Abstract
Few routinely available biomarkers are clinically useful in assessing dogs with chronic enteropathy (CE) and aid in CE subclassification. The diagnostic potential of the blood neutrophil-to-lymphocyte ratio (NLR) has not been evaluated in canine CE. We evaluated the NLR in 93 dogs with CE (no steroid treatment for ≥2 wk prior) and tested for an association with clinical, clinicopathologic, and histologic characteristics and also with CE subclassification. NLR was significantly higher in CE dogs with severe clinical disease than dogs with mild clinical disease (p = 0.047). Hypoalbuminemia (p < 0.001), but not hypocobalaminemia, was associated with higher NLRs. NLR was correlated with fecal alpha1-proteinase inhibitor concentrations (ρ = 0.47) and the serum-to-fecal alpha1-proteinase inhibitor ratio (ρ = –0.48; both p < 0.001) but not with serum or fecal inflammatory markers nor with the overall histologic score (all p > 0.05). Dogs with steroid- or other immunosuppressant-responsive (IRE) or nonresponsive enteropathy (NRE) had significantly higher NLRs (median: 7.3) than dogs with food-responsive enteropathy (FRE; median: 3.0; p = 0.003), and a NLR ≥5.5 best distinguished both groups of dogs. No difference in NLR was detected between dogs with IRE and dogs diagnosed with NRE. These findings suggest that leukogram changes (i.e., NLR) could be clinically useful in canine CE, and that neutrophils might play a role in the systemic inflammatory response associated with canine CE. The NLR can be easily assessed on routine hematology and can potentially aid in the subclassification of dogs with CE based on the response to treatment.
Keywords: canine, food-responsive enteropathy, inflammation, inflammatory bowel disease, immunosuppressant-responsive enteropathy, steroid-responsive enteropathy, stress leukogram
Introduction
Chronic enteropathies (CEs) comprise an important group of diseases in dogs, causing chronic gastrointestinal signs for ≥3 wk, with the common denominator of chronic inflammation that can affect some or all segments of the gastrointestinal tract.44 The pathogenesis of these conditions is complex, and an exaggerated immune reaction to luminal (i.e., dietary and/or microbial) antigens appears to play a role in the pathogenesis of CE.30
The diagnosis of CE requires exclusion of other diseases that can mimic CE (e.g., endoparasites, exocrine pancreatic insufficiency, atypical hypoadrenocorticism, gastrointestinal neoplasia).44 Further classification of CE is done retrospectively based on the response to sequential treatment trials as food-responsive enteropathy (FRE) if dogs respond to an elimination diet, antibiotic-responsive enteropathy (ARE) for dogs with a durable response to antibiotic treatment, steroid- of other immunosuppressant-responsive (IRE) or -refractory enteropathy (nonresponsive enteropathy, NRE) if corticosteroids and/or other immunosuppressive medications are needed.10,13 The existence of true ARE has been a matter of debate.10,13
Clinical, clinicopathologic, endoscopic, and histologic characteristics can be similar in dogs with different types of CE. The definitive diagnosis of CE requires histologic evaluation of endoscopic or surgical tissue biopsies of the intestine10 and, if necessary, also immunohistochemical or other molecular tests. Current consensus regarding the diagnostic algorithm for CE in dogs includes elimination dietary trials prior to more invasive testing.10,13 Thus, dogs with a complete and durable response to an elimination diet (i.e., dogs classified as FRE) might not undergo more invasive testing. Several biomarkers have been evaluated in dogs with CE, but few such markers that can be routinely measured (e.g., serum cobalamin and albumin, fecal calprotectin) appear to be of clinical utility in dogs with CE and can potentially aid in CE subclassification based on the response to treatment (i.e., diagnosis of FRE vs. IRE or NRE based on fecal calprotectin concentrations).23,24
Characteristic leukogram changes expressed as the neutrophil-to-lymphocyte ratio (NLR) have been shown to have diagnostic and prognostic potential in humans with inflammatory bowel disease (IBD),1,5 showing a correlation of the NLR with several clinical parameters and demonstrating that the NLR serves as a potentially useful prognostic marker in human IBD. The NLR also appears to be useful in diagnosing dogs with hypoadrenocorticism46 and has been evaluated in canine atopic dermatitis,35 septic and noninfectious systemic inflammatory response syndrome,27 and various types of cancer.14,34,36,39,41 However, the NLR has not been evaluated in dogs with CE. Thus, we aimed to evaluate the clinical utility of the NLR as a diagnostic marker in dogs with CE. We hypothesized that the NLR correlates (1) with clinical, laboratory, and histological parameters, and (2) with the response to different forms of treatment (i.e., disease subclassification: FRE vs. IRE or NRE) in dogs with CE. Our secondary aim was to determine a reference interval for NLR from healthy controls.
Materials and methods
Sample population
We included data from 93 dogs diagnosed with CE (Fig. 1) enrolled at 2 different veterinary centers: the Gastrointestinal Laboratory at the Small Animal Veterinary Teaching Hospital at Texas A&M University (TAMU, College Station, TX, USA; 2008–2015, cases from the TAMU Small Animal Veterinary Teaching Hospital or other tertiary veterinary centers across the United States) and the Department for Small Animals at the University of Leipzig (UL, Germany; 2013–2018). Data from some of the dogs were reported previously.23
Figure 1.
Inclusion flowchart for evaluation of the blood neutrophil-to-lymphocyte ratio (NLR) as a diagnostic marker in dogs with chronic enteropathy. Of the 287 dogs considered for inclusion in the study, we included 93 dogs. I. Data from all 93 dogs were included in the first part of the study (I.a–c), but prospective canine chronic enteropathy clinical activity index [CCECAI] scores (I.b) and histologic lesion scores (I.c) were only available from a subset of those dogs. II. Complete follow-up information used for the second part of the study (II) was available from 41 dogs.
The collection of specimens from dogs with CE for the study of several biomarkers was approved by the Clinical Research Review Committee (CRRC approval TAMU 2009-06, approved 01-15-2009), the Institutional Animal Care and Use Committee at TAMU (IACUC approval TAMU 2012-083, approved 05-22-2012), and the Veterinary Authority of the German Free State of Saxony (TVV 06/17, approved 04-11-2017). Written informed consent was obtained from the owner of each dog that was prospectively enrolled in the first part of the study (correlation analyses: part I.a–c, n = 69; Fig. 1). A standard study questionnaire completed by the owner and/or the attending veterinarian was used to evaluate each dog’s health, medication (including preventive drugs and vaccinations), and dietary history (Suppl. Table 1); a modified questionnaire translated into German was used for dogs prospectively enrolled at UL and included in part I.a–c of the study (n = 5). Follow-up information from cases enrolled at TAMU (part II; n = 17) included completing a follow-up study questionnaire (Suppl. Table 2). Data extracted from the standard patient medical records at the UL College of Veterinary Medicine were used for the additional dogs with CE that were retrospectively included in the second part of the study (follow-up for subclassification of CE: part II, n = 24; Fig. 1).
As an inclusion criterion, dogs must have been diagnosed with CE based on the current World Small Animal Veterinary Association (WSAVA) consensus statement.10,44 Dogs were excluded from further consideration in our study if they had received corticosteroids and/or other anti-inflammatory or immunosuppressant medication within 2 wk before diagnostic evaluation or if the information about previous administration of such medications was not available.
Dogs prospectively included in the first part of the study (Fig. 1) had a canine chronic enteropathy clinical activity index (CCECAI) score calculated at first presentation by either a veterinarian or veterinary staff as a semi-objective determination of the clinical disease severity (part I.b; n = 65).2 The CCECAI evaluates 9 clinical parameters: attitude or activity, food intake, frequency of vomiting, frequency of defecation, stool consistency, weight loss, pruritus, serum albumin concentration, and peripheral edema or ascites. Individual criteria are graded from 0 to 3 (0 = normal, 1 = slightly abnormal, 2 = moderately abnormal, and 3 = severely abnormal), and the cumulative CCECAI score can range from 0 to 27 (cumulative score: 0–3 = clinically insignificant disease; 4–5 = mild disease; 6–8 = moderate disease; 9–11 = severe disease; ≥12 = very severe disease).2 Single whole blood (EDTA was used as an anticoagulant), serum, and urine specimens were collected from each dog at first presentation for diagnostic workup. Fecal samples (aliquots of ~1 g) were collected on 3 consecutive days. Endoscopic (n = 61) or surgical (n = 5) gastrointestinal tissue biopsies were obtained from 66 dogs (Fig. 1, part I.c).
Follow-up data (part II) were included from dogs (n = 41) that were clinically re-evaluated by the attending veterinarian (n = 36) or through telephone follow-up consultation with the referring veterinarian (n = 5) to determine whether dogs were ultimately diagnosed with FRE or IRE or NRE. Diagnosis of FRE was based on the complete clinical response to an elimination diet trial using a novel protein and/or hydrolyzed protein diet or an ultra-low-fat diet,10,37 with a minimum follow-up time of 4 wk. Dogs that had histologic evidence of gastroenteritis and required anti-inflammatory and/or immunosuppressive treatment were categorized as IRE or NRE.10 Dogs were classified as IRE if the CCECAI score at the time of follow-up decreased by ≥75% or if clinical signs resolved (complete responders),23 with a minimum follow-up time of 4 wk. Dogs were also classified as IRE if there was only a partial clinical response (minimum follow-up time of 3 wk) as assessed by a change in CCECAI scores <25% or lack of complete resolution of clinical signs (partial responders).23 Dogs in the NRE group showed no clinical response to treatment (minimum follow-up time of 7 d) as assessed by a change in CCECAI scores <25% or no clinical improvement.23
Data from 44 healthy pet dogs enrolled in a blood donor program and presented at UL (2012–2019) for their annual check-up and vaccination, or pre-screening for inclusion in the blood donor program, served as a retrospective control group to establish a reference interval (RI) for NLR.
Sample analyses
Whole blood was used for routine hematology, which was performed at the Texas Veterinary Medical Diagnostic Laboratory (Cell-Dyn; Abbott), for cases enrolled at TAMU, or the Small Animal Veterinary Diagnostic Laboratory at the UL College of Veterinary Medicine (ProCyte Dx; Idexx), for dogs enrolled at UL. Neutrophil counts (RIs: TAMU = 3.0–11.5 × 109/L, UL = 3.0–11.6 × 109/L) and lymphocyte counts (RIs: TAMU = 1.0–4.8 × 109/L, UL = 1.0–5.1 × 109/L) were extracted from the routine hematologic profile, and the NLR was calculated as (neutrophil count)/(lymphocyte count).
Serum (fasted sample) was used for a serum chemistry profile (LiquiColor, Sirrus clinical chemistry analyzer, Stanbio Laboratory for TAMU cases; Fuji DRI-CHEM NX500i, Scil for UL cases) and for measurement of serum cobalamin (Immulite 2000, vitamin B12; Siemens), folate (Immulite 2000, folic acid; Siemens), canine-specific pancreatic lipase (Spec cPL assay; Idexx), and C-reactive protein (Phase CRP; Tri-Delta) concentrations. Serum concentrations of the pro-inflammatory molecules calprotectin (RI: 0.6–11.8 mg/L) and S100A12 (RI: 49–320 μg/L), as well as the anti-inflammatory decoy receptor sRAGE (soluble receptor for advanced glycation end products), were determined by species-specific ELISAs (only TAMU cases).20,22,25
Fecal concentrations of calprotectin (preliminary RI: 3.2–18.4 μg/g) and S100A12 (RI: 2–484 ng/g) were also measured by species-specific ELISAs (only TAMU cases),22,25 and the 3-d mean fecal concentrations of both analytes were calculated18 and used for statistical analyses. Alpha1-proteinase inhibitor (α1PI) concentrations in serum (RI: 732–1,800 mg/L), fecal extracts (RI for the 3-d sample mean: 2.2–13.9 μg/g), and the serum-to-fecal α1PI ratio were determined as markers of gastrointestinal protein loss (only TAMU cases).17,19,21
Urine samples were used for routine urinalysis (performed at the TAMU Clinical Pathology Laboratory for cases enrolled at TAMU or the Small Animal Veterinary Diagnostic Laboratory at UL for dogs enrolled from UL) and, if indicated, for further testing (i.e., urine culture, urine protein-to-creatinine ratio).
Histologic evaluation of gastrointestinal tissue biopsies was performed by 1 of 8 board-certified pathologists using the WSAVA Gastrointestinal Standardization grading system.11,44 The severity of structural and inflammatory lesions in the duodenum, ileum, and colon were recorded based on a 4-point grading scheme (0 = normal, 1 = mild lesions, 2 = moderate lesions, 3 = severe lesions; Suppl. Table 3),11,44 and the maximum and mean cumulative lesion scores (calculated as the sum of individual lesion scores from each segment) were considered for statistical analysis. Gastric biopsies were evaluated to exclude neoplasia.3
Statistical analyses
Data were analyzed for the assumptions of normality and equal variances using a Shapiro–Wilk W and Levene test, respectively. Summary statistics for continuous variables are presented as medians and interquartile ranges (IQRs), and for categorical data are reported as counts (n) and percentages. Nonparametric group comparisons of continuous variables were performed using a Wilcoxon rank-sum test (2 groups, unpaired data) or a Kruskal–Wallis test (≥3 groups, unpaired data) with a Dunn post-hoc test for joint ranking. Associations between continuous variables were tested by calculating a Spearman correlation coefficient ρ. Multivariate analyses using a standard least squares model for selected variables served to test for a confounding effect using 2 different hematology analyzers in our study. The null hypothesis was rejected whenever p ≤ 0.05, and a Holm sequential Bonferroni correction28 was applied for multiple comparisons of paired data, with consideration of the numbers of categories (pcorr = unadjusted p × [n − k + 1], where n is the number of hypotheses tested, and k is the ordered rank of the uncorrected p values). Receiver operating characteristic (ROC) curves were constructed, and the likelihood ratio was used to determine the optimal cutoff for calculating the sensitivity and specificity of the NLR to distinguish dogs based on dichotomous outcomes. A commercial statistical software package (JMP v.13.1.0; SAS Institute) was used for all statistical analyses.
A RI for the NLR in dogs was established using the robust method after Box–Cox transformation (Reference Value Advisor v.2.1; http://www.biostat.envt.fr/reference-value-advisor) of the single-sample NLR (using the ProCyte Dx) determined in the 44 healthy adult pet dogs that were of various breeds and ages (median age: 4.2 y, range: 1.0–9.3 y; 28 males, 16 females).15,16
Results
The NLR was 0.2–54.0 (median: 5.5) in the 93 dogs with CE included in our study (Table 1); NLRs varied within this group of dogs, but most dogs had an NLR <10. Age was moderately correlated with the magnitude of the NLR (Table 2). Most dogs with CE had a normal neutrophil count (n = 68; 73%) and also a normal lymphocyte count (n = 69; 74%). The most common abnormality for each cell count was neutrophilia (n = 22; 24%) and lymphopenia (n = 23; 25%), respectively. Mild neutropenia was seen in 3 dogs (3%), and lymphocytosis in 1 dog (1%).
Table 1.
Characteristics of 93 dogs with chronic enteropathy included in our study.
| Group characteristic | n | Value |
|---|---|---|
| Age (y), median | 93 | 6.4 (3.8–9.0) |
| Sex, male/female | 93 | 54/39 |
| Bodyweight (kg), median | 93 | 19.0 (7.2–30.6) |
| Breed, n | 93 | |
| Purebred dogs | 77 (83%) | |
| German Shepherd dog | 10 (11%) | |
| Yorkshire Terrier | 5 (6%) | |
| West Highland White Terrier | 4 (4%) | |
| Mixed-breed dogs | 16 (17%) | |
| CCECAI score, median | 65 | 8 (5–10) |
| Clinical disease severity, n | ||
| Mild clinical disease (CCECAI score: 4–5) | 19 (29%) | |
| Moderate clinical disease (CCECAI score: 6–8) | 17 (26%) | |
| Severe clinical disease (CCECAI score: 9–11) | 19 (29%) | |
| Very severe clinical disease (CCECAI score: ≥12) | 10 (16%) | |
| Serum albumin concentration (g/L), median | 91 | 27 (20–32) |
| Hypoalbuminemia (<24 g/L), n | 37 (41%) | |
| Mild hypoalbuminemia (15–20 g/L) | 12 (13%) | |
| Moderate hypoalbuminemia (12–14.9 g/L) | 4 (4%) | |
| Severe hypoalbuminemia (<12 g/L) | 5 (5%) | |
| Serum cobalamin concentration (ng/L), median | 84 | 345 (205–609) |
| Hypocobalaminemia (<221 pmol/L [<300 ng/L]), n | 33 (39%) | |
| Serum folate concentration (µg/L), median | 79 | 12.2 (9.3–16.8) |
| Hypofolatemia (<17.5 nmol/L [<7.7 μg/L]), n | 12 (15%) | |
| Hyperfolatemia (>55.5 nmol/L [>24.4 μg/L]), n | 2 (3%) | |
| Neutrophil count (×109/L), median | 93 | 6.98 (4.84–11.26) |
| Lymphocyte count (×109/L), median | 1.30 (1.01–2.04) | |
| Neutrophil-to-lymphocyte ratio (NLR), median | 5.5 (3.0–11.0) | |
| Serum CRP concentration (mg/L), median | 61 | 4.5 (1.0–31.8) |
| Fecal calprotectin concentration (µg/g), median | 35 | 12.7 (0.3–67.4) |
| Serum calprotectin concentration (mg/L), median | 28 | 5.6 (4.0–7.7) |
| Fecal S100A12 concentration (ng/g), median | 43 | 226 (17–1,047) |
| Serum S100A12 concentration (µg/L), median | 53 | 162 (109–250) |
| Fecal α1PI concentration (µg/g), median | 55 | 12.2 (5.8–31.6) |
| Serum α1PI concentration (mg/L), median | 59 | 1,185 (953–1,523) |
| Serum Spec cPL concentration (µg/L), median | 81 | 72 (35–233) |
CCECAI = canine chronic enteropathy clinical disease activity index2; CRP = C-reactive protein; Spec cPL = specific canine pancreatic lipase.
Numbers in parentheses are interquartile ranges unless indicated otherwise by a percentage sign.
Table 2.
Association between neutrophil-to-lymphocyte ratio (NLR) and demographic and clinicopathologic parameters in 93 dogs with chronic enteropathy.
| Parameter correlated with NLR | Spearman ρ | p value | pcorr value |
|---|---|---|---|
| Patient characteristics | |||
| Age | 0.40 | <0.0001 | <0.0003 |
| Body weight | −0.01 | 0.9432 | NS |
| Clinical disease severity (CCECAI score) | 0.41 | 0.0008 | 0.0024 |
| Clinicopathologic variables | |||
| Biomarkers of gastrointestinal tract function | |||
| Serum cobalamin concentration | −0.18 | 0.109 | NS |
| Serum folate concentration | −0.11 | 0.327 | NS |
| Biomarkers of systemic inflammation or pancreatic disease | |||
| Serum CRP concentration | 0.26 | 0.0478 | 0.0956 |
| Serum calprotectin concentration | 0.30 | 0.120 | NS |
| Serum S100A12 concentration | 0.29 | 0.0323 | 0.0969 |
| Serum sRAGE concentration | −0.43 | 0.0344 | 0.138 |
| Serum Spec cPL concentration | 0.52 | <0.0001 | <0.0005 |
| Biomarkers of gastrointestinal inflammation | |||
| Fecal calprotectin concentration | 0.26 | 0.139 | NS |
| Fecal S100A12 concentration | 0.24 | 0.119 | NS |
| Biomarkers of gastrointestinal protein loss | |||
| Serum albumin concentration | −0.36 | 0.0005 | 0.0010 |
| Fecal α1PI concentration | 0.47 | 0.0003 | 0.0009 |
| Serum α1PI concentration | −0.10 | 0.451 | NS |
| Serum-to-fecal α1PI ratio | −0.48 | 0.0003 | 0.0012 |
α1PI = alpha1-proteinase inhibitor; CCECAI = canine chronic enteropathy clinical activity index2; CRP = C-reactive protein; Spec cPL = specific canine pancreatic lipase; NS = not significant; pcorr = Holm–Bonferroni corrected p value (n = 2, 3, or 4); sRAGE = soluble receptor of advanced glycation end products.
In the healthy control group (n = 44), the NLR range was 1.0–4.7 (median: 2.0). The RI for the NLR was 1.0–4.1, and the NLRs were also moderately correlated with age (ρ = 0.47, p = 0.001) in healthy dogs. Applying the upper reference limit of 4.1, an increased NLR (>4.1) was detected in 57 of 93 (61%) dogs with CE.
Association of NLR with the severity of clinical disease and histological lesions
NLR was moderately positively correlated with CCECAI scores (Table 2) and differed significantly (p = 0.026) among all 4 disease severity groups (Table 3, Fig. 2A). Dogs with very severe clinical disease (CCECAI score ≥12) had significantly higher NLRs compared to dogs with mild clinical disease (p = 0.047).
Table 3.
Association of neutrophil-to-lymphocyte ratio (NLR) with clinical and histologic disease severity in 91 dogs with chronic enteropathy.
| Parameter | NLR, median | p value |
|---|---|---|
| Clinical disease severity (CCECAI score; n = 65) | ||
| Mild clinical disease (CCECAI score: 4–5) | 3.7 (2.2–7.0) | 0.026 |
| Moderate clinical disease (CCECAI score: 6–8) | 3.7 (2.3–8.3) | |
| Severe clinical disease (CCECAI score: 9–11) | 4.9 (4.6–13.1) | |
| Very severe clinical disease (CCECAI score: ≥12) | 12.7 (5.7–33.8) | |
| Histologic lesion severity | ||
| Mild histologic lesions (grade 1) | 4.6 (1.9–9.6) | 0.265 |
| Moderate histologic lesions (grade 2) | 6.9 (3.9–9.4) | |
| Severe histologic lesions (grade 3) | 5.3 (3.3–17.3) | |
| Presence and severity of lacteal dilation and/or crypt abscesses | ||
| Mild histologic lesions (grade 1) | 5.2 (2.1–10.6) | 0.285 |
| Moderate histologic lesions (grade 2) | 5.8 (3.1–9.4) | |
| Severe histologic lesions (grade 3) | 6.9 (4.0–11.6) | |
| Prognostic clinicopathologic variables | ||
| Normoalbuminemia | 3.7 (2.6–8.3) | 0.0007 |
| Hypoalbuminemia | 7.1 (4.9–15.7) | |
| Serum albumin concentration >20 g/L | 4.5 (2.7–7.7) | <0.0001 |
| Serum albumin concentration 15–20 g/L | 8.0 (6.1–17.0) | |
| Serum albumin concentration 12–14.9 g/L | 9.2 (6.5–11.3) | |
| Serum albumin concentration <12 g/L | 22.8 (18.4–38.2) | |
| Normocobalaminemia* | 5.7 (2.9–10.5) | 0.312 |
| Hypocobalaminemia† | 5.9 (3.7–13.4) | |
CCECAI = canine chronic enteropathy clinical disease activity index2.
Numbers in parentheses are interquartile ranges.
Defined as a serum cobalamin concentration ≥300 ng/L.
Defined as a serum cobalamin concentration <221 pmol/L (<300 ng/L).
Figure 2.
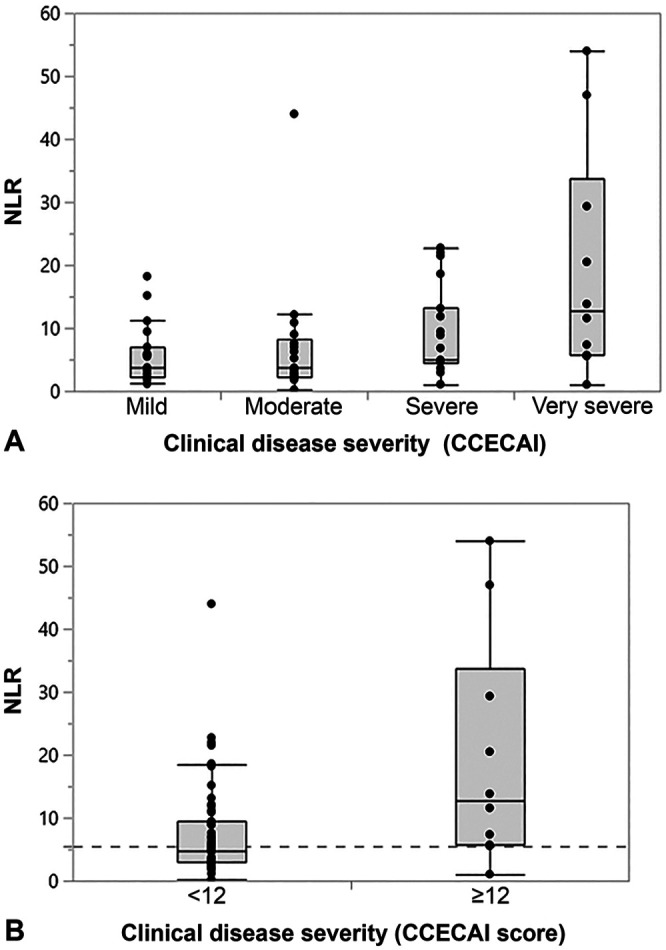
Association of neutrophil-to-lymphocyte ratio (NLR) with the severity of chronic enteropathy in 93 dogs. A. The NLR differed significantly among the disease severity groups (p = 0.0263). Dogs with very severe clinical disease (canine chronic enteropathy clinical activity index [CCECAI] score ≥12) had significantly higher NLRs than dogs with mild clinical signs (p = 0.0468). No significant differences were detected among other of these subgroups of dogs. B. The NLR was significantly higher in dogs with very severe clinical signs (CCECAI score ≥12; median: 12.7, interquartile range [IQR]: 5.7–33.8) than in dogs with a CCECAI score <12 (median: 4.9, IQR: 2.9–9.4; p = 0.0138), with an optimal cutoff NLR of 5.5 (dashed gray line) to distinguish the 2 groups of dogs.
NLR was strongly correlated with the blood neutrophil count in the group of dogs with a CCECAI score ≥12 (ρ = 0.88, p = 0.001) and also in the group of dogs with a CCECAI score < 12 (ρ = 0.72, p < 0.0001), and was strongly but inversely correlated with the lymphocyte count in both groups of dogs (ρ = –0.72, p = 0.018; ρ = –0.71, p < 0.0001, respectively). An NLR of 5.5 was the optimal cutoff for the dichotomous distinction of dogs with very severe clinical signs (CCECAI score ≥ 12) from dogs with a CCECAI score < 12 (Fig. 2B), with a sensitivity of 90% and a specificity of 58% (area under the ROC curve [AUC]: 75%).
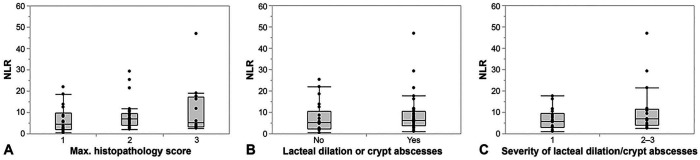
NLR was not correlated with the mean cumulative histologic lesion score from all intestinal segments evaluated (ρ = 0.07, p = 0.597) nor was it associated with the maximum histologic lesion severity (Fig. 3A, Table 3). There was also no correlation between the amount of neutrophilic infiltration in any of the intestinal segments and the NLR nor the blood neutrophil count (all p > 0.05). However, none of the dogs had a polymorphonuclear-dominated inflammatory infiltrate in any of the intestinal segments evaluated.
Figure 3.
Association of neutrophil-to-lymphocyte ratio (NLR) with the severity of histologic lesions in 66 dogs. A. The NLR did not differ among dogs with chronic enteropathy with a maximum histologic severity grade interpreted as either mild, moderate, or severe (p = 0.265). B. NLRs were only slightly higher in dogs with histologic lesions typically seen with protein-losing enteropathy (PLE) compared to dogs with no such lesions and C. in dogs with more severe lesions of PLE compared to dogs with mild lacteal dilation and/or crypt abscesses. However, both differences did not reach statistical significance (p = 0.300 and p = 0.198).
Magnitude of the NLR was slightly higher in dogs with microscopic lesions typically seen in dogs with protein-losing enteropathy (PLE; i.e., lacteal dilation and/or crypt abscesses), and particularly in dogs with more severe such lesions (histologic lesion score ≥2). However, these associations were not statistically significant (Fig. 3B, 3C; Table 3). Neither NLRs nor neutrophil or lymphocyte counts differed between dogs with characteristics of a PLE [i.e., (1) moderate or severe lacteal dilation and/or crypt abscesses, and/or (2) serum albumin concentration <20 g/L) and those without (p = 0.384, 0.062, and 1.000, respectively)].
Association of NLR with prognostic clinicopathologic factors
A weak inverse correlation was detected between NLR and serum albumin concentration (Table 2). Dogs with hypoalbuminemia (defined as a serum albumin concentration <24 g/L) had significantly higher NLRs than normoalbuminemic dogs (Table 3, Fig. 4A), with a sensitivity of 78% and a specificity of 61% (AUC = 71%) to distinguish hypoalbuminemic dogs with CE from those dogs with normoalbuminemia when using a cutoff NLR of 4.9. Severe hypoalbuminemia (serum albumin concentration <12 g/L) was associated with significantly higher NLRs than having a serum albumin concentration ≥12 g/L (Table 3, Fig. 4B). Hypoalbuminemia was not associated with hyperglobulinemia (p = 0.742), and there was a positive correlation between serum albumin and serum globulin concentrations (ρ = 0.63; p < 0.0001).
Figure 4.
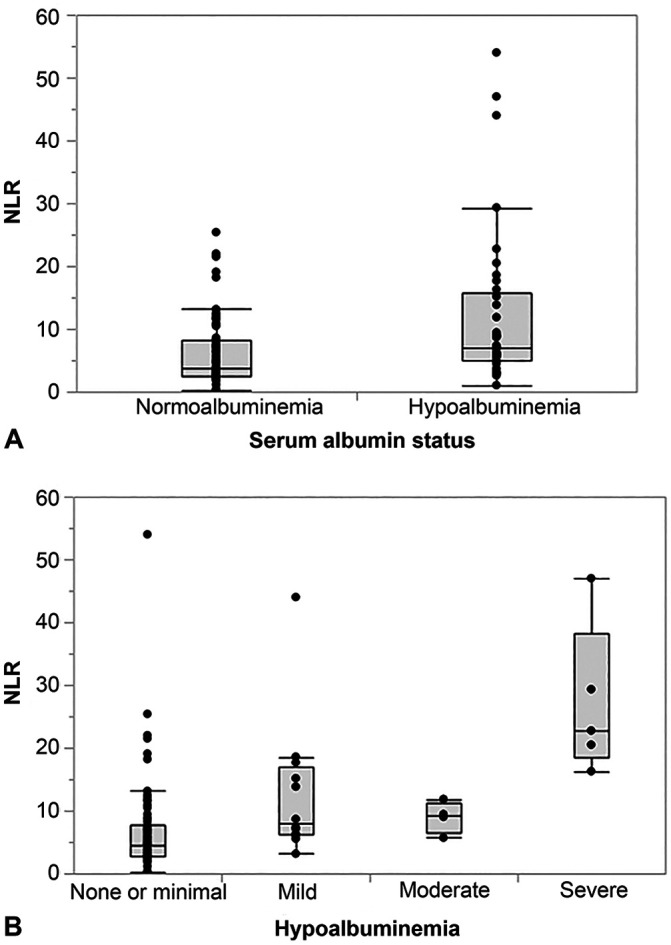
Association of the serum albumin status and neutrophil-to-lymphocyte ratio (NLR) in 91 dogs with chronic enteropathy. A. Dogs with hypoalbuminemia (serum albumin concentration <24 g/L) had significantly higher NLRs (median: 7.1, interquartile range [IQR]: 4.9–15.7) compared to dogs with normoalbuminemia (median: 3.7, IQR: 2.6–8.3; p = 0.0007). B. The NLR differed significantly among the hypoalbuminemia severity groups (p < 0.0001). Dogs with none or minimal hypoalbuminemia (serum albumin concentration >20 g/L; median: 4.5, IQR: 2.7–7.7) had significantly lower NLRs compared to dogs with mild (serum albumin concentration 15–20 g/L; median: 8.0, IQR: 6.1–17.0; p = 0.0254) or very severe (serum albumin concentration <12 g/L; median: 22.8, IQR: 18.4–38.2; p = 0.0012) hypoalbuminemia. No significant differences were detected among any other of these subgroups of dogs.
No differences were detected between the NLRs in dogs with hypocobalaminemia (defined as a serum cobalamin concentration <221 pmol/L [<300 ng/L]31) and normocobalaminemic dogs (p = 0.312; Table 2, Fig. 5).
Figure 5.
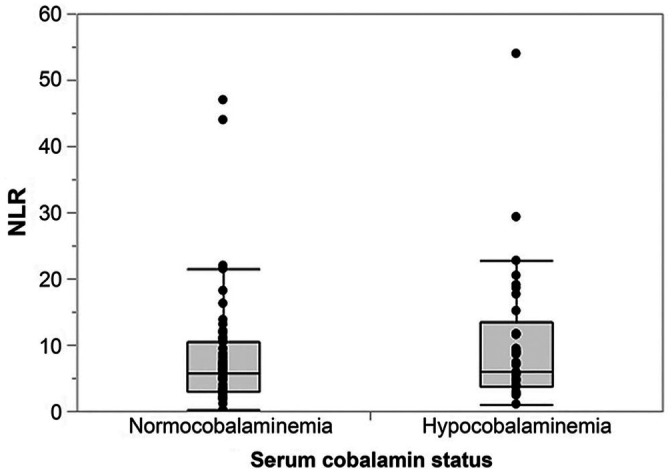
Association of serum cobalamin status and neutrophil-to-lymphocyte ratio (NLR) in 84 dogs with chronic enteropathy. No difference in NLR was detected between dogs with hypocobalaminemia (serum cobalamin concentration <221 pmol/L [<300 ng/L]; median: 5.9, interquartile range [IQR]: 3.7–13.4) and normocobalaminemic dogs (median: 5.7, IQR: 2.9–10.5; p = 0.312).
Association of NLR with biomarkers of inflammation and gastrointestinal protein loss
Of the circulating biomarkers of inflammation that we evaluated, serum CRP and S100A12 concentrations were weakly positive, and serum sRAGE was moderately negatively correlated with the NLR in univariate analysis, but there was no statistical significance after correction for multiple comparisons (Table 2).
Fecal calprotectin and S100A12 concentrations were also not significantly correlated with the corresponding NLR, but serum Spec cPL concentration was moderately correlated with NLR (Table 2). In addition to the serum albumin concentration, fecal α1PI concentrations (positively) and the serum-to-fecal α1PI ratio (inversely) were moderately correlated with the NLR (Table 2).
Neutrophil counts, but not lymphocyte counts, were correlated with serum S100A12 (ρ = 0.50, p = 0.0001), calprotectin (ρ = 0.48, p = 0.0096), and CRP concentrations (ρ = 0.30, p = 0.018) and with fecal S100A12 (ρ = 0.33, p = 0.0328) and calprotectin concentrations (ρ = 0.39, p = 0.0225) in dogs with CE.
Association of NLR with CE subclassification
A diagnosis of FRE was established in 12 dogs, and 29 dogs were categorized as IRE or NRE. The follow-up time was 29–2,945 d (median: 171 d) in dogs with FRE, and 7–586 d (median: 69 d) in dogs with IRE or NRE. Dogs with FRE received either a hydrolyzed diet (n = 8, of which 3 dogs were initially given a novel protein diet), a novel protein diet (n = 3), or an ultra-low-fat diet (n = 1); 3 of the dogs had also received antimicrobial treatment (metronidazole and/or tylosin). Monotherapy with prednisone or prednisolone was most common (n = 22) in dogs with IRE or NRE, followed by the combination of prednisolone with cyclosporine or chlorambucil (n = 4); 2 dogs were treated with budesonide (n = 2) and 1 dog with cyclosporine (n = 1). Sixteen dogs with IRE or NRE underwent 1 dietary trial (with a novel protein, hydrolyzed protein, or ultra-low-fat diet), and 13 dogs had at least 2 dietary trials performed; 18 of the dogs had received antimicrobial treatment (metronidazole and/or tylosin). In the group of dogs classified as IRE or NRE, clinical response to immunomodulatory treatment could be reliably assessed in 24 dogs: 12 dogs (50%) entered clinical remission, 6 dogs (25%) showed a partial response, and 6 dogs (25%) did not respond to treatment. Thus, 18 dogs (75%) were classified as IRE and 6 dogs (25%) as NRE.
CCECAI scores were higher in dogs with IRE or NRE (median: 9, IQR: 6–11) than in dogs with FRE (median: 6, IQR: 3–7), but the difference did not reach statistical significance (p = 0.0991). CCECAI scores were also not significantly different between the IRE and NRE group of dogs (p = 0.2393). Dogs with IRE or NRE were older (median: 7.6 y, IQR: 3.5–9.8 y) than dogs with FRE (median: 4.3 y, IQR: 1.5–8.3 y), and dogs classified as NRE were older (median: 9.8 y, IQR: 6.8–12.2 y) than dogs with IRE (median: 6.9 y, IQR: 3.0–9.4 y), but both differences were not significant (p = 0.0691 and p = 0.0891, respectively).
Neutrophil counts were significantly higher in dogs with IRE or NRE than in dogs with FRE (p = 0.0080), whereas lymphocyte counts were not significantly different between FRE and IRE or NRE dogs (p = 0.0882). Dogs diagnosed with IRE or NRE had significantly higher NLRs (p = 0.0033) than those that were classified as having FRE (Fig. 6). This difference remained if dogs with characteristics of a PLE [i.e., 1) moderate or severe lacteal dilation and/or crypt abscesses, and/or 2) serum albumin concentration <20 g/L] were excluded from the analysis (p = 0.0326) and also if dogs with serum Spec cPL concentration ≥400 μg/L (consistent with a diagnosis of pancreatitis) were excluded from the analysis (p = 0.0492). An NLR of 5.5 was determined to be the optimal cutoff for the separation of dogs with IRE or NRE and dogs diagnosed with FRE, with a sensitivity of 76% and a specificity of 83% (AUC = 80%). Ten dogs (83%) with FRE had a NLR < 5.5, and 22 dogs (76%) with IRE or NRE had a NLR ≥ 5.5.
Figure 6.
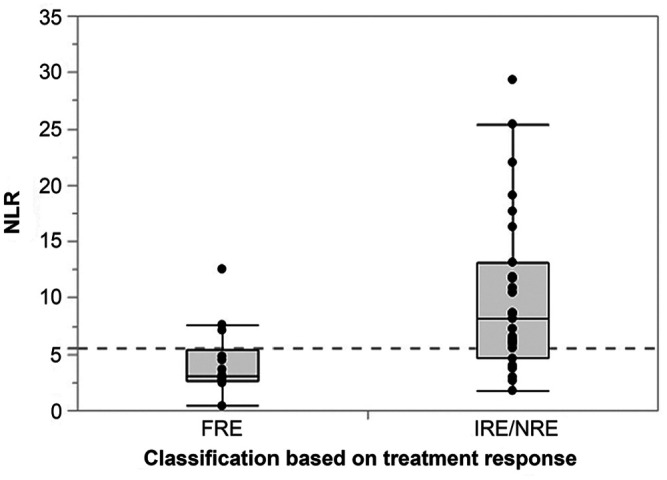
Disease subclassification and neutrophil-to-lymphocyte ratio (NLR) in 41 dogs with chronic enteropathy. Dogs that were diagnosed with steroid- or other immunosuppressant-responsive or -refractory enteropathy (IRE or NRE, n = 29) had significantly higher NLRs (median: 7.3, interquartile range [IQR]: 5.1–12.5, range: 1.8–29.3) than dogs that were classified as food-responsive (FRE, n = 12; median: 3.0, IQR: 2.7–4.7, range: 0.4–12.5; p = 0.0033), with an optimal cutoff NLR of 5.5 (dashed gray line) to distinguish the 2 groups of dogs.
There was no difference in the NLR between dogs with IRE (median: 7.7, IQR: 5.3–12.1, range: 1.8 –29.3; n = 18) and dogs with NRE (median: 6.2, IQR: 3.7–16.6, range: 3.0–17.7; n = 6; p = 0.6648). NLR was ≥5.5 in 14 dogs with IRE (78%) and 4 dogs with IRE or NRE (67%). There were no differences in neutrophil or lymphocyte counts, or any other clinicopathologic or histologic variables between dogs with IRE and those dogs classified as NRE (all p > 0.05).
Investigation for a confounding effect of the different hematology analyzers used at TAMU and UL on variables that affect NLR (CCECAI score, hypoalbuminemia, disease subclassification), neutrophil count (CCECAI score, disease subclassification), and lymphocyte count (CCECAI score) revealed no confounding effect on the association between CCECAI score and NLR (p = 0.521), neutrophil count (p = 0.250), and lymphocyte count (p = 0.0608); between hypoalbuminemia and NLR (p = 0.504); and between disease subclassification and NLR (p = 0.399) and neutrophil count (p = 0.498).
Discussion
Dogs in our study appeared to have only slightly higher NLR values and a larger variation of the NLRs than human patients with active IBD.1,5 The NLR was strongly correlated with the neutrophil count in dogs with CE and very severe clinical disease. In contrast, the correlation between NLR and lymphocyte counts in clinically severely affected dogs, and the correlations in dogs with less severe clinical signs, were only moderate. This might explain the slightly higher NLRs (calculated as the ratio of both variables) in dogs with CE compared to people.1,5 Further, it suggests species-specific differences in leukogram changes. However, interleukin-8 (the primary regulator of neutrophil responses and recruitment4) is not dysregulated in canine CE.6
CE is characterized by changes localized to the intestinal wall. However, this condition can also be associated with systemic effects, such as an increase in inflammatory markers (e.g., serum CRP concentration) or hypoalbuminemia (e.g., from PLE). Characteristic leukogram changes, reflected by an increase in the NLR, in dogs with CE in our study suggest that canine CE is associated with systemic stress (stress leukogram) and/or inflammatory response (inflammatory leukogram). This could reflect an increased production of endogenous cortisol associated with chronic inflammation, and it suggests that neutrophils might play a role in the systemic inflammatory response associated with canine CE. Neutrophils are an essential element of innate immunity and, beyond immune surveillance, are essential to bridge innate and adaptive (T cell–mediated) immune responses. Increased neutrophil counts in dogs with CE could also result from TNF-α– or NF-κB–mediated inhibition of neutrophil apoptosis,6,33 interleukin-17–mediated increase in granulopoiesis,43 increased recruitment and decreased clearance associated with chronic sterile inflammation,12,26 or TLR4 ligand–mediated platelet activation.8 In line with this, TLR4 ligands calprotectin and S100A12 were also correlated with the neutrophil counts (but not NLR) in our study. Also, despite canine CE (and particularly IRE or NRE) being a diagnosis by exclusion, an effect of comorbidity on increased NLR values (e.g., concurrent pancreatitis) cannot be excluded in all of the dogs in our study. Increased serum Spec cPL concentrations (compatible with a diagnosis of pancreatitis) were also associated with increased NLRs and with a diagnosis of IRE or NRE (p = 0.0060) and have been shown previously to be a negative prognostic factor in dogs with CE.32 In our study, dogs included were not allowed to have received treatment with a steroid or other anti-inflammatory or immunosuppressant medication for ≥2 wk before diagnostic evaluation and inclusion. Therefore, the leukogram changes seen (i.e., increased NLR) cannot be explained by the administration of exogenous steroids. However, stress associated with the hospital visit cannot be excluded as a potential confounding factor.
There was moderate correlation between the NLR and the clinical severity of CE as assessed by the CCECAI score in the dogs in our study, but there was also a significant degree of overlap in the NLR between various CCECAI scores. This finding agrees with the results of a study in people,1 showing that the NLR is associated with the clinical activity of Crohn disease. The cutoff to distinguish dogs with a CCECAI score > 12 from dogs with mild or moderate clinical signs (NLR of 5.5) was also higher than those cutoffs determined in humans (Crohn disease: 3.2, ulcerative colitis: 3.1) to distinguish patients with active IBD from patients in clinical remission.1
Lymphoplasmacytic enteritis is the most common histologic form of canine CE, and severe inflammation can cause protein-losing enteropathy (PLE). We found no significant correlation between the NLR and the overall severity of histologic lesions (comprised of structural and inflammatory changes). NLRs only tended to be higher in dogs with histologic lesions characteristic of a PLE, i.e., lacteal dilation and/or crypt abscesses. This finding is interesting, given the inverse correlation of the NLR with serum albumin concentration and the serum-to-fecal α1PI ratio, the positive correlation between NLR and fecal α1PI concentrations in our study, and also the fact that hypoalbuminemia was previously shown to be a negative prognostic factor in dogs with CE.2 Lymphopenia is also a common finding in dogs with intestinal lymphangiectasia and PLE,9,13 and this could further contribute to an increase in the NLR in affected dogs. Lack of a significant relationship between the NLR and the presence of histologic lesions typically seen with PLE could be explained by the fact that lymphangiectasia can either be caused by idiopathic inflammation compromising lymphatic flow or obstructing lymphatic vessels within the intestinal mucosa, or can be a primary condition that in turn might result in intestinal mucosal infiltration of inflammatory cells.9,13 Hypocobalaminemia, another negative prognostic factor in dogs with CE,2,31 was not associated with a high NLR in our study. However, we cannot exclude the possibility of supplementation of cobalamin in all of the dogs before enrollment in the study.
Clinical severity of CE in dogs can be characterized by clinical, histologic, and endoscopic findings.10,44 Also, noninvasive variables such as serum CRP, fecal calprotectin, or fecal α1PI concentrations might be useful surrogate markers to diagnose, characterize, and/or monitor this condition.24 Weak-to-moderate correlations between the NLR and other biomarkers of systemic inflammation (i.e., serum S100A12 and CRP concentrations, serum sRAGE concentration) that did not remain significant after correction for multiple comparisons might indicate spatial and/or temporal differences in the expression of these markers. This is further supported by the lack of a correlation with fecal calprotectin and S100A12 concentrations as markers of gastroenteritis. Similar to our findings, there was no significant correlation between NLR and CRP in human patients with IBD.1
Eliminating a diagnosis of FRE as a cause of the chronic gastrointestinal signs requires thorough dietary elimination trials, which is time-consuming and can be frustrating for both the client and the veterinarian.10 Further patient evaluation, if dietary trials have failed, includes the histologic examination of gastrointestinal biopsies to document inflammation, further characterizing the histologic picture, and excluding a diagnosis of alimentary lymphoma.10,44 Thus, markers that can help differentiate IRE or NRE from FRE early in the diagnostic process of canine CE would be very useful in clinical practice. Such markers might also guide the clinician’s decision whether a dietary trial should be performed first or if further testing (i.e., intestinal biopsy) should be pursued, followed by a more aggressive approach to treatment. Our study showed that NLR values in dogs diagnosed with IRE or NRE were significantly higher and more variable than in dogs with FRE, and that an NLR of ≥5.5 detected dogs with IRE or NRE with moderate sensitivity and specificity. However, the possibility of an additive effect of concurrent pancreatitis cannot be excluded definitively. Thus, the NLR might be a further surrogate marker to help differentiate FRE from IRE or NRE. Lack of a difference in NLR between dogs with IRE and dogs classified as NRE in our study contrasts with the utility of the NLR to predict the response to immunomodulatory treatment in human patients with IBD.5 Further studies are warranted to determine whether NLR could be useful to differentiate between CE and alimentary lymphoma in dogs.
A significant correlation between NLR and the patient’s age was observed in dogs with CE and in healthy controls in our study. In contrast, the effect of age on NLR values was not seen in a previous study evaluating hematologic and serum biochemical variables in puppies.40 The age effect seen in our study might be explained by the increased probability of metabolic and inflammatory diseases with increasing age. An effect of breed on NLR has not been reported and cannot be evaluated based on the results of our study given the large number of canine breeds represented. Further, an effect of season on the neutrophil count has been demonstrated in dogs.42 The possibility of this effect on the NLR cannot be excluded entirely from our study, although we saw no seasonal differences in the NLRs among CE dogs and healthy controls (data not shown). Also, an effect of exercise, which has been demonstrated to cause an increase in both neutrophil and lymphocyte counts,29 appears to be unlikely.
We acknowledge that the clinical and partially retrospective nature of this investigation is a limitation. Another shortcoming of our study is that not all white blood cell counts were manually differentiated, thus inherent errors in automated cell counting cannot be excluded. For this reason, platelet counts or the platelet-to-lymphocyte ratios (PLRs) were also not evaluated. Furthermore, the study includes data obtained from 2 different hematology analyzers. A correlation and regression (Passing–Bablok and/or Deming) and/or agreement (kappa) analysis could have determined the agreement between both methods, but patient samples could not be tested on both machines. However, the RIs determined for the neutrophil (TAMU: 3.0–11.5 × 109/L; UL: 3.0–11.6 × 109/L) and lymphocyte (TAMU: 1.0–4.8 × 109/L, UL: 1.0–5.1 × 109/L) counts used for the 2 machines were almost identical, and multivariate analyses for selected variables detected no confounding effect for using 2 different hematology analyzers in our study. The severity of endoscopic lesions in the duodenum was also shown to be associated with a negative prognosis.2 However, we did not include or evaluate endoscopic lesion scores given the inclusion of dogs from several veterinary centers. Thus, the relationship between NLR and the severity of endoscopic lesions in dogs with CE warrants further study. Further, gastrointestinal biopsies were evaluated by several pathologists rather than a single pathologist. This presents a limitation of our study because of the known interobserver variability among pathologists evaluating intestinal biopsies,45 even using the WSAVA guidelines.11,44 The absence of ileal biopsies in some of the dogs (6 of 27 dogs [22%] in which a lower gastrointestinal endoscopy was performed) is another limitation of our study because histologic lesion severity can differ between intestinal segments (e.g., duodenum and ileum).7,38 Also, diffuse gastrointestinal neoplasia (e.g., alimentary lymphoma) cannot be ruled out in some of the dogs in our study in which gastrointestinal biopsies were not performed (e.g., with a complete response to an elimination diet). Lastly, the reduced numbers in some comparisons and the lack of complete information from some dogs are limitations of our clinical study.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_1040638721992057 for Blood neutrophil-to-lymphocyte ratio (NLR) as a diagnostic marker in dogs with chronic enteropathy by Anja Becher, Jan S. Suchodolski, Jörg M. Steiner and Romy M. Heilmann in Journal of Veterinary Diagnostic Investigation
Acknowledgments
Part of the data was presented as a poster at the 29th Annual Congress of the European College of Veterinary Internal Medicine (ECVIM), Milano, Italy (19–21 September 2019), for which the 2019 ECVIM-ESCG poster award was received.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support from a formal grant for the research, authorship, and/or publication of this article. Materials and supplies for the study were generously provided by the Gastrointestinal Laboratory at Texas A&M University; publication costs were paid by the Leipzig University Department for Small Animals.
ORCID iD: Romy M. Heilmann  https://orcid.org/0000-0003-3485-5157
https://orcid.org/0000-0003-3485-5157
Supplementary material: Supplementary material for this article is available online.
Contributor Information
Anja Becher, Department for Small Animals, Veterinary Teaching Hospital, College of Veterinary Medicine, University of Leipzig, Leipzig, Germany.
Jan S. Suchodolski, Gastrointestinal Laboratory, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX
Jörg M. Steiner, Gastrointestinal Laboratory, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX
Romy M. Heilmann, Department for Small Animals, Veterinary Teaching Hospital, College of Veterinary Medicine, University of Leipzig, Leipzig, Germany.
References
- 1. Acarturk G, et al. Neutrophil-to-lymphocyte ratio in inflammatory bowel disease—as a new predictor of disease severity. Bratisl Lek Listy 2015;116:213–217. [DOI] [PubMed] [Google Scholar]
- 2. Allenspach K, et al. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med 2007; 21:700–708. [DOI] [PubMed] [Google Scholar]
- 3. Allenspach K, et al. Correlating gastrointestinal histopathologic changes to clinical disease activity in dogs with idiopathic inflammatory bowel disease. Vet Pathol 2019;56:435–443. [DOI] [PubMed] [Google Scholar]
- 4. Altstaedt J, et al. Cytokine production of neutrophils in limited to interleukin-8. Immunology 1996;89:563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertani L, et al. Novel prognostic biomarkers of mucosal healing in ulcerative colitis patients treated with anti-TNF: neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Inflamm Bowel Dis 2020;26:1579–1587. [DOI] [PubMed] [Google Scholar]
- 6. Buono A, et al. Serum IL-2, IL-6, IL-8, and TNF-α concentrations in dogs with chronic enteropathies. J Vet Intern Med 2018;32:2234. Abstract. [Google Scholar]
- 7. Casamian-Sorrosal D, et al. Comparison of histopathologic findings in biopsies from the duodenum and ileum of dogs with enteropathy. J Vet Intern Med 2010;24:80–83. [DOI] [PubMed] [Google Scholar]
- 8. Clark SR, et al. Platelet TLR-4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007; 13:463–469. [DOI] [PubMed] [Google Scholar]
- 9. Craven MD, et al. Comparative pathophysiology and management of protein-losing enteropathy. J Vet Intern Med 2019;33: 383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dandrieux JR. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J Small Anim Pract 2016;57:589–599. [DOI] [PubMed] [Google Scholar]
- 11. Day MJ, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008;138(Suppl 1):S1–S43. [DOI] [PubMed] [Google Scholar]
- 12. Dumusc SD, et al. Cyclooxygenase-2 and lipoxygenase in dogs with chronic enteropathies. J Vet Intern Med 2014;28:1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erdmann C, et al. Chronisch-entzündliche Darmerkrankungen beim Hund – diagnostische und therapeutische Aspekte [Diagnostic and therapeutic approach to chronic inflammatory enteropathies in dogs]. Tierarztl Prax Ausg K Kleintiere Heimtiere 2017;45:317–327. [DOI] [PubMed] [Google Scholar]
- 14. Fernández R, et al. Comparison of two melphalan protocols and evaluation of outcome and prognostic factors in multiple myeloma in dogs. J Vet Intern Med 2018;32:1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedrichs KR, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 2012;41:441–453. [DOI] [PubMed] [Google Scholar]
- 16. Geffré A, et al. Reference value advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet Clin Pathol 2011;40:107–112. [DOI] [PubMed] [Google Scholar]
- 17. Heilmann RM, et al. Development and analytical validation of a radioimmunoassay for the measurement of alpha1-proteinase inhibitor concentrations in feces from healthy puppies and adult dogs. J Vet Diagn Invest 2011;23:476–485. [DOI] [PubMed] [Google Scholar]
- 18. Heilmann RM, et al. Development and analytical validation of an enzyme-linked immunosorbent assay for the quantification of canine calprotectin in serum and feces from dogs. J Vet Intern Med 2011;25:693. [Google Scholar]
- 19. Heilmann RM, et al. Serum alpha1-proteinase inhibitor concentrations in healthy dogs–method validation and determination of reference interval and intra-individual variation. Vet Clin Pathol 2013;42:190–195. [DOI] [PubMed] [Google Scholar]
- 20. Heilmann RM, et al. Systemic levels of the anti-inflammatory decoy receptor soluble RAGE (receptor for advanced glycation end products) are decreased in dogs with inflammatory bowel disease. Vet Immunol Immunopathol 2014;161:184–192. [DOI] [PubMed] [Google Scholar]
- 21. Heilmann RM, et al. Serum and fecal canine α1-proteinase inhibitor concentrations reflect the severity of intestinal crypt abscesses and/or lacteal dilation in dogs. Vet J 2016;207:131–139. [DOI] [PubMed] [Google Scholar]
- 22. Heilmann RM, et al. Validation of an enzyme-linked immunosorbent assay (ELISA) for the measurement of canine S100A12. Vet Clin Pathol 2016;45:135–147. [DOI] [PubMed] [Google Scholar]
- 23. Heilmann RM, et al. Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. J Vet Intern Med 2018;32:679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heilmann RM, et al. Clinical utility of currently available biomarkers in inflammatory enteropathies of dogs. J Vet Intern Med 2018;32:1495–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heilmann RM, et al. Biological variation of serum canine calprotectin concentrations as measured by ELISA in healthy dogs. Vet J 2019;247:61–64. [DOI] [PubMed] [Google Scholar]
- 26. Heilmann RM, et al. Mucosal expression of S100A12 (calgranulin C) and S100A8/A9 (calprotectin) and correlation with serum and fecal concentrations in dogs with chronic inflammatory enteropathy. Vet Immunol Immunopathol 2019;211:64–74. [DOI] [PubMed] [Google Scholar]
- 27. Hodgeson N, et al. Utility and prognostic significance of neutrophil-to-lymphocyte ratio in dogs with septic peritonitis. J Am Anim Hosp Assoc 2018;54:351–359. [DOI] [PubMed] [Google Scholar]
- 28. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- 29. Ilkiw JE, et al. Hematologic, biochemical, blood-gas, and acid-base values in greyhounds before and after exercise. Am J Vet Res 1989;50:583–586. [PubMed] [Google Scholar]
- 30. Jergens AE, et al. Inflammatory bowel disease in veterinary medicine. Front Biosci 2012;4:1404–1419. [DOI] [PubMed] [Google Scholar]
- 31. Kather S, et al. Review of cobalamin status and disorders of cobalamin metabolism in dogs. J Vet Intern Med 2020;34:13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kathrani A, et al. Elevated canine pancreatic lipase immunoreactivity concentration in dogs with inflammatory bowel disease is associated with a negative outcome. J Small Anim Pract 2009;50:126–132. [DOI] [PubMed] [Google Scholar]
- 33. Luckschander N, et al. Activation of nuclear factor-kappaB in dogs with chronic enteropathies. Vet Immunol Immunopathol 2010;133:228–236. [DOI] [PubMed] [Google Scholar]
- 34. Macfarlane L, et al. Diagnostic value of neutrophil-lymphocyte and albumin-globulin ratios in canine soft tissue sarcoma. J Small Anim Pract 2016;57:135–141. [DOI] [PubMed] [Google Scholar]
- 35. Martins GC, et al. Clinical-pathological and immunological biomarkers in dogs with atopic dermatitis. Vet Immunol Immunopathol 2018;205:58–64. [DOI] [PubMed] [Google Scholar]
- 36. Mutz M, et al. Prognostic value of baseline absolute lymphocyte concentration and neutrophil/lymphocyte ratio in dogs with newly diagnosed multi-centric lymphoma. Vet Comp Oncol 2015;13:337–347. [DOI] [PubMed] [Google Scholar]
- 37. Nagata N, et al. Clinical characteristics of dogs with food-responsive protein-losing enteropathy. J Vet Intern Med 2020;34:659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Procoli F, et al. Comparison of histopathologic findings in duodenal and ileal endoscopic biopsies in dogs with chronic small intestinal enteropathies. J Vet Intern Med 2013;27:268–274. [DOI] [PubMed] [Google Scholar]
- 39. Rejec A, et al. Evaluation of complete blood count indices (NLR, PLR, MPV/PLT, and PLCRi) in healthy dogs, dogs with periodontitis, and dogs with oropharyngeal tumors as potential biomarkers of systemic inflammatory response. J Vet Dent 2017;34:231–240. [DOI] [PubMed] [Google Scholar]
- 40. Rørtveit R, et al. Age-related changes in hematologic and serum biochemical variables in dogs aged 16–60 days. Vet Clin Pathol 2015;44:47–57. [DOI] [PubMed] [Google Scholar]
- 41. Skor O, et al. Pretreatment leukocyte ratios and concentrations as predictors of outcome in dogs with cutaneous mast cell tumours. Vet Comp Oncol 2017;15:1333–1345. [DOI] [PubMed] [Google Scholar]
- 42. Sothern RB, et al. Circannual variations in baseline blood values of dogs. Chronobiol Int 1993;10:364–382. [DOI] [PubMed] [Google Scholar]
- 43. Tan W, et al. IL-17f/IL-17R interaction stimulates granulopoiesis in mice. Exp Hematol 2008;36:1417–1427. [DOI] [PubMed] [Google Scholar]
- 44. Washabau RJ, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]
- 45. Willard MD, et al. Interobserver variation among histopathologic evaluations of intestinal tissues from dogs and cats. J Am Vet Med Assoc 2002;220:1177–1182. [DOI] [PubMed] [Google Scholar]
- 46. Zeugswetter FK, Schwendenwein I. Diagnostic efficacy of the leukogram and the chemiluminometric ACTH measurement to diagnose canine hypoadrenocorticism. Tierarztl Prax Ausg K Kleintiere Heimtiere 2014;42:223–230. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_1040638721992057 for Blood neutrophil-to-lymphocyte ratio (NLR) as a diagnostic marker in dogs with chronic enteropathy by Anja Becher, Jan S. Suchodolski, Jörg M. Steiner and Romy M. Heilmann in Journal of Veterinary Diagnostic Investigation