensor to obtain their current glucose value and glucose level trends.24 Moreover, this device’s warm-up period was reduced to a single hour and the implanted sensor’s lifespan was extended to 14 days. Perhaps of paramount importance, the FreeStyle Libre completely eliminated the need for initial and subsequent finger-stick calibrations.24 Nevertheless, the user is advised to perform blood glucose testing in the event that the sensor measurements are unreliable, such as in instance of dehydration.
With the release of secondary transmitters such as Dexcom Share and MiniMed Connect in 2015, compatibility with mobile devices became an essential feature for CGM devices. While these accessories worked in conjunction with company-released apps to enable the user to view their glucose values on their mobile phones, it was not until Dexcom released the G5 that the need to carry a separate receiver was eliminated.
In May 2016, Eversense (Senseonics) received CE mark, introducing a CGM that included the only implantable glucose sensor with a 90-day lifespan.24 One year later, Senseonics launched the Eversense XL in 2017, which advertised a sensor lifespan of 180 days.25 To this day, the Eversense XL remains the CGM with the long-lasting glucose sensor available on the market. Table 1 provides a summary of the current FDA-approved CGM systems including compatible products offered.
Table 1.
Currently Available Continuous Glucose Monitoring Diabetes Management Systems.
| Company name | Product(s) | Classification type of device | Calibration frequency | FDA approval year | Compatibility |
|---|---|---|---|---|---|
| Medtronic | iPro 2 | Professional CGM | 3-4/day | 2011 | Sof-Sensor, Enlite sensor, iPro2 Recorder (MMT-7741) |
| Minimed 630G | Insulin pump/artificial pancreas | 3-4/day | 2016 | Smart Guard, 630G Insulin Pump, Enlite Sensor, Guardian Sensor 3, Guardian Link Transmitter System, CareLink, Bayer’s CONTOUR NEXT LINK 2.4 Wireless Meter, Bayer’s CONTOUR NEXT Test Strips | |
| Minimed 670G | Insulin pump (manual mode)/artificial pancreas | 3-4/day | 2016 | Smart Guard, Guardian Sensor 3, Guardian Link Transmitter, CONTOUR NEXT LINK 2.4 Glucose Meter | |
| Guardian Connect | Real-time display CGM | 3-4/day | 2018 | Guardian Sensor 3 (MMT-7020), Guardian Transmitter (MMT-7821), Guardian Connect App (CSS7200) | |
| Dexcom | G4 PLATINUM | Real-time display CGM | 2/day | 2012 | G4 Sensor, G4 Receiver, G4 Transmitter |
| G5 | Real-time display CGM | 2/day | 2016 | G5 Sensor, G5 Receiver, G5 Transmitter, G5 Mobile App, t:slim X2 Insulin Pump | |
| G6 | Real-time display CGM | None | 2018 | G6 Sensor, G6 Receiver, G6 Transmitter, G6 Mobile App, t:slim X2 Insulin Pump | |
| Abbott Diabetes Care | FreeStyle Libre | Flash glucose monitoring | None | 2017 | FreeStyle Sensor, FreeStyle Reader, FreeStyle Libre Data Management Software, Built-In Glucose Meter, FreeStyle Precision Neo Blood Glucose Test Strips |
| Senseonics | Eversense | Implant | 2/day | 2018 | Eversense Sensor, Eversense Transmitter, MMA Software |
| Tandem | t:slim X2 | Insulin pump | 2/day | 2015 | Dexcom G4 Platinum CGM, Dexcom G5 CGM, Dexcom G6 CGM |
Abbreviation: CGM, continuous glucose monitoring.
This table depicts FDA-approved and currently available continuous glucose monitoring systems, differentiated by leading companies targeting the diabetes care market including their respective products offered. Calibration frequency is described as the amount of times a manual calibration is required for the respective device. Calibration is usually accomplished in the form of a finger prick. Compatibility with the particular continuous glucose monitoring system includes other sensor devices, receivers, transmitters, applications/software, glucose meters, and insulin infusion pumps.
Future directions include the development of “artificial pancreas” in which CGM devices and automated insulin dosing (AID) systems are linked to function beyond the current three-day limitations. Early in 2018, the Dexcom G6 became the first CGM to be approved by the FDA for integration into AID systems. At that time, the G6 glucose sensor had already been incorporated into closed-loop systems like the Diabeloop and Tandem’s t:slim X2 (Table 1). Dexcom G6 CGM functions without the need for start-up calibration or confirmatory finger sticks and operates on a ten-day lifespan. FDA has since categorized Dexcom G6 as an integrated or interoperable device based on the performance data meeting, special controls established for interoperable CGM. In the future, in conjunction with research organization Verily, Dexcom is expected to launch G7. Most notably, the device is being advertised as “thinnest CGM ever.”26 This device purports to have a lifespan of 14 to 15 days and function without a separate receiver, which may result in a less expensive CGM device.
Limitations for the Current Transdermal CGM Devices: Tissue Perspective
The host response to any respective glucose sensor will define sensor sensitivity, sensor performance, and ultimately sensor longevity. As such, one significant limitation of all implantable glucose monitoring devices is the foreign body response (FBR), which is an inflammatory reaction stimulated by the host’s immune system in response to a foreign substance. An FBR commences when macrophages, particularly pro-inflammatory M1 macrophages, are recruited to the sensor site through the skin’s vasculature. Recruitment of macrophages to the device location significantly affects the accuracy of the glucose sensor given that the metabolically active inflammatory cells consume interstitial glucose.27-29 This results in glucose gradient in which the glucose concentration adjacent to the sensor is vastly different from the true serum glucose concentration.28 Inflammatory cells are also responsible for the recruitment of fibroblasts, which are intimately associated with the FBR. Fibroblasts produce fibrous tissue that encapsulates the device in order to sequester it from the remainder of the body. Nevertheless, the lifespan of transdermal sensor devices has increased from 3 to 14 days over the past decade by addressing the FBR through advances in sensor chemistry, sensor coatings, and improved implantation techniques.30 Initial sensor biocompatibility studies first examined the toxicity of sensor materials followed by analyses of FBRs as in vivo lifespan increased from a few days to weeks.31 The root cause of these diverse tissue injuries, seven or more days postimplantation, is inflammation and fibrosis. Both of these tissue reactions compromise sensor function in vivo: inflammation by inducing sensor damage and/or glucose consumption at the sensor site and fibrosis by inducing blood vessel regression thus compromising diffusion in the interstitial fluid of the sensors located in the subcutaneous space.9,29,32-36 Given the need to increase sensor performance usage time beyond its current FDA-approved lifespan, transdermal sensors designed to last beyond one to two weeks as developed by DexCom, Abbott Diabetes Care, and Medtronic/MiniMed must address additional parameters. These include ensuring adequate skin adhesion of these devices while simultaneously preventing injury to the epithelial dermis layer. Other limitations may arise from the persistent open wound at the sensor insertion site, as well as “micromovement” of the sensor within the implantation site.37,38 In these situations, the implanted sensor not only prevents wound closure but sensor wearer’s activities could result in continuous localized micromovements and shear forces. The net result of this is mechanical tissue destruction at the poles of the implanted sensor. This triggers the recurrent episodes of acute inflammation and subsequent chronic inflammation leading to fibrosis, which results in sensor performance degradation. Eventually, the micromovement and tissue damage could create a “funnel” that channels debris and provides a culture medium for bacteria along the sides of the shaft of the sensor. Cell detritus, bacteria, and their byproducts then migrate into the deeper tissues along the shaft of the sensor and eventually emerge at the sensor tip. In turn, this drainage may lead to additional tissue inflammation, fibrosis, and degraded sensor performance. More concerning is the possibility of infiltrating bacteria at the insertion site posing a risk for biofilm formation as the push for longer insertion time past two weeks increases. Biofilms are microenvironments held together by a “sugary-sticky” substance secreted by bacteria, designated extracellular polymeric substances (EPS).39 These EPS provide protection to the bacteria resulting in damage to the host’s surrounding tissue. Once a biofilm forms, ultimately the only solution is to remove the sensor device, which creates a fibrotic tissue site unavailable for future CGM implantations. As such, further advances will likely require addressing tissue biocompatibility including localized inflammation at implantation sites that would permit recurrent implantations at that site.
Overcoming the Foreign Body Reaction
The key to achieving long-term sensor performance requires the successful integration of the device into the surrounding tissue by mitigating the effects of the FBR. Previous efforts in this regard have focused on implanted synthetic polymer coatings with or without concomitant use of anti-inflammatory agents.40-47 At present, the only FDA-approved implantable device is Eversense (Senseonics, Germantown, MD, United States), with a lifespan of 180 days.25 Eversense, with a diameter of 3.5 mm and a length of 18.3 mm, is implanted in the subcutaneous tissue with the assistance of healthcare providers. The longevity of the Eversense is most likely achieved through the synergy of the anti-inflammatory corticosteroid dexamethasone and the polyhydroxyethyl methacrylate (PHEMA)-based hydrogel coating (Figure 1; CE mark).25 Cell viability studies cultured on PHEMA- and polyethylene glycol-based hydrogel supported its use as superior material for implant material coatings.
More recent research has been directed toward developing device coatings that are less likely to incite a robust FBR. Cell and Molecular Engineering LLC (CMTE, Avon, CT, United States) aims to incorporate tissue response modifiers into basement membrane matrix coatings in an attempt to induce tissue tolerance33,48 and allow repetitive use of the same insertion site. Clinical Sensors, Inc. (Research Triangle, NC, United States) employed a different approach by designing a nitric oxide-releasing polymer sensor coating designed to enhance sensor accuracy and longevity.49 However, as the half-life of NO is relatively short in the physiological state, NO donors are needed to secure a prolonged and secure NO delivery.50
Emerging CGM Technology
Biorasis, Inc. (Storrs/Mansfield, CT, United States), Profusa (South San Francisco, CA, United States), and Laxmi Therapeutic Devices INC (Goleta, CA, United States) are all emerging businesses directed at developing CGM systems using the concept of microminiaturization, whereas Biorasis and Profusa aiming at a totally implantable sensor device. Biorasis and Profusa sensor devices are in the dimension of 5 mm in length and 500 µm in diameter such that the device may be inserted without medical personnel. It is well established that the degree of tissue reaction correlates with device size.51 Thus, the small size of these devices should minimize the associated FBR. Profusa’s sensor technology is based on fluorescent, nonenzymatic boronic acid possibly incorporating a microporous gel, similar to Helionics (Seattle, WA, United States) uniform pore size technology. The polymorphous biomaterial is composed of uniformly sized pores believed to facilitate neovascularization while limiting inflammatory cell migration. One potential limitation is that these devices would remain in situ once the device exceeded its useful lifespan. Although the FBR may be greatly reduced, experimental data would need to establish the safety and efficacy of this approach. As it is known that the insertion trauma negatively impacts a FBR, Laxmi Therapeutic Devices INC’s CGM approach targets a smaller needle size transcutaneous device in an effort to reduce FBR. The device is described as a “strip on a chip” and samples glucose analyte using magnetic actuation by dipping individual “hair-like” thin needles into the skin.
In order to circumvent the FBR, a joint project between Novartis (Basel, Switzerland) unit Alcon and Google (Menlo Park, CA, United States) designated Verily spent significant effort developing a glucose-sensing contact lens in 1994. Since measuring the glucose level in tears subsequently proved unreliable, the project was abandoned in November 2018. Verily has since partnered with Dexcom. These companies are projecting the launch of G7, a significantly smaller CGM version of Dexcom’s current G6 device. Software incorporation for G7 is partnered with Ondue (Newton, MA, United States), a virtual diabetes tool, designed to provide patients with guidance on routine diabetic management.
As CGM devices are not necessarily covered by private or governmental insurance policies, it is critical that cost reductions are considered for future devices entering the diabetes market. Other issues to consider are scalable manufacturing processes, a short sensor lag time, and the inclusion of reliable algorithms, which match the data coming from the sensor. Specifically, accuracy, reliability, and usability need to match or exceed the current products already on a competing diabetes market.
Future Requirements for Advanced CGM Technology
Given the rise in diabetes and its associated diabetic complications, an unsustainable increase in healthcare expenditure will be superimposed on an industry that consumes nearly 20% of the gross domestic product. A concomitant shortage of healthcare providers will exacerbate this situation. Diabetes requires intensive management to normalize glucose in an effort to avoid short- and long-term complications, healthcare expenditures, and premature mortality. Self-management includes glucose monitoring, exogenous insulin replacement, diet, and exercise. Such self-management requires complex problem solving, social supports, and effective access to quality healthcare resources. Currently, all sensor technology is aimed at a “one-size fits all” approach. This approach does not take into account the special needs of subpopulations such as senior citizens, ethnic minorities, children, adolescents, and people with limited resources. Future diabetes technology will have to address these issues. As such, the forthcoming sensor technology will need to be applicable across a wide spectrum of the diabetes population. Ideal devices would be properly sized for the specific user, easy to implement, and affordably priced. Such devices could be coupled with behavioral interventions that promote patient and family centered care along with decision support tools for clinicians. While there have been great advancements in CGM technology, biological, pharmacological, and socioeconomic factors have limited their use in widespread clinical practice.
Long-term sensor performance requires the successful integration of the device into the surrounding tissue by mitigating the effects of the host tissue response. All sensors elicit a host response that is determined by the sensor’s composition, size and shape, implantation process, and host variables (e.g. age, body mass index, and comorbidities). At the cellular level, innate immune cells including neutrophils, monocytes, macrophages (including subpopulations), mast cells, dendritic cells, and adaptive immune cells such as T cells are among the important cells to consider when designing the next generation of totally implantable CGM systems. Future studies will need to demonstrate the ability of sensor coatings with or without the inclusion of tissue response modifiers to control target inflammatory cell function in an effort to promote tissue integration of implantable glucose sensors.
Conclusion
Over the past two decades, CGM has revolutionized diabetes management such that it is now widely accepted. The next wave of invention will need to focus on combating the foreign body reaction in an effort to improve the tolerability of totally implantable devices. Previous efforts have focused on implanted synthetic polymer coatings with or without concomitant use of anti-inflammatory agents to combat the host tissue response to the implanted sensor device. Efforts directed at drug or biological protein integration have been hindered by pharmacokinetics (eg, rapid release kinetics with short dosing timelines) as well as diverse/nonspecific side effects. Alternative methods that employ cytokines and growth factors have been limited by their short duration of efficacy. It is likely that other means will be required to mitigate the effects of the host tissue response. In addition, diabetes technology should aim to be user-friendly, inexpensive, require minimal involvement of healthcare providers, and provide long-lasting lifespans.
Acknowledgments
We would like to thank Mr Adam Ferguson for assistance with the image timeline.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: UK is a cofounder and co-owner of the small business Cell and Molecular Tissue Engineering, LLC, Avon, CT, United States.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ulrike Klueh  https://orcid.org/0000-0003-1104-7704
https://orcid.org/0000-0003-1104-7704
References
- 1. Clement S. Medical management of the diabetic patient. Clin Podiatr Med Surg. 2019;36:349-354. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. National diabetes statistics report, 2017. Available at: http://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
- 3. Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patton SR. Adherence to glycemic monitoring in diabetes. J Diabetes Sci Technol. 2015;9(3):668-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Role of continuous glucose monitoring in diabetes treatment. Arlington, VA: American Diabetes Association; 2018. [PubMed] [Google Scholar]
- 6. Gerritsen M, Jansen JA, Kros A, et al. Influence of inflammatory cells and serum on the performance of implantable glucose sensors. J Biomed Mater Res. 2001;54(1):69-75. [DOI] [PubMed] [Google Scholar]
- 7. Gerritsen M, Jansen JA, Lutterman JA. Performance of subcutaneously implanted glucose sensors for continuous monitoring. Neth J Med. 1999;54(1):167-179. [DOI] [PubMed] [Google Scholar]
- 8. Gerritsen M. Problems associated with subcutaneously implanted glucose sensors. Diabetes Care. 2000;23(2):143–145. [DOI] [PubMed] [Google Scholar]
- 9. Wisniewski N, Klitzman B, Miller B, Reichert WM. Decreased analyte transport through implanted membranes: differentiation of biofouling from tissue effects. J Biomed Mater Res. 2001;57(4):513-521. [DOI] [PubMed] [Google Scholar]
- 10. Abel PU, von Woedtke T. Biosensors for in vivo glucose measurement: can we cross the experimental stage. Biosens Bioelectron. 2002;17(11-12):1059-1070. [DOI] [PubMed] [Google Scholar]
- 11. Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A view beyond HbA1c: role of continuous glucose monitoring. Diabetes Ther. 2019;10(3):853-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wan W, Skandari MR, Minc A, et al. Cost-effectiveness of continuous glucose monitoring for adults with type 1 diabetes compared with self-monitoring of blood glucose: the DIAMOND randomized trial. Diabetes Care. 2018;41(6):1227-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaugule S, Graham C. Cost-effectiveness of G5 Mobile continuous glucose monitoring device compared to self-monitoring of blood glucose alone for people with type 1 diabetes from the Canadian societal perspective. J Med Econ. 2017;20(11):1128-1135. [DOI] [PubMed] [Google Scholar]
- 14. Health Quality Ontario. Continuous monitoring of glucose for type 1 diabetes: a health technology assessment. Ont Health Technol Assess Ser. 2018;18(2):1-160. [PMC free article] [PubMed] [Google Scholar]
- 15. Huang ES, O’Grady M, Basu A, et al. The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2010;33(6):1269-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Lorenzo B, Rivero-Santana A, Vallejo-Torres L, et al. Cost-effectiveness analysis of real-time continuous monitoring glucose compared to self-monitoring of blood glucose for diabetes mellitus in Spain. J Eval Clin Pract. 2018;24(4):772-781. [DOI] [PubMed] [Google Scholar]
- 17. Acerini C. The rise of technology in diabetes care. Not all that is new is necessarily better. Pediatr Diabetes. 2016;17(3):168-173. [DOI] [PubMed] [Google Scholar]
- 18. Mariani HS, Layden BT, Aleppo G. Continuous glucose monitoring: a perspective on its past, present, and future applications for diabetes management. Clin Diabetes. 2017;35(1):60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12(2):181–187. [DOI] [PubMed] [Google Scholar]
- 20. Vettoretti M, Cappon G, Acciaroli G, Facchinetti A, Sparacino G. Continuous glucose monitoring: current use in diabetes management and possible future applications. J Diabetes Sci Technol. 2018;12(5):1064-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medtronic. Innovation milestones. Available at: https://www.medtronicdiabetes.com/about-medtronic-innovation/milestone-timeline2019.
- 22. Gilligan BJ, Shults MC, Rhodes RK, Updike SJ. Evaluation of a subcutaneous glucose sensor out to 3 months in a dog model. Diabetes Care. 1994;17(8):882–887. [DOI] [PubMed] [Google Scholar]
- 23. Updike SJ, Shults MC, Gilligan BJ, Rhodes RK. A subcutaneous glucose sensor with improved longevity, dynamic range, and stability of calibration. Diabetes Care. 2000;23(2):208-214. [DOI] [PubMed] [Google Scholar]
- 24. Petrie JR, Peters AL, Bergenstal RM, Holl RW, Fleming GA, Heinemann L. Improving the clinical value and utility of CGM systems: issues and recommendations: a joint statement of the european association for the study of diabetes and the American diabetes association diabetes technology working group. Diabetes Care. 2017;40(12):1614-1621. [DOI] [PubMed] [Google Scholar]
- 25. Aronson R, Abitbol A, Tweden KS. First assessment of the performance of an implantable continuous glucose monitoring system through 180 days in a primarily adolescent population with type 1 diabetes. Diabetes Obes Metab. 2019;21(7):1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levine BB, Brown A. What’s coming from dexcom in 2020? A low-cost, slimmer, fully disposable CGM. Available at: https://shares/a3cxAD2018.
- 27. Klueh U, Czajkowski C, Ludzinska I, Qiao Y, Frailey J, Kreutzer DL. Impact of CCL2 and CCR2 chemokine/receptor deficiencies on macrophage recruitment and continuous glucose monitoring in vivo. Biosens Bioelectron. 2016;86:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klueh U, Frailey JT, Qiao Y, Antar O, Kreutzer DL. Cell based metabolic barriers to glucose diffusion: macrophages and continuous glucose monitoring. Biomaterials. 2014;35(10):3145-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klueh U, Liu Z, Feldman B, et al. Metabolic biofouling of glucose sensors in vivo: role of tissue microhemorrhages. J Diabetes Sci Technol. 2011;5(3):583-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koh A, Nichols SP, Schoenfisch MH. Glucose sensor membranes for mitigating the foreign body response. J Diabetes Sci Technol. 2011;5(5):1052-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nichols SP, Koh A, Storm WL, Shin JH, Schoenfisch MH. Biocompatible materials for continuous glucose monitoring devices. Chem Rev. 2013;113(4):2528-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klueh U, Kaur M, Qiao Y, Kreutzer DL. Critical role of tissue mast cells in controlling long-term glucose sensor function in vivo. Biomaterials. 2010;31(16):4540-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klueh U, Ludzinska I, Czajkowski C, Qiao Y, Kreutzer DL. Crosslinked basement membrane-based coatings enhance glucose sensor function and continuous glucose monitoring in vivo. J Biomed Mater Res A. 2018;106(1):7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wisniewski NA, Klueh U, Stenken J. Interstitial fluid physiology as it relates to glucose monitoring technologies: symposium introduction. J Diabetes Sci Technol. 2011;5(3):579-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Novak MT, Reichert WM. Modeling the physiological factors affecting glucose sensor function in vivo. J Diabetes Sci Technol. 2015;9(5):993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novak MT, Yuan F, Reichert WM. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal Bioanal Chem 2010;398(4):1695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor-tissue interface-effects of motion, pressure, and design on sensor performance and foreign body response-part II: examples and application. J Diabetes Sci Technol. 2011;5(3):647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor-tissue interface-effects of motion, pressure, and design on sensor performance and the foreign body response-part I: theoretical framework. J Diabetes Sci Technol. 2011;5(3):632-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4:e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling acute inflammation with fast releasing dexamethasone-PLGA microsphere/pva hydrogel composites for implantable devices. J Diabetes Sci Technol. 2007;1(1):8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J Biomed Mater Res. 2002;61(2):180-187. [DOI] [PubMed] [Google Scholar]
- 42. Klueh U, Kaur M, Montrose DC, Kreutzer DL. Inflammation and glucose sensors: use of dexamethasone to extend glucose sensor function and life span in vivo. J Diabetes Sci Technol. 2007;1(4):496-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol Ther. 2004;6(6):887-897. [DOI] [PubMed] [Google Scholar]
- 44. Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117(1):68-79. [DOI] [PubMed] [Google Scholar]
- 45. Vallejo-Heligon SG, Brown NL, Reichert WM, Klitzman B. Porous, Dexamethasone-loaded polyurethane coatings extend performance window of implantable glucose sensors in vivo. Acta Biomater. 2016;30:106-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y, Papadimitrakopoulos F, Burgess DJ. Polymeric “smart” coatings to prevent foreign body response to implantable biosensors. J Control Release. 2013;169(3):341-347. [DOI] [PubMed] [Google Scholar]
- 47. Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J Biomed Mater Res A. 2007;81(4):858-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Klueh U, Qiao Y, Czajkowski C, Ludzinska I, Antar O, Kreutzer DL. Basement membrane-based glucose sensor coatings enhance continuous glucose monitoring in vivo. J Diabetes Sci Technol. 2015;9(5): 957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cha KH, Wang X, Meyerhoff ME. Nitric oxide release for improving performance of implantable chemical sensors - a review. Appl Mater Today. 2017;9:589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wo Y, Brisbois EJ, Bartlett RH, Meyerhoff ME. Recent advances in thromboresistant and antimicrobial polymers for biomedical applications: just say yes to nitric oxide (NO). Biomater Sci. 2016;4(8):1161-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ward WK, Slobodzian EP, Tiekotter KL, Wood MD. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials. 2002;23(21):4185-4192. [DOI] [PubMed] [Google Scholar]