Abstract
Study Objectives
Disturbances of rest–activity rhythms are associated with higher body mass index (BMI) in adults. Whether such relationship exists in children is unclear. We aimed to examine cross-sectional associations of rest–activity rhythm characteristics with BMI z-score and obesity-related inflammatory markers in school-age children.
Methods
Participants included 411 healthy children (mean ± SD age 10.1 ± 1.3 years, 50.8% girls) from a Mediterranean area of Spain who wore wrist accelerometers for 7 consecutive days. Metrics of rest–activity rhythm were derived using both parametric and nonparametric approaches. Obesity-related inflammatory markers were measured in saliva (n = 121).
Results
In a multivariable-adjusted model, higher BMI z-score is associated with less robust 24-h rest–activity rhythms as represented by lower relative amplitude (–0.16 [95% CI –0.29, –0.02] per SD, p = 0.02). The association between BMI z-score and relative amplitude persisted with additional adjustment for sleep duration, and attenuated after adjustment for daytime activity level. Less robust rest–activity rhythms were related to increased levels of several salivary pro-inflammatory markers, including C-reactive protein, which is inversely associated with relative amplitude (–32.6% [–47.8%, –12.9%] per SD), independently of BMI z-score, sleep duration, and daytime activity level.
Conclusion
Blunted rest–activity rhythms are associated with higher BMI z-score and salivary pro-inflammatory markers already at an early age. The association with BMI z-score seem to be independent of sleep duration, and those with pro-inflammatory markers further independent of BMI z-score and daytime activity. Novel intervention targets at an early age based on improving the strength of rest–activity rhythms may help to prevent childhood obesity and related inflammation.
Clinical Trials Registration
Keywords: rest, activity rhythm, school-aged children, body mass index, inflammation
Statement of Significance.
Disturbances in rest–activity rhythms are positively associated with body mass index (BMI) z-score and pro-inflammatory markers in adults. However, little is known about such associations in school-aged children. In a cross-sectional study, we found that disrupted rest–activity rhythms, as represented by low relative amplitude, show strong associations with high BMI z-score and levels of C-reactive protein, independent of sleep duration and/or daytime activity levels. Novel intervention targets at an early age based on improving the strength of 24 h rest–activity rhythms may help to prevent childhood obesity and related inflammation.
Introduction
Childhood is a critical period in the development and persistence of obesity and related co-morbidities into adulthood [1]. Thus, identifying modifiable risk factors is of urgent public health importance. In parallel with the childhood obesity epidemic, there has been an increase in the prevalence of lifestyle factors that may disturb the circadian system in school-aged children, including artificial light, digital media, demanding school schedules, and extracurricular activities [2–5]. Since the circadian system plays a major role in regulating sleep–wake cycles as well as various metabolic outputs, circadian disturbances may have deleterious health consequences. Indeed, mounting evidence supports a strong association of lifestyle factors that are conducive of circadian disruption with obesity [6–12]. However, whether such relationship already exists in pediatric populations is unclear. Circadian disturbance may increase inflammation. Experimentally induced circadian disruption in laboratory setting led to elevated pro-inflammatory markers in healthy adults [13–16]. Moreover, low-grade systemic inflammation is a characteristic of obesity. Pro-inflammatory markers, such as C-reactive protein (CRP) are increased in obese adults as well as in obese adolescents [17–19]. Such chronic subclinical activation of the immune system predicts the increased risks for metabolic syndromes [20], and is considered to play a role in the pathogenesis of atherosclerosis from early ages [21], suggesting that preventive measures should start early in life. However, it is unknown whether circadian disturbances are associated with increased pro-inflammatory markers at early ages, and whether such association is mediated by body mass index (BMI) or obesity level.
The rest–activity cycle, which is in part a behavioral manifestation of the circadian function in response to environmental cycles, encompasses major daily activities such as sleep timing, sleep regularity, and daytime physical activity [22, 23]. The rapid development of wearable devices has provided an objective measurement of 24-h rest–activity rhythms with low participant burden in free-living conditions. However, research has mostly been conducted in adults [23–28]. For the few studies in adolescents, only limited aspects of rest–activity cycles have been assessed [29, 30]. Thus, more comprehensive approaches are needed to identify rest–activity metrics and their association with BMI in pediatric populations. Furthermore, no previous study has examined the link between obesity, rest–activity rhythms, and pro-inflammatory markers in children. The scarcity of research in this field could be partly due to the invasive and stressful nature of venipuncture [31]. Noninvasive methods such as salivary biomarkers have been proposed as alternatives to blood collection [32, 33].
To address the aforementioned knowledge gaps, we conducted a cross-sectional study in a cohort of school-aged children in a Mediterranean area of Spain [34]. We obtained metrics of rest–activity rhythms by wrist-worn accelerometer to examine potential associations with BMI z-score and salivary pro-inflammatory markers. Secondarily, we examined whether these associations were modified by: (1) total daytime motor activity and/or total sleep duration; and (2) BMI z-score (for pro-inflammatory markers). Understanding which metrics of rest–activity rhythms associate with health-related outcomes and whether associations are modified by conventional risk factors such as sleep duration and physical activity level, can provide insight into mechanisms and novel intervention targets.
Methods
Study population
Healthy children of ages between 8 and 12 years (n = 411, 209 girls and 202 boys, 93.2% Caucasian, and 6.8% other races/ethnicities) from a Mediterranean area of Spain were recruited as previously described [34] (ONTIME-Jr population; ClinicalTrials.gov ID: NCT02895282). Central and rural school centers were chosen with the goal of having a greater heterogeneity in the sample. The study was approved by the Ethics Committee of the University of Murcia (ID: 1868/2018).
Details of the study protocol and recruitment/retention procedures are available elsewhere [34]. Data collection was carried out during the academic terms between 2014 and 2016, which started in October 2014 and was completed in May 2016. The study was approved by the steering committees of the schools. A comprehensive verbal description of the nature, purpose, and procedures of the study was given to both the children and their parents. For all the children enrolled in this study, written consents were received from their parents. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Assessment of rest–activity rhythms
Children wore a triaxial accelerometer (HOBO Pendant G Acceleration Data Logger UA-04-64, Onset Computer, Bourne, MA, USA) on the non-dominant wrist with its x-axis parallel to the humerus bone for 24 h per day (except when bathing or swimming) for 7 consecutive days. This accelerometer sensor was programed to record data on motor activity and body position every 30 s, as previously described [34, 35]. In brief, motor activity was expressed as the accumulative changes in three-axis tilt with respect to the previous point and expressed as Δ degrees per minute. Body position was calculated as the angle between x-axis of the accelerometer and the horizontal plane, where 0° represents the arm in a horizontal position and 90° when vertical. This method has been previously validated with the commonly used wrist-worn Actiwatch accelerometer, and almost all the parameters derived from the rhythm showed high correlations between both devices [35]. Because the device used in the current study integrates the activity sensor together with a temperature sensor, we were able to use both the motor activity and temperature readings to estimate wear time. Nonwear time was defined as an interval in which motor activity readings were lower than 4°/min and the skin temperature readings were out of the physiological range (i.e. <28°C). A valid day was defined as a day with less than 8 hours of non-wear time. All the children included in the analysis had 7 days of validated recording.
Rest–activity rhythm parameters were derived using both parametric and nonparametric methods (Table 1):
Table 1.
24-h rest–activity rhythm parameters
| Acrophase | The time of peak activity |
|---|---|
| Amplitude | Difference between the model fitted peak value and the mesor |
| Mesor | The mean activity of the fitted 24-h pattern |
| Relative amplitude | Refers to the difference between the maximum (or minimum) value of the fitted cosine function and mesor and is a normalized measure of rhythm strength accounting for average activity levels |
| Circadian Function Index (CFI) | A numerical index that determines the circadian robustness, based on three circadian parameters: interday stability, intraday variability, and relative amplitude. CFI oscillates between 0 (absence of circadian rhythmicity) and 1 (robust circadian rhythm) |
| L5 midpoint | Timing of the least active 5-h period, an indicator of midpoint of sleep |
| M10 midpoint | Timing of the most active 10-h period |
| Interday stability | An estimate of how closely the 24-h rest–activity rhythm follows the 24-h light–dark cycle. Determines the consistency of the 24 h rhythmic pattern over days. A stable rhythm is characterized by a 24-hour profile that remains very similar from day to day |
| Intraday variability | Fragmentation of the rhythm. Its values oscillate between 0 when the wave is perfectly sinusoidal and 2 when the wave describes a Gaussian noise |
(1) Parametric cosinor analysis [36]: A single-component cosine curve representing a 24-h period was fitted to the data, using regression analysis. The regression model can be described as: Y(t) = M + A*cos(2πt/T + φ) + e(t) where M is the mesor, φ is the acrophase, and A is the amplitude (Table 1). Relative amplitude was calculated as the ratio of amplitude and mesor.
(2) Nonparametric analysis [37] was also used in consideration that the daily rest–activity rhythm does not perfectly follow the sinusoidal curve. As described in Table 1, we estimated: (a) intradaily variability; (b) interdaily stability; (c) timing of most active 10-h episode (M10 midpoint); (d) timing of the least active 5-h episode (L5 midpoint); and (e) Circadian Function Index (CFI) [38].
All of the rhythmic parameters were obtained using an integrated package for temporal series analysis Kroniwizard (https://kronowizard.um.es/kronowizard) (Chronobiology Laboratory, University of Murcia, Spain, 2015).
Anthropometric measurements
As previously described [34], body weight was measured in barefooted subjects wearing light clothes using a digital scale accurate to the nearest 0.1 kg. Height was determined using a portable stadiometer (rank, 0.14–2.10). Height and weight measurements were obtained at the beginning of the appointment always in the morning at the same time of day (around 10 am). Child overweight and obesity was determined by age and sex specific cut off points of BMI based on international data [39], including two underweighted participants grouped into “normal weight” category [40]. We calculated BMI and age- and sex-specific z scores using the addWGSR function of the R “zscorer” package [41] which calculates BMI z-score based on the WHO Growth Reference [42, 43].
Saliva sample collection and inflammatory markers
Saliva samples were collected in the morning (approximately 9 am) of Sunday after overnight fast. To collect saliva (n = 121), a salivette, which is a small cotton swab inside a standard centrifugation tube, was used (Sarstedt, Barcelona, Spain). All samples were maintained refrigerated at 4°C until delivered (1 day) to the laboratory and then stored at –80°C until analyzed.
Interleukin (IL)1b, IL6, IL8, monocyte chemotractant protein 1 (MCP1), tumor necrosis factor alpha (TNFα) were analyzed using commercially available kits (MILLIPLEX MAP Human Adipokine Magnetic Bead Panel 2 – Endocrine Multiplex Assay; Life Science, Darmstadt, Germany) according to manufacturer’s indications. C-reactive protein (CRP) was analyzed using a commercially available kit (MILLIPLEX MAP Human – CRP Assay; Life Science, Darmstadt, Germany) according to manufacturer’s indications. IgA was evaluated with a commercial ELISA kit (Bethyl, Montgomery, TX, USA) in an automated biochemistry analyzer [44]. The values for all the assays were calculated based on a standard curve constructed for the assay.
Covariates
Information on sociodemographic factors (age, school, grade, sex) was assessed using self-administered questionnaires. Total sleep duration was estimated from a formula integrating the objectively measured motor activity, body position, and wrist skin temperature, as previously described [38]. Missing sleep duration data (n = 30) was imputed using mean value. Average daytime motor activity level was derived from the accelerometer as described above and calculated as average accumulative degree changes in three-axis tilt per minute during the most active 10 h during the wake period (i.e. nonsleep time), which shows a decent correlation with motor activity measured by wrist-worn Actiwatch [26, 35].
Statistical analysis
The primary outcome was BMI z-score. To facilitate comparison of the strength of the association between different rest–activity rhythm parameters and the BMI z-score, we examined associations between 1 standard deviation unit (SD) increase in each rest–activity rhythm parameter and the BMI z-score using linear regression models. The secondary outcomes were inflammatory markers which were transformed using the natural logarithm for all of the analyses, and expressed as percentage change per 1 SD increase in each rest–activity rhythm parameter. Models with BMI z-score, IgA, or CRP as outcome were adjusted for age, sex, school, and grade. Models with IL8, IL1b, TNFα, or MCP1 as outcome, were adjusted for age, sex, and grade, since those measures were only available in one school. To examine if sleep duration or daytime activity level accounts for the influence of rest–activity rhythm characteristics, we further adjusted for total sleep duration and daytime motor activity level in multivariable models. For pro-inflammatory markers, we also adjusted for BMI z-score to examine the associations with rest–activity rhythm characteristics. We performed regressions for multiple exposure and outcome variables so false positive may be a concern. However, many of our variables were highly correlated; therefore, we did not control for multiple comparisons using conventional methods such as Bonferroni corrections, because such methods assume independence among tests [45]. Instead, we presented confidence intervals and p-values for all the associations and evaluated our results based on both effect sizes and measures of statistical significance. We completed the statistical analyses using R 3.5.1.
Results
Study participant characteristics are presented in Table 2. Parameters of rest–activity distribution are presented in Figure S1.
Table 2.
Participant characteristics, overall, and by category of body mass index
| Overall | Normal weight | Overweight | Obese | p value | |
|---|---|---|---|---|---|
| N | 411 | 276 | 93 | 42 | |
| Age, years | 10.1 (1.25) | 10.1 (1.24) | 10.1 (1.15) | 9.90 (1.51) | 0.59 |
| Sex, boys/girls, n | 202/209 | 135/141 | 43/50 | 24/18 | 0.5 |
| Body mass index, z-score | 0.86 (1.36)[-3.35,5.46] | 0.15 (0.98)[-3.35, 2.71] | 1.93 (0.38)[1.25, 2.78] | 3.14 (0.64)[2.21, 5.46] | <0.0001 |
| Rest–activity rhythm characteristics | |||||
| Mesor | 46.6 (6.1)[21.7, 61.3] | 47.1 (5.98)[21.7, 61.3] | 46.2 (6.05)[22.9, 57.4] | 44.4 (6.19)[28.6, 56.1] | 0.05 |
| Acrophase, clock hour | 15.0 (0.69)[13.3, 16.9] | 15.0 (0.69)[13.4, 16.9] | 15.1 (0.65)[13.3, 16.6] | 15.2 (0.73)[13.6, 16.6] | 0.05 |
| Relative amplitude | 0.84 (0.05)[0.62, 1.00] | 0.84 (0.04)[0.68, 1.00] | 0.84 (0.04)[0.72, 0.97] | 0.83 (0.07)[0.62, 0.93] | 0.62 |
| Interdaily stability | 0.38 (0.08)[0.21, 1.04] | 0.39 (0.07)[0.21, 1.00] | 0.38 (0.08)[0.22, 0.98] | 0.38 (0.12)[0.22, 1.04] | 0.12 |
| Intradaily variability | 0.87 (0.06)[0.60, 1.05] | 0.86 (0.06)[0.60, 1.03] | 0.87 (0.07)[0.65, 1.05] | 0.88 (0.06)[0.72, 1.03] | 0.24 |
| M10 midpoint, clock hour | 15.2 (1.32)[12.0, 19.0] | 15.2 (1.32)[12.0, 19.0] | 15.2 (1.29)[12.4, 19.0] | 15.2 (1.38)[12.3, 18.1] | 0.91 |
| L5 midpoint, clock hour | 2.5 (1.2)[0.6, 6.0] | 3.4 (1.2)[0.6, 6.0] | 3.8 (1.05)[1.6, 5.5] | 3.5 (1.11)[1.5, 5.6] | 0.03 |
| CFI | 0.60 (0.04)[0.47, 0.82] | 0.60 (0.04)[0.51, 0.81] | 0.60 (0.04)[0.51, 0.79] | 0.59 (0.05)[0.47, 0.82] | 0.29 |
| Other lifestyle factors | |||||
| Total sleep duration, h | 7.68 (0.68)[6.45, 12.6] | 7.68 (0.59)[6.45, 9.77] | 7.69 (0.77)[6.48, 12.2] | 7.65 (0.99)[6.47, 12.6] | 0.63 |
| Daytime motor activity, ∆°/min | 73.4 (10.0)[38.2, 98.4] | 74.5 (9.8)[38.2, 98.4] | 72.4 (9.5)[38.6, 90.7] | 68.5 (11.2)[43.8, 86.5] | 0.001 |
Data are n or mean (SD) [range]. p values obtained with the Kruskal–Wallis test for continuous variables and χ2 test for categorical variables.
M10 midpoint, timing of most active 10-h episode; L5 midpoint, timing of the least active 5 h episode; CFI, circadian function index.
We found that several characteristics of the rest–activity rhythm, such as mesor, relative amplitude and CFI, were significant and inversely associated with BMI z-score (p = 0.03, p = 0.02, p = 0.03, respectively) while intradaily variability was positively associated with BMI z-score (a trend, p = 0.08) (Table 3, Figure 1A and B). Specifically, every 1 SD increase in relative amplitudes associated with 0.16 (95% CI: –0.29, –0.02) decrease in BMI z-score. A similar decrease in BMI z-score was observed with 1 SD increase in CFI (β–0.16 [–0.3, –0.02]). One SD increase in mesor was associated with –0.16 (–0.31, –0.02) change in BMI z-score, while 1 SD increase in intradaily variability associated with 0.12 (–0.02, 0.26) increase in BMI z-score. Further adjustment for total sleep duration had minimal impact on the findings regarding mesor, relative amplitude, and CFI. However, additional adjustment for daytime motor activity attenuated the three associations (Table 3).
Table 3.
Continuous associations with body mass index z-score per 1 standard deviation increase in rest–activity parameters
| Beta coefficient (95% confidence interval); p value | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Mesor | –0.16 (–0.31, –0.02) | –0.17 (–0.32, –0.03) | 0.32 (–0.05, 0.69) |
| p = 0.03 | p = 0.02 | p = 0.09 | |
| Acrophase | 0.06 (–0.07, 0.20) | 0.06 (–0.08, 0.19) | 0.06 (–0.08, 0.19) |
| p = 0.38 | p = 0.41 | p = 0.42 | |
| Relative amplitude | –0.16 (–0.29, –0.02) | –0.16 (–0.3, –0.02) | –0.12 (–0.26, 0.01) |
| p = 0.02 | p = 0.03 | p = 0.08 | |
| Interdaily stability | –0.08 (–0.22, 0.05) | –0.08 (–0.22, 0.06) | 0.06 (–0.11, 0.22) |
| p = 0.23 | p = 0.25 | p = 0.51 | |
| Intradaily variability | 0.12 (–0.02, 0.26) | 0.12 (–0.02, 0.26) | 0.1 (–0.03, 0.24) |
| p = 0.08 | p = 0.10 | p = 0.14 | |
| M10 midpoint | –0.06 (–0.19, 0.08) | –0.06 (–0.19, 0.08) | –0.04 (–0.18, 0.09) |
| p = 0.42 | p = 0.39 | p = 0.52 | |
| L5 midpoint | 0.06 (–0.07, 0.19) | 0.05 (–0.08, 0.18) | 0.03 (–0.1, 0.16) |
| p = 0.40 | p = 0.43 | p = 0.63 | |
| CFI | –0.16 (–0.3, –0.02) | –0.16 (–0.3, –0.01) | –0.07 (–0.23, 0.09) |
| p = 0.03 | p = 0.03 | p = 0.41 | |
(Model 1) Adjusted for age, sex, school, and grade. (Model 2) Model 1 + total sleep duration. (Model 3) Model 1 + daytime motor activity.
M10 midpoint, timing of most active 10-h episode; L5 midpoint, timing of the least active 5 episode; CFI, circadian function index.
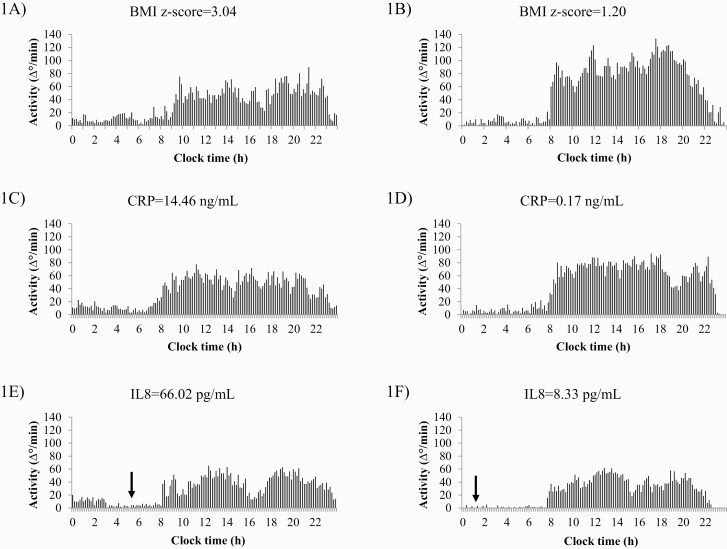
Figure 1.
Representative example of 7-day average actigraphs of several individual cases with high and low BMI z-score, C-reactive protein (CRP) and interleukin 8 (IL8). (A) represents an obese child (code 470; BMI z-score = 3.04) with a low relative amplitude (RA = 0.69) and a high fragmentation (intradaily variability = 1.01). (B) Represents a normal weight child (code 304; BMI z-score = 1.20) with a high relative amplitude (RA = 0.87) and low fragmentation (intradaily variability = 0.71). (C) represents a child (code 405) with a high C-reactive protein (CRP = 14.46 ng/mL) and a low relative amplitude (RA = 0.73). (D) represents to a child (code 422) with a low C-reactive protein (CRP = 0.17 ng/mL) and a high relative amplitude (RA = 0.88). (E) represents a child (code 455) with a high interleukin 8 (IL8 = 66.02 pg/mL) and a later L5 midpoint (05:37 am). (F) represents a child (code 416) with a low interleukin 8 (IL8 = 8.33 pg/mL) and an early L5 midpoint (01:07 am). The arrow represents L5 midpoint in (E) and (F).
We next examined several inflammatory markers in a subset of 121 children who provided saliva samples (Table 4). Among those, CRP levels were inversely associated with relative amplitudes in which 1 SD increase of relative amplitude was linked to 32.6% decrease (–47.8%, –12.9%; p = 0.002, Figure 1C and D) in CRP levels. Associations remained significant after further adjustment for total sleep duration or daytime motor activity level (–33.5% [–49.3%, –12.8%] per SD, p = 0.003; –32.2% [–47.8%, –12.0%] per SD, p = 0.01, respectively), and BMI z-score (both p < 0.02). IL8 and IL1b levels were both positively and significantly associated with L5 midpoint with 1 SD increase in L5 midpoint correlated with 30.8% (3.6%, 65.2%, p = 0.02) increase in IL8 and 43.4% (5.6%, 94.9%, p = 0.02, Figure 1E and F) increase in IL1b. Importantly, the association of L5 midpoint with IL8 or IL1b was independent of total sleep duration, daytime motor activity level, and BMI z-score (all p < 0.05). Mesor was inversely associated with IgA with 1 SD increase associated with –24.0% (–40.7%, –2.7%, p = 0.03) change in concentrations of IgA, which was independent of total sleep duration and BMI z-score (all p < 0.05), while additional adjustment of daytime motor activity level slightly attenuated the association (p = 0.06). Mesor, interdaily stability and intradaily variability were inversely associated with MCP1 (–31.5% [–44.6%, –15.3%], p = 0.0008, –33.3% [–50.1%, –10.7%], p = 0.007 and –22.0% [–36.4%, –4.4%], p = 0.02, respectively). Moreover, the associations of mesor or interdaily stability with MCP1 were independent of total sleep duration and BMI z-score (all p < 0.01), and the association of intradaily variability with MCP1 was independent of total sleep duration, daytime motor activity level, and BMI z-score (all p < 0.05).
Table 4.
Percentage change of inflammatory marker concentration per 1 standard deviation increase in rest–activity parameters
| IgA (n = 121) | CRP (n = 118) | IL8 (n = 57) | IL1b (n = 60) | TNFα (n = 59) | MCP1 (n = 60) | |
|---|---|---|---|---|---|---|
| Mesor | –24 (–40.7, –2.7) | –4.5 (–31.9, 33.9) | 10.8 (–13.8, 42.5) | –10 (–36.6, 27.8) | 6.6 (–14.5, 32.8) | –31.5 (–44.6, –15.3) |
| p < 0.05 b, d | p = 0.79 | p = 0.42 | p = 0.55 | p = 0.56 | p < 0.01 a, c | |
| Acrophase | 7.6 (–15.9, 37.7) | –10 (–35, 24.8) | –6.1 (–28.1, 22.7) | –5.2 (–33.4, 35) | –3.5 (–23.4, 21.5) | –14.9 (–32.6, 7.5) |
| p = 0.56 | p = 0.52 | p = 0.64 | p = 0.76 | p = 0.76 | p = 0.17 | |
| Relative amplitude | 2.9 (–15.8, 25.6) | –32.6 (–47.8, –12.9) | 6.5 (–12.3, 29.4) | 16.1 (–11, 51.6) | –0.7 (–16.3, 17.7) | 3.2 (–13.8, 23.6) |
| p = 0.78 | p < 0.02 a, b, c, d | p = 0.52 | p = 0.26 | p = 0.93 | p = 0.73 | |
| Interdaily stability | 6.2 (–17.3, 36.6) | 0.2 (–28.4, 40.1) | 0.9 (–27.9, 41.3) | –11.9 (–44.5, 39.8) | –17.8 (–38.4, 9.7) | –33.3 (–50.1, –10.7) |
| p = 0.63 | p = 0.99 | p = 0.96 | p = 0.58 | p = 0.18 | p < 0.01 a, c | |
| Intradaily variability | –9.7 (–27.3, 12.2) | 19.7 (–10.7, 60.4) | –9.9 (–28.3, 13.3) | –6.6 (–32.2, 28.6) | –8.6 (–25.1, 11.6) | –22 (–36.4, –4.4) |
| p = 0.35 | p = 0.23 | p = 0.37 | p = 0.67 | p = 0.37 | p < 0.05 a, b, c, d | |
| M10 midpoint | –3.2 (–21.8, 19.7) | –16.1 (–36.6, 11.1) | 5.4 (–16.7, 33.5) | –13.8 (–37.1, 18.3) | 0.7 (–18, 23.7) | –8.3 (–25.9, 13.3) |
| p = 0.76 | p = 0.22 | p = 0.65 | p = 0.35 | p = 0.95 | p = 0.41 | |
| L5 midpoint | 10.1 (–11.7, 37.1) | 14.8 (–14.6, 54.3) | 30.8 (3.6, 65.2) | 43.4 (5.6, 94.9) | 11.5 (–9, 36.6) | 16.3 (–5.9, 43.7) |
| p = 0.39 | p = 0.36 | p < 0.05 a, b, c, d | p < 0.05 a, b, c, d | p = 0.29 | p = 0.16 | |
| CFI | 8.5 (–14.1, 37.1) | –23.7 (–44.1, 4.1) | 8.8 (–16.3, 41.5) | 8 (–24.7, 55) | –5.4 (–24.7, 19) | –5.9 (–26.1, 19.9) |
| p = 0.49 | p = 0.09 | p = 0.52 | p = 0.67 | p = 0.63 | p = 0.62 |
Data are expressed as mean (95 CI). Unit is %. (Model 1) the default model is adjusted for age, sex, grade, and school (school for IgA and CRP only). If significance was found in the default model, further adjustments were made as following: a(Model 2) Model 1 + total sleep duration. b(Model 3) Model 1 + daytime motor activity. c(Model 4) Model 2 + BMI z-score. d(Model 5) Model 3 + BMI z-score.
M10 midpoint, timing of most active 10-h episode; L5 midpoint, timing of the least active 5 h episode; CFI, circadian function index.
Discussion
In this cohort of school-aged children, less robust 24-h rest–activity rhythms, as represented by higher mesor, lower relative amplitude, and lower Circadian Functional Index (CFI), was associated with higher BMI z-score. For the first time, in a subset of children with saliva samples, we uncovered that many aspects of rest–activity rhythms were significantly associated with several pro-inflammatory markers, including CRP. Importantly, these associations remained significant after adjustment for potential mediating factors such as total sleep duration, daytime motor activity level, and obesity level (BMI z-score), suggesting that the robustness of rest–activity rhythms is related to inflammation independently of these traditional risk factors.
An increasing number of studies have linked the strength and regularity of daily rest–activity rhythms to health outcomes in community-dwelling middle-aged and older adults [23–25, 27, 28]. However, this is an emerging area of research in pediatric populations, thus few studies are directly comparable. In a recent cross-sectional study, Quante et al. examined four characteristics of the rest–activity rhythm using the nonparametric method and found that the relative amplitude was negatively associated with BMI z-score in adolescents, independent of parent-reported sleep duration, and physical activity level [29]. In our study, we also found that the relative amplitude had the strongest associations with BMI z-score. We further included the parametric method and additional metrics to characterize the regularity (interdaily stability) and fragmentation (intradaily variability) of the rest–activity rhythm, thus generating a more complete picture. In addition to objectively measured sleep duration, we further adjusted for objectively measured daytime activity level (instead of parent-reported in the previous study). Interestingly, while the significant associations were independent of sleep duration, they were attenuated after adjustment for daytime activity level. Future studies are needed to test whether daytime activity is a mediating factor for the association between the parameters of rest–activity rhythm and BMI z-score. If so, promoting daytime physical activity could be an effective strategy to boost rest–activity rhythm and help prevent excess weight gain in school-aged children. Our results are also consistent with our previous study in adolescents showing that intradaily variability positively associated with obesity, although only waking hours activity was available to derive the metrics in that study [30]. Moreover, the current study is focused on a younger pediatric population (7–12 years old) while both previous studies included adolescents (11–17.5 years old). Adolescents are especially susceptible to disruption in rest–activity rhythm due to the dramatic pubertal transformation in body composition and a biologically-mediated tendency to eveningness [46–48]. Our results indicate that children at a younger age may already face the deleterious metabolic consequences of a weak rest–activity rhythm, pointing out a need to start behavioral interventions early in life.
As far as we are aware, this is the first study investigating the association between the rest–activity rhythm characteristics and inflammatory markers in a pediatric population of school-age children. In a subset of 118 children, we found that a lower relative amplitude was associated with higher salivary CRP levels, independent of sleep duration, daytime activity, and BMI z-score. Circulating CRP is a sensitive marker for low-grade systemic inflammation that has been shown to be a powerful predictor for the development of type 2 diabetes and cardiovascular diseases in apparently healthy adults [49–51]. Elevated CRP concentrations have been associated with higher adiposity and lower aerobic fitness in children [52, 53]. Since salivary CRP has shown to have a moderate-to-strong correlation with plasma CRP, its determination in saliva has been proposed as a noninvasive alternative method to assess subclinical systemic inflammation [32, 54].
The observed associations cannot determine causality. However, our results suggest that a weakened rest–activity rhythm may contribute to low-grade inflammation in children, which could increase the risk of metabolic syndromes in later life [55]. Similarly, in a smaller subset, salivary IL8 and IL1b increased with later sleep timing (L5 midpoint) an indicator of whether a person goes to bed earlier or later in the day. These associations were independent of sleep duration, daytime activity level, and BMI z-score. MCP1, is a chemokine which plays a pivotal role in the development of atherosclerosis [56]. Our results in school-age children show that salivary MCP1 level was negatively associated with the 24-h average activity level (mesor) and the regularity and fragmentation of the rest–activity rhythms. Such results were consistent with previous findings from randomized controlled studies demonstrating that increased physical activity can reduce MCP1 levels in human [57, 58]. Overall, our results revealed that different characteristics of the rest–activity rhythms were associated with specific pro-inflammatory markers in children. These associations cannot be fully explained by sleep duration, daytime activity and BMI z-score, thus pointing out an independent contribution of the rest–activity rhythms characteristics.
A potential explanation to these findings could be that weakened rest–activity rhythms lead to increased BMI z-score and pro-inflammatory markers, although the reverse causation could be also true (higher BMI z-score leads to weakened rhythms). Previous literature has shown that circadian disruption results in dysregulation of energy metabolism and inflammatory responses, independently from sleep duration as demonstrated by highly-controlled human in-laboratory protocols [10, 11, 14–16, 59–62] which is consistent with our findings. However, these in-laboratory studies often represented dramatic forms of circadian disruption including arrhythmic locomotor activity in animals or inverted behavioral cycles in human. Our results indicate that even mild-to-moderate disruption in rest–activity rhythms, as shown in the current school-age children population, is associated with adverse metabolic consequences.
This research has multiple strengths: first, our observations in a unique, large, school-aged pediatric population under the real-life setting identified the inverse association of relative amplitude of rest–activity rhythms with BMI z-score and salivary CRP levels, which supports the utility of wearable devices as a tool for large pediatric cohorts to measure exposures related to daily rest–activity rhythms. Second, we used both parametric and nonparametric methods to quantify different domains of the rest–activity rhythms and tested their associations with health outcomes such as BMI z-score. Such approach provides insights into which rhythm parameters may be the strongest biomarkers for certain health outcomes in children. Third, for the first time, we revealed that the strength and regularity of the rest–activity rhythms are inversely associated with pro-inflammatory markers, highlighting the potential of salivary biomarkers to be an easy and non-invasive alternative method to blood samples in children.
Our study also has several limitations. Due to the cross-sectional design, causal inferences cannot be made. Future studies are needed to test the prospective relationship between the rest–activity rhythm and health outcomes in children as well as the multiple potential mechanisms of these associations. In addition, while the rest–activity rhythm is partially under the regulation of the circadian system, it is not a direct assessment of the circadian rhythm. However, previous research has found that rest–activity rhythms associate with the timing of light exposure (the strongest Zeitgeber of the circadian system) and amplitude of melatonin secretion (classical circadian phase marker) [63, 64]. These results suggested that altered rest–activity rhythms do, to some degree, reflect circadian disruption, thus can be used as a relevant biological sign for circadian function. Another potential limitation could be that we did not correct for multiple comparisons considering that many exposures and outcomes were correlated with each other. While this may increase the likelihood of type 1 error, it is consistent with many other prior similar studies [23–25, 29, 65]. Future studies are needed to confirm our findings. It is also pointed out that the current methods used to measure sleep duration has not been validated with polysomnography in children. However, the results were consistent with results obtained by sleep diaries, supporting the validity of this objective measure. Last, we recognize that wrist-worn device is not as accurate as hip-worn device in estimating physical activity.
Conclusion
We found that disrupted rest–activity rhythms associate with higher BMI z-score and salivary pro-inflammatory markers in school-aged children. Such associations with BMI z-score seem to be independent of sleep duration, those with pro-inflammatory markers further independent of daytime motor activity levels. Among different metrics of the rest–activity rhythms, relative amplitude, the rhythm strength adjusted for total 24-h activity level, showed strong independent associations with both BMI z-score and salivary CRP levels, and thus may be a potential target for intervention. Future research should examine the underlying mechanisms of these associations and develop effective countermeasures to improve the strength of the rest–activity rhythms in order to prevent childhood obesity and related inflammation.
Supplementary Material
Acknowledgments
This work has been supported in part by The Spanish Government of Investigation, Development and Innovation (SAF2017-84135-R) including European Regional Development Fund (FEDER) co-funding; The Autonomous Community of the Region of Murcia through the Seneca Foundation (20795/PI/18) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK105072 granted to M.G. J.Q. was supported in part by the American Diabetes Association (award 1-17-PDF-103) and by the National Institutes of Health (NIH) (grants K99HL148500 and R01DK102696). F.A.J.L.S. was supported in part by the NIH (grants R01HL118601, R01DK099512, R01DK102696, R01DK105072, and R01HL140574).
Author Contributors
J.Q. conceptualized and designed the study, performed data analysis, drafted the initial manuscript, and reviewed and revised the manuscript. N.M.L. designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. A.T. performed assays to measure the obesity-related inflammatory markers. R.R. recruited children for the study and collected samples. F.A.J.L.S. conceptualized and designed the study, and critically reviewed the manuscript for important intellectual content. M.G. conceptualized and designed the study, coordinated and supervised data collection, and reviewed and revised the manuscript.
Disclosure Statement
Financial disclosure: F.A.J.L.S. received speaker fees from Bayer Healthcare, Sentara Healthcare, Philips, Kellogg Company, Vanda Pharmaceuticals, and Pfizer. The other authors have indicated they have no financial relationships relevant to this article to disclose.
Non-financial disclosure: The authors have no non-financial disclosure relevant to this article to disclose.
Conflict of interest statement. None declared.
Data Availability
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Ludwig DS. Childhood obesity—the shape of things to come. N Engl J Med. 2007;357(23):2325–2327. [DOI] [PubMed] [Google Scholar]
- 2. Cain N, et al. . Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11(8):735–742. [DOI] [PubMed] [Google Scholar]
- 3. Crowley SJ, et al. . Increased sensitivity of the circadian system to light in early/mid-puberty. J Clin Endocrinol Metab. 2015;100(11):4067–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LeBourgeois MK, et al. . Digital media and sleep in childhood and adolescence. Pediatrics. 2017;140(Suppl 2):S92–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansen M, et al. . The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115(6):1555–1561. [DOI] [PubMed] [Google Scholar]
- 6. Roenneberg T, et al. . Social jetlag and obesity. Curr Biol. 2012;22(10):939–943. [DOI] [PubMed] [Google Scholar]
- 7. Sun M, et al. . Meta-analysis on shift work and risks of specific obesity types. Obes Rev. 2018;19(1):28–40. [DOI] [PubMed] [Google Scholar]
- 8. Pan A, et al. . Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruiz-Lozano T, et al. . Evening chronotype associates with obesity in severely obese subjects: interaction with CLOCK 3111T/C. Int J Obes (Lond). 2016;40(10):1550–1557. [DOI] [PubMed] [Google Scholar]
- 10. Qian J, et al. . Sex differences in the circadian misalignment effects on energy regulation. Proc Natl Acad Sci USA. 2019;116(47):23806–23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McHill AW, et al. . Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci USA. 2014;111(48):17302–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turek FW, et al. . Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morris CJ, et al. . Circadian misalignment increases C-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms. 2017;32(2):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wright KP Jr, et al. . Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leproult R, et al. . Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris CJ, et al. . Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci USA. 2016;113(10):E1402–E1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herder C, et al. . Low-grade inflammation, obesity, and insulin resistance in adolescents. J Clin Endocrinol Metab. 2007;92(12):4569–4574. [DOI] [PubMed] [Google Scholar]
- 18. Kim CS, et al. . Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond). 2006;30(9):1347–1355. [DOI] [PubMed] [Google Scholar]
- 19. Visser M, et al. . Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–2135. [DOI] [PubMed] [Google Scholar]
- 20. Calder PC, et al. . Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(Suppl 3):S5–S 78. [DOI] [PubMed] [Google Scholar]
- 21. Järvisalo MJ, et al. . Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol. 2002;22(8):1323–1328. [DOI] [PubMed] [Google Scholar]
- 22. van Someren EJ, et al. . Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol Psychiatry. 1996;40(4):259–270. [DOI] [PubMed] [Google Scholar]
- 23. Cespedes Feliciano EM, et al. . Actigraphy-derived daily rest-activity patterns and body mass index in community-dwelling adults. Sleep. 2017;40(12). doi: 10.1093/sleep/zsx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rogers-Soeder TS, et al. ; Osteoporotic Fractures in Men Study Research Group. Rest-activity rhythms and cognitive decline in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2018;66(11):2136–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luik AI, et al. . Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 2013;30(10):1223–1230. [DOI] [PubMed] [Google Scholar]
- 26. Mitchell JA, et al. . Variation in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiol Int. 2017;34(8):1042–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sohail S, et al. . Irregular 24-hour activity rhythms and the metabolic syndrome in older adults. Chronobiol Int. 2015;32(6):802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao Q, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Cross-sectional and prospective associations of rest-activity rhythms with metabolic markers and type 2 diabetes in older men. Diabetes Care. 2020;43(11):2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quante M, et al. . Association of daily rest-activity patterns with adiposity and cardiometabolic risk measures in teens. J Adolesc Health. 2019;65(2):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garaulet M, et al. ; HELENA study group. Fragmentation of daily rhythms associates with obesity and cardiorespiratory fitness in adolescents: the HELENA study. Clin Nutr. 2017;36(6):1558–1566. [DOI] [PubMed] [Google Scholar]
- 31. Hands C, et al. . Evaluating venepuncture practice on a general children’s ward. Paediatr Nurs. 2010;22(2):32–35. [DOI] [PubMed] [Google Scholar]
- 32. Tvarijonaviciute A, et al. . Saliva as a non-invasive tool for assessment of metabolic and inflammatory biomarkers in children. Clin Nutr. 2019;39(8):2471–2478. [DOI] [PubMed] [Google Scholar]
- 33. Lindsay A, et al. . Realising the potential of urine and saliva as diagnostic tools in sport and exercise medicine. Sports Med. 2017;47(1):11–31. [DOI] [PubMed] [Google Scholar]
- 34. Barraco GM, et al. . Circadian health differs between boys and girls as assessed by non-invasive tools in school-aged children. Clin Nutr. 2019;38(2):774–781. [DOI] [PubMed] [Google Scholar]
- 35. Bonmati-Carrion MA, et al. . Validation of an innovative method, based on tilt sensing, for the assessment of activity and body position. Chronobiol Int. 2015;32(5):701–710. [DOI] [PubMed] [Google Scholar]
- 36. Naitoh P, et al. . Circadian rhythms determined by cosine curve fitting: analysis of continuous work and sleep-loss data. Behav Res Methods Instrum Comput. 1985;17:630–641. [Google Scholar]
- 37. Van Someren EJ, et al. . Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505–518. [DOI] [PubMed] [Google Scholar]
- 38. Ortiz-Tudela E, et al. . A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput Biol. 2010;6(11):e1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cole TJ, et al. . Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rahman SA, et al. . Functional decoupling of melatonin suppression and circadian phase resetting in humans. J Physiol. 2018;596(11):2147–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Myatt M, et al. . zscorer: child anthropometry z-score calculator. R package version 0.3.1. Edition, 2019. [Google Scholar]
- 42. World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. World Health Organization; 2006. [Google Scholar]
- 43. de Onis M, et al. . Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lopez-Jornet P, et al. . Oral lichen planus: salival biomarkers cortisol, immunoglobulin A, adiponectin. J Oral Pathol Med. 2016;45(3):211–217. [DOI] [PubMed] [Google Scholar]
- 45. Moskvina V, et al. . On multiple-testing correction in genome-wide association studies. Genet Epidemiol. 2008;32(6):567–573. [DOI] [PubMed] [Google Scholar]
- 46. Carskadon MA, et al. . Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21(8):871–881. [DOI] [PubMed] [Google Scholar]
- 47. Cespedes Feliciano EM, et al. . Chronotype, social jet lag, and cardiometabolic risk factors in early adolescence. JAMA Pediatr. 2019;173(11):1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qian J, et al. . Sex-dependent link between circadian misalignment and adiposity. Nat Rev Endocrinol. 2020;16(1):13–15. [DOI] [PubMed] [Google Scholar]
- 49. Pradhan AD, et al. . C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. [DOI] [PubMed] [Google Scholar]
- 50. Ridker PM, et al. . Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98(8):731–733. [DOI] [PubMed] [Google Scholar]
- 51. Yudkin JS, et al. . C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–978. [DOI] [PubMed] [Google Scholar]
- 52. Wärnberg J, et al. ; AVENA Study Group. Inflammatory proteins are related to total and abdominal adiposity in a healthy adolescent population: the AVENA Study. Am J Clin Nutr. 2006;84(3):505–512. [DOI] [PubMed] [Google Scholar]
- 53. Steene-Johannessen J, et al. . Adiposity, aerobic fitness, muscle fitness, and markers of inflammation in children. Med Sci Sports Exerc. 2013;45(4):714–721. [DOI] [PubMed] [Google Scholar]
- 54. Ouellet-Morin I, et al. . Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain Behav Immun. 2011;25(4):640–646. [DOI] [PubMed] [Google Scholar]
- 55. Wärnberg J, et al. . Low-grade inflammation and the metabolic syndrome in children and adolescents. Curr Opin Lipidol. 2008;19(1):11–15. [DOI] [PubMed] [Google Scholar]
- 56. Namiki M, et al. . Local overexpression of monocyte chemoattractant protein-1 at vessel wall induces infiltration of macrophages and formation of atherosclerotic lesion: synergism with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2002;22(1):115–120. [DOI] [PubMed] [Google Scholar]
- 57. Adamopoulos S, et al. . Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22(9):791–797. [DOI] [PubMed] [Google Scholar]
- 58. Trøseid M, et al. . Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J. 2004;25(4):349–355. [DOI] [PubMed] [Google Scholar]
- 59. Castanon-Cervantes O, et al. . Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185(10):5796–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gibbs J, et al. . An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med. 2014;20(8):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qian J, et al. . Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes Metab. 2018;20(10):2481–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morris CJ, et al. . Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab. 2016;101(3):1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Quante M, et al. . Zeitgebers and their association with rest-activity patterns. Chronobiol Int. 2019;36(2):203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Corbalán-Tutau MD, et al. . Differences in daily rhythms of wrist temperature between obese and normal-weight women: associations with metabolic syndrome features. Chronobiol Int. 2011;28(5):425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Musiek ES, et al. . Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol. 2018;75(5):582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.