Abstract
As one of the most important cancer treatment strategies, conventional chemotherapy has substantial side effects and leads easily to cancer treatment failure. Therefore, exploring and developing more efficient methods to enhance cancer chemotherapy is an urgently important problem that must be solved. With the development of nanotechnology, nanomedicine has showed a good application prospect in improving cancer chemotherapy. In this review, we aim to present a discussion on the significant research progress in nanomedicine for enhanced cancer chemotherapy. First, increased enrichment of drugs in tumor tissues relying on different targeting ligands and promoting tissue penetration are summarized. Second, specific subcellular organelle-targeted chemotherapy is discussed. Next, different combinational strategies to reverse multidrug resistance (MDR) and improve the effective intracellular concentration of therapeutics are discussed. Furthermore, the advantages of combination therapy for cancer treatment are emphasized. Finally, we discuss the major problems facing therapeutic nanomedicine for cancer chemotherapy, and propose possible future directions in this field.
Keywords: chemotherapy, combination therapy, nanocarriers, nanomedicine, cancer therapy
Introduction
Conventional chemotherapy is a crucial component of cancer treatments for various cancer types, and the treatment strategy is to use toxic drugs to kill cancer cells 1, 2. Many chemotherapeutic drugs have been discovered or synthesized since World War II 3. Although there have been great breakthroughs in cancer treatment, cancer remains a major life-threatening disease worldwide. For instance, 18.1 million new cancer cases, and 9.6 million cancer deaths occurred in 2018 4. At present, the low accumulation/retention of drugs in the tumor is acknowledged as a factor leading to the failure of clinical chemotherapy against cancer. Furthermore, chemotherapy usually induces multidrug resistance (MDR), which refers to a resistance phenotype, and cancer cells become resistant to different drugs with varying structures and molecular resemblances 5. Therefore, exploring and developing more efficient and simpler cancer treatment methods have important research significance and clinical value.
In recent years, nanocarrier-based drug delivery systems (NDDSs) (e.g., polymeric micelles, liposomes, and organic/inorganic nanoparticles) have attracted substantial interest in cancer therapeutics because of their special physical and chemical properties 6, 7. In contrast to anticancer drugs without carriers, NDDSs can deliver higher doses of drug to tumor tissue via enhanced permeability and retention (EPR) effects and decrease the adverse effects of high doses 8. To date, many nanoformulations have been approved for clinical applications in cancer chemotherapy, and several nanomedicines are undergoing clinical trials (see Table 1). To further utilize the advantages of NDDSs, researchers have been exploring and fabricating many functionalized NDDSs by 1) modifying the surface of nanocarriers with targeting ligands on to improve their enrichment in tumor tissues 26 and 2) endowing NDDSs with specific responsiveness for drug release (pH 27, enzymes 28, glutathione (GSH) 29 and temperature 30) via in vivo and in vitro stimulation. Multifunctional NDDSs have shown good prospects in solving the problems of low drug delivery efficiency and unsatisfactory anticancer effects (especially for treatment of MDR tumors), laying a foundation for application of NDDSs in clinical practice. In addition, due to the limitations of single chemotherapy regiments, combined treatment strategies based on NDDSs are also emerging 31, 32.
Table 1.
Clinically approved or under clinical trial nanomedicines
| Product | Drug | Carrier components | Company | Stage | Ref. |
|---|---|---|---|---|---|
| Nab-Paclitaxel (Abraxane) | PTX | Human serum albumin | Abraxis BioScience | FDA and EMA approved | 9 |
| Genexol-PM | PTX | Micelle: mPEG-PDLLA | Samyang Biopharm | Approved in Korea | 10 |
| Apealea | PTX | Micelle: two isoforms of N-retinoyl-L-cysteic acid Methyl ester sodium salt | Oasmia Pharmaceutical | EMA approved | 11 |
| Lipusu | PTX | Liposome: lecithin/cholesterol | Nanjing Luye Sike Pharmaceutical Co. | Phase IV | 12 |
| Doxil | DOX | Liposome: HSPC, cholesterol, mPEG-DSPE | Johnson &Johnson | FDA and EMA approved | 13 |
| Myocet | DOX | Liposome: phosphatidylcholine, cholesterol | Teva | EMA approved | 14 |
| ThermoDox | DOX | Thermosensitive liposomal doxorubicin | MedKoo Biosciences Inc. | Phase III completed | 15 |
| Nanoparticle generator | DOX | Porous silicon microparticle with polymeric doxorubicin | / | Planning of phase I | 16 |
| NC-6004 | Cisplatin | Micelle: PEG-P(Glu) | Nano Carrier Co. | Phase I/II | 17 |
| Lipoplatin | Cisplatin | Liposome: SPC/cholesterol/DPPG/mPEGDSPE | Regulon Inc. | Phase II/III | 18 |
| CRLX101 | CPT | PEG-modified β-cyclodextrin | Cerulean Pharma Inc. | Phase II | 19 |
| NKTR-102 | Irinotecan | PEG (four-arm) conjugation | Nektar Therapeutics | Phase II | 20 |
| Onivyde | Irinotecan | Liposome: DSPC, cholesterol, mPEG-DSPE | Merrimack Pharmaceuticals | Phase II/III | 21 |
| DOTAP: Chol-TUSC2 | TUSC2 | DOTAP: Chol | Genprex, Inc. | Phase I/II | 22 |
| Mepact | Mifamurtide | Liposome: POPC,OOPS | Takeda Pharmaceutical | EMA approved | 23 |
| Marqibo | Vincristine sulfate | Liposome: sphingomyelin, cholesterol | Talon Therapeutics | FDA approved | 24 |
| Vyxeos | Cytarabine and daunorubicin |
Liposome: DSPC,DSPG, cholesterol | Jazz Pharmaceuticals | FDA and EMA approved | 25 |
In this review, we aim to present a discussion on the significant research progress in improving cancer chemotherapy based on nanomedicine (Figure 1). This review is generally divided into five parts. In the first part, methods to increase enrichment of drugs in tumor tissues relying on different targeting ligands (targeting tumor blood vessels or cell membranes) and promoting tissue penetration are summarized. In the second part, specific subcellular organelle-targeted chemotherapy is discussed. In the third part, different combinational strategies to reverse MDR and improve the effective intracellular concentration of therapeutics are discussed. In the fourth part, the advantages of combination therapy (e.g., chemotherapy combined with phototherapy, chemodynamic therapy, gas therapy, immunotherapy and multiple therapies) for cancer treatment are emphasized. Finally, we discuss the major problems therapeutic nanomedicine facing in cancer chemotherapy, and propose possible future directions in this field.
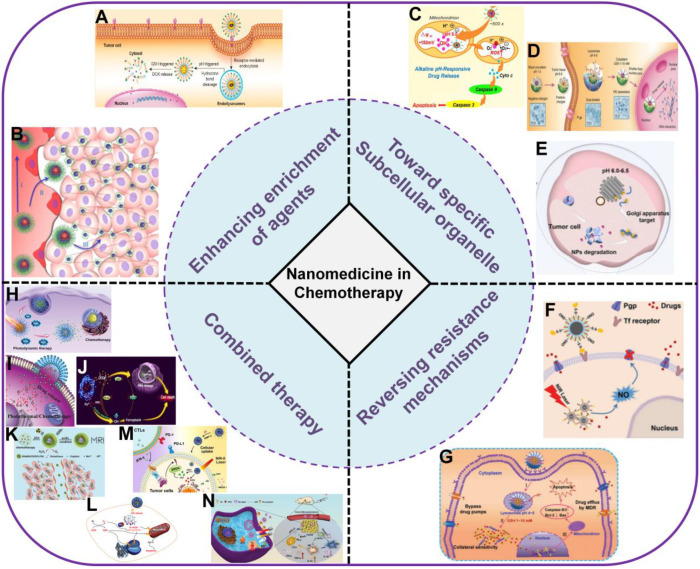
Figure 1.
Schematic illustration of nanocarrier-based drug delivery systems (NDDS) for improving cancer chemotherapy based on different strategies. (A) Targeted drug delivery 45. Copyright 2016, ACS Publications, (B) Promoting tissue penetration 59. Copyright 2016, ACS Publications, (C) Mitochondria-targeted chemotherapy 74. Copyright 2018, Elsevier, (D) Nucleus-targeted chemotherapy 78. Copyright 2015, Wiley-VCH, (E) Golgi-targeted chemotherapy 80. Copyright 2019, ACS Publications, (F) Inhibition of P-gp 97. Copyright 2017, Wiley-VCH, (G) π-π stacked dual anticancer drug 98. Copyright 2016, Wiley-VCH, (H) Combination with PDT 136. Copyright 2020, Wiley-VCH, (I) Combination with PTT 142. Copyright 2020, Wiley-VCH, (J) Combination with CDT 113. Copyright 2020, ACS Publications, (K) Combination with radiotherapy 114. Copyright 2017, Wiley-VCH, (L) Combination with gas therapy 163. Copyright 2018, ACS Publications, (M) Combination with immunotherapy 166. Copyright 2021, Elsevier, (N) Multiple combination therapy 133. Copyright 2019, Wiley-VCH.
Enhancing enrichment of chemotherapy agents in tumor tissue
Targeted drug delivery
The ultimate goal of NDDSs is to achieve targeted drug therapy. In the past decade, to improve drug delivery efficiency, NDDSs with active targeting functions has become a heavily researched topic. To deliver more drugs to tumor tissue/cells, NDDSs can be modified with different targeting ligands on their surfaces, which allow them to specifically target tumor blood vessels or tumor cells.
Targeting tumor blood vessels
In the rapid growth process of tumor tissue, specific antigens or receptors are abnormally expressed on the surface of tumor vascular endothelial cells, while they are less or even unexpressed on the surface of blood vessels in normal tissues 33, 34. Therefore, researchers have grafted corresponding antibodies or ligands onto the surface of NDDSs to increase their enrichment in tumor blood vessels and achieve targeted delivery of drugs.
Because arginine/glycine/aspartic acid (RGD) can specifically bind to the integrin receptor αvβ3, which is overexpressed in tumor neovascularization 35-37, it is often used as a tumor vascular targeting ligand and used to modify on the surface of nanocarriers to achieve tumor-targeted therapy. For example, Schiffelers et al. 38 reported liposomes with cyclic 5mr RGD (c(RGDf(ε-S-acetyl-thioacetyl)K) peptides that could target integrin αvβ3 on tumor vascular endothelial cells. The results of in vitro experiments confirmed that the modified liposomes significantly increased drug accumulation in tumor tissues compared to the non-RGD-modified liposomes. In addition, endothelial growth factor receptor 1 (VEGFR-1/Flt-1) and receptor 3 (VEGFR-3) are highly expressed on various tumor vascular endothelial cells. Therefore, VEGFR-1 and VEGFR-3 are also used as targeting ligands for promoter-targeted delivery. For example, Wang et al. 39 reported new tumor blood vessel-targeting nanoparticles, vincristine-loaded and F56-peptide conjugated nanoparticles (named F56-VCR-NPs); the F56 peptide has high affinity and specific VEGFR-1 binding ability and can achieve a high degree of cell internalization. In vitro and in vivo experimental results confirmed that F56-VCR-NP accurately targeted neovascularization in colorectal cancer, inducing tumor vascular endothelial cells to internalize nanoparticles, and significantly prolonged the survival time of mice without significant toxicity. The Esbp peptide (DITWDQLWDLMK) can also be used as a vascular targeting ligand due to its high affinity for E-selectin 40. For example, Shamay et al. 41 synthesized N-(2-hydroxypropyl) methacrylamide (HPMA) copolymers conjugated to Esbp peptide and equipped with doxorubicin (DOX) (P-(Esbp)-DOX). The results of in vivo experiments showed that P-(Esbp)-DOX significantly reduced tumor growth rate and prolonged the survival rate of lung cancer mice compared to those treated with a copolymer (P-DOX) or free DOX. In addition to the vascular targeting ligands mentioned above, KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK peptide (F3), a 31-amino acid peptide, can also be used as a tumor-targeting peptide due to its preferential targeting of tumor blood vessels and tumor cells 42 and is usually grafted onto the surface of nanoparticles to target the tumor vasculature and increase the accumulation of nanoparticles in tumor blood vessels.
Targeting tumor cell membranes
Compared with normal cells, certain specific receptors or antigens are overexpressed on tumor cell membranes. Therefore, surface modification of nanocarriers with cell membrane targeting ligands can endow them with active targeting capabilities. Cell membrane targeting ligands mainly include folic acid (FA) 43, hyaluronic acid (HA) 44, phenylboronic acid (PBA) 45, aptamers 46, and peptides 47, et al.
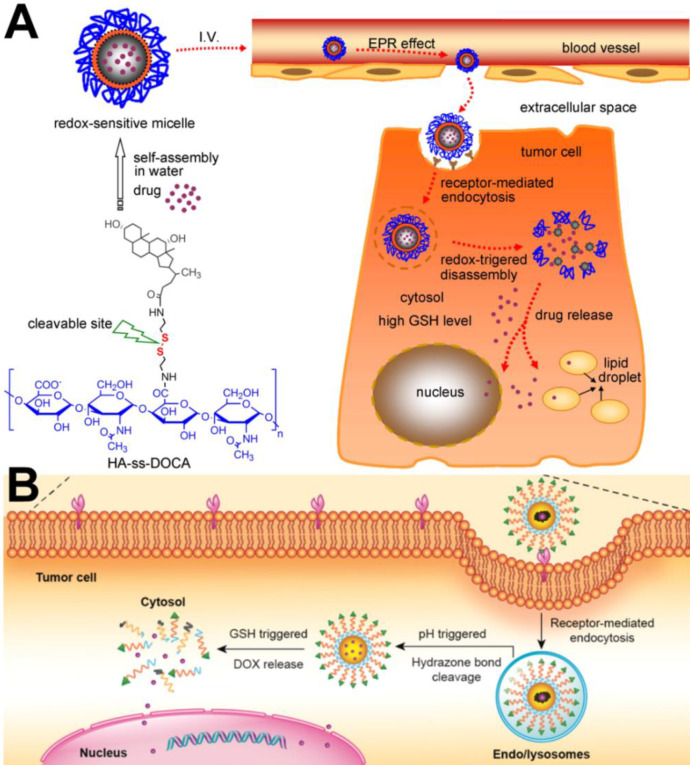
As the most commonly used cell membrane targeting ligand, FA can bind to the folate receptor, which is overexpressed on the membrane surface of a variety of tumor cells (e.g., breast cancer, ovarian cancer, and osteosarcoma cells). Zhou et al. 48 designed and developed actively targeted prodrug polymer micelles. First, FA was conjugated to the end of hydrophilic chain segments of the amphiphilic copolymer polyethylene glycol-b-polycaprolactone (PEG-b-PCL). DOX was then grafted on the end of the hydrophobic chain segments through an acid-sensitive bond, and FA-PEG-b-PCL-hyd-DOX micelles were finally prepared. Flow cytometry (FACS) and laser confocal microscopy (CLSM) confirmed that the FA-modified prodrug micelles could be internalized in a large amount by 4T1 tumor cells, and in vivo experimental studies showed that the FA-modified prodrug micelles could increase DOX enrichment in tumor tissues and exhibit better antitumor activity than micelles without FA modification. Similarly, HA, another commonly used targeting ligand, can specifically bind to the overexpressed CD44 receptor on the cell membrane and increase the cell membrane targeting ability of nanocarriers 49. For example, Li et al. 50 successfully developed a targeted cell drug delivery system based on redox sensitivity: hyaluronic acid-deoxycholic acid conjugate (HA-ss-DOCA) (Figure 2A). It was confirmed that HA-ss-DOCA micelles could be internalized in a large amount by human breast adenocarcinoma cells (MDA-MB-231) through endocytosis mediated via the HA-CD44 receptor. As a new targeting ligand, PBA can bind to the overexpressed sialic acid receptor and is often used to modify the surface of nanocarriers. Zhou et al. 35 designed a PBA-modified prodrug micelle (PBA-PEG-SS-PCL-hyd-DOX) with a GSH/acid response performance (Figure 2B). PBA increased internalization of the prodrug micelles by HepG2 cells and the cytotoxicity of DOX. In addition, Tang et al. 51 prepared PBA-modified magnetic mesoporous silicon nanoparticles. In vivo and in vitro experimental results confirmed that an external magnetic field and PBA could not only increase enrichment of DOX-loaded nanoparticles in tumor tissues but also increase the amount of DOX in HepG2 cells. Thus, compared with other control groups, external magnetic field and PBA achieved better antitumor effects of PBA-modified magnetic mesoporous silicon nanoparticles.
Figure 2.
(A) Illustration of the self-assembly and intracellular trafficking pathway of redox-sensitive HA-ss-DOCA micelles 50. Copyright 2012, Elsevier. (B) PBA ligand-mediated endocytosis and Intracellular drug release triggered by GSH 45. Copyright 2016, ACS Publications.
Promoting tissue penetration
NDDSs can not only improve the solubility of chemotherapeutics but can also reduce the toxicity of systemic chemotherapy to normal tissues. Currently, commonly used chemotherapy drugs include DOX, cisplatin, paclitaxel (PTX), and camptothecin (CPT). At present, chemotherapeutic drugs are mainly loaded in NDDSs in two forms: physical loading and chemical grafting. For example, Kinoh et al. 52 linked the epirubicin (Epi) to polyaspartic acid through a pH-sensitive hydrazide bond, and then loaded staurosporine via intermolecular interactions to obtain dual-drug-loaded copolymer micelles. Although nanomedicines have often shown greatly enhanced therapeutic efficacy in preclinical studies compared with traditional small molecule drugs, their efficacy in the clinical setting is suboptimal due to the heterogeneity of the EPR effect and the biological barriers of tumors hindering effective penetration of NPs 53. It has been found that the extracellular matrix (ECM) 54 and a high interstitial fluid pressure (IFP) 55 in tumor tissues form a primary biological barrier to prevent nanomedicines from penetrating into tumor tissues, which is the reason why we frequently observe that nanomedicines are located around the tumor blood vessel walls of tumors and rarely diffuse deeper into tumor tissues 56. Tumor penetration of nanomedicines is highly dependent on their physicochemical characteristics (size, surface charge, and particle shape), therefore, it is very helpful to explore effective strategies to enhance therapeutic tumor penetration by tuning these factors.
Switchable size
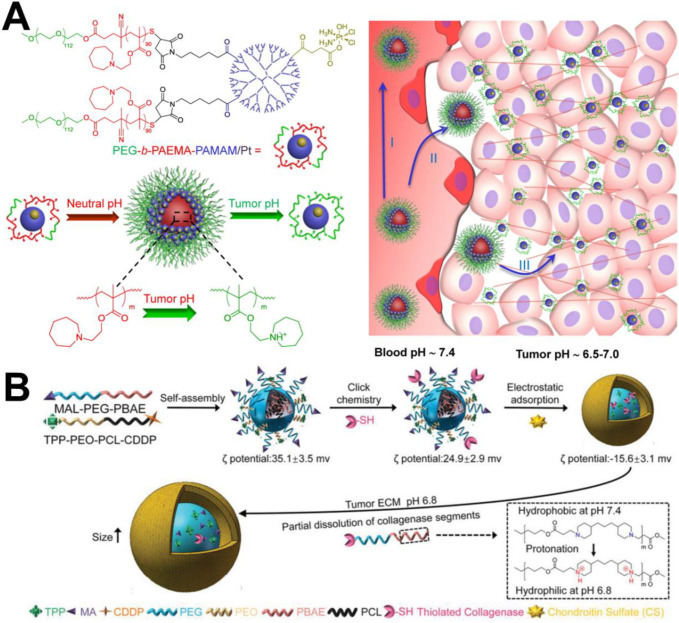
It has been reported that particles with small diameter show higher tumor penetration efficiency than large particles 57, but with a smaller particle size, less retention of particles in tumor tissue occurs because they are able to re-enter the bloodstream or are filtered by the renal system. Therefore, to solve this problem, it may be an efficient strategy to design nanocarriers with changeable particle size to achieve enhanced tumor penetration. For example, Wang et al. 58 designed and synthesized the polymer prodrug PCL-CDM-PAMAM/Pt conjugated with cisplatin and the amphiphilic copolymer PEG-b-PCL. The two polymer chains formed micelles via self-assembly. At physiological pH, the micelles hold a size of approximately 100 nm and have a high propensity for long blood circulation and enhanced tumor accumulation through the EPR effect. An acidic environment (pH 6.8) triggers the release of small dendrimers of polyamide-amine (PAMAM) prodrugs that enable deep and uniform tumor penetration to reach more cancer cells. Similarly, this research group 59 also prepared PEG-b-PAEMA-PAMAM/Pt nanoparticles with the ability to penetrate tumor tissue. The difference was that the polyethyl methacrylate (PAEMA) in the nanoparticle structure was hydrophobic under physiological conditions and could become hydrophilic under acidic conditions. Therefore, the size of the nanoparticles was approximately 80 nm in the blood circulation and decreased after arriving at the tumor microenvironment because PAEMA changed from hydrophilic to hydrophobic, which was conducive to tissue penetration (Figure 3A).
Figure 3.
(A) Schematic illustration of the self-assembly of PEG-b-PAEMA-PAMAM/Pt into the pH-sensitive cluster nanobombs (SCNs/Pt) at neutral pH and the disintegration of SCNs/Pt into small particles at tumor acidic pH 59. Copyright 2016, ACS Publications. (B) Fabrication and response of size-changeable collagenase-modified nanoscavenger (CS/Col-TCPPB NPs) 66. Copyright 2020, Wiley-VCH.
Switchable surface charge
Surface charge is another influential physicochemical characteristic of nanomedicines due to their penetration into tumors. Some studies have shown that cationic nanoparticles target tumor endothelial cells and exhibit higher vascular permeability than neutral or anionic nanoparticles 53. However, cationic nanoparticles easily adhere to the tumor ECM, decreasing their effective diffusivity 60. To prolong blood circulation and promote efficient tumor uptake/penetration, nanoparticles (NPs) that are neutral or have a negative surface charge are preferred. Once NPs reach tumor tissue, positive charges are necessary to enhance tumor retention and cellular internalization through strong electrostatic interactions with negatively charged cell membranes. Therefore, designing nanocarriers with a switchable surface charge is an important way to address the above question.
Chen et al. 61 reported a pH-responsive zwitterionic poly(carboxybetaine) (PCB)-like zwitterion-modified nanomedicine with zwitterionic-to-cationic (ZTC) charge conversion ability (denoted as ZTC-NMs) for CPT delivery. ZTC-NMs showed high stability during blood circulation due to their nanosized diameter and PCB-like zwitterionic surface modification. After entering tumor tissue, the amide bond formed between 2,3-dimethylmaleic anhydride (DMMA) and the amino group responded to the acidic tumor microenvironment and achieved acid-responsive cleavage, leading to ZTC surface charge conversion of the ZTC-NMs. Then, the highly positive quaternary ammonium salt could induce rapid internalization of the NPs by tumor cells through effective electrostatic interactions with negatively charged cell membranes. Therefore, the ZTC charge conversion property can improve the cellular internalization efficiency of NMs and thus promote efficient drug penetration.
Particle shape
In addition to the above strategies, morphology can also improve penetration of nanocarriers into tumor tissues and enhance the effect of chemotherapy. For example, Zeng et al. 67 designed a worm-like drug-loaded micelle (RNW) with tumor targeting and pH responsiveness. The drug-loaded micelles could not only actively target tumor cells, but also had strong tumor penetration and on-demand drug release capability. The system was formed from a pH-responsive amphiphilic copolymer of methoxypoly (ethylene glycol)-block-poly(2-diisopropyl methacrylate) (mPEG-b-PDPA), and disulfide-linked RGD-targeted cytotoxic drug (DM1) conjugates (RGD-SS-DM1). Drug-loaded micelles have the following advantages: 1) they can accurately target brain tumors due to their worm-like structure with the ability to pass through the blood-brain barrier; 2) they have better tumor penetration and internalization efficiency by tumor cells; and 3) they respond to the tumor microenvironment (acidic and reducing substances) and then release drugs as needed. The results of in vivo experiments confirmed that this system had a good inhibitory effect in an in-situ glioma model with an inhibition rate of 88.9%.
Scrapable extracellular matrix
The extracellular matrix (ECM) (e.g., HA and collagen fibre) has become one of the most important factors that can seriously prevent deep penetration of NPs in the intercellular space. Therefore, researchers have been studying how to degrade the extracellular matrix and increase the penetration of NPs. Certain exogenous enzymes can consume tumor matrix components and can be used to improve the penetration of NPs. For example, Zhou et al. 62 designed HPEG-PH20-NPs nanocarriers containing human hyaluronidase PH20 (rHuPH20). It confirmed that the HPEG-PH20-NPs had a good ability to remove hyaluronic acid in vitro. In addition, in vivo antitumor experiments confirmed that DOX-loaded HPEG-PH20-NPs could better inhibit the growth of 4T1 breast cancer tumors. In another report, Liu et al. 63 used hyaluronidase to degrade hyaluronic acid in the tumor microenvironment, which improved tissue permeability to improve drug diffusion. Recombinant long-acting hyaluronidase was constructed through genetic engineering technology to improve the bioavailability of subcutaneously administered of macromolecules, and to increase the therapeutic effect of anticancer drugs in vivo. It was verified that collagenase could break down the ECM and enhance the interstitial diffusion rate of nanocarriers in tumor tissue 64. For example, Dong et al. 65 proposed a strategy that employed nitric oxide (NO) to activate endogenous matrix metalloproteinases (MMP-1 and MMP-2) and to induce collagen consumption to improve drug penetration in solid tumors. In their report, mesoporous silica nanoparticles (MSNs) were used as a NDDSs to load DOX and NO donors (S-nitrosothiol) simultaneously, obtaining DN@MSNs. NO-loaded MSNs could induce MMP activation, which led to collagen degradaion in the tumor extracellular matrix, thereby enhancing the penetration of both the nanovehicle and DOX into the tumor tissue and significantly improving the antitumor effect of chemotherapy with no obvious systemic side effects. Xu et al. 66 developed novel size-changeable collagenase-modified polymer micelles (named CS/Col-TCPPB) to simultaneously enhance penetration and retention of nanocarriers in deep tumor tissue to enhance cancer therapeutic efficiency. The preparation process can be found in Figure 3B. After CS/Col-TCPPB arrived at the tumor site, the PBAE segments turned from hydrophobic to hydrophilic due to protonation of the tertiary amino group in the acidic tumor environment (pH 6.8), thereby exposing collagenase, promoting enzymatic digestion of collagen fibres, and enhancing the intratumoral penetration of the drug-loaded nanoparticles.
Chemotherapy towards specific subcellular organelles
To achieve the therapeutic effects of chemotherapeutic drugs according to their respective mechanisms of action, it is well known that almost all of them must be targeted to specific sites of action 68. For example, DOX can induce cancer cell apoptosis by inhibiting the activity of topoisomerase Ⅱ and damaging DNA, while PTX can cause cell death by inhibiting the microtubule depolymerization in the cytoplasm 69. Therefore, to obtain a satisfactory therapeutic outcome, the ideal approach is to ensure that the appropriate therapeutic agents with optimal concentration can be located at the right place. For these reasons, simply delivering therapeutic agents into tumor tissues or cells is not sufficient enough to obtain the desired therapeutic effect. To tackle this problem, it is particularly important to achieve targeted delivery of therapeutic agents to subcellular organelles, which is the best strategy to completely eradicate tumors and prevent tumor recurrence, invasion, and metastasis 70, 71. At present, research on targeted subcellular organelle delivery based on NDDSs mainly includes targeting mitochondria and nuclei.
Mitochondria-targeted chemotherapy
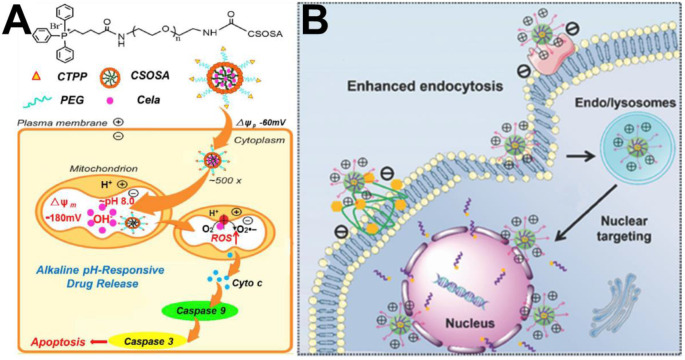
In the past several decades, anticancer strategies based on targeting mitochondria have received much attention due to their crucial functions in the cell. To date, triphenylphosphine (TPP) is the most commonly used functional group for transporting mitochondriotoxic agents to mitochondria because it can embed into the mitochondrial membrane 72. Yu et al. 73 constructed a pillar arene-based rotaxane (R1) by using tetraphenylethene (TPE) and TPP moieties as stoppers; the TPE unit acted as the aggregation-induced emission (AIE) reagent, and the TPP group was used as a mitochondria-targeted unit. DOX was introduced into R1 through acid-sensitive bonds to form fluorescence resonance transfer (FRET)-capable DOX-loaded nanoparticles. In vitro cell experiments confirmed that large amounts of DOX could be released from the DOX-loaded nanoparticles and enriched in mitochondria to kill cancer cells after nanoparticles are internalized by HeLa cells. In another report, Tan et al. 74 produced mitochondria-targeted nanocarriers (CTPP-CSOSA) by choosing the lipophilic cation (4-carboxybutyl) triphenylphosphonium bromide (a type of TPP cation, CTPP) to modify glucolipid-like conjugates (CSOSA). Celastrol-loaded micelles (CTPP-CSOSA/Cela) selectively targeted mitochondria and responded to the mitochondrial alkaline pH environment (pH 8.0) and released Cela, which induced reactive oxygen species (ROS) generation, further activating a cascade of Caspase 9 and Caspase 3 reactions and promoting tumor cell apoptosis by regulating mitochondrial signalling pathways (Figure 4A).
Figure 4.
(A) The schematic illustration of drug delivery system with mitochondrial alkaline pH-responsive release 74. Copyright 2018, Elsevier. (B) The schematic illustration of Tat-mediated enhanced endocytosis into tumor cells and nuclear targeting 77. Copyright 2018, Wiley-VCH.
Nucleus-targeted chemotherapy
Because the final destination of many first-line chemotherapeutics (e.g., DOX, cisplatin, and CPT) is DNA or its associated enzymes in the nucleus, these drugs must be transported into cellular nuclei to exert their anticancer effect 70. Unfortunately, an agent transported from the cytoplasm into the nucleus must pass through the nuclear pore complexes (NPCs) 75, and only sufficiently small molecules can enter nuclei through passive diffusion, especially in proliferating cells. Therefore, the development of nuclear-targeted drug delivery mostly relies on nanocarriers with nuclear accumulation capacity. At present, the design ideas for nuclear-targeted nanocarriers can be summarized into the following two types: 1) modification of nanocarriers with nuclear targeting peptides to facilitate nuclear enrichment and 2) preparation of nanocarriers with large-to-small size-changeable performance after internalization by cancer cells to activate nuclear entry.
The positively charged TAT peptide has been demonstrated to promote nuclear delivery of TAT-modified nanoparticles, which significantly enhances nuclear drug delivery 76. For example, Zhou et al. 77 designed multifunctional micelles with high nuclear targeting of therapeutics, which were constructed from poly(ethylene glycol)-poly(ε-caprolactone) with 2,3-dimethylmaleic anhydride-TAT decoration (PECL/DA-TAT). As shown in Figure 4B, in a mildly acidic environment (pH 6.8), these micelles facilitated cell internalization and subsequent nuclear targeting of the chemotherapeutic 10-hydroxycamptothecin, and obviously enhanced cytotoxicity against 4T1 and A549 cells.
In addition, as confirmed in previous studies, small-sized NPs (<50 nm) have the advantage of passing through NPCs and efficient nuclear uptake. Therefore, depending on the specific microenvironment in the cancer cell, size-changeable nanocarriers are a promising drug delivery system to actively transport chemotherapeutic drugs to cancer cell nuclei. That is, the size of the nanoparticles at the initial stage should be large enough to reduce renal clearance and maintain a good EPR effect, but once internalized by the cancer cell, the NPs are able to decrease to a smaller size for nuclear uptake. For example, in a “proof-of-concept” study, Zhou et al. 78 designed mPEG-PLA-ss-PEI-DMMA (PELEss-DA) polymer micelles as drug delivery systems with variable sizes from large into small to facilitate nuclear entry and release of therapeutics in the nucleoplasm. In this well-defined core-corona structure, a polylactide (PLA) segment was used as the core, and two water-soluble polymers, namely methoxy poly(ethyleneglycol) (mPEG) and polyethyleneimine (PEI), were used as the corona material. Specifically, the positive charges of PEI were masked through amidation to ensure good stability and long blood circulation of the carriers under physiological conditions (pH 7.4). Due to the charge reversal and subsequent size enlargement in acidic pH tumor tissues, greater cell internalization and faster lysosome escape via the proton sponge effect of PEI occurred. Then, due to deshielding of the PEI shell via the cleavage of disulfide bonds by intracellular GSH, a sufficiently small PELEss-DA micelle was produced, which could effectively transport the drug into the cell nucleus.
In addition, external stimuli-triggers (e.g., light and ultrasound) have also been used to change the size of nanocarriers to achieve drug delivery into the nucleus. For example, Tan et al. 79 developed a size-photocontrollable nanoplatform via DNA hybridization, in which a small nucleus-uptake nanodrug system (DOX-loaded gold NPs) was assembled onto a larger cell-targeted near-infrared light (NIR)-responsive silver-gold nanorod (NR). In vitro experimental results showed that the photothermal effect of the NR under NIR irradiation caused DNA dehybridization and release of the NPs, which further entered the nuclei using the advantage of their small particle size. Therefore, this nanoplatform promoted accumulation of the anticancer drug DOX at its target site.
Others
Because endo/lysosome, the golgi apparatus, endoplasmic reticulum (ER), and other subcellular organelles have certain roles in cancer cells, they can also be studied as chemotherapy targets. For example, Gong et al. 80 developed a Golgi apparatus-targeting prodrug nanoparticle system by synthesizing retinoic acid (RA)-conjugated chondroitin sulfate (CS) (CS-RA). The prodrug nanoparticles appeared to accumulate in the Golgi apparatus in cancer cells and improve RA release in an acidic environment. In addition, DOX and cisplatin can be directly transported into the ER, lead to cancer cell death via severe ER stress. Similarly, PTX can induce lysosomal membrane permeabilization (LMP) and activate the lysosomal cell death pathway in cancerous cell, therefore it can be transported into lysosomes to kill cancer cells. In our opinion, this study area will open a new paradigm for precise and high-performance cancer therapy by exploring new subcellular-targeted chemotherapies.
Reversing resistance mechanisms
MDR encompasses a broad spectrum of defence mechanisms by cancer cells, which makes them resistant to one or more chemotherapeutic drugs by decreasing uptake, increasing efflux, inactivating drugs, activating DNA repair mechanisms, upregulating metabolism, and/or stimulating detoxification pathways 81. Therefore, the failure of chemotherapy against cancer often occurs because of MDR 82, 83. To overcome MDR, the traditional approach is using higher doses or greater frequencies of chemotherapeutic agents 84. Although these measures can improve antitumor efficacy to a certain extent, they are far from optimal due to the serious side effects or toxicity to healthy tissues and organs caused by nonspecific treatments. Hence, there is a strong incentive to develop other optimized strategies to overcome MDR to maximize the therapeutic index of anticancer drugs.
At present, research has focused on investigating the molecular pathways mediating MDR in an effort to develop rational strategies for intervention. Overexpression of the ATP-binding cassette (ABC) is the major molecular mechanism of MDR. In particular, P-glycoprotein (P-gp) is one of the main ABC transporter proteins and is overexpressed on the cell-membrane. P-gp has the ability to pump cytotoxic agents out of cells 84. Therefore, overcoming the P-gp-mediated MDR mechanism is one of most widely employed strategies to improve the effect of chemotherapeutics on MDR tumors 85. Although NPs can enhance chemotherapeutic uptake by cells via phagocytosis, which can bypass the efflux action of P-gp, and active targeting ligands on the surface of nanoparticles can also facilitate the evasion of the P-gp pathway via receptor-mediated endocytosis 84, these measures cannot fundamentally solve the problem of MDR. Consequently, combination chemotherapy with P-gp inhibitors is considered the most reliable strategy and is applied for treatment of multidrug resistant tumors.
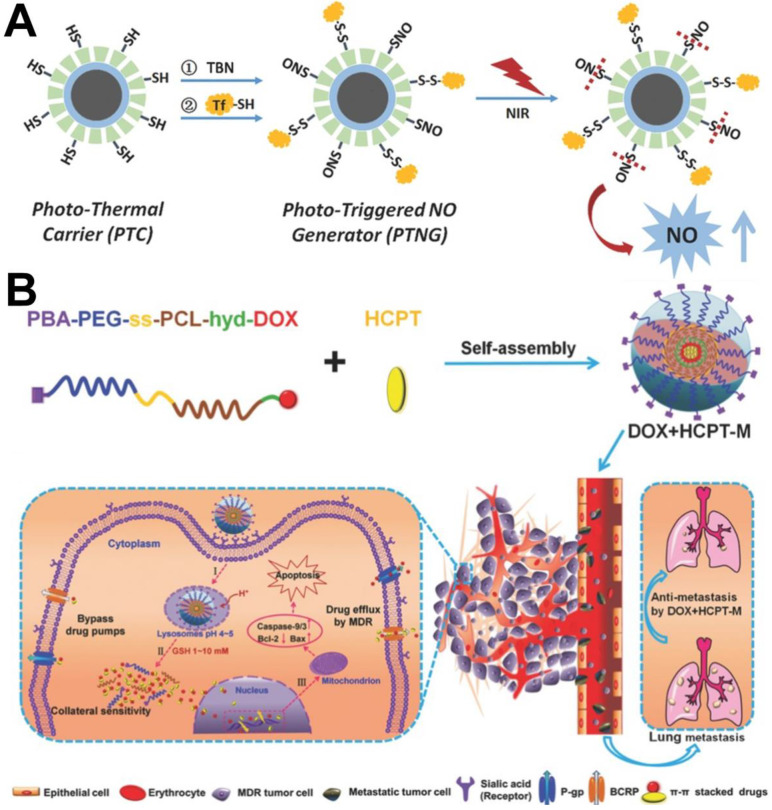
P-gp inhibitors can be classified into organic small molecules (e.g. cyclosporin A 86, vitamin E 87, verapamil hydrochloride 88 and curcumin 89), small interfering RNA (siRNA) 90, gas molecules (H2 92 and NO 93) and ions (e.g. Ca2+ 91). Among them, siRNA and gas molecules are widely used to overcome MDR. For example, Cheng et al. 94 successfully developed a multifunctional nanoplatform (M-R@D-PDA-PEG-FA-D) that integrates chemotherapy/photothermal therapy/gene therapy. Because the nanocarrier could simultaneously deliver siRNA and DOX into MCF-7/ADR cells, in vitro western blotting results showed that the expression of P-gp protein could be effectively downregulated by siRNA, resulting in higher concentrations of DOX in the MCF-7/ADR cancer cells to significantly improve chemotherapy. In addition, because NO can inhibit expression of the P-gp, it is also often used as a P-gp inhibitor to reverse MDR tumors 95. For example, Chen et al. 96 designed a nanomicelle mPEG-PLGA containing both BNN6 and DOX; BNN6 could decompose and produce NO to inhibit DOX efflux mediated by P-gp under light conditions. In another report, Yang et al. 97 designed NPs that could reverse MDR by inhibiting overexpression of P-gp via NO. The difference in this design was that the NO donor (nitrosothiol, SNO) was connected to the surface of the nanoparticle through a chemical bond and could be decomposed to produce NO via heat (Figure 5A).
Figure 5.
(A) Schematic illustration of the fabrication of phototriggered NO nanogenerators (PTNGs) 97. Copyright 2017, Wiley-VCH. (B) Schematic illustration of combination of DOX and HCPT using a multifunctional micelle to combat MDR and lung metastases 98. Copyright 2016, Wiley-VCH.
In addition to using P-gp inhibitors, Zhou et al. 98 developed another new strategy to evade drug pumps recognition by co-delivering π-π stacked dual anticancer drugs (DOX and CPT). As shown in Figure 5B, the DOX prodrug copolymer PBA-PEG-ss-PCL-hyd-DOX, which was synthesized by conjugating DOX to the hydrophobic chain of the polymer backbone via a pH-responsive hydrazine bond, could encapsulate HCPT via π-π stacking between DOX and HCPT to obtain dual drug-loaded micelles named DOX+HCPT-M. The results of in vitro experiments verified that the released drugs with complex aromatic π-π conjugated structures could evade recognition by drug pumps due to a slight change in the molecular structure of the drugs and display high therapeutic efficacy in MCF-7/ADR cancer cells.
Combined chemotherapy
A single therapeutic modality is one reason for the undesirable outcomes of clinical chemotherapy, and it is difficult to achieve satisfactory anticancer effects. Compared to monochemotherapy, combination regimens can target different therapeutic pathways in cancer cells and lower drug doses to reduce side effects. Therefore, in recent years, combination approaches for enhancing cancer chemotherapy have been explored, such as chemotherapy combined with photodynamic therapy, photothermal therapy, chemodynamic therapy, radiotherapy, gas therapy, and immunotherapy and multiple therapy approaches (see Table 2).
Table 2.
Summary of chemotherapy-based combined cancer treatment
| Combinational type | Agent regimen | Nanoplatform | Cancer type | Ref. | |
|---|---|---|---|---|---|
| In vitro | In vivo | ||||
| Combination with PDT | CPT + PtNP | CPT-TK-HPPH/Pt NP | CT26 | CT26 | 134 |
| Cisplatin + Ce6 | PCT@HCCT | 4T1 | 4T1 | 68 | |
| TPZ + porphyrinic MOFs | TPZ/UCSs | CT26 | CT26 | 135 | |
| CPT + PPa | MPEG-(TK-CPT)-PPa | HCT116 | HCT116 | 136 | |
| DOX + Ps | DOX-loaded; H-LTDC | HCT-116 | HCT-116 | 101 | |
| TPZ + ICG | iNP/IZ | 4T1 | 4T1 | 103 | |
| TPZ +Ce6 | Lip/Ce6/TPZ-PmiRNA | MCF-7 | MCF-7 | 102 | |
| TPZ +Ce6 | TPZ/AI-NVs | HepG2 | HepG2 | 138 | |
| Combination with PTT | SN38-Nif + ZrC NSs | ZrC@prodrug | A549 and SMMC-7721 | SMMC-7721 | 139 |
| DOX + polyaniline | PANI-ES@AOT-V-D | MCF-7 and Hela | MCF-7 | 140 | |
| DXL + AuNPs | Au/Fe3O4/PVA-10%DXL | MCF-7 | MCF-7 | 141 | |
| DOX + PDA | F@PDA-TPP/SS/DOX | B16F10 | B16F10 | 106 | |
| DOX + Bi2Se3 | Bi2Se3/DOX@MPs | H22 | H22 | 142 | |
| DOX + iron oxide nanoparticles | DOX/MNP-PMs | A549 | / | 143 | |
| DOX + MPN | MSN@MPN@DOX | A549 | / | 144 | |
| DOX + B nanosheets | DOX-17AAG@B-PEG-cRGD | MDA-MB-231 | MDA-MB-231 | 145 | |
| DOX + Cu2-xSe | PT-V@TPDOX | MCF-7 and MCF-7/ADR | MCF-7/ADR | 72 | |
| Pt(IV) + IR780 | Pt-I-IR780 NPs | 4T1 | 4T1 | 146 | |
| Tyroservatide + PpIX | PpIX NAs | 4T1, MCF-7, A2780/Taxol and MCF-7/DOX | 4T1 | 147 | |
| DOX/TAX + PpIX | PM-DOX-TAX | MDA-MB-231 | MDA-MB-231 | 105 | |
| DOX + IR820 | LA-IR820/DOX ND | HepG2 | / | 148 | |
| Combination with CDT | TMZ + MnO | iRPPA@TMZ/MnO | C6 | C6 | 149 |
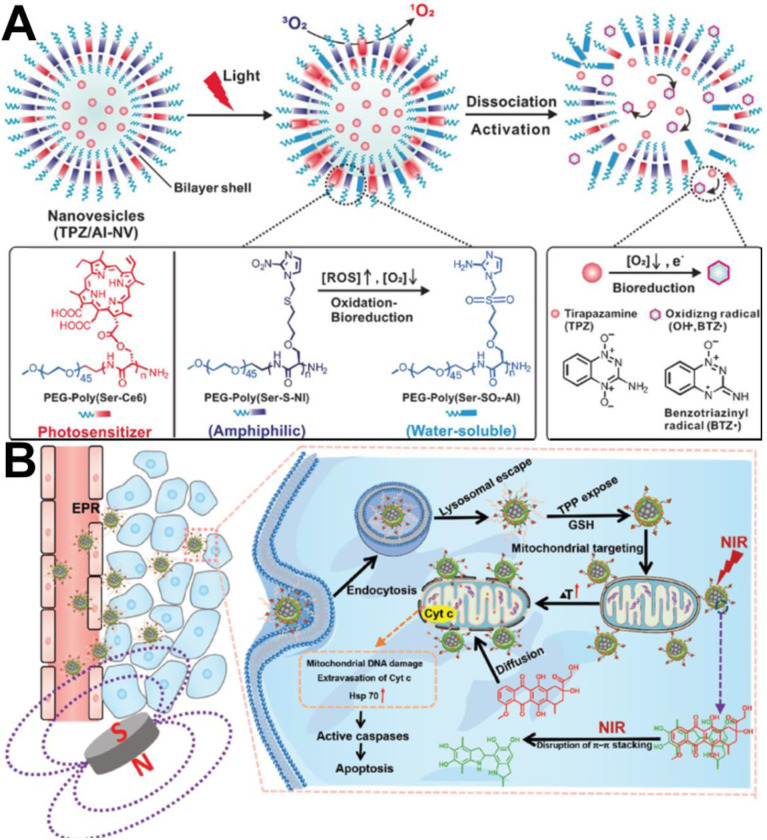
| Cisplatin + Fe(III) | PtH@FeP | 4T1 | 4T1 | 113 | |
| TPZ + Fe(III) | HGTFT | 4T1 | 4T1 | 150 | |
| DOX + Cu (Ⅱ) | DOX@Cu2O-PEG NCs | MCF-7 | MCF-7 | 151 | |
| Cisplatin + EGCG | PTCG NPs |
HepG2 | HepG2 | 112 | |
| DOX + MIL-100 | DMH NPs | MCF-7 | MCF-7 | 108 | |
| Platinum(IV) + MnO2 | UCMnPt | HepG2 | HepG2 | 152 | |
| DOX + MnO2 | AMSNs/DOX | Huh7 | Huh7 | 153 | |
| CPT + MnO2 | MS@MnO2-CPT | U87MG | U87MG | 154 | |
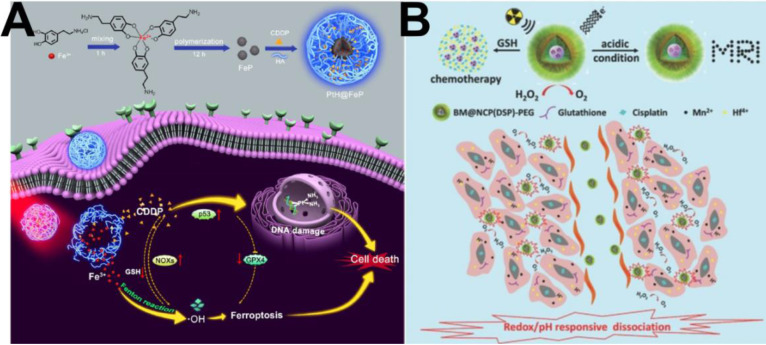
| CPT + Iron oxide NPs | LaCIONPs | A549 | A549 | 155 | |
| platinum(IV) + Fe3+ | Fe-DSCP-PEG-cRGD | C6 | C6 | 156 | |
| DOX + PB | DOX-Au@PB | SMMC-7721 | SMMC-7721 | 157 | |
| DOX + copper/iron(II) | Cu-Fe-MSNs-DOX | Hela | / | 158 | |
| DOX+ MnO2 | MnO2@HMCuS-DOX | MCF-7 | / | 159 | |
| Cisplatin + Fe2/3+ | Pt&Fe3O4@PP | U87 MG | U87 MG | 160 | |
| Vitamin k3 + Cu2+ | Vk3@MOF-199 NPs | 4T1 | 4T1 | 161 | |
| Combination with radiotherapy | DSP + Hf | BM@NCP(DSP)-PEG | 4T1 | 4T1 | 114 |
| Combination with gas therapy | PTX + S-nitrosoalbumin | Ptx@AlbSNO | 4T1 | 4T1 | 115 |
| DOX + NTC | NO-M@DOX | MCF-7/ADR | MCF-7/ADR | 162 | |
| DOX + GSNO | DOX@GSNO-HAPNs | MCF-7 | MCF-7 | 163 | |
| DOX + DEA/NO | DOX@HA-DNB-DEA/NO | SMMC-772 | SMMC-772 | 164 | |
| Combination with immunotherapy | CUR + NLG919 | PEG-CDM-PEI-P(CURDT) | B16F10 | B16F10 | 126 |
| DOX + R837 | PPP-DOX + ACP-R837 | 4T1 | 4T1 | 127 | |
| DTX + GM-CSF | gETL NPs | CT26 | CT26 | 165 | |
| DOX + JQ1 | IRG@JQ1/DOX | 4T1 | 4T1 | 166 | |
| SF + M1-type macrophages | M1/SLNPs | Hepa1-6 | Hepa1-6 | 167 | |
| DOX + siRNA | PEG@D:siRNA | CT26 | CT26 | 124 | |
| DOX + anti-PD-L1 | CM@MON@DOX | 4T1 | 4T1 | 168 | |
| PTX + anti-PD-1 | sAMcP | B16F10 | B16F10 | 125 | |
| Oxaliplatin + ApoA1 mimic peptide | TA-OBL | 4T1 | 4T1 | 169 | |
| DOX +CpG | LMWH/PPD/CpG | B16F10 | B16F10 | 127 | |
| GEM + 1MT | GEM-1MT NPs | B16F10 | B16F10 | 170 | |
| Multiple combination therapy | SN38 + PDA + PZM | FA-PPSM | Eca-109 | Eca-109 | 171 |
| DOX + γ-radiation + Seleninic acid | PSeR/DOX NPs | MDA-MB-231 | MDA-MB-231 | 172 | |
| PTX + IR780 + S-nitrosothiol | HSA-NO | 4T1 | 4T1 | 173 | |
| DOX + Ce6 | PDPC | MCF-7/ADR | MCF-7/ADR | 130 | |
| DOX + BP | BP-DOX | 4T1 | 4T1 | 131 | |
| DOX + BP + aPPL | BP-DcF + aPL | MC-38 | MC-38 | 132 | |
| DTX + PpIX + CpG | FA-CD@PP-CpG | 4T1 | 4T1 | 133 | |
| R837 + IR820 + 1MT | HA/IR820@ZIF-8 + MAN/(R837+1 MT)@ZIF-8 | B16F10 | B16F10 | 174 | |
Combination with photodynamic therapy (PDT)
Chemotherapy combined with PDT is one of the most common strategies to improve anticancer effects. Because PDT functions as a cancer treatment strategy and is associated with cytotoxic ROS, which are produced by photosensitizers (e.g., porphyrin, chlorine e6, indocyanine green, and Rose Bengal) under specific light irradiation 99, 100, coadministration of photosensitizers and chemotherapeutic drugs is involved. For example, Park et al. 101 designed a highly tumor-specific light- triggered drug delivery system (H-LTDC) composed of chondroitin sulfate (CS) and pheophorbide-a (photosensitizer) joined through covalent bonding. Under 670 nm laser irradiation, DOX-loaded H-LTDC could generate ROS to induce degradation of CS, leading to DOX release for chemotherapy. Furthermore, the generated ROS could also be used to kill cancer cells. In particular, although the hypoxic environment of tumor tissue affects PDT efficacy, it is good for several hypoxia-activated prodrugs. For example, tirapazamine (TPZ) exhibits highly selective cytotoxicity toward hypoxic cancer cells. Therefore, combining PDT and TPZ-mediated hypoxia-activated chemotherapy could be promising for enhanced anticancer therapy 102. To date, researchers have used the characteristics of nanomaterials to design different nanomedicines containing TPZ. For example, Wang et al. 103 developed iRGD-modified nanoparticles for simultaneous tumor delivery of the photosensitizers indocyanine green (ICG) and TPZ. In vitro and in vivo experimental results showed that the nanoparticles could significantly improve penetration in both 3D tumor spheroids and orthotopic breast tumors. In addition, under NIR irradiation, ICG-mediated photodynamic therapy induced oxygen consumption and aggravated the hypoxic environment of cancer cells, which further activated the anticancer activity of the codelivered TPZ for a synergistic cell-killing effect.
Subsequently, Qian et al. 104 designed a polymeric nanovesicle (TPZ/AI-NV), which was assembled by combining the photosensitizer chlorine e6 (Ce6)-modified diblock copolymer PEG-Poly(Ser-Ce6) and 2-nitroimidazole (NI) with the thioether-modified diblock copolymer PEG-Poly(Ser-S-NI), to enhance the hypoxia-activatable chemotherapy, as shown in Figure 6A. Compared to the above design, the difference is that the photosensitizer was Ce6, and it was grafted with the main chain of the polymer through a covalent bond, which greatly increased the drug loading capacity and prevented early release of Ce6. In addition, to better release TPZ in cancer cells, the hypoxia-sensitive 2-nitroimidazole (NI) structure was introduced into the backbone. In vitro and in vivo experiments indicated that this nanovesicle efficiently induced apoptotic cell death and significantly inhibited tumor growth.
Figure 6.
(A) Formation and mechanism of TPZ/AI-NV for dissociation of vehicles and simultaneous activation of bioreductive prodrug 104. Copyright 2017, Wiley-VCH. (B) Schematic of F@PDA-TPP/SS/DOX for synergism of PTT and chemotherapy 106. Copyright 2018, Wiley-VCH.
Combination with photothermal therapy (PTT)
Chemotherapy combined with PTT is another common combination therapy strategy and shows great potential to optimize cancer therapy. PTT functions as a physical treatment modality because it is associated with hyperthermia induced by laser irradiation, which increases the local temperature to cause cellular damage in tumors. Various photothermal agents can induce hyperthermia, such as metal-based nanomaterials, carbon-based nanomaterials, magnetic nanoparticles, and organic dyes. When co-administrated with chemotherapeutic agents, these photothermal agents can enhance chemotherapeutic outcomes because hyperthermia can increase vascular permeability within tumors to promote intratumoral transport of drugs 5. For example, Su et al. 105 designed a novel porphyrin-based micelle, which was formed via self-assembly of a hybrid amphiphilic polymer mPEG-PLGA-porphyrin, and the micelle could be used to load DOX and TAX simultaneously via an improved double-emulsion method. Under NIR irradiation, the dual-drug-loaded micelles could generate high heat and release dual drugs to co-kill the cancer cells. More importantly, the combined strategy of PTT and chemotherapy conferred great chemosensitivity to cancer cells and achieved tumor regression using approximately 1/10 of the traditional drug dosage.
In addition to the use of photothermal agents, nanocarriers themselves also have photothermal effects, which can be used for delivering chemotherapeutic drugs to achieve synergism between PTT and chemotherapy. For example, Wang et al. 106 reported a multistage targeting strategy using magnetic composite nanoparticles to provide synergistic PTT and chemotherapy. The magnetic composite nanoparticles (named F@PDA-TPP/SS) were composed of four units: Fe3O4 colloidal nanocrystal clusters (Fe3O4 CNCs) as a core, a polydopamine (PDA) inner shell as the PTT agent functionalized with triphenylphosphonium (TPP) for mitochondrial targeting, and the mPEG outer shell linked by disulfide bonds (ss). DOX was loaded in F@PDA-TPP/SS via π-π stacking between the aromatic regions of PDA and DOX. Under NIR irradiation, the nanoparticles not only rapidly produced a large amount of heat for PTT, but also triggered rapid drug release for chemotherapy at the same time (Figure 6B).
Combination with chemodynamic therapy (CDT)
In recent years, CDT has become an interesting research topic due to its advantages of higher tumor specificity and selectivity, low systemic toxicity, and few side effects. Most importantly, compared to PDT/PTT, CDT does not require a specific stimulation in the treatment process 107. Furthermore, the combination of chemotherapy and CDT can not only reduce the side effects of chemotherapeutic drugs, but can also enhance CDT efficacy. For example, Xue et al. 108 reported a DOX-loaded and modified HA metal-organic framework (MOF) material (named MIL-100@DOX-HA, DMH) for combined CDT and chemotherapy against cancer. Because of the high DOX loading efficiency, DMH could achieve better chemotherapy effects and could produce a large amount of toxic •OH for CDT through Fenton reactions. Therefore, DMH NPs have immense potential for reducing systemic toxicity and improving the therapeutic effect of DOX against breast cancer.
Similarly, due to its good killing effect on cancer cells, the cell death pathway iron-based ferroptosis has received widespread attention in recent years 109. To the best of our knowledge, Fenton/Fenton-like reactions have been illuminated as a clear mechanism to induce ferroptosis in tumor cells through ROS upregulating 110, 111. In particular, the activated cisplatin can specifically elevate the intracellular H2O2 level through cascade reactions. Therefore, cisplatin prodrug-based chemotherapy is an ideal treatment partner for CDT because of its ability to supply H2O2 for the Fenton reaction, thereby obtaining synergetic CDT-chemotherapy 112. For example, Yu et al. 113 successfully prepared a core-shell platform from HA-cisplatin (PtH) cross-linked complexes placed onto a Fe(III)-polydopamine (FeP) core to obtain PtH@FeP for combined CDT-chemotherapy. As shown in Figure 7A, suppression of GPX4 and activation of NOXs subsequently resulted in a high level of H2O2 and lipid peroxidation owing to the participation of CDDP, indirectly resulting in the ferroptosis effect. In addition, the FeP core endowed the nanocarriers with CDT capability, which not only exerted direct tumor elimination activity but also benefited ferroptosis and CDDP cytotoxicity. This work developed a promising strategy for designing ferroptosis-assisted multidrug chemotherapy by amplifying intratumoral oxidative stress. In short, the combination of CDT and chemotherapy can realize a satisfactory treatment performance through a significant synergistic effect and thus has broad potential applications.
Figure 7.
(A) Schematic illustration of PtH@FeP-mediated antitumor synergistic therapy by combining CDDP-induced apoptosis and CDT-based ferroptosis 113. Copyright 2020, ACS Publications. (B) Scheme illustrating the redox/pH responsive behaviors of BM@NCP(DSP)-PEG composite nanoparticles in the tumor microenvironment for cancer chemoradiotherapy 114. Copyright 2017, Wiley-VCH.
Combination with radiotherapy
Radiotherapy is one of the most widely used and effective approaches for cancer treatment in clinic, therefore, the combination of chemotherapy and radiotherapy has also been reported. For example, Liu et al. 114 used the coordination principle of metal ions and organic molecules to synthesize a nanometal complex carrier named BM@NCP (DSP)-PEG containing both a cisplatin prodrug and the radioactive element Hf. The agent Hf ion and the cisplatin prodrug were attached to the surface of bovine serum albumin (BSA) stabilized manganese dioxide (MnO2) nanoparticles via metal coordination, and then, the nanoparticles were modified with PEG to increase their biocompatibility. As shown in Figure 7B, the MnO2 core could catalyze in situ generation of O2 from decomposition of the endogenous H2O2 produced in tumor cells, to overcome the hypoxic environment of tumor tissues and enhance the effect of radiotherapy. In addition, the cisplatin prodrug was cleaved under the condition of a high GSH concentration and then produced a chemotherapeutic effect. The acidic environment also promoted the production of Mn2+ for imaging effects. Therefore, this system contains three functions: imaging functions based on radiotherapy and chemotherapy.
Combination with gas therapy
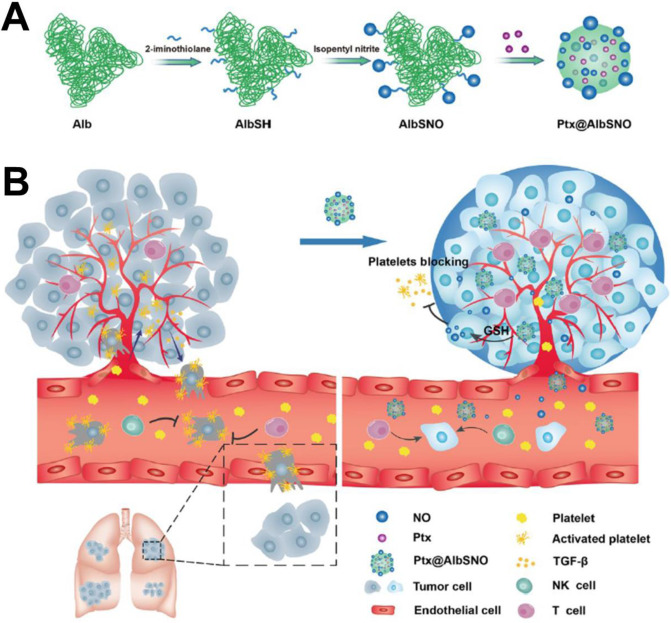
Among new therapies, gas therapy (e.g., NO, hydrogen sulfide, and carbon monoxide) has been developed as an emerging “green” approach for cancer therapy because it does not induce drug resistance and has minimal side effects in normal tissues 115. However, gas is a concentration-dependent “double-edged sword” in the body, which may limit the effect of gas treatment alone. In recent years, gas therapy-based combined cancer therapy, called gas-chemotherapy, has emerged and significantly improves antitumor efficiency by generating synergistic effects. For example, Li et al. 116 designed a tumor microenvironment-responsive NO release nanoparticle (named Ptx@AlbSNO) that was able to specifically and safely codeliver a NO donor and PTX into tumor tissues. As shown in Figure 8, the Ptx@AlbSNO NPs could respond to GSH in the tumor cell and specifically release NO and PTX for synergistic cytotoxicity to suppress primary tumor growth. Furthermore, the released NO inhibits platelet aggregation and adhesion to prevent tumor cell epithelial-mesenchymal transition (EMT), thereby preventing platelet adhesion around circulating tumor cells (CTCs) and reducing distant metastasis. Therefore, NO-based nanoplatforms provide a simple strategy to improve cancer treatment effects.
Figure 8.
(A) Schematic of AlbSNO and Ptx@AlbSNO preparation. (B) Tumor-specific release of NO from Ptx@AlbSNO possesses the dual functions of simultaneously preventing tumor metastasis and reversing tumor immunosuppression by blocking platelets 116. Copyright 2020, ACS Publications.
Combination with gene therapy
Gene therapy, such as plasmid DNA (pDNA), siRNA, and microRNA (miRNA), has been introduced in combination with chemotherapy because it could downregulate/replace disordered genes or silence unwanted gene expression in tumor 117. For example, Kang et al. 118 explored the novel concept of engineering blood exosomes as co-delivery nanosystems, which can efficiently co-loading of DOX and nucleic acids (chol-miR21i) for cancer therapy. They demonstrated that this blood exosome-based nanosystem co-delivered DOX and chol-miR21i into tumor cells with superior tumor accumulation and improved cytosolic release, and the released drugs and RNAs simultaneously interfere with nuclear DNA activity and downregulate the expression of oncogenes, thus remarkably inhibiting the growth of tumors and alleviating side effects.
Combination with immunotherapy
To the best of our knowledge, cancer immunity consists of five steps (called the cancer-immunity cycle) 119: 1) antigen production by cancer cells; 2) antigen delivery by antigen-presenting cells (APCs); 3) activation of T cells by antigens; 4) trafficking and infiltration of T cells to tumors; and 5) recognition and killing of tumor cells by cytotoxic T cells. Therefore, each step can be used as a potential therapeutic target to activate cancer immunotherapy. At present, the combination of chemotherapy and immunotherapy based on NDDSs is a promising approach for improving antitumor efficiency and has been widely studied in preclinical and clinical research because NDDSs can easily be internalized by immune cells and can re-educate the tumor microenvironment (TEM) due to their special physical and chemical properties, thus boosting the immune system 120, 121.
In recent years, immune checkpoint blockers, such as those inducing cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockade, programmed death 1/programmed death ligand 1 (PD-1/PD-L1) blockade, and indoleamine 2,3-dioxygenase (IDO) blockade, have often been used as immunotherapeutic agents combined with chemotherapy to enhance the effectiveness of cancer treatment 122, 123. For example, Wang et al. 124 developed a facile carrier-free nanoassembly of an acid-activatable DOX prodrug and siRNA for combined induction of immunogenic cell death (ICD) and reversal of immunosuppression. As shown in Figure 9A, the carrier-free nanoassembly loaded with the two drugs was formed via cooperative π-π stacking and electrostatic interactions and termed PEG@D:siRNA. When PEG@D:siRNA was internalized by cancer cells, it released DOX and siRNA in the acidic endosomal/lysosomal compartments, and the released DOX induced ICD in tumor cells, and then provoked an antitumor immune response. At the same time, the released siRNA efficiently suppressed upregulation of the immunosuppressive gene PD-L1 during DOX-based chemotherapy, propagating antitumor immunity from ICD and enhancing cancer therapy. Similarly, Shuai et al. 125 reported pH and MMP-2 dual-sensitive polymeric micelles that could co-deliver PTX and anti-PD-1 antibody (aPD-1) for synergistic cancer chemo-immunotherapy. Both in vitro and in vivo experiments showed an enhanced antitumor effect of PTX and aPD-1 in combination chemo-immunotherapy. In another study, Yang et al. 126 reported dual pH/redox-responsive shrinkage and charge-reversal micelles that could co-deliver IDO inhibitors (NLG919) and curcumin (CUR). After the micelles were internalized by cancer cells, CUR and NLG919 were rapidly released from the micelles in the enriched GSH cellular microenvironment, leading to chemotherapy and an improved immune response for tumor therapy.
Figure 9.
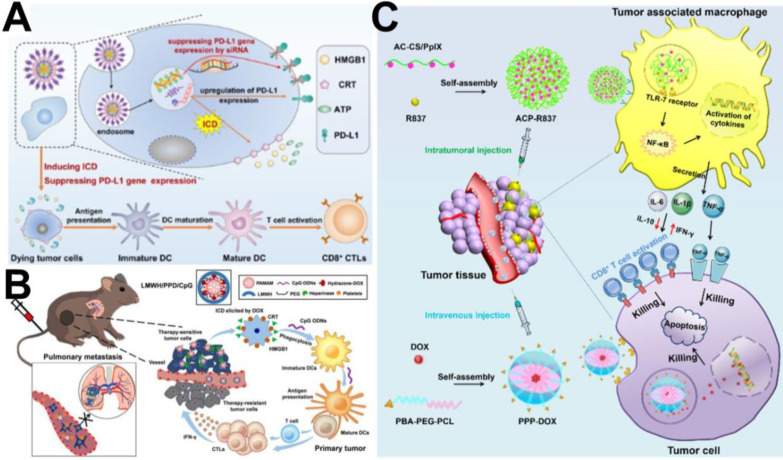
(A) Schematic illustration of the carrier-free nanoassembly PEG@D:siRNA for combinationally inducing ICD and reversing immunosuppression 124. Copyright 2020, Elsevier. (B) Schematic illustration of LMWH/PPD/CpG to inhibit melanoma primary tumor and pulmonary metastasis 127. Copyright 2019, Ivyspring International Publisher. (C) Schematic illustration of the enhanced cancer chemo-immunotherapy resulting from intratumorally and intravenously injected nanomedicines 128. Copyright 2019, Elsevier.
In addition, several studies have indicated that immunoadjuvants (e.g., CpG, R837, and R848) can observably enhance the tumor response to chemotherapy-elicited immune responses by improving the recognition and delivery of cancer antigens by DCs. For example, He et al. 127 designed a PAMAM-based chemo-immunotherapy nanoparticle (LMWH/PPD/CpG) that could co-deliver DOX and immunoadjuvant (cytosine-phosphate-guanine oligonucleotides, CpG ONDs) for synergistic treatment of metastatic melanoma. DOX was conjugated to the amino-terminated PAMAM dendrimer by pH-sensitive hydrazine, and LMWH/PPD/CpG was added as a negatively charged anti-metastatic low molecular weight heparin (LMWH) coating on the surface of PAMAM. As shown in Figure 9B, DOX elicited tumor-specific immune responses via ICD, and the CpG ODNs further improved immunological effects by enhancing the maturation of DCs and then increasing the level of cytolytic T lymphocytes. R837 is also used as an immune-adjuvant to modulate the tumor-associated macrophages (TAMs) to improve tumor chemo-immunotherapy. For example, Zhou et al. 128 designed two types of targeting micelles to separately deliver DOX and R837 to tumor cells and TAMs via intravenous injection and intratumoral injection, respectively, for enhanced cancer chemo-immunotherapy against breast cancer. As shown in Figure 9C, the immune-stimulating micelle (ACP-R837) could activate tumor-associated macrophages and promote the secretion of cytokines, leading to an augmented antitumor immune response. The chemotherapeutic micelle (PPP-DOX) could induce tumor cell death through chemotherapeutic toxicity.
Multiple combination therapy
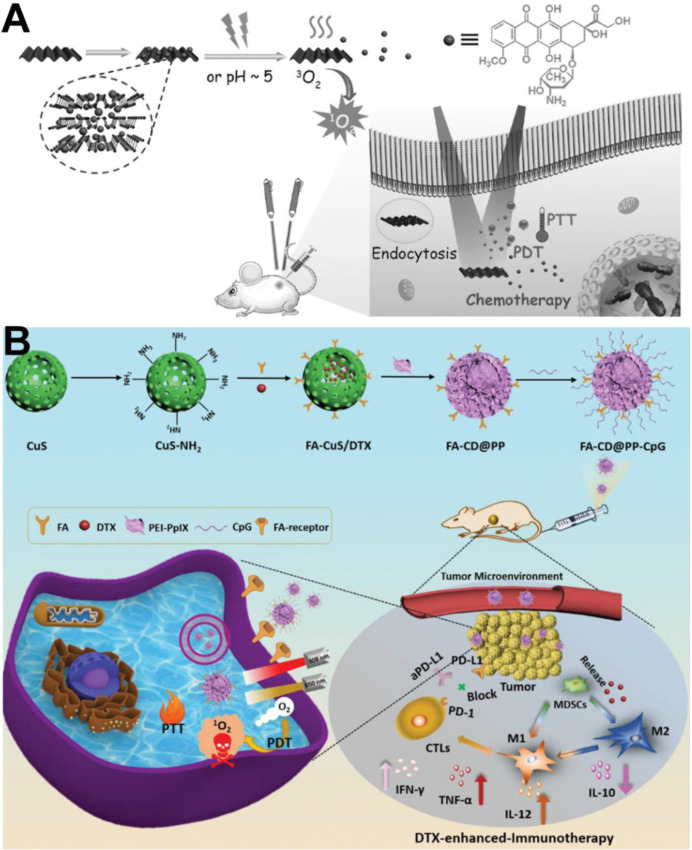
To increase anticancer efficiency and tackle the difficulty of cancer recurrence, chemotherapy-based multi-therapy has been widely reported 129. Recently, the PTT/PDT/chemotherapy tri-modal anticancer strategy has been reported to improve therapeutic outcome and decrease drug dosage. For example, Li et al. 130 developed a multifunctional nanoplatform with PTT/PDT/chemotherapy tri-synergistic functions. This nanoplatform consisted of a pH-responsive diblock copolymer, Ce6, and the prodrug DOX and could reverse MDR in MCF-7/ADR tumor-bearing nude mice. Under NIR laser irradiation, Ce6 generated ROS for PDT and triggered the release of DOX for chemotherapy. The micelle could also efficiently convert NIR light to local heat for PTT and simultaneously enhance DOX penetration into tumors to improve the effectiveness of chemotherapy. In addition, because black phosphorus (BP) has both PTT and PDT effects, DOX-loaded BP can be used in multiple anticancer therapies via PDT/PTT/chemotherapy. For example, Guo et al. 131 reported an interesting concept of a BP nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy against cancer. In this system, BP not only effectively loaded higher amounts of DOX and showed pH-/photoresponsive drug release, but also generated 1O2 and possessed NIR photothermal activity (Figure 10A). Therefore, the tri-modal therapeutic system displayed dramatic therapeutic outcomes in 4T1-tumor bearing mice.
Figure 10.
(A) Schematic illustration of BP-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer 131. Copyright 2017, Wiley-VCH. (B) Rational design and synthesis of FA-CD@PP-CpG nanocomposites (top), its application in cancer treatment (left), and illustration of FA-CD@PP-CpG for docetaxel-enhanced immunotherapy (right) 133. Copyright 2019, Wiley-VCH.
In addition, a PTT/PDT/chemotherapy/immunotherapy anticancer strategy has been reported. Jong et al. 132 reported BP flakes with plug-and-play and ultrasonic bubble bursting features, which can load DOX, PD-L1, and siRNA to fabricate BP-DcF@sPL nanoplatforms for chemo-photo-immunotherapy. In vivo antitumor results showed that ABP-DcF@sPL significantly inhibited tumor growth and prolonged the survival period in a C57BL/6 mouse model with MC-38 tumor xenografts. Similarly, metallic material-derived nanoparticles can be used as photosensitizers due to their PTT and PDT effects for cancer immunotherapy via ICD. For example, Dong et al. developed multifunctional FA-CuS/DTX@PEI-PpIX-CpG nanoparticles (named FA-CD@PP-CpG NPs) for synergistic PDT/PTT/chemotherapy/immunotherapy 133. As shown in Figure 10B, FA-CD@PP-CpG promoted infiltration of cytotoxic T lymphocytes (CTLs) to improve the efficacy of aPD-L1, suppressed myeloid-derived suppressor cells (MDSCs), and effectively polarized marrow-derived suppressor cells (MDSCs) toward the M1 phenotype to reduce tumor burden, further enhancing the antitumor efficacy. Therefore, FA-CD@PP-CpG nanocomposite is an efficient synergistic therapeutic modality in multi-combination therapy for anticancer treatment.
Conclusions and future directions
Chemotherapy serves as one of the most important cancer treatment modalities and has been extensively used in clinic. However, conventional chemotherapeutics usually induce serious side effects due to their low half-lives in the blood and rapid distribution in healthy tissues and organs. Therefore, exploring and developing more efficient methods to enhance cancer chemotherapy is an urgent problem that must be solved. With the development of nanomedicine, multifunctional nanocarriers have showed a good application prospect in improving cancer chemotherapy. In this review, we systematically summarized different strategies to improve chemotherapy effects based on nanomedicine, including enhancement of chemotherapeutic drugs at tumor sites, chemotherapy towards specific subcellular organelles, reversal of resistance mechanisms, and combined chemotherapy. Despite the rapid development of nanotechnology and the development of multifunctional nanomedicines, the relevant research is still in its infancy. To reach clinical translation of research results as soon as possible, many key problems must be solved.
First, although NDDSs can improve enrichment of therapeutic drugs at tumors via passive targeting (EPR effect) or active targeting (modified with a targeting ligand), less than 0.7% of the injected dose of chemotherapeutic drugs accumulate in tumors 175, 176. The requirement of high accumulation in tumors seems to be a limiting factor in the development of nanomedicine. To address this bottleneck, a potent strategy will be to augment the permeability and retention of nanocarriers by developing NDDSs with step-by-step targeting capability, which can selectively increase vascular permeability, promote penetration of drug-loaded nanoparticles into tumor tissues, and target tumor cells and their subcellular organelles.
Second, assembling a reasonable combination of different treatment methods based on nanomedicine to achieve effective cancer treatment is still a challenge. Although combined chemotherapy has shown a significant therapeutic effect, related research is still in the early stage, and many important problems must be solved. For example, the mechanisms underlying combination therapy are still unclear or ambiguous. To deeply explain the mechanism of action of these combination therapies, it is necessary to select a variety of detection and analysis methods. In addition, because of tumor heterogeneity and differences in primary sites, the most reasonable combination strategies should be determined according to the different tumor types. Nanomedicine with diagnostic and therapeutic functions to achieve effective and successful cancer treatment can also be designed.
Third, although the reported nanomedicines have demonstrated excellent tumor therapeutic effects in vivo based on the current tumor models, pharmaceutical researchers and regulatory agencies recognize that results from preclinical animal models frequently fail to predict drug responses in humans 177, and thus, few of these nanomedicines have entered clinical studies. This actuality may be because animal models constructed for in vivo antitumor efficacy evaluation are not good substitutes for clinical tumor characteristics. Therefore, to make results more meaningful, it is important to explore and develop new animal models that are more consistent with human clinical characteristics. In future studies, humanized animal model may be a good choice and should be widely used in preclinical evaluation of nanomedicine. In addition, if possible, animal models of different species, especially large animals (e.g., pigs and monkeys) that have immune systems similar to the human immune system, should be used for in vivo evaluation, so that the results can be more useful for clinical application.
Fourth, the safety of nanomedicine must be carefully considered. More studies on clinical safety, pharmacokinetics, biostability, and overall therapeutic effects should be intensively and extensively explored.
In summary, nanotechnology is an effective tool to enhance cancer chemotherapy, but there is still a long way to go, and more key challenges must be addressed before widespread clinical use can be achieved. More multidisciplinary and multiteam cooperation is required to promote clinical translation of nanomedicine-based cancer chemotherapy.
Acknowledgments
This project is supported by the National Natural Science Foundation of China (Grant NO. 51703189), the Sichuan Science and Technology Program (Grant NO. 2019YJ0245), and the Fundamental Research Funds for the Central Universities (Grant NO. 2682019CX76).
References
- 1.Gilman A. The initial clinical trial of nitrogen mustard. Am J Surg. 1963;105:574–8. doi: 10.1016/0002-9610(63)90232-0. [DOI] [PubMed] [Google Scholar]
- 2.Qin S, Zhang A, Cheng S, Rong L, Zhang X. Drug self-delivery systems for cancer therapy. Biomaterials. 2017;112:234–47. doi: 10.1016/j.biomaterials.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Penny LK, Wallace HM. The challenges for cancer chemoprevention. Chem Soc Rev. 2015;44:8836–47. doi: 10.1039/c5cs00705d. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Qin S, Cheng Y, Lei Q, Zhang A, Zhang X. Combinational strategy for high-performance cancer chemotherapy. Biomaterials. 2018;171:178–97. doi: 10.1016/j.biomaterials.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Majumder J, Taratula O, Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv Drug Deliv Rev. 2019;144:57–77. doi: 10.1016/j.addr.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Yana L, Wang X, Zhu S, Chen C, Gu Z. et al. Progress, challenges, and future of nanomedicine. Nano Today. 2020;35:101008. [Google Scholar]
- 8.Zhang X, Wang Y, Wei G, Zhao J, Yang G, Zhou S. Stepwise dual targeting and dual responsive polymer micelles for mitochondrion therapy. J Control Release. 2020;322:157–69. doi: 10.1016/j.jconrel.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 9.U.S. FDA. ABRAXANE®for Injectable Suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound) 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021660s037lbl.pdf.
- 10.Lee S, Kim Y, Cho CH, Kim YT, Kim SM, Hur SY. et al. An open-label, randomized, parallel, phase II trial to evaluate the efficacy and safety of a cremophor-free polymeric micelle formulation of paclitaxel as first-line treatment for ovarian cancer: a korean gynecologic oncology group study (KGOG-3021) Cancer Res Treat. 2018;50:195–203. doi: 10.4143/crt.2016.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36:1150–63. doi: 10.1007/s12325-019-00909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: what has been done and the challenges remain ahead. Int J Pharm. 2017;526:474–495. doi: 10.1016/j.ijpharm.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Silverman L, Barenholz Y. In vitro experiments showing enhanced release of doxorubicin from Doxil1 in the presence of ammonia may explain drug release at tumor site. Nanomedicine. 2015;11:1841–50. doi: 10.1016/j.nano.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Swenson C, Perkins W, Roberts P, Janoff AS. Liposome technology and the development of MyocetTM (liposomal doxorubicin citrate) Breast. 2001;10:1–7. [Google Scholar]
- 15.Dou Y, Hynynen K, Allen C. To heat or not to heat: challenges with clinical translation of thermosensitive liposomes. J Control Release. 2017;249:63–73. doi: 10.1016/j.jconrel.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Xu R, Zhang G, Mai J, Deng X, Segura-Ibarra V, Wu S. et al. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol. 2016;34:414–8. doi: 10.1038/nbt.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deshmukh AS, Chauhan PN, Noolvi MN, Chaturvedi K, Ganguly K, Shukla SS. et al. Polymeric micelles: basic research to clinical practice. Int J Pharm. 2017;532:249–68. doi: 10.1016/j.ijpharm.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Stathopoulos G, Antoniou D, Dimitroulis J, Stathopoulos J, Marosis K, Michalopoulou P. Comparison of liposomal cisplatin versus cisplatin in non-squamous cell non-small-cell lung cancer. Cancer Chemother Pharmacol. 2011;68:945–50. doi: 10.1007/s00280-011-1572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark AJ, Wiley DT, Zuckerman JE, Webster P, Chao J, Lin J. et al. CRLX101 nanoparticles localize in human tumors and not in adja- cent, nonneoplastic tissue after intravenous dosing. Proc Natl Acad Sci U S A. 2016;113:3850–4. doi: 10.1073/pnas.1603018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neal JW, Wakelee H, Padda SK, Bertrand S, Acevedo B, Tisch AH. et al. PS01. 04: a Phase II study of etirinotecan pegol (NKTR-102) in patients with refractory brain metastases and advanced lung cancer. J Thorac Oncol. 2016;11:271–2. [Google Scholar]
- 21.Adiseshaiah PP, Crist RM, Hook SS, McNeil SE. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat Rev Clin Oncol. 2016;13:750–65. doi: 10.1038/nrclinonc.2016.119. [DOI] [PubMed] [Google Scholar]
- 22.Lu C, Stewart DJ, Lee JJ, Ji L, Ramesh R, Jayachandran G. et al. Phase I clinical trial of systemically administered TUSC2 (FUS1)-nanoparticles mediating functional gene transfer in humans. Plos One. 2012;7:34833. doi: 10.1371/journal.pone.0034833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan S, Mackay A. Summary of Product Characteristics. Springer. 2010.
- 24.Bedikian AY, Vardeleon A, Smith T, Campbell S, Namdari R. Pharmacokinetics and urinary excretion of vincristine sulfate liposomes injection in metastatic melanoma patients. J Clin Pharmacol. 2006;46:727–37. doi: 10.1177/0091270006288953. [DOI] [PubMed] [Google Scholar]
- 25.Pillai G. Applications Targeted Nano Drugs Delivery Systems. Elsevier. 2019.
- 26.Rawal S, Patel MM. Threatening cancer with nanoparticle aided combination oncotherapy. J Control Release. 2019;301:76–109. doi: 10.1016/j.jconrel.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Yang W, Cui J, Li X, Dou Y, Su L. et al. pH- and NIR light responsive nanocarriers for combination treatment of chemotherapy and photodynamic therapy. Biomater Sci. 2016;4:338–45. doi: 10.1039/c5bm00328h. [DOI] [PubMed] [Google Scholar]
- 28.Huang P, Gao Y, Lin J, Hu H, Liao H, Yan X. et al. Tumor-specific formation of enzyme-instructed supramolecular self-assemblies as cancer theranostics. ACS Nano. 2015;9:9517–27. doi: 10.1021/acsnano.5b03874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu S, Ding J, He C, Cao Y, Xu W, Chen X. Disulfide cross-linked polyurethane micelles as a reduction-triggered drug delivery system for cancer therapy. Adv Healthc Mater. 2014;3:752–60. doi: 10.1002/adhm.201300308. [DOI] [PubMed] [Google Scholar]
- 30.Chen K, Chaung E, Wey S, Lin K, Cheng F, Lin C. et al. Hyperthermia-mediated local drug delivery by a bubble-generating liposomal system for tumor-specific chemotherapy. ACS Nano. 2014;8:5105–15. doi: 10.1021/nn501162x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Su H, Cai J, Cheng D, Ma Y, Zhang J. et al. A multifunctional platform for tumor angiogenesis-targeted chemo-thermal therapy using polydopamine-coated gold nanorods. ACS Nano. 2016;10:10404–17. doi: 10.1021/acsnano.6b06267. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei G, Wang Y, Huang X, Hou H, Zhou S. Peptide-based nanocarriers for cancer therapy. Small Methods. 2018;2:1700358. [Google Scholar]
- 35.Boohaker RJ, Lee MW, Vishnubhotla P, Perez JM, Khaled AR. The use of therapeutic peptides to target and to kill cancer cells. Curr Med Chem. 2012;19:3794–804. doi: 10.2174/092986712801661004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei G, Wang Y, Huang X, Yang G, Zhao J, Zhou S. Enhancing the accumulation of polymer micelles by selectively dilating tumor blood vessels with NO for highly effective cancer treatment. Adv Healthc Mater. 2018;7:1801094. doi: 10.1002/adhm.201801094. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Zhang L, Cai Y, Yang Y, Qiu L, Shen Y. et al. Bioengineered human serum albumin fusion protein as target/enzyme/pH three-stage propulsive drug vehicle for tumor therapy. ACS Nano. 2020;14:17405–18. doi: 10.1021/acsnano.0c07610. [DOI] [PubMed] [Google Scholar]
- 38.Samanta S, Sistla R, Chaudhuri A. The use of RGDGWK-lipopeptide to selectively deliver genes to mouse tumor vasculature and its complexation with p53 to inhibit tumor growth. Biomaterials. 2010;31:1787–97. doi: 10.1016/j.biomaterials.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Zhao M, Liu Y, Luan X, Guan Y, Lu Q. et al. Suppression of colorectal cancer subcutaneous xenograft and experimental lung metastasis using nanoparticle-mediated drug delivery to tumor neovasculature. Biomaterials. 2014;35:1215–26. doi: 10.1016/j.biomaterials.2013.08.091. [DOI] [PubMed] [Google Scholar]
- 40.Martens CL, Cwirla SE, Lee RY-W, Whitehorn E, Chen EY-F, Bakker A. et al. Peptides which bind to E-selectin and block neutrophil adhesion. J Biol Chem. 1995;270:21129–36. doi: 10.1074/jbc.270.36.21129. [DOI] [PubMed] [Google Scholar]
- 41.Shamay Y, Raviv L, Golan M, Voronov E, Apte RN, David A. Inhibition of primary and metastatic tumors in mice by E-selectin-targeted polymer-drug conjugates. J Control Release. 2015;217:102–12. doi: 10.1016/j.jconrel.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Porkka K, Laakkonen P, Hoffman JA, Bernasconi M, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc Natl Acad Sci U S A. 2002;99:7444–9. doi: 10.1073/pnas.062189599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahir M, Bhattacharya S, Karmakar S, Mukhopadhyay A, Mukherjee S, Ghosh S. et al. Tailored-CuO nanowire decorated with folic acid mediated coupling of the mitochondrial-ROS generation and miR425-PTEN axis in furnishing potent anti-cancer activity in human triple negative breast carcinoma cells. Biomaterials. 2016;76:115–32. doi: 10.1016/j.biomaterials.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 44.Yin T, Liu J, Zhao Z, Zhao Y, Dong L, Yang M. et al. Redox sensitive hyaluronic acid-decorated graphene oxide for photothermally controlled tumor-cytoplasm-selective rapid drug delivery. Adv Funct Mater. 2017;27:1604620. [Google Scholar]
- 45.Wang Y, Zhang L, Zhang X, Wei X, Tang Z, Zhou S. Precise polymerization of a highly tumor microenvironment-responsive nanoplatform for strongly enhanced intracellular drug release. ACS Appl Mater Interfaces. 2016;8:5833–46. doi: 10.1021/acsami.5b11569. [DOI] [PubMed] [Google Scholar]
- 46.Deshayes S, Cabral H, Ishii T, Miura Y, Kobayashi S, Yamashita T. et al. Phenylboronic acid-installed polymeric micelles for targeting sialylated epitopes in solid tumors. J Am Chem Soc. 2013;135:15501–7. doi: 10.1021/ja406406h. [DOI] [PubMed] [Google Scholar]
- 47.Lazarovits J, Chen YY, Sykes EA, Chan WCW. Nanoparticle-blood interactions: the implications on solid tumour targeting. Chem Commun. 2015;51:2756–67. doi: 10.1039/c4cc07644c. [DOI] [PubMed] [Google Scholar]
- 48.Guo X, Li D, Yang G, Shi C, Tang Z, Wang J. et al. Thermo-triggered drug release from actively targeting polymer micelles. ACS Appl Mater Interfaces. 2014;6:8549–59. doi: 10.1021/am501422r. [DOI] [PubMed] [Google Scholar]
- 49.Yin S, Gao Y, Zhang Y, Xu J, Zhu J, Zhou F. et al. Reduction/oxidation-responsive hierarchical nanoparticles with self-driven degradability for enhanced tumor penetration and precise chemotherapy. ACS Appl Mater Interfaces. 2020;12:18273–91. doi: 10.1021/acsami.0c00355. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Huo M, Wang J, Zhou J, Mohammad JM, Zhang Y. et al. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials. 2012;33:2310–20. doi: 10.1016/j.biomaterials.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 51.Tang Z, Gao Y, Li D, Zhou S. Controllably switched drug Release from successively dual-targeted nanoreservoirs. Adv Healthc Mater. 2017;6:1600919. doi: 10.1002/adhm.201600919. [DOI] [PubMed] [Google Scholar]
- 52.Nishiyama N, Cabral H, Kataoka K. Nanomedicines eradicating cancer stem-like cells in vivo by pH-triggered intracellular cooperative action of loaded drugs. ACS Nano. 2016;10:5643–55. doi: 10.1021/acsnano.6b00900. [DOI] [PubMed] [Google Scholar]
- 53.Sun Q, Ojha T, Kiessling F, Lammers T, Shi Y. Enhancing tumor penetration of nanomedicines. Biomacromolecules. 2017;18:1449–59. doi: 10.1021/acs.biomac.7b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Liu Y, Gao X, Li X, Niu X, Yuan Zhi. et al. Near-infrared-light induced nanoparticles with enhanced tumor tissue penetration and intelligent drug release. Acta Biomater. 2019;90:314–23. doi: 10.1016/j.actbio.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Wang G, Wu B, Li Q, Chen S, Jin X, Liu Y. et al. Active transportation of liposome enhances tumor accumulation, penetration, and therapeutic efficacy. Small. 2020;16:2004172. doi: 10.1002/smll.202004172. [DOI] [PubMed] [Google Scholar]
- 56.Stapleton S, Milosevic M, Tannock IF, Allen C, Jaffray DA. The intra-tumoral relationship between microcirculation, interstitial fluid pressure and liposome accumulation. J Control Release. 2015;211:163–70. doi: 10.1016/j.jconrel.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Huang P, Chen X. Hierarchical targeting strategy for enhanced tumor tissue accumulation/retention and cellular internalization. Adv Mater. 2016;28:7340–64. doi: 10.1002/adma.201601498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H-J, Du J-Z, Du X-J, Xu C-F, Sun C-Y, Wang H-X. et al. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc Nati Acad Sci U S A. 2016;113:4164–4169. doi: 10.1073/pnas.1522080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H-J, Du J-Z, Liu J, Du X-J, Shen S, Zhu Y-H. et al. Smart superstructures with ultrahigh pH-sensitivity for targeting acidic tumor microenvironment: instantaneous size switching and improved tumor penetration. ACS Nano. 2016;10:6753–61. doi: 10.1021/acsnano.6b02326. [DOI] [PubMed] [Google Scholar]
- 60.Stylianopoulos T, Poh M, Insin N, Fukumura D, Munn LL, Jain RK. et al. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys J. 2010;99:1342–9. doi: 10.1016/j.bpj.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S, Zhang F, Yu G, Wang Z, Jacobson O, Ma Y. et al. Zwitterionic-to-cationic charge conversion polyprodrug nanomedicine for enhanced drug delivery. Theranostics. 2020;10:6629–37. doi: 10.7150/thno.47849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou H, Fan Z, Deng J, Lemons PK, Arhontoulis DC, Bowne WB. et al. Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016;16:3268–77. doi: 10.1021/acs.nanolett.6b00820. [DOI] [PubMed] [Google Scholar]
- 63.Liu S, Wei W, Xie B, Yue H, Ni D, Bao Y. et al. Breaching the hyaluronan barrier with PH20-Fc facilitates intratumoral permeation and enhances antitumor efficiency: A comparative investigation of typical therapeutic agents in different nanoscales. Adv Healthc Mater. 2016;5:2872–81. doi: 10.1002/adhm.201600528. [DOI] [PubMed] [Google Scholar]
- 64.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–503. [PubMed] [Google Scholar]
- 65.Dong X, Liu HJ, Feng HY, Yang SC, Liu XL, Lai X. et al. Enhanced drug delivery by nanoscale integration of a nitric oxide donor to induce tumor collagen depletion. Nano Lett. 2019;19:997–1008. doi: 10.1021/acs.nanolett.8b04236. [DOI] [PubMed] [Google Scholar]
- 66.Xu F, Huang X, Wang Y, Zhou S. A size-changeable collagenase-modified nanoscavenger for increasing penetration and retention of nanomedicine in deep tumor tissue. Adv Mater. 2020;32:1906745. doi: 10.1002/adma.201906745. [DOI] [PubMed] [Google Scholar]
- 67.Zeng L, Zou L, Yu H, He X, Cao H, Zhang Z. et al. Treatment of malignant brain tumor by tumor-triggered programmed wormlike micelles with precise targeting and deep penetration. Adv Funct Mater. 2016;26:4201–12. [Google Scholar]
- 68.Wei G, Wang Y, Huang X, Yang G, Zhao J, Zhou S. Induction of mitochondrial apoptosis for cancer therapy via dual-targeted cascade-responsive multifunctional micelles. J Mater Chem B. 2018;6:8137–47. doi: 10.1039/c8tb02159g. [DOI] [PubMed] [Google Scholar]
- 69.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–64. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 70.Chen W, Luo G, Zhang X. Recent advances in subcellular targeted cancer therapy based on functional materials. Adv Mater. 2019;31:1802725. doi: 10.1002/adma.201802725. [DOI] [PubMed] [Google Scholar]
- 71.Guo X, Wei X, Chen Z, Zhang X, Yang G, Zhou S. Multifunctional nanoplatforms for subcellular delivery of drugs in cancer therapy. Prog Mater Sci. 2020;107:100599. [Google Scholar]
- 72.Ji C, Cheng W, Hu Y, Liu Y, Liu F, Yin M. A nano vector with photothermally enhanced drug release and retention to overcome cancer multidrug resistance. Nano Today. 2021;36:101020. [Google Scholar]
- 73.Yu G, Wu D, Li Y, Zhang Z, Shao L, Zhou J. et al. A pillar[5]arene-based [2]rotaxane lights up mitochondria. Chem Sci. 2016;7:3017–24. doi: 10.1039/c6sc00036c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan Y, Zhu Y, Zhao Y, Wen L, Meng T, Liu X. et al. Mitochondrial alkaline pH-responsive drug release mediated by celastrol loaded glycolipid-like micelles for cancer therapy. Biomaterials. 2018;154:169–81. doi: 10.1016/j.biomaterials.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 75.Meinema AC, Laba JK, Hapsari RA, Otten R, Mulder FA, Kralt A. et al. Long unfolded linkers facilitate membrane protein import through the nuclear pore complex. Science. 2011;333:90–3. doi: 10.1126/science.1205741. [DOI] [PubMed] [Google Scholar]
- 76.Pan ZZ, Wang HY, Zhang M, Lin TT, Zhang WY, Zhao PF. et al. Nuclear-targeting TAT-PEG-Asp8 doxorubicin polymeric nanoassembly to overcome drug-resistant colon cancer. Acta Pharmacol Sin. 2016;37:1110–20. doi: 10.1038/aps.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jing Y, Xiong X, Ming Y, Zhao J, Guo X, Yang G. et al. A multifunctional micellar nanoplatform with pH-triggered cell penetration and nuclear targeting for effective cancer therapy and inhibition to lung metastasis. Adv Healthc Mater. 2018;7:1700974. doi: 10.1002/adhm.201700974. [DOI] [PubMed] [Google Scholar]
- 78.Guo X, Wei X, Jing Y, Zhou S. Size changeable nanocarriers with nuclear targeting for effectively overcoming multidrug resistance in cancer therapy. Adv Mater. 2015;27:6450–6. doi: 10.1002/adma.201502865. [DOI] [PubMed] [Google Scholar]
- 79.Qiu L, Chen T, Öçsoy I, Yasun E, Wu C, Zhu G. et al. A cell-targeted, size-photocontrollable, nuclear uptake nanodrug delivery system for drug-resistant cancer therapy. Nano Lett. 2014;15:457–63. doi: 10.1021/nl503777s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li H, Zhang P, Luo J, Hu D, Huang Y, Zhang Z. et al. Chondroitin sulfate-linked prodrug nanoparticles target the golgi apparatus for cancer metastasis treatment. ACS Nano. 2019;13:9386–96. doi: 10.1021/acsnano.9b04166. [DOI] [PubMed] [Google Scholar]
- 81.Kim J, Yung BC, Kim WJ, Chen X. Combination of nitric oxide and drug delivery systems: tools for overcoming drug resistance in chemotherapy. J Control Release. 2017;263:223–30. doi: 10.1016/j.jconrel.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li R, Xie Y. Nanodrug delivery systems for targeting the endogenous tumor microenvironment and simultaneously overcoming multidrug resistance properties. J Control Release. 2017;251:49–67. doi: 10.1016/j.jconrel.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 83.Jin W, Wang Q, Wu M, Li Y, Tang G, Ping Y. et al. Lanthanide-integrated supramolecular polymeric nanoassembly with multiple regulation characteristics for multidrug-resistant cancer therapy. Biomaterials. 2017;129:83–97. doi: 10.1016/j.biomaterials.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 84.Li R, Xie Y. Nanodrug delivery systems for targeting the endogenous tumor microenvironment and simultaneously overcoming multidrug resistance properties. J. Control Release. 2017;251:49–67. doi: 10.1016/j.jconrel.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 85.Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev. 2013;65:1866–79. doi: 10.1016/j.addr.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soma CE, Dubernet C, Bentolila D, Benita S, Couvreur P. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin A in polyalkylcyanoacrylate nanoparticles. Biomaterials. 2000;21:1–7. doi: 10.1016/s0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 87.Zhu H, Chen H, Zeng X, Wang Z, Zhang X, Wu Y. et al. Co-delivery of chemotherapeutic drugs with vitamin E TPGS by porous PLGA nanoparticles for enhanced chemotherapy against multi-drug resistance. Biomaterials. 2014;35:2391–400. doi: 10.1016/j.biomaterials.2013.11.086. [DOI] [PubMed] [Google Scholar]
- 88.Song XR, Cai Z, Zheng Y, He G, Cui FY, Gong DQ. et al. Reversion of multidrug resistance by co-encapsulation of vincristine and verapamil in PLGA nanoparticles. Eur J Pharm Sci. 2009;37:300–5. doi: 10.1016/j.ejps.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 89.Duan J, Mansour HM, Zhang Y, Deng X, Chen Y, Wang J. et al. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm. 2012;426:193–201. doi: 10.1016/j.ijpharm.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 90.Wang S, Liu X, Chen S, Liu Z, Zhang X, Liang XJ. et al. Regulation of Ca2+ signaling for drug-resistant breast cancer therapy with mesoporous silica nanocapsule encapsulated doxorubicin/siRNA cocktail. ACS Nano. 2019;13:274–83. doi: 10.1021/acsnano.8b05639. [DOI] [PubMed] [Google Scholar]
- 91.Liu J, Zhu C, Xu L, Wang D, Liu W, Zhang K. et al. Nanoenabled intracellular calcium bursting for safe and efficient reversal of drug resistance in tumor cells. Nano Lett. 2020;20:8102–11. doi: 10.1021/acs.nanolett.0c03042. [DOI] [PubMed] [Google Scholar]
- 92.Sun R, Liu X, Li G, Wang H, Luo Y, Huang G. et al. Photoactivated H2 nanogenerator for enhanced chemotherapy of bladder cancer. ACS Nano. 2020;14:8135–48. doi: 10.1021/acsnano.0c01300. [DOI] [PubMed] [Google Scholar]
- 93.Kim J, Yung BC, Kim WJ, Chen X. Combination of nitric oxide and drug delivery systems: tools for overcoming drug resistance in chemotherapy. J Control Release. 2017;263:223–30. doi: 10.1016/j.jconrel.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng W, Nie J, Gao N, Liu G, Tao W, Xiao X. et al. A multifunctional nanoplatform against multidrug resistant cancer: merging the best of targeted chemo/gene/photothermal therapy. Adv Funct Mater. 2017;27:1704135. [Google Scholar]
- 95.Wei G, Yang G, Wei B, Wang Y, Zhou S. Near-infrared light switching nitric oxide nanoemitter for triple-combination therapy of multidrug resistant cancer. Acta Biomater. 2019;100:365–77. doi: 10.1016/j.actbio.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Fan J, He Q, Liu Y, Zhang F, Yang X, Wang Z. et al. Light-responsive biodegradable nanomedicine overcomes multidrug resistance via NO-enhanced chemosensitization. ACS Appl Mater Interfaces. 2016;8:13804–11. doi: 10.1021/acsami.6b03737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo R, Tian Y, Wang Y, Yang W. Near-infrared laser-triggered nitric oxide nanogenerators for the reversal of multidrug resistance in cancer. Adv Funct Mater. 2017;27:1606398. [Google Scholar]
- 98.Wei X, Wang Y, Xiong X, Guo X, Zhang L, Zhang X. et al. Codelivery of a π-π stacked dual anticancer drug combination with nanocarriers for overcoming multidrug resistance and tumor metastasis. Adv Funct Mater. 2016;26:8266–80. [Google Scholar]
- 99.Zhou Y, Ren X, Hou Z, Wang N, Jiang Y, Luan Y. Engineering a photosensitizer nanoplatform for amplified photodynamic immunotherapy via tumor microenvironment modulation. Nanoscale Horiz. 2021;6:120–31. doi: 10.1039/d0nh00480d. [DOI] [PubMed] [Google Scholar]
- 100.Shang Q, Zhou S, Jiang Y, Wang D, Wang J, Song A. et al. Rational design of a robust antibody-like small-molecule inhibitor nanoplatform for enhanced photoimmunotherapy. ACS Appl Mater Interfaces. 2020;12:40085–93. doi: 10.1021/acsami.0c11156. [DOI] [PubMed] [Google Scholar]
- 101.Park W, Bae B, Na K. A highly tumor-specific light-triggerable drug carrier responds to hypoxic tumor conditions for effective tumor treatment. Biomaterials. 2016;77:227–34. doi: 10.1016/j.biomaterials.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 102.Zhang K, Zhang Y, Meng X, Lu H, Chang H, Dong H. et al. Light-triggered theranostic liposomes for tumor diagnosis and combined photodynamic and hypoxia-activated prodrug therapy. Biomaterials. 2018;185:301–9. doi: 10.1016/j.biomaterials.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y, Xie Y, Li J, Peng ZH, Sheinin Y, Zhou J. et al. Tumor-penetrating nanoparticles for enhanced anticancer activity of combined photodynamic and hypoxia-activated therapy. ACS Nano. 2017;11:2227–38. doi: 10.1021/acsnano.6b08731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qian C, Feng P, Yu J, Chen Y, Hu Q, Sun W. et al. Anaerobe-inspired anticancer nanovesicles. Angew Chem Inter Ed. 2017;56:2588–93. doi: 10.1002/anie.201611783. [DOI] [PubMed] [Google Scholar]
- 105.Su S, Ding Y, Li Y, Wu Y, Nie G. Integration of photothermal therapy and synergistic chemotherapy by a porphyrin self-assembled micelle confers chemosensitivity in triple-negative breast cancer. Biomaterials. 2016;80:169–78. doi: 10.1016/j.biomaterials.2015.11.058. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, Wei G, Zhang X, Huang X, Zhao J, Guo X. et al. Multistage targeting strategy using magnetic composite nanoparticles for synergism of photothermal therapy and chemotherapy. Small. 2018;14:1702994. doi: 10.1002/smll.201702994. [DOI] [PubMed] [Google Scholar]
- 107.Wang X, Zhong X, Liu Z, Cheng L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today. 2020;35:100946. [Google Scholar]
- 108.Xue T, Xu C, Wang Y, Wang Y, Tian H, Zhang Y. Doxorubicin-loaded nanoscale metal-organic framework for tumor-targeting combined chemotherapy and chemodynamic therapy. Biomater Sci. 2019;7:4615–23. doi: 10.1039/c9bm01044k. [DOI] [PubMed] [Google Scholar]
- 109.Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater. 2018;30:1704007. doi: 10.1002/adma.201704007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH. et al. The role of ferroptosis in ionizing radiation induced cell death and tumor suppression. Cell Res. 2020;30:146–62. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater. 2019;31:1904197. doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- 112.Ren Z, Sun S, Sun R, Cui G, Hong L, Rao B. et al. A metal-polyphenol-coordinated nanomedicine for synergistic cascade cancer chemotherapy and chemodynamic therapy. Adv Mater. 2020;32:1906024. doi: 10.1002/adma.201906024. [DOI] [PubMed] [Google Scholar]
- 113.Chen G, Yang Y, Xu Q, Ling M, Lin H, Ma W. et al. Self-amplification of tumor oxidative stress with degradable metallic complexes for synergistic cascade tumor therapy. Nano Lett. 2020;20:8141–50. doi: 10.1021/acs.nanolett.0c03127. [DOI] [PubMed] [Google Scholar]
- 114.Liu J, Chen Q, Zhu W, Yi X, Yang Y, Dong Z. et al. Nanoscale-coordination-polymer-shelled manganese dioxide composite nanoparticles: A multistage redox/pH/H2O2-responsive cancer theranostic nanoplatform. Adv Funct Mater. 2017;27:1605926. [Google Scholar]
- 115.Yu L, Hu P, Chen Y. Gas-generating nanoplatforms: material chemistry, multifunctionality, and gas therapy. Adv Mater. 2018;30:1801964. doi: 10.1002/adma.201801964. [DOI] [PubMed] [Google Scholar]
- 116.Xu Y, Liu J, Liu Z, Ren H, Yong J, Li W. et al. Blockade of platelets using tumor-specific NO releasing nanoparticles prevents tumor metastasis and reverses tumor immunosuppression. ACS Nano. 2020;14:9780–95. doi: 10.1021/acsnano.0c01687. [DOI] [PubMed] [Google Scholar]
- 117.Qin S-Y, Cheng Y-J, Lei Q, Zhang A-Q, Zhang X-Z. Combinational strategy for high performance cancer chemotherapy. Biomaterials. 2018;171:178–97. doi: 10.1016/j.biomaterials.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 118.Zhan Q, Yi K, Qi H, Li S, Li X, Wang Q. et al. Engineering blood exosomes for tumor targeting efficient gene/chemo combination therapy. Theranostics. 2020;10:7889–905. doi: 10.7150/thno.45028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 120.Zang X, Zhao X, Hu H, Qiao M, Deng Y, Chen D. Nanoparticles for tumor immunotherapy. Eur J Pharm Biopharm. 2017;115:243–56. doi: 10.1016/j.ejpb.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 121.Mu W, Chu Q, Liu Y, Zhang N. A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nano-Micro Lett. 2020;12:142. doi: 10.1007/s40820-020-00482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X. et al. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano. 2017;11:4463–74. doi: 10.1021/acsnano.7b00715. [DOI] [PubMed] [Google Scholar]
- 124.Chen S, Li D, Du X, He X, Huang M, Wang Y. et al. Carrier-free nanoassembly of doxorubicin prodrug and siRNA for combinationally inducing immunogenic cell death and reversing immunosuppression. Nano Today. 2020;35:100924. [Google Scholar]
- 125.Su Z, Xiao Z, Wang Y, Huang J, An Y, Wang Y. et al. Codelivery of anti-PD-1 antibody and paclitaxel with matrix metalloproteinase and pH dual-sensitive micelles for enhanced tumor chemoimmunotherapy. Small. 2020;16:1906832. doi: 10.1002/smll.201906832. [DOI] [PubMed] [Google Scholar]
- 126.Dai L, Li X, Yao M, Niu P, Yuan X, Li K. et al. Programmable prodrug micelle with size-shrinkage and charge-reversal for chemotherapy-improved IDO immunotherapy. Biomaterials. 2020;241:119901. doi: 10.1016/j.biomaterials.2020.119901. [DOI] [PubMed] [Google Scholar]
- 127.Xia C, Yin S, Xu S, Ran G, Deng M, Mei L. et al. Low molecular weight heparin-coated and dendrimer-based core-shell nanoplatform with enhanced immune activation and multiple anti-metastatic effects for melanoma treatment. Theranostics. 2019;9:337–54. doi: 10.7150/thno.29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wei X, Liu L, Li X, Wang Y, Guo X, Zhao J. et al. Selectively targeting tumor-associated macrophages and tumor cells with polymeric micelles for enhanced cancer chemo-immunotherapy. J Control Release. 2019;313:42–53. doi: 10.1016/j.jconrel.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 129.Kemp JA, Shim MS, Heo CY, Kwon YJ. "Combo" nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv Drug Deliv Rev. 2016;98:3–18. doi: 10.1016/j.addr.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 130.Wang T, Wang D, Yu H, Wang M, Liu J, Feng B. et al. Intracellularly acid-switchable multifunctional micelles for combinational photo/chemotherapy of the drug-resistant tumor. ACS Nano. 2016;10:3496–508. doi: 10.1021/acsnano.5b07706. [DOI] [PubMed] [Google Scholar]
- 131.Chen W, Ouyang J, Liu H, Chen M, Zeng K, Shen J. et al. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv Mater. 2017;29:1603864. doi: 10.1002/adma.201603864. [DOI] [PubMed] [Google Scholar]
- 132.Ou W, Byeon JH, Thapa RK, Ku SK, Yong CS, Kim JO. Plug-and-play nanorization of coarse black phosphorus for targeted chemo-photoimmunotherapy of colorectal cancer. ACS Nano. 2018;12:10061–74. doi: 10.1021/acsnano.8b04658. [DOI] [PubMed] [Google Scholar]
- 133.Chen L, Zhou L, Wang C, Han Y, Lu Y, Liu J. et al. Tumor-targeted drug and CpG delivery system for phototherapy and docetaxel-enhanced immunotherapy with polarization toward M1-type macrophages on triple negative breast cancers. Adv Mater. 2019;31:1904997. doi: 10.1002/adma.201904997. [DOI] [PubMed] [Google Scholar]
- 134.Hao Y, Chen Y, He X, Yu Y, Han R, Yang C. et al. Polymeric nanoparticles with ROS-responsive prodrug and platinum nanozyme for enhanced chemophotodynamic therapy of colon cancer. Adv Sci. 2020;7:2001853. doi: 10.1002/advs.202001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shao Y, Liu B, Di Z, Zhang G, Sun L, Li L. et al. Engineering of upconverted metal-organic frameworks for near-infrared light-triggered combinational photodynamic/chemo-immunotherapy against hypoxic tumors. J Am Chem Soc. 2020;142:3939–46. doi: 10.1021/jacs.9b12788. [DOI] [PubMed] [Google Scholar]
- 136.Chu B, Qu Y, He X, Hao Y, Yang C, Li Y, ROS-responsive camptothecin prodrug nanoparticles for on-demand drug Release and combination of chemotherapy and photodynamic therapy. Adv Funct Mater. 2020. 2005918.
- 137.Cheng Z, Cheng Y, Chen Q, Li M, Wang J, Liu H. et al. Self-assembly of pentapeptides into morphology-adaptable nanomedicines for enhanced combinatorial chemo-photodynamic therapy. Nano Today. 2020;33:100878. [Google Scholar]
- 138.Qian C, Feng P, Yu J, Chen Y, Hu Q, Sun W. et al. Anaerobe-inspired anticancer nanovesicles. Angew Chem Int Ed. 2017;56:2588–93. doi: 10.1002/anie.201611783. [DOI] [PubMed] [Google Scholar]
- 139.Liu Q, Xie Z, Qiu M, Shim I, Yang Y, Xie S, Prodrug-loaded zirconium carbide nanosheets as a novel biophotonic nanoplatform for effective treatment of cancer. Adv Sci. 2020. 2001191. [DOI] [PMC free article] [PubMed]
- 140.Zhang Y, Wang Y, Yang X, Yang Q, Li J, Tan W. Polyaniline nanovesicles for photoacoustic imaging-guided photothermal-chemo synergistic therapy in the second near-infrared window. Small. 2020;16:2001177. doi: 10.1002/smll.202001177. [DOI] [PubMed] [Google Scholar]
- 141.Taheri-Ledari R, Zhang W, Radmanesh M, Mirmohammadi SS, Maleki A, Cathcart Nicole. et al. Multi-stimuli nanocomposite therapeutic: docetaxel targeted delivery and synergies in treatment of human breast cancer tumor. Small. 2020;16:2002733. doi: 10.1002/smll.202002733. [DOI] [PubMed] [Google Scholar]
- 142.Wang D, Yao Y, He J, Zhong X, Li B, Rao S. et al. Engineered cell-derived microparticles Bi2Se3/DOX@MPs for imaging guided synergistic photothermal/low-dose chemotherapy of cancer. Adv Sci. 2020;7:1901293. doi: 10.1002/advs.201901293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim H, Kim E, Jeong SW, Ha T, Park S, Lee SG. et al. Magnetic nanoparticle-conjugated polymeric micelles for combined hyperthermia and chemotherapy. Nanoscale. 2015;7:16470. doi: 10.1039/c5nr04130a. [DOI] [PubMed] [Google Scholar]
- 144.Yang B, Zhou S, Zeng J, Zhang L, Zhang R, Liang K. et al. Super-assembled core-shell mesoporous silica-metal-phenolic network nanoparticles for combinatorial photothermal therapy and chemotherapy. Nano Res. 2020;13:1013–9. [Google Scholar]
- 145.Fu Z, Williams GR, Niu S, Wu J, Gao F, Zhang X. et al. Functionalized boron nanosheets as an intelligent nanoplatform for synergistic low-temperature photothermal therapy and chemotherapy. Nanoscale. 2020;12:14739. doi: 10.1039/d0nr02291h. [DOI] [PubMed] [Google Scholar]
- 146.Zhang J, Zhao B, Chen S, Wang Y, Zhang Y, Wang Y. et al. Near-infrared light irradiation induced mild hyperthermia enhances glutathione depletion and DNA interstrand cross-Link formation for efficient chemotherapy. ACS Nano. 2020;14:14831–45. doi: 10.1021/acsnano.0c03781. [DOI] [PubMed] [Google Scholar]
- 147.Ren C, Wang Z, Zhang X, Gao J, Gao Y, Zhang Y. et al. Construction of all-in-one peptide nanomedicine with photoacoustic imaging guided mild hyperthermia for enhanced cancer chemotherapy. Chem Eng J. 2021;405:127008. [Google Scholar]
- 148.Jiang Y, Huang C, Luan Y. Lactosylated IR820/DOX co-assembled nanodrug for synergetic antitumour therapy. Int J Nanomedicine. 2020;15:4431–40. doi: 10.2147/IJN.S247617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tan J, Duan X, Zhang F, Ban X, Mao J, Cao M. et al. Theranostic nanomedicine for synergistic chemodynamic therapy and chemotherapy of orthotopic glioma. Adv Sci. 2020;7:2003036. doi: 10.1002/advs.202003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guo Y, Jia H, Zhang X, Zhang X, Sun Q, Wang S. et al. A glucose/oxygen-exhausting nanoreactor for starvation-and hypoxia-activated sustainable and cascade chemo-chemodynamic therapy. Small. 2020;16:2000897. doi: 10.1002/smll.202000897. [DOI] [PubMed] [Google Scholar]
- 151.Wu H, Chen F, You C, Zhang Y, Sun B, Zhu Q. Smart porous core-shell cuprous oxide nanocatalyst with high biocompatibility for acid-triggered chemo/chemodynamic synergistic therapy. Small. 2020;16:2001805. doi: 10.1002/smll.202001805. [DOI] [PubMed] [Google Scholar]
- 152.Ding B, Shao S, Jiang F, Dang P, Sun C, Huang S. et al. MnO2-Disguised upconversion hybrid nanocomposite: An ideal architecture for tumor microenvironment-triggered UCL/MR bioimaging and enhanced chemodynamic therapy. Chem Mater. 2019;31:2651–60. [Google Scholar]
- 153.Wang S, Li F, Qiao R, Hu X, Liao H, Chen L. et al. Arginine-rich manganese silicate nanobubbles as a ferroptosis-inducing agent for tumor-targeted theranostics. ACS Nano. 2018;12:12380–92. doi: 10.1021/acsnano.8b06399. [DOI] [PubMed] [Google Scholar]
- 154.Lin LS, Song J, Song L, Ke K, Liu Y, Zhou Z. et al. Simultaneous fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew Chem Inter Ed. 2018;57:4902–6. doi: 10.1002/anie.201712027. [DOI] [PubMed] [Google Scholar]
- 155.Wang S, Wang Z, Yu G, Zhou Z, Jacobson O, Liu Y. et al. Tumor-specific drug release and reactive oxygen species generation for cancer chemo/chemodynamic combination therapy. Adv Sci. 2019;6:1801986. doi: 10.1002/advs.201801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu J, Wu M, Pan Y, Duan Y, Dong Z, Chao Y. et al. Biodegradable nanoscale coordination polymers for targeted tumor combination therapy with oxidative stress amplification. Adv Funct Mater. 2020;30:1908865. [Google Scholar]
- 157.Hang L, Li H, Zhang T, Men D, Zhang C, Gao P. et al. Au@prussian blue hybrid nanomaterial synergy with a chemotherapeutic drug for tumor diagnosis and chemodynamic therapy. ACS Appl Mater Interfaces. 2019;11:39493–502. doi: 10.1021/acsami.9b13470. [DOI] [PubMed] [Google Scholar]
- 158.Liu CG, Han YH, Zhang JT, Kankala RK, Wang SB, Chen A-Z. Rerouting engineered metal dependent shapes of mesoporous silica nanocontainers to biodegradable Janus-type (sphero-ellipsoid) nanoreactors for chemodynamic therapy. Chem Eng J. 2019;370:1188–99. [Google Scholar]
- 159.Gu D, An P, He X, Wu H, Gao Z, Li Y. et al. A novel versatile yolk-shell nanosystem based on NIR elevated drug release and GSH depletion-enhanced fenton-like reaction for synergistic cancer therapy. Colloids Surf B Biointerfaces. 2020;189:110810. doi: 10.1016/j.colsurfb.2020.110810. [DOI] [PubMed] [Google Scholar]
- 160.Gao Z, He T, Zhang P, Li X, Zhang Y, Liu J. et al. Polypeptide-based theranostics with tumor-microenvironment-activatable cascade reaction for chemo-ferroptosis combination therapy. ACS Appl Mater Interfaces. 2020;12:20271–80. doi: 10.1021/acsami.0c03748. [DOI] [PubMed] [Google Scholar]
- 161.Tian H, Zhang M, Jin G, Jiang Y, Luan Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H2O2 in tumor microenvironment for amplified cancer therapy. J Colloid Interface Sci. 2021;587:358–66. doi: 10.1016/j.jcis.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 162.Gao L, Dong B, Zhang J, Chen Y, Qiao H, Liu Z. et al. Functional biodegradable nitric oxide donor-containing polycarbonate-based micelles for reduction-triggered drug release and overcoming multidrug resistance. ACS Macro Lett. 2019;8:1552–8. doi: 10.1021/acsmacrolett.9b00758. [DOI] [PubMed] [Google Scholar]
- 163.Kim DE, Kim CW, Lee HJ, Min KH, Kwack KH, Lee H-W. et al. Intracellular NO-releasing hyaluronic acid-based nanocarriers: A potential chemosensitizing agent for cancer chemotherapy. ACS Appl Mater Interfaces. 2018;10:26870–81. doi: 10.1021/acsami.8b06848. [DOI] [PubMed] [Google Scholar]
- 164.Hou L, Zhang Y, Yang X, Tian C, Yan Y, Zhang H. et al. Intracellular NO-generator based on enzyme trigger for localized tumor-cytoplasm rapid drug release and synergetic cancer therapy. ACS Appl Mater Interfaces. 2019;11:255–68. doi: 10.1021/acsami.8b17750. [DOI] [PubMed] [Google Scholar]
- 165.Lv Q, Cheng L, Lu Y, Zhang X, Wang Y, Deng J. et al. Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv Sci. 2020;7:2000515. doi: 10.1002/advs.202000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou F, Gao J, Xu Z, Li T, Gao A, Sun F. et al. Overcoming immune resistance by sequential prodrug nanovesicles for promoting chemoimmunotherapy of cancer. Nano Today. 2021;36:101025. [Google Scholar]
- 167.Hou T, Wang T, Mu W, Yang R, Liang S, Zhang Z. et al. Nanoparticle-loaded polarized-macrophages for enhanced tumor targeting and cell-chemotherapy. Nano-Micro Lett. 2021;13:6. doi: 10.1007/s40820-020-00531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shao D, Zhang F, Chen F, Zheng X, Hu H, Yang C, Biomimetic diselenide-bridged mesoporous organosilica nanoparticles as an X-ray-responsive biodegradable carrier for chemo-immunotherapy. Adv Mater. 2020. 2004385. [DOI] [PubMed]
- 169.Li J, Wang H, Wang Y, Gong X, Xu X, Sha X. et al. Tumor-activated size-enlargeable bioinspired lipoproteins access cancer cells in tumor to elicit anti-tumor immune responses. Adv Mater. 2020;32:2002380. doi: 10.1002/adma.202002380. [DOI] [PubMed] [Google Scholar]
- 170.Zhou S, Shang Q, Wang N, Li Q, Song A, Luan Y. Rational design of a minimalist nanoplatform to maximize immunotherapeutic efficacy: Four birds with one stone. J. Control Release. 2020;328:617–30. doi: 10.1016/j.jconrel.2020.09.035. [DOI] [PubMed] [Google Scholar]
- 171.Chen J, Zhou L, Wang C, Sun Y, Lu Y, Li R. et al. A multifunctional SN38-conjugated nanosystem for defeating myelosuppression and diarrhea induced by irinotecan in esophageal cancer. Nanoscale. 2020;12:21234. doi: 10.1039/d0nr06266a. [DOI] [PubMed] [Google Scholar]
- 172.Gao S, Li T, Guo Y, Sun C, Xianyu B, Xu H. Selenium-containing nanoparticles combine the NK cells mediated immunotherapy with radiotherapy and chemotherapy. Adv Mater. 2020;32:1907568. doi: 10.1002/adma.201907568. [DOI] [PubMed] [Google Scholar]
- 173.Xu Y, Ren H, Liu J, Wang Y, Meng Z, He Z. et al. A switchable NO-releasing nanomedicine for enhanced cancer therapy and inhibition of metastasis. Nanoscale. 2019;11:5474–88. doi: 10.1039/c9nr00732f. [DOI] [PubMed] [Google Scholar]
- 174.Zhang H, Zhang J, Li Q, Song A, Tian H, Wang J. et al. Site-specific MOF-based immunotherapeutic nanoplatforms via synergistic tumor cells-targeted treatment and dendritic cells targeted immunomodulation. Biomaterials. 2020;245:119983. doi: 10.1016/j.biomaterials.2020.119983. [DOI] [PubMed] [Google Scholar]
- 175.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak FH. et al. Analysis of nanoparticle delivery totumours. Nat Rev Mater. 2016;1:16014. [Google Scholar]
- 176.Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2:17024. doi: 10.1038/natrevmats.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ingber DE. Is it time for reviewer 3 to request human organ chip experiments instead of animal validation studies? Adv Sci. 2020;7:2002030. doi: 10.1002/advs.202002030. [DOI] [PMC free article] [PubMed] [Google Scholar]