Abstract
We investigated the occurrence and pathologic findings of transmissible viral proventriculitis (TVP) associated with the chicken proventricular necrosis virus (CPNV) in commercial broiler chickens in southeastern Brazil. Seventy-three broilers, 25–36 d old, with a history of reduced growth, were referred to our veterinary pathology services from 2013 to 2017. Broilers were clinically examined, weighed, and euthanized for postmortem examination. Broilers of different ages with proventricular histologic lesions were positive for CPNV by RT-PCR; however, the intensity of histologic lesions was higher among 33-d-old animals, and viral RNA detection was more frequent among those that were 28 d old. In the proventriculi of 35 of 73 (48%) broilers, lesions were characterized by glandular epithelial necrosis, lymphoplasmacytic and histiocytic infiltrates, and metaplasia of glandular epithelium to ductal epithelium. In 24 of 73 (36%) broilers with histologic TVP-compatible lesions, CPNV was detected by RT-PCR for the viral protein 1 (VP1) gene. Broilers with histologic lesions were lighter than expected compared to the Cobb 500 standard weight. TVP has not been reported previously in broiler chickens in Brazil, to our knowledge.
Keywords: birnavirus, broiler chickens, chicken proventricular necrosis virus, histopathology, RT-PCR, transmissible viral proventriculitis
Transmissible viral proventriculitis (TVP) is an emerging infectious disease with a poorly known etiopathogenesis. It affects mainly broiler chickens,6,16 but is also reported in broiler breeder and layer chickens.12,15 TVP is characterized macroscopically by enlargement, wall thickening, and pallor of the proventriculus.1,5,11 Microscopically, it is characterized by specific lesions, including necrosis of oxynticopeptic cells, lymphocytic infiltrates, and ductal epithelial hyperplasia with metaplasia of glandular epithelium to ductal epithelium.2,6,7
The clinical signs of the disease are nonspecific and characterized by impaired growth, poor feed conversion,2,19 nonuniformity of the flock,2,16,19 and impaired digestion.1,5,14 Mortality in flocks with TVP is reported to be 3.03–4.49%.6 The disease has been reported in broilers 13–59 d old7 and in broiler breeders 9–20 wk old.15
Initially, TVP was associated with other viruses, such as infectious bursal disease virus.17 Later, researchers identified a virus named R11/3. This virus was isolated from broilers with TVP, inoculated into specific pathogen–free (SPF) chickens, and the disease was reproduced for the first time.10,11 The virus was later named chicken proventricular necrosis virus (CPNV), and physical and genomic analysis led to its identification as a birnavirus.10 Nevertheless, questions still remain regarding the role of other agents in the development of TVP.7 Experimental infections with CPNV were unable to reproduce the impaired growth seen in natural cases, thus reinforcing the idea that additional factors could be involved in the pathogenesis of TVP.8,9
TVP has economic implications because it can increase costs by decreasing production indicators in affected flocks2,8,12 and increasing the rate of stunted broilers.16 Since its first identification in 2005,8 TVP has been reported in many countries,9,13,16 but not in Brazil, to our knowledge. We investigated the occurrence and pathologic findings of TVP in broilers confirmed with reverse-transcription PCR (RT-PCR) for CPNV.
Our study included 73 broilers from different mixed flocks grown in conventional houses in southeastern Brazil. All broilers were vaccinated at the hatchery against Marek disease, infectious bronchitis, infectious bursal disease, and Newcastle disease. Samples included 10 broilers from retrospective cases (2013, 2016, and 2017) and 63 broilers from prospective cases (2017). Retrospective cases consisted of 2 pools of proventriculi samples fixed in 10% neutral-buffered formalin (2013 and 2016), and another pool of fixed and frozen proventriculi (2017). The 63 prospective cases consisted of stunted broilers from 6 different flocks or houses from the same producer. The populations were 17,000–40,000 birds per house, and we examined 10–12 broilers from each of these flocks. All 73 broilers were sampled at 25–36 d old, and all the flocks had a previous history of impaired growth and thickening of the proventricular wall. Clinically, stunting was the main sign in these broilers. In addition, the broilers had ruffled feathers (12 of 73) and poor feathering (6 of 73). Broilers from the prospective study were weighed, clinically examined, and postmortem examined in the Animal Pathology Sector of the Universidade Federal de Minas Gerais (Belo Horizonte, Minas Gerais, Brazil). The broilers’ weights were compared to the standard weight of Cobb 500 mixed flocks.4 Data regarding age, vaccination schedule, management, production data, mortality rate, and clinical signs were obtained from the owner.
The project was approved by the Committee on Ethics in the Use of Animals (CEUA), protocol 169/2017, and all of the owners of the farms signed a consent form. Broilers were euthanized following the guidelines from the Brazilian Federal Council of Veterinarians, resolution 1000/2012.
During postmortem examination, larynx, trachea, lung, heart, proventriculus, gizzard, intestine, pancreas, bursa of Fabricius, thymus, spleen, liver, and kidneys were fixed in 10% neutral-buffered formalin, processed routinely for histopathology, and stained with hematoxylin and eosin. The proventriculus from each broiler was sectioned longitudinally, and the thickness of the wall was measured at the central portion before placement in formalin. In addition, 2 transverse sections of the proventriculus were collected and formalin-fixed. Microscopically, the proportion of affected versus unaffected glands of the mucosa was examined and scored. For scoring the proventricular lesions, a representative section from each broiler was selected.
Proventricular lesions were scored based on the distribution and intensity of glandular epithelial necrosis and the interstitial or deep glandular histiocytic and lymphoplasmacytic infiltrations as follows: mild (+), occasional glandular epithelial necrosis and inflammatory infiltrate affecting 25% of the glands; moderate (++), multifocal glandular necrosis and inflammatory infiltrate with 25–50% of glands affected; marked (+++), diffuse epithelial glandular necrosis and inflammatory infiltrate with >50% of glands affected. The intensity and distribution of ductal metaplasia of the glandular epithelium were associated with necrotic and inflammatory lesions and classified as mild (occasional inflammatory cells), moderate (cells near the surface of the glands), and marked (cells reaching the base of the glands). The lymphoid follicles and plasma cells in the lamina propria of the mucosa (particularly near the surface) were not considered inflammatory infiltrates given that they are normally found in healthy broilers.
Samples of the proventriculi collected from the 63 broilers in the prospective sampling were placed in microtubes and immediately frozen in liquid nitrogen. Total RNA extraction was performed using the silica and guanidine lysis buffer protocol.3 Four proventriculi from the retrospective samples stored at –80°C were also used, totaling 67 proventriculi analyzed by RT-PCR. The proventriculi of the other 6 broilers were received fixed in formalin, and no frozen samples were available for RT-PCR.
The concentration and purity of the RNA extracted were assessed (NanoDrop 2000 spectrophotometer; Thermo Fisher). RT-PCR reactions were performed (one-step kit, Access RT-PCR system A1250; Promega), following the manufacturer’s instructions. Detection and amplification of the CPNV viral protein 1 (VP1) gene were performed using the oligonucleotides and protocols described previously.10 The positive control was obtained from a proventricular sample previously confirmed by partial Sanger sequencing of the VP1 gene.10 As a negative control, similar reactions were performed with all of the reagents and without template cDNA. The final product of each reaction was subjected to electrophoresis in 1.5% agarose gel containing ethidium bromide along with a molecular weight marker of 100 bp (LowRanger 100 bp DNA ladder; Norgen).
The chi-square test or Fisher exact test was used as appropriate to evaluate the association between the presence and intensity of the proventricular histologic lesions and RT-PCR viral detection. The effect of the histologic lesions on proventricular wall thickness was evaluated by ANOVA, including the linear effect of age as a covariate. To study the effect of intensity of the histologic lesions, gross lesions, and molecular detection on weight, a repeated-measure mixed model was fit in which “flock” was considered a random variable. The effect of age on the difference between the observed and expected weights of Cobb 500 strain, proventricular wall thickness, RT-PCR results, and histologic lesion scores was investigated by ANOVA. For all analyses, the significance level of ≤5% (SAS Institute, 2018) was used.
The examined broilers were grouped according to age and the histologic lesions compatible with TVP, which had different intensities for various ages (25–36 d old). Lesions were more frequent in 33- and 36-d-old broilers than in those <30 d old. Two broilers were excluded from this analysis because their age was not known, thus totaling 71 broilers. Twenty-one (30%) broilers positive by RT-PCR were 25–36 d old, and 13 of 21 (62%) had histologic TVP lesions. No association was observed between age and RT-PCR results (p = 0.79). Positive and negative broilers had average ages of 31.2 and 30.9 d, respectively. An association was observed between histologic lesions and age (p = 0.04), in which broilers with histologic TVP lesions had an average age of 32 d, and broilers without lesions were on average 30 d old. Therefore, it is more likely to find histologic lesions in 32-d-old broilers. However, age did not influence proventricular histologic lesion scores (p = 0.11); different lesion intensities were present in all age groups and showed no pattern.
Weekly mortality rates were 0.5–4.0%, with an average of 1.4%. At the end of 4 wk, cumulative mortality was 2.2–7.4%, with an average of 5.4%. Nevertheless, it was not possible to correlate mortality rates to the presence of histologic TVP lesions because this analysis would require differentiation between flocks with and without histologic lesions and all sampled flocks had broilers with TVP.
All 63 broilers referred to our pathology service had body weights below the Cobb 500 standard weight for mixed flocks.4 The broilers weighed 0.50–1.84 kg and were an average of 1.60 kg below the Cobb 500 standard. The presence and absence of histologic TVP lesions and the body weights were compared among these broilers. The average weight of broilers with histologic lesions was 1.04 kg, whereas the average weight of those without histologic lesions was 1.06 kg. Thus, broilers with histologic lesions were, on average, 1.15 kg below the Cobb 500 standard weight, and broilers without lesions were 0.99 kg below. Nevertheless, the presence or absence of histologic lesions showed no significant influence on the weight (p = 0.58).
Macroscopically, the main proventricular change was thickening of the mucosa, albeit not always associated with lumen dilation and undigested content. Five of 73 (7%) broilers had proventriculus with a thickened wall. In the mucosa, the papillae were flattened in 3 of 8 broilers, which also had mucosal gland dilation, and miliary white areas in the mucosa (Suppl. Figs. 1–4). The enlargement of the proventriculus was associated with lumen and isthmus dilation (Fig. 1). The presence or absence of histologic lesions interfered with the proventricular thickness (p = 0.002). Broilers with histologic TVP-compatible lesions had an average thickness of 0.578 cm; broilers without histologic lesion had an average thickness of 0.505 cm. Other findings included dyspigmentation of feet, legs, and skin (52 of 73), necrotic pododermatitis (36 of 73), periorbital swelling (3 of 73), airsacculitis (3 of 73), and fibrinous pericarditis (1 of 73).
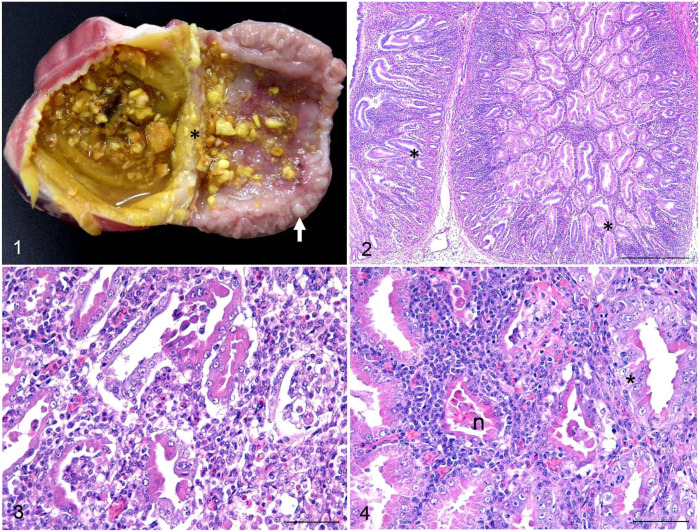
Figures 1–4.
Transmissible proventriculitis in broiler chickens. Figure 1. Longitudinal section of the proventriculus and ventriculus. Dilation of the lumen and isthmus (asterisk) with diffuse thickening of the proventricular mucosa in association with white nodules in the mucosa (arrow). Figure 2. Proventricular mucosa of a 20-d-old broiler with marked inflammatory infiltrate in the interstitium and in the lumen of glands. There is also loss of glandular epithelium and ductal epithelial hyperplasia (*). H&E. Bar = 500 µm. Figure 3. High magnification of Figure 3. Necrosis and loss of glandular cells, replacement of glandular epithelial cells with ductal epithelium (ductal metaplasia), and diffuse infiltrate of lymphocytes and histiocytes in the interstices and in the lumens of acini. H&E. Bar = 50 µm. Figure 4. Proventricular mucosa of a 32-d-old broiler. Marked ductal metaplasia (*) is seen at the base of the gland, associated with epithelial necrosis (n) and infiltration by lymphocytes and histiocytes. H&E. Bar = 50 µm.
Microscopically, 35 of 73 (48%) broilers had TVP-compatible histologic lesions in the proventriculus. Of those, 21 (60%) had mild (+) proventricular lesions, 13 (37%) had moderate (++) lesions, and 1 (3%) had marked (+++) lesions. The presence of histologic lesions compatible with TVP was significantly associated with positive RT-PCR results by the chi-square test (p = 0.04). Of the 63 prospective samples tested, 21 were positive by RT-PCR, and proventricular lesions were observed in 13 of the 21 (62%). From the 42 RT-PCR–negative proventriculi, 27 (64%) had no histologic lesions. No significant association was observed between histologic lesions and the presence of gross proventricular lesions (p = 0.38).
RT-PCR was positive in 3 proventriculi from the 10 tested retrospective cases, resulting in a total of 24 (36%) positive RT-PCR results. Histologic TVP-compatible lesions were present in 6 of the 10 retrospective samples.
Proventricular histologic lesions were characterized by oxynticopeptic cell necrosis of the submucosal proventricular glands, inflammatory infiltrates, and ductal epithelial hyperplasia with metaplasia of glandular epithelium to ductal epithelium. The inflammatory infiltrates of lymphocytes, histiocytes, and plasma cells were the most evident lesions in the proventriculi, followed by necrosis and metaplasia (Figs. 2–4). Glandular ectasia was associated with necrotic epithelial cells and histocytes inside the lumen. Moderate-to-marked reactive follicular aggregates of lymphocytes were also present (Table 1). A case with marked TVP-compatible lesions also had mild fibroplasia. Intranuclear inclusion bodies were not present. Lymphoid depletion was found in the bursa of Fabricius of 3 broilers; there were no lesions in the thymus and spleen. Enteritis caused by Eimeria spp. was found in 2 broilers.
Table 1.
Frequency by type and distribution of histologic lesions in the proventriculi of broiler chickens with transmissible viral proventriculitis.
| Proventricular histologic lesions | Frequency |
|---|---|
| Lymphoplasmacytic and histiocytic infiltrate affecting >50% of the glands (marked) | 1/73 (1) |
| Ductal metaplasia of the glandular epithelium | 11/73 (15) |
| Interglandular mild number of follicular aggregates of lymphocytes | 12/73 (16) |
| Lymphoplasmacytic and histiocytic infiltrate affecting 25–50% of the glands (moderate) | 13/73 (18) |
| Lymphoplasmacytic and histiocytic infiltrate affecting up to 25% of the glands (mild) | 21/73 (29) |
| Glandular epithelial necrosis | 22/73 (30) |
| Glandular ectasia | 24/73 (33) |
| Moderate to marked number of follicular aggregates of lymphocytes | 24/73 (33) |
Numbers in parentheses are percentages.
We defined proventriculitis in broilers by histologic lesions in the proventriculus and confirmed the viral etiology in 36% of the cases by RT-PCR for the VP1 gene of CNPV. These results are similar to other studies,1,6,7,11,16 in which similar lesions have been reported as TVP cases in different countries.1,10,14,19
The majority of TVP cases documented in the literature are in broiler chickens.6,14 Our cases occurred in broilers housed in conventional houses without optimal biosecurity practices. The transmission of the TVP agent is not fully comprehended (vertical, horizontal, or both), but oral and ocular infections have been reported.11 Also, an experimental study that propagated the virus by amniotic inoculation of embryonated chicken eggs caused proventricular TVP lesions.8 Therefore, in our study, the poor biosecurity measures may have favored the entrance of the virus via people, fomites, vehicles, food, or other animals. However, the breed, environmental factors, concurrent diseases, and host immunity should also be considered in the onset of the disease.
We found the higher percentage of broilers with histologic TVP-compatible lesions among 29- to 36-d-old animals. The most severe lesions were identified in 33-d-old broilers. These data differed from other documented studies, in which 18- to 31-d-old broilers were affected.1,11,16,19 However, only broilers up to 31 d old were examined in those studies.1,16,19 We found that the disease can manifest later given that some of our older broilers had lesions in the initial stage. Experimental and natural TVP studies have identified CPNV by RT-PCR in broilers at 14–31 d old1,11 and 21 and 49 d old.7 Nonetheless, an experimental study that inoculated the virus in 14-d-old broilers and tested for CPNV up to 49 d old only detected the virus until 28 d old.11
Although there is no confirmation that TVP leads to death or stunting, broilers with TVP histologic lesions in our study were 1.038 kg below the Cobb 500 standard weight, although only stunted broilers were examined. We have not studied flocks unaffected by TVP to allow weight comparisons. The proventriculus is responsible for chemical digestion,20 and it is therefore expected that lesions in this organ might lead to decreased productivity and quality of health. Reports on TVP have shown worse feed conversion, reduced weight gain, and poor uniformity in natural cases of the disease.16,19 Nonetheless, the disease did not lead to a decrease in production indicators in SPF chickens.8,9
We identified gross lesions similar to those reported in other studies, such as proventricular enlargement, thickening of the wall, glandular dilation,8,9 flattening of the mucosa, and circular white areas in the mucosa.5,16,19 Nevertheless, only 5 of 35 proventriculi with histologic lesions had macroscopic thickening of the wall, and some broilers had typical histologic lesions and no thickening of the proventricular wall, thus highlighting the importance of histologic examination to support the diagnosis. We did not find any other studies evaluating the thickening of the proventricular wall by macroscopic measures.
The main histologic lesions found in the mucosa of the proventriculi from broilers in our study were lymphoplasmacytic and histiocytic infiltrates, glandular epithelial necrosis, glandular ectasia, and interglandular follicular aggregates of lymphocytes, similar to previous reports.2,6,11 Ductal metaplasia of the glandular epithelium, seen in the base of the glands in association with necrosis and inflammation, was present in the proventriculi of only 15% of the broilers. Other reports that showed higher frequencies of metaplasia may have examined a greater number of proventriculi with chronic lesions. Lesions such as activated follicular aggregates of lymphocytes7 and glandular ectasia9 have also been documented in TVP cases.
We identified viral RNA of CPNV in the proventriculi without histologic lesions. This result raises the question of whether this virus could persist in the proventriculus, although subclinical infection may not be ruled out. Studies indicate that CPNV has an incubation period of 2–3 d,10,11 and the sensitivity and specificity of the RT-PCR for CPNV were 88% and 83%, respectively, in frozen proventriculi.11 We did not find any studies on the persistence of the virus in the proventriculus of healthy broilers.
Our study is in accord with others that presented CPNV as the agent of TVP cases,1,6,7,11,16 although viral detection was not confirmed for all of the cases diagnosed as TVP.6,7 Some researchers have raised questions about the role of other viruses in the disease.12,13 Nevertheless, these studies did not look for CPNV and only examined the proventriculus content, instead of searching for these viruses in the proventricular mucosa and in situ to associate the virus with histologic lesions. Some data reinforce CPNV as the TVP etiologic agent, such as the experimental reproduction of the disease9 and the identification of the CPNV VP1 gene by RT-PCR in the proventricular tissue of broilers in different studies.1,7,11
Other viruses, such as picornavirus12 and gyrovirus,13 have also been associated with TVP. However, they have not been used to reproduce the disease and were not identified in situ in association with lesions. Therefore, it is not possible to determine whether they cause TVP.13,18
TVP apparently caused a loss of performance in broilers with proventricular lesions in our study. Therefore, we recommend investigation for TVP in flocks >20 d old showing impaired growth. Moreover, additional investigations are needed encompassing flocks from different regions to determine the incidence and prevalence of TVP in Brazil.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211004106 for Retrospective and prospective studies of transmissible viral proventriculitis in broiler chickens in Brazil by Philipe A. Leão, Camila I. Amaral, Willian H. M. Santos, Matheus V. L. Moreira, Leticia B. de Oliveira, Erica A. Costa, Mauricio Resende, Raphael Wenceslau and Roselene Ecco in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank the Coordenação de aperfeiçoamento de Pessoal de Nível Superior–Brazil (CAPES)–Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (research fellowship), and Pró-reitoria de Pesquisa da Universidade Federal de Minas Gerais.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Our study was funded by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
ORCID iDs: Raphael Wenceslau  https://orcid.org/0000-0002-0034-1484
https://orcid.org/0000-0002-0034-1484
Roselene Ecco  https://orcid.org/0000-0002-8052-5389
https://orcid.org/0000-0002-8052-5389
Supplementary material: Supplementary material for this article is available online.
Contributor Information
Philipe A. Leão, Departments of Veterinary Clinic and Surgery, Veterinary School, Universidade Federal de Minas Gerais–UFMG, Belo Horizonte, Minas Gerais, Brazil
Camila I. Amaral, Departments of Veterinary Clinic and Surgery, Veterinary School, Universidade Federal de Minas Gerais–UFMG, Belo Horizonte, Minas Gerais, Brazil
Willian H. M. Santos, Departments of Veterinary Clinic and Surgery, Veterinary School, Universidade Federal de Minas Gerais–UFMG, Belo Horizonte, Minas Gerais, Brazil
Matheus V. L. Moreira, Departments of Veterinary Clinic and Surgery, Veterinary School, Universidade Federal de Minas Gerais–UFMG, Belo Horizonte, Minas Gerais, Brazil
Leticia B. de Oliveira, Departments of Veterinary Clinic and Surgery, Veterinary School, Universidade Federal de Minas Gerais–UFMG, Belo Horizonte, Minas Gerais, Brazil
Erica A. Costa, Preventive Veterinary Medicine, Veterinary School, Universidade Federal de Minas Gerais–UFMG, Belo Horizonte, Minas Gerais, Brazil
Mauricio Resende, Preventive Veterinary Medicine, Veterinary School, Universidade Federal de Minas Gerais–UFMG, Belo Horizonte, Minas Gerais, Brazil.
Raphael Wenceslau, Departments of Veterinary Clinic and Surgery, Veterinary School, Universidade Federal de Minas Gerais–UFMG, Belo Horizonte, Minas Gerais, Brazil.
Roselene Ecco, Departments of Veterinary Clinic and Surgery, Veterinary School, Universidade Federal de Minas Gerais–UFMG, Belo Horizonte, Minas Gerais, Brazil.
References
- 1. Allawe AB, et al. Detection of transmissible viral proventriculitis in Iraq. J Entomol Zool Stud 2017;5:974–978. [Google Scholar]
- 2. Bayyari GR, et al. Experimental reproduction of proventriculitis using homogenates of proventricular tissues. Poult Sci 1995;74:1799–1809. [DOI] [PubMed] [Google Scholar]
- 3. Boom R, et al. Rapid and simple method for purification of nucleic acids. Clin Microbiol 1990;28:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. COBB. Cobb500: broiler performance & nutrition supplement. Cobb Vantress, 2018. https://www.cobb-vantress.com/assets/Cobb-Files/product-guides/bdc20a5443/70dec630-0abf-11e9-9c88-c51e407c53ab.pdf
- 5. Goodwin MA, et al. Viral proventriculitis in chickens. Avian Pathol 1996;25:369–379. [DOI] [PubMed] [Google Scholar]
- 6. Grau-Roma L, et al. Detection of transmissible viral proventriculitis (TVP) and chicken proventricular necrosis virus (CPNV) in the United Kingdom. Avian Pathol 2017;46:68–75. [DOI] [PubMed] [Google Scholar]
- 7. Grau-Roma L, et al. Retrospective study on transmissible viral proventriculitis and chicken proventricular necrosis virus (CPNV) in the UK. Avian Pathol 2020;49:99–105. [DOI] [PubMed] [Google Scholar]
- 8. Guy JS, et al. Partial characterization of an adenovirus-like virus isolated from broiler chickens with transmissible viral proventriculitis. Avian Dis 2005;49:344–351. [DOI] [PubMed] [Google Scholar]
- 9. Guy JS, et al. Experimental reproduction of transmissible viral proventriculitis by infection of chickens with a novel adenovirus-like virus (isolate R11/3). Avian Dis 2007;51:58–65. [DOI] [PubMed] [Google Scholar]
- 10. Guy JS, et al. Physical and genomic characteristics identify chicken proventricular necrosis virus (R11/3 Virus) as a novel birnavirus. Avian Dis 2011;55:2–7. [DOI] [PubMed] [Google Scholar]
- 11. Guy JS, et al. Detection of R11/3 virus in experimental and naturally-occurring cases of transmissible viral proventriculitis using a reverse-transcriptase polymerase chain reaction procedure. Avian Dis 2011;55:70–75. [DOI] [PubMed] [Google Scholar]
- 12. Kim HR, et al. Identification of a picornavirus from chickens with transmissible viral proventriculitis using metagenomic analysis. Arch Virol 2015;160:701–709. [DOI] [PubMed] [Google Scholar]
- 13. Li G, et al. Emergence of gyrovirus 3 in commercial broiler chickens with transmissible viral proventriculitis. Transbound Emerg Dis 2018;65:1–5. [DOI] [PubMed] [Google Scholar]
- 14. Marguerie J, et al. Birnavirus-associated proventriculitis in French broiler chickens. Vet Rec 2011;169:394–396. [DOI] [PubMed] [Google Scholar]
- 15. Marusak RA, et al. Transmissible viral proventriculitis identified in broiler breeder and layer hens. Avian Dis 2012;56:757–759. [DOI] [PubMed] [Google Scholar]
- 16. Noiva R, et al. Runting stunting syndrome associated with transmissible viral proventriculitis in broiler chickens. Avian Dis 2015;59:384–387. [DOI] [PubMed] [Google Scholar]
- 17. Pantin-Jackwood MJ, Brown TP. Infectious bursal disease virus and proventriculitis in broiler chickens. Avian Dis 2003;47:681–690. [DOI] [PubMed] [Google Scholar]
- 18. Pantin-Jackwood MJ, et al. Reproduction of proventriculitis in commercial and specific-pathogen-free broiler chickens. Avian Dis 2005;49:352–360. [DOI] [PubMed] [Google Scholar]
- 19. Śmialek M, et al. Identification of transmissible viral proventriculitis (TVP) in broiler chickens in Poland. Pol J Vet Sci 2017;20:417–420. [DOI] [PubMed] [Google Scholar]
- 20. Zhang H, et al. Microstructure features of proventriculus and ultrastructure of the gastric gland cells in Chinese Taiho black-bone silky fowl (Gallus gallus domesticus Brisson). Anat Histol Embryol 2016;45:1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211004106 for Retrospective and prospective studies of transmissible viral proventriculitis in broiler chickens in Brazil by Philipe A. Leão, Camila I. Amaral, Willian H. M. Santos, Matheus V. L. Moreira, Leticia B. de Oliveira, Erica A. Costa, Mauricio Resende, Raphael Wenceslau and Roselene Ecco in Journal of Veterinary Diagnostic Investigation