Abstract
Study Objectives
Patients with obstructive sleep apnea (OSA) exhibit heterogeneous heart rate variability (HRV) during wakefulness and sleep. We investigated the influence of OSA severity on HRV parameters during wakefulness in a large international clinical sample.
Methods
1247 subjects (426 without OSA and 821 patients with OSA) were enrolled from the Sleep Apnea Global Interdisciplinary Consortium. HRV parameters were calculated during a 5-minute wakefulness period with spontaneous breathing prior to the sleep study, using time-domain, frequency-domain and nonlinear methods. Differences in HRV were evaluated among groups using analysis of covariance, controlling for relevant covariates.
Results
Patients with OSA showed significantly lower time-domain variations and less complexity of heartbeats compared to individuals without OSA. Those with severe OSA had remarkably reduced HRV compared to all other groups. Compared to non-OSA patients, those with severe OSA had lower HRV based on SDNN (adjusted mean: 37.4 vs. 46.2 ms; p < 0.0001), RMSSD (21.5 vs. 27.9 ms; p < 0.0001), ShanEn (1.83 vs. 2.01; p < 0.0001), and Forbword (36.7 vs. 33.0; p = 0.0001). While no differences were found in frequency-domain measures overall, among obese patients there was a shift to sympathetic dominance in severe OSA, with a higher LF/HF ratio compared to obese non-OSA patients (4.2 vs. 2.7; p = 0.009).
Conclusions
Time-domain and nonlinear HRV measures during wakefulness are associated with OSA severity, with severe patients having remarkably reduced and less complex HRV. Frequency-domain measures show a shift to sympathetic dominance only in obese OSA patients. Thus, HRV during wakefulness could provide additional information about cardiovascular physiology in OSA patients.
Clinical Trial Information:
A Prospective Observational Cohort to Study the Genetics of Obstructive Sleep Apnea and Associated Co-Morbidities (German Clinical Trials Register - DKRS, DRKS00003966) https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00003966
Keywords: obstructive sleep apnea, heart rate variability, wakefulness, autonomic nervous activity, time domain analysis, frequency domain analysis, nonlinear dynamic analysis
Statement of Significance.
Obstructive sleep apnea (OSA) is strongly associated with cardiovascular morbidity and mortality. One possible mechanism explaining this association is abnormal autonomic nervous control during wakefulness and sleep. Measurement of heart rate variability (HRV) is a widely accepted approach to assess the autonomic modulation. However, little is known about the impact of OSA severity on linear and nonlinear HRV metrics during wakefulness. This research study examined the association between HRV parameters during wakefulness and OSA and its severity in a large international clinical sample. Among all patients, time-domain and nonlinear HRV parameters significantly differ among OSA severity groups, with more severe patients demonstrating the largest reductions in HRV. Frequency domain measurements suggested a shift to sympathetic dominance in severe OSA among obese patients, but not in non-obese. Thus, HRV provides information regarding cardiovascular physiology related to OSA, without additional testing beyond the polysomnography.
Introduction
Heart rate variability (HRV) is a simple and non-invasive tool for the assessment of autonomic nervous activity. HRV is quantified by identifying variations between successive heart beats, measured as RR intervals, derived from electrocardiography (ECG) [1]. Using the standard deviation of normal-to-normal intervals (SDNN), a standard HRV metric, Hillebrand et al. [2] suggested that cardiovascular morbidity increased with reduction in HRV, with a 1% decrease in HRV translating to about a 1% increase in fatal and non-fatal cardiovascular events. Thus, HRV may represent a marker of underlying cardiovascular risk.
Obstructive sleep apnea (OSA) is a common sleep disorder that has also been associated with increased cardiovascular disease risk [3]. Patients with OSA who have abnormal cardiac electrophysiologic changes during wakefulness and sleep are at a higher risk of developing cardiac events [4–6]. Moreover, Gami et al. [7] found that the incidence of nocturnal sudden death was more likely to occur in patients with severe OSA. While the severity of OSA is commonly determined by the apnea-hypopnea index (AHI) [8], this likely does not comprehensively characterize disease severity and treatment response [9, 10]. Additional measures in combination with AHI, such as HRV, may help to refine our characterization of disease severity and assess the susceptibility to cardiovascular complications.
Supporting this concept, early small-scale studies indicated that the severity of OSA has a detrimental impact on HRV indices [11–14]. However, mechanisms by which OSA severity affects HRV during wake and sleep ultimately remain unclear. Current studies, mainly focusing on HRV around apneic episodes, during sleep or across 24-hours, demonstrate that patients with OSA have altered autonomic regulation [14–17]. Limited data are available in patients with OSA addressing abnormalities of autonomic control and cardiac impairment during wakefulness before sleep.
We predict that HRV during wakefulness may represent a non-invasive approach to characterize the underlying interaction between OSA and autonomic modulation. Currently, the three established methods to quantify HRV are: (1) time-domain analysis for quantifying the magnitudes of variation; (2) frequency-domain analysis for revealing the underlying autonomic components; and (3) nonlinear analysis for displaying dynamic complexity of heartbeats [1]. Each of these metrics captures unique aspects of HRV. Previous findings describing differences among OSA severity groups (based on AHI) during wakefulness are limited to either a small-scale study or use of only one or two of these analytical approaches [11–14].
Thus, the relationship between OSA severity and multiple HRV metrics during wakefulness remains an open question. Towards this end, the present study utilizes data among individuals without OSA and with different severity of OSA from a large international clinical sample, the Sleep Apnea Global Interdisciplinary Consortium (SAGIC), to evaluate differences in HRV measures with a normal breathing pattern during wakefulness immediately preceding the overnight sleep study.
Methods
Study population
All subjects (426 individuals without OSA and 821 patients with OSA, based on AHI ≥ 5) were recruited as part SAGIC [18, 19]. Individuals without OSA (age 46.2 ± 14.5 years, 39.9% males) and OSA patients (53.3 ± 13.8 years, 59.4% males) included in this present study underwent overnight PSG at one of seven sleep centers (University of Pennsylvania, United States; The Ohio State University, United States; Royal North Shore Hospital, Sydney and Sir Charles Gairdner Hospital, Perth, Australia; Charité University Hospital, Germany; Instituto do Sono/AFIP, Brazil; and Chang Gung Memorial Hospital, Taiwan). Before the overnight sleep study, demographic, and anthropometric profiles were assessed. Clinical data of each subject were collected from medical interviews and questionnaires. Demographic and anthropometric characteristics included age, sex, body mass index (BMI), clinical symptoms, previous medical history and current medication usage. A total of 1,297 subjects with HRV measurements and available data on AHI, age, sex, BMI, and race/ethnicity were included in our eligible study sample. Of these, 50 individuals with over 10% ectopy time in 5-minute HRV analysis (no normal-to-normal [NN] time > 30 seconds) were excluded from the analysis sample, resulting in a final sample of 1247 participants: 426 without OSA (AHI < 5 events/hour), 319 mild OSA (5 ≤ AHI < 15 events/hour), 184 moderate OSA (15 ≤ AHI < 30 events/hour), and 318 severe OSA (AHI≥30 events/hour). Every participant provided informed consent and the experimental procedures as part of SAGIC were approved by the ethics committees of each institution.
ECG recording and HRV measurements
ECG acquisition
A standardized operating procedure for ECG recording was introduced across all centers in order to collect comparable data. Individual sleep labs followed standard clinical procedures prior to completing sleep studies; all sites except Sydney and Perth site informed patients to avoid alcohol and caffeinated beverages, such as tea and coffee during the day of study. ECG measures were obtained from a single-channel ECG using modified lead II (Supplementary Figure S1). The sampling rate used for ECG collection in each sleep center ranged from 128 to 512 Hz; 62.7% of all samples had a sampling rate <256 Hz (e.g. 128Hz [11.3%], 200Hz [51.2%] or 250Hz [0.2%]), while the remaining were recorded at either 256Hz [28.0%] or 512Hz [9.3%]. All participants were required to maintain relaxed tidal breathing for 15 minutes during wakefulness before the initiation of the overnight PSG. Electroencephalogram (EEG) was used to identify whether subjects were awake during the ECG acquisition. All subjects were awake without any abnormal breathing (e.g. OSA and central sleep apnea) during the measurement of HRV. R peaks were detected in the ECG using the Pan Tompkins algorithm [20]. The RR interval series was obtained as a series of time differences between consecutive R peaks. Then, as previously described, an adaptive filtering algorithm was applied to automatically detect and remove artifacts and additional ectopic values from the RR intervals [21]. Finally, HRV was analyzed for each subject from a selected 5-minute segment prior to sleep onset, as 5 minutes is considered a preferred time window for short-term time-domain, frequency-domain, and nonlinear dynamic HRV measurements [1, 22], with less than 10% ectopy time from the RR interval time series using Matlab R2018a (The MathWorks Inc., Natick, MA).
Linear methods
The time-domain parameters are based on statistical measures derived from RR intervals and the differences between them [1]. The parameters obtained in this study included standard deviation of the normal-to-normal interval (SDNN), SD of the 1-minute average of NN intervals over five minutes (SDANN1), square root of the mean squared differences of successive NN intervals (RMSSD), and NN>50ms counts divided by the total number of all NN intervals in percentage (pNN50), which are commonly used to analyze HRV.
The frequency-domain parameters allow the quantification of the periodic components in the HRV signal [1]. In this study, 5-minute total power (5-min TP, 0.0001–0.4 Hz), power in low frequency (LF power, 0.04–0.15 Hz) and high frequency (HF power, 0.15–0.4 Hz) ranges, low frequency expressed in normalized units [see below] (LF nu), high frequency expressed in normalized units [see below] (HF nu), and low-frequency to high-frequency power ratio (LF/HF) were calculated [1]. LF nu and HF nu are calculated as relative proportions, equal to LF/(LF+HF)x100 and HF/(LF+HF)x100, respectively. Power in ultra-low frequency (0.0001–0.003 Hz) and very low frequency (0.003–0.04 Hz) ranges are not reported in this study as those data obtained from short-term ECG recordings (e.g. 5 minutes) are inaccurate to elaborate the power spectral density [1]. The HF component mainly represents parasympathetic activity and the LF component reflects both sympathetic and vagal activity mainly mediated by baroreflexes [1, 23]. The LF/HF ratio is considered a measure of sympathovagal balance [1].
Nonlinear dynamic methods
Nonlinear phenomena also occur in the HRV signal. We used Shannon entropy (ShanEn) and symbolic dynamics measures to identify monotonous and complex presences of heartbeats [21]. A higher Shannon entropy indicates that there is more irregularity in HRV. Symbolic dynamics transforms a time series into a symbol sequence in order to analyze the coarse dynamic behavior. In this study, the RR intervals are transformed into a symbolic dynamics representation using 3-symbol (w = 3) words and an alphabet composed of four symbols [21] (Supplementary Figure S2). Then, the dynamics of the symbols sequence is analyzed by two parameters—the frequency of word distribution of Shannon entropy (Fwshannon) and forbidden words (Forbword). The Fwshannon measures complexity of the corresponding tachograms (Supplementary Figure S3), with larger values meaning higher complexity. Forbword is the number of words of length 3 that never or only seldom occur. Larger values of Forbword indicate a higher stability, since the number of forbidden words will be low when the time series is highly irregular [21]. Additional details are described in the Supplementary Material.
Polysomnography and Scoring
A standard overnight polysomnography was performed using AASM criteria for evaluation of OSA. Signals included EEG, electrooculogram (EOG), ECG as described above, chin electromyogram (EMGchin), thoracoabdominal movements bands, body position sensor, pulse oximetry, and airflow (thermistor and nasal pressure) after skin preparation with gel and alcohol. Patients were encouraged to lie in the supine position. Sleep apnea episodes were defined as ≥90% reduction of baseline airflow amplitude for ≥10 seconds and hypopnea episodes were defined as ≥30% reduction of airflow with ≥4% desaturation for ≥10 seconds, respectively [24]. Patients were diagnosed as without OSA (AHI < 5 events/hour), mild OSA (AHI ≥ 5 and <15 events/hour), moderate OSA (AHI ≥ 15 and <30 events/hour), and severe OSA (AHI ≥ 30 events/hour).
Statistical Analysis
Quantitative data were presented as mean ± SD and compared among groups using analysis of variance (ANOVA). Categorical data were expressed as number (percentages) and compared among groups using chi-squared tests. To evaluate differences in HRV variables among OSA severity groups, we used ANOVA (unadjusted) or analysis of covariance (ANCOVA) controlling for important clinical covariates, including age, sex, BMI, race/ethnicity, presence of comorbidities (hypertension, hyperlipidemia, coronary artery disease, heart failure, stroke and diabetes), SAGIC site, and ECG sampling rate and time from end of ECG to sleep onset [18, 19]. To better understand potential modifying effects of obesity on observed among group associations, analyses were performed in the full sample and stratified by obesity (defined as BMI ≥ 27 kg/m2 among participants of self-reported Asian ethnicity and BMI ≥ 30 kg/m2 among others) [25]. Statistical interaction tests evaluating the significance of an [obesity (1 = obese, 0 = non-obese)] by [OSA severity group] product term in models including the main effects were performed to formally test for differences by obesity status.
In addition to analyses among groups, we utilized Pearson’s linear and partial correlations (adjusted for the same covariates) to assess the relationship between continuous AHI or Epworth Sleepiness Scale (ESS) and HRV measures. Based on observed non-normality in variable distributions, SDNN, SDANN1, RMSSD, 5-min TP and LF/HF were natural log-transformed in all analyses. Similarly, the natural log of AHI + 1 was used to assess continuous associations with HRV measures.
Statistical significance was based on a domain-specific Bonferroni correction, including p < 0.0125 for time-domain measures, p < 0.0083 for frequency-domain measures and p < 0.0167 for nonlinear domain measures. If significant or nominal (p < 0.05) differences among groups were observed in overall ANOVA/ANCOVA analyses, between-group comparisons were performed, using a Bonferroni corrected threshold of p < 0.0083 for statistical significance. In all analyses, p < 0.05 was considered nominal evidence of an association. SAS version 9.4 (SAS Institute, Cary, NC) and Stata/SE 14.2 (StataCorp LLC, College Station, TX) were used to perform statistical analyses.
Results
Characteristics of participants
Comparisons of baseline characteristics among OSA severity groups are shown in Table 1. Significant differences were observed among groups for each demographic and anthropometric variable (all p < 0.0001). Similarly, differences were observed in nearly all comorbidities, with higher prevalence among patients with OSA. In general, patients with OSA were older, more obese, and more likely to be male compared to patients without OSA. There was no significant difference in the time from end of 5-minute ECG recording to sleep onset (p = 0.554). A higher proportion of severe OSA patients had recordings performed at 512 Hz. All of these measures, except AHI, were included as covariates in adjusted comparisons of HRV measures among groups.
Table 1.
Baseline characteristics of patients with and without obstructive sleep apnea
| Variable | No OSA | Mild OSA | Moderate OSA | Severe OSA | p-value |
|---|---|---|---|---|---|
| AHI < 5 | 5 ≤ AHI < 15 | 15 ≤ AHI < 30 | AHI ≥ 30 | ||
| N | 426 | 319 | 184 | 318 | |
| Age, years | 46.2 ± 14.5 | 53.1 ± 13.5 | 55.1 ± 13.9 | 52.5 ± 14.0 | <0.0001 |
| Male, N (%) | 170 (39.9) | 168 (52.7) | 99 (53.8) | 221 (69.5) | <0.0001 |
| BMI, kg/m2 | 27.4 ± 5.6 | 30.8 ± 7.4 | 32.6 ± 7.2 | 33.3 ± 8.8 | <0.0001 |
| AHI, events/hour | 1.7 ± 1.5 | 9.5 ± 2.9 | 21.0 ± 4.3 | 59.0 ± 24.1 | <0.0001 |
| Ethnicity, N (%) | |||||
| Caucasian | 158 (37.1) | 124 (38.9) | 74 (40.2) | 118 (37.1) | <0.0001 |
| Asian | 11 (2.6) | 18 (5.6) | 15 (8.2) | 96 (30.2) | |
| C/S American | 205 (48.1) | 129 (40.4) | 61 (33.2) | 72 (22.6) | |
| Afr./Afr. American | 11 (2.6) | 18 (5.6) | 13 (7.1) | 12 (3.7) | |
| Others | 41 (9.6) | 30 (9.4) | 21 (11.4) | 20 (6.3) | |
| Site, N (%) | |||||
| Berlin | 22 (5.2) | 25 (7.8) | 12 (6.5) | 18 (5.7) | <0.0001 |
| Brazil | 241 (56.6) | 156 (48.9) | 75 (40.8) | 92 (28.9) | |
| OSU | 25 (5.9) | 28 (8.8) | 18 (9.8) | 41 (12.9) | |
| Penn | 13 (3.1) | 19 (6.0) | 16 (8.7) | 13 (4.1) | |
| Perth | 40 (9.4) | 20 (6.3) | 23 (12.5) | 17 (5.3) | |
| Sydney | 79 (18.5) | 67 (21.0) | 27 (14.7) | 44 (13.8) | |
| Taiwan | 6 (1.4) | 4 (1.3) | 13 (7.1) | 93 (29.2) | |
| Comorbidity, N (%) | |||||
| Hypertension | 84 (19.7) | 100 (31.3) | 75 (40.8) | 127 (39.9) | <0.0001 |
| CAD | 10 (2.3) | 21 (6.6) | 16 (8.7) | 28 (8.8) | 0.0007 |
| Heart failure | 6 (1.4) | 8 (2.5) | 8 (4.3) | 18 (5.7) | 0.008 |
| Stroke | 7 (1.6) | 9 (2.8) | 4 (2.2) | 16 (5.0) | 0.048 |
| High cholesterol | 88 (20.7) | 100 (31.3) | 79 (42.9) | 126 (39.6) | <0.0001 |
| Diabetes | 18 (4.2) | 42 (13.2) | 17 (9.2) | 35 (11.0) | 0.0002 |
| ECG Latency*, minutes | 12.4 ± 20.3 | 12.3 ± 19.8 | 11.2 ± 14.0 | 10.7 ± 15.3 | 0.554 |
| Sampling rate, N (%) | |||||
| 128 Hz | 46 (10.8) | 39 (12.2) | 28 (15.2) | 28 (8.8) | <0.0001 |
| 200 Hz | 262 (61.5) | 181 (56.7) | 87 (47.3) | 108 (34.0) | |
| 250 Hz | 0 (0.0) | 0 (0.0) | 1 (0.5) | 2 (0.6) | |
| 256 Hz | 112 (26.3) | 95 (29.8) | 55 (29.9) | 87 (27.4) | |
| 512 Hz | 6 (1.4) | 4 (1.3) | 13 (7.1) | 93 (29.2) |
Continuous data presented as mean ± SD and categorical data as frequency and percentage; Abbreviations: BMI = body mass index, AHI = apnea-hypopnea index, OSU = The Ohio State University, Penn = University of Pennsylvania, C/S = Central/South, Afr. = African, CAD = coronary artery disease; *Time (minutes) from end of 5-minute ECG recording to sleep onset.
Linear and nonlinear metrics among OSA severity groups
Comparisons of HRV measurements among groups are presented unadjusted (Table 2) and controlling for clinical covariates (Table 3 and Figure 1).
Table 2.
Unadjusted heart rate variability parameters in healthy subjects and patients with obstructive sleep apnea
| Variable | Mean ± SD | ||||
|---|---|---|---|---|---|
| No OSA | Mild OSA | Moderate OSA | Severe OSA | p-value† | |
| AHI < 5 | 5 ≤ AHI < 15 | 15 ≤ AHI < 30 | AHI ≥ 30 | ||
| Time domain measures | |||||
| SDNN, ms | 48.4 ± 25.9 | 44.8 ± 24.6j | 41.5 ± 22.0d, l | 35.8 ± 19.5f | <0.0001 ‡ |
| SDANN1, ms | 23.8 ± 19.3 | 22.3 ± 18.2j | 20.7 ± 15.2l | 17.0 ± 12.8f | <0.0001 ‡ |
| RMSSD, ms | 29.5 ± 17.2 | 26.7 ± 16.2a, g, j | 23.6 ± 14.7d, l | 20.5 ± 12.8f | <0.0001 ‡ |
| pNN50, % | 10.1 ± 13.1 | 8.1 ± 12.1a, j | 6.4 ± 10.7d | 4.7 ± 8.5f | <0.0001 |
| Frequency domain measures | |||||
| 5-min TP, ms2 | 312.4 ± 371.7 | 271.4 ± 364.8b, i | 259.6 ± 292.0c | 208.1 ± 246.7f | <0.0001 ‡ |
| LF Power, ms2 | 82.2 ± 91.4 | 67.1 ± 86.3a | 66.7 ± 96.3c | 59.4 ± 79.1f | 0.0039 |
| HF Power, ms2 | 39.1 ± 48.9 | 29.5 ± 41.7b, i | 23.8 ± 29.2d | 22.4 ± 29.0f | <0.0001 |
| LF/HF | 3.22 ± 3.13 | 3.63 ± 3.55 | 4.11 ± 4.07 | 3.85 ± 4.44 | 0.178‡ |
| LF nu | 0.663 ± 0.176 | 0.676 ± 0.190 | 0.693 ± 0.196 | 0.677 ± 0.191 | 0.293 |
| HF nu | 0.337 ± 0.176 | 0.324 ± 0.190 | 0.307 ± 0.196 | 0.323 ± 0.191 | 0.293 |
| Nonlinear dynamic measures | |||||
| Shannon entropy | 2.06 ± 0.48 | 1.99 ± 0.48j | 1.93 ± 0.50d, l | 1.80 ± 0.48f | <0.0001 |
| Fwshannon | 2.75 ± 0.53 | 2.66 ± 0.52a, g, j | 2.56 ± 0.56d | 2.46 ± 0.54f | <0.0001 |
| Forbword | 31.5 ± 11.1 | 34.3 ± 11.2b, j | 35.9 ± 10.9d | 37.5 ± 9.6f | <0.0001 |
p-values in bold statistically significant after domain-specific Bonferroni correction. SDNN = standard deviation of normal to normal (NN) interval; SDANN1 = standard deviation of the 1-minute average of NN intervals; RMSSD = square root of the mean squared differences of successive NN intervals; pNN50 = NN > 50 ms counts divided by the total number of all NN intervals in percentage; 5-min TP = total power in five minutes; LF power = power in low frequency range; HF power = power in high frequency range; LF/HF = ratio between low frequency and high frequency power; LF nu = normalized low frequency power; HF nu = normalized high frequency power; Fwshannon = frequency of word distribution of Shannon entropy; Forbword = forbidden word.
† p-value from analysis of variance (ANOVA) test comparing measures among OSA severity groups.
‡Analysis performed on natural log transformed outcome; ap < 0.05 (mild vs. control); bp < 0.0083 (mild vs. control); cp < 0.05 (moderate vs. control); dp < 0.0083 (moderate vs. control); ep < 0.05 (severe vs. control); fp < 0.0083 (severe vs. control); gp < 0.05 (mild vs. moderate); hp < 0.0083 (mild vs. moderate); ip < 0.05 (mild vs. severe); jp < 0.0083 (mild vs. severe); kp < 0.05 (moderate vs. severe); lp < 0.0083 (moderate vs. severe).
Table 3.
Adjusted comparison of heart rate variability parameters in individual without and with obstructive sleep apnea
| Variable | Covariate adjusted mean and 95% confidence interval† | p-value | |||
|---|---|---|---|---|---|
| No OSA | Mild OSA | Moderate OSA | Severe OSA | ||
| AHI < 5 | 5 ≤ AHI < 15 | 15 ≤ AHI < 30 | AHI ≥ 30 | ||
| Time domain measures | |||||
| SDNN, ms | 46.2 (43.8, 48.6) | 45.1 (42.5, 47.6)j | 43.4 (40.1, 46.7)l | 37.4 (34.5, 40.3)f | 0.0001 ‡ |
| SDANN1, ms | 22.8 (21.0, 24.6) | 22.3 (20.4, 24.2)j | 21.5 (19.3, 24.0)k | 18.0 (15.9, 20.0)f | 0.0051 ‡ |
| RMSSD, ms | 27.9 (26.3, 29.6) | 27.1 (25.4, 28.8)j | 24.8 (22.6, 27.1)c,k | 21.5 (19.6, 23.4)f | <0.0001 ‡ |
| pNN50, % | 9.2 (8.0, 10.4) | 8.4 (7.2, 9.7)j | 7.2 (5.6, 8.9) | 5.1 (3.7, 6.5)f | 0.0008 |
| Frequency domain measures | |||||
| 5-min TP, ms2 | 276.0 (241.8, 310.1) | 277.9 (242.0, 313.7) | 289.4 (242.0, 336.5) | 233.1 (192.7, 273.3) | 0.126‡ |
| LF Power, ms2 | 71.4 (62.5, 80.4) | 69.2 (59.8, 78.5) | 76.4 (63.0, 87.7) | 66.5 (56.0, 77.1) | 0.726 |
| HF Power, ms2 | 33.4 (29.3, 37.5) | 31.7 (27.4, 36.0) | 28.3 (22.6, 34.0) | 25.1 (20.3, 29.9) | 0.098 |
| LF/HF | 3.31 (2.91, 3.70) | 3.58 (3.16, 3.99) | 4.10 (3.54, 4.63) | 3.8 (3.3, 4.3) | 0.240‡ |
| LF nu | 0.661 (0.642, 0.680) | 0.671 (0.651, 0.692) | 0.693 (0.666, 0.720) | 0.684 (0.661, 0.707) | 0.280 |
| HF nu | 0.339 (0.320, 0.358) | 0.329 (0.308, 0.349) | 0.307 (0.280, 0.334) | 0.316 (0.293, 0.339) | 0.280 |
| Nonlinear dynamic measures | |||||
| Shannon entropy | 2.01 (1.96, 2.05) | 2.01 (1.96, 2.06)j | 1.98 (1.91, 2.05)l | 1.83 (1.77, 1.89)f | <0.0001 |
| Fwshannon | 2.68 (2.62, 2.73) | 2.69 (2.64, 2.75)j | 2.62 (2.54, 2.69)k | 2.50 (2.43, 2.56)f | 0.0001 |
| Forbword | 33.0 (32.0, 34.1) | 33.7 (32.6, 34.8)j | 34.7 (33.3, 36.3) | 36.7 (35.4, 38.0)f | 0.0006 |
p-values in bold statistically significant after domain-specific Bonferroni correction. SDNN: Standard deviation of normal to normal (NN) interval; SDANN1: Standard deviation of the 1-min average of NN intervals; RMSSD: Square root of the mean squared differences of successive NN intervals; pNN50: NN>50ms counts divided by the total number of all NN intervals in percentage; 5-min TP: Total power over five minutes in log transformation; LF power: Power in low frequency range; HF power: Power in high frequency range; LF/HF: Ratio between low frequency and high frequency in log transformation; LF nu: Normalized low frequency power; HF nu: Normalized high frequency power; Fwshannon: frequency of word distribution of Shannon entropy; Forbword: Forbidden word.
†Least squares mean and 95% confidence interval, adjusted for age, gender, BMI, race/ethnicity, site, comorbidities (hypertension, CAD, heart failure, stroke, high cholesterol and diabetes), ECG sampling rate and time from end of ECG recording to sleep onset.
‡Analysis performed on natural log transformed outcome; ap < 0.05 (mild vs. control); bp < 0.0083 (mild vs. control); cp < 0.05 (moderate vs. control); dp < 0.0083 (moderate vs. control); ep < 0.05 (severe vs. control); fp < 0.0083 (severe vs. control); gp < 0.05 (mild vs. moderate); hp < 0.0083 (mild vs. moderate); ip < 0.05 (mild vs. severe); jp < 0.0083 (mild vs. severe); kp < 0.05 (moderate vs. severe); lp < 0.0083 (moderate vs. severe).
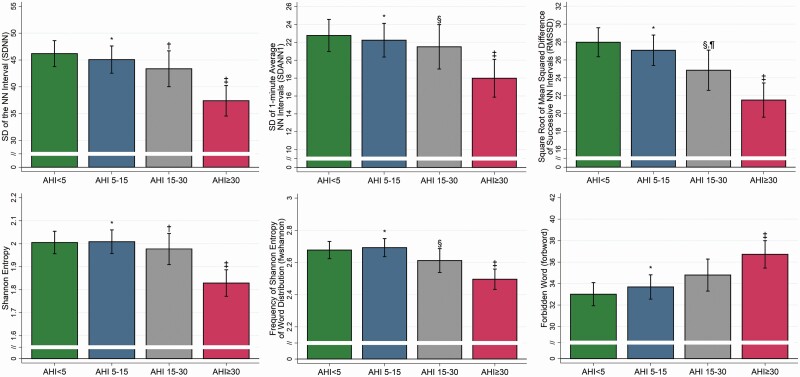
Figure 1.
Time-domain and nonlinear HRV measures among OSA severity groups. The covariate adjusted mean and 95% confidence interval for time-domain and nonlinear HRV measures are shown across OSA severity groups ranging from no OSA (AHI<5; green), mild OSA (AHI 5-15; blue), moderate OSA (AHI 15-30; grey) and severe OSA (AHI ≥ 30; red). We observed significant differences among OSA severity groups for both domains, including in SDNN (p < 0.0001), SDANN1 (p = 0.003), and RMSSD (p < 0.0001), Shannon entropy (p < 0.0001), Fwshannon (p < 0.0001), and Forbword (p = 0.0004). Overall, reduced and less complex HRV measures were observed among patients with more severe OSA. *p < 0.0083 (mild vs. severe); †p < 0.0083 (moderate vs. severe); ‡p < 0.0083 (severe vs. control); §p < 0.05 (moderate vs. severe); ¶p < 0.05 (moderate vs. control).
In time-domain measures of HRV, unadjusted analyses showed a significant reduction in all measurements (SDNN, SDANN1, RMSSD, and pNN50) with increased OSA severity (Table 2). After controlling for clinical covariates, differences in all measures remained significant (see Table 3; p ≤ 0.005). For each HRV measure, patients with severe OSA had lower values compared to patients with no, mild or moderate OSA (Table 3 and Figure 1). These results indicate that more severe OSA is associated with lower HRV parameters in the time-domain.
In frequency-domain measures, differences in 5-minute TP (p < 0.0001), LF power (p = 0.0039) and HF power (p < 0.0001) were observed across all groups in unadjusted analyses (Table 2). However, after adjustment for clinical covariates, there were no differences in these measures among AHI categories within the full sample (Table 3).
Finally, for nonlinear HRV parameters, there was a loss of HRV complexity in patients with more severe OSA compared to those without OSA. Patients with severe OSA had significantly lower ShanEn and Fwshannon values and higher values in Forbword (Figure 1). Results were statistically significant in both unadjusted (Table 2) and covariate adjusted (Table 3 and Figure 1) comparisons.
HRV measures among OSA severity groups in obese and non-obese subjects
In addition to including BMI as a covariate in the analysis of HRV among OSA severity groups, we also performed additional analysis by performing separate analyses in obese and non-obese subjects (see Methods). Results are shown in Supplementary Tables S1 (unadjusted) and S2 (covariate adjusted). In covariate-adjusted analyses (Supplementary Table S2), associations between time-domain and nonlinear HRV measures were consistent in non-obese and obese participants, with a decrease in the overall HRV and less complex HRV measures among more severe patients. On the other hand, results show evidence of differences in the relationship between frequency domain measures and OSA severity based on obesity status. Specifically, there was evidence of significant effect modification for LF power (p = 0.009), LF/HF (p = 0.009), LF nu (p = 0.014) and HF nu (p = 0.014). For each measure, nominal differences (p < 0.05) were observed among OSA severity groups for obese patients, but no associations seen in the non-obese. Overall, OSA severity was associated with a shift to sympathetic dominance among obese patients, as shown by a higher LF/HF ratio (p = 0.017) and increased LF (p = 0.042), but not in non-obese subjects. Overall, these results provide sufficient confidence that measures of HRV are related to OSA status regardless of obesity.
Association between HRV measures and continuous AHI
In addition to comparisons of HRV measures among OSA severity groups, we assessed the relationship with continuous AHI (Table 4). Consistent with differences among groups, in adjusted analyses increased AHI was significantly associated with lower values of all time-domain measures, including SDNN (ρ = −0.11, p < 0.0001), SDANN1 (ρ = −0.09, p = 0.002), RMSSD (ρ = −0.15, p < 0.0001) and pNN50 (ρ = −0.11, p = 0.0001). Similarly, more severe OSA was associated with a loss of HRV complexity, including negative correlations with ShanEn (ρ = −0.11, p = 0.0001) and Fwshannon (ρ = -0.11, p = 0.0001) and positive correlations with Forbword (ρ = 0.12, p < 0.0001) in adjusted analyses. There were no statistically significant correlations between AHI and frequency-domain measures (Table 4).
Table 4.
Pearson’s correlations between HRV measurements and ln(AHI+1)
| Variable | Unadjusted | Adjusted† | ||
|---|---|---|---|---|
| ρ | p | ρ | p | |
| Time domain measures | ||||
| SDNN‡, ms | −0.21 | <0.0001 | −0.11 | <0.0001 |
| SDANN1‡, ms | −0.16 | <0.0001 | −0.09 | 0.0020 |
| RMSSD‡, ms | −0.25 | <0.0001 | −0.15 | <0.0001 |
| pNN50, % | −0.19 | <0.0001 | −0.11 | 0.0001 |
| Frequency domain measures | ||||
| 5-min TP‡, ms2 | −0.14 | <0.0001 | −0.04 | 0.137 |
| LF Power, ms2 | −0.11 | 0.0002 | −0.01 | 0.673 |
| HF Power, ms2 | −0.18 | <0.0001 | −0.07 | 0.013 |
| LF/HF‡ | 0.06 | 0.047 | 0.05 | 0.054 |
| LF nu | 0.05 | 0.090 | 0.05 | 0.057 |
| HF nu | −0.05 | 0.090 | −0.05 | 0.057 |
| Nonlinear dynamic measures | ||||
| Shannon entropy | −0.21 | <0.0001 | −0.11 | 0.0001 |
| Fwshannon | −0.23 | <0.0001 | −0.11 | 0.0001 |
| Forbword | 0.23 | <0.0001 | 0.12 | <0.0001 |
Results in bold statistically significant after domain-specific Bonferroni correction. SDNN: Standard deviation of normal to normal (NN) interval; SDANN1: Standard deviation of the 1-min average of NN intervals; RMSSD: Square root of the mean squared differences of successive NN intervals; pNN50: NN>50ms counts divided by the total number of all NN intervals in percentage; 5-min TP: Total power over five minutes in log transformation; LF power: Power in low frequency range; HF power: Power in high frequency range; LF/HF: Ratio between low frequency and high frequency in log transformation; LF nu: Normalized low frequency power; HF nu: Normalized high frequency power; Fwshannon: frequency of word distribution of Shannon entropy; Forbword: Forbidden word.
†Pearson’s partial correlation adjusted for age, gender, BMI, race/ethnicity, site, comorbidities (hypertension, CAD, heart failure, stroke, high cholesterol and diabetes), ECG sampling rate and time from end of ECG recording to sleep onset.
‡Analysis performed on natural log transformed outcome.
Association between HRV measures and Epworth Sleepiness Scale
As an exploratory analysis, we also examined whether HRV during wakefulness associated with the subjective sleepiness measured by the Epworth Sleepiness Scale (ESS) (Supplementary Table S3). In covariate adjusted analyses, higher ESS was statistically significantly associated with lower RMSSD (ρ = −0.10, p = 0.004) and lower Fwshannon (ρ = −0.09, p = 0.005). Correlations were consistent for other time domain and nonlinear dymanic measures, although results did not reach statistical significance after Bonferroni correction. Thus, results support some correlation between ESS and wake HRV, albeit not as strong as observed with AHI.
Discussion
We have demonstrated that a 5-minute ECG recording during wakefulness provides comprehensive insights regarding variation in HRV patterns with OSA severity. Our results indicate that time-domain and nonlinear measures of HRV during the wake period are valuable markers of OSA severity, providing additional information related to cardiovascular physiology. OSA patients showed reduced HRV compared to non-OSA patients. OSA patients exhibit significantly lower values of time-domain HRV measures and less complex dynamic HRV compared to patients without OSA after adjusting for relevant covariates. Importantly, our data demonstrated that OSA severity independently contributes to lower variation of heart rate in time-series and dynamic analysis, particularly, in severe OSA patients. Moreover, our results suggest that frequency-domain HRV measurements demonstrate a shift to sympathetic dominance in obese participants with more severe OSA, but not in the non-obese. Our analysis evaluated all aspects of HRV and in much larger sample than prior studies [11–13], and provides important evidence of OSA-related differences in previously identified cardiovascular risk endpoints [3, 26].
The observed associations were found using HRV during wakefulness immediately prior to the sleep study. This approach provides a readily obtainable metric of HRV that can be readily incorporated into clinical practice. Moreover, HRV changes in OSA patients during sleep is influenced by sleep stages, apneas and respiratory event-related blood gas disturbances [16, 27, 28]. Analyses focused on the residual effect of the spontaneous autonomic nervous system variability in OSA patients during resting periods of wakefulness, overcomes these complex and dynamic physiological differences across the night. Importantly, our study identified meaningful relationships with HRV measures during wake, demonstrating their potential utility.
Time-domain measures
We observed a decrease in overall HRV, quantified as SDNN and SDANN1, from mild to severe OSA. This is in agreement with previous findings that OSA is associated with abnormal cardiac autonomic modulation that continues during wakefulness (when there are no respiratory events) [11–13]. In the present study, RMSSD also significantly differed based on OSA severity. Notably, there is a decline of 9 ms with RMSSD in severe OSA compared to controls. Previous studies have suggested that an RMSSD < 25 ms or a reduction in RMSSD > 8 ms could be a risk indicator for coronary heart disease in the general population without recognized cardiovascular disease [29, 30]. These findings may be associated pathophysiologically with the increased cardiovascular risk observed in patients with severe OSA in longitudinal studies [31]. Similarly, pNN50 is significantly reduced in severe OSA, which indicated less variability in cardiac rhythm.
Frequency domain measures
We found a relationship between OSA severity and HRV frequency-domain measures in unadjusted analyses, but not after adjustment for covariates. However, more in-depth analyses showed that the relationship between the measures and OSA severity was modified by obesity status. In particular, differences in the ratio between low and high frequency power suggest a shift to sympathetic dominance in more severe OSA among obese participants, but not in the non-obese. Prior studies found elevated sympathetic tone directly measured by MSNA during wakefulness in small samples of OSA patients [11, 32, 33]. Somers et al. [32] suggested respiratory event-induced hypoxia and enhanced chemoreflex sensitivity might contribute. Carlson et al. [33] showed that augmented awaking sympathetic activity is related to an impaired baroreflex sensitivity in an OSA population. However, unchanged or decreased wake LF during daytime wakefulness when using HRV has also been observed [34–37]. Further research is warranted, particularly regarding the modifying effects of obesity.
The changes in spectral components of HRV in OSA are mostly attributed to abnormal autonomic activity, mediated by the cardiovascular sympathetic and parasympathetic nervous system due to complex interaction between respiratory disturbances and hemodynamics [32]. Spectral HRV patterns vary from daytime to nighttime in OSA patients [38]. Moreover, abnormal autonomic activity likely contributes to progressively higher cardiovascular-related morbidity and mortality in the general population [39, 40]. Ultimately, differences between our study and prior studies reporting spectral findings [11–13, 34, 36] could be explained by differences in underlying disease severity and the HRV analysis approach. Previous results likely reflect the spectral HRV patterns under the restrictive conditions tested. Specifically, none of those studies compared the spectral measures among controls and the full range of OSA severity groups. Also, our study is the only one reporting statistical comparison results with robust covariate adjustment.
Nonlinear measures
To the best of our knowledge, we are the first to study nonlinear wake HRV features in relation to OSA severity. In particular, Shannon entropy and symbolic dynamics during wakefulness across AHI groups has not previously been studied. These metrics are important when considering relationships to cardiovascular disease. Nonlinear HRV measures quantify the randomness of beat-to-beat dynamic properties and cardiac autonomic functioning. Stein et al. [41] found that time and frequency analyses sometimes fail to identify abnormal HRV patterns in individuals with erratic cardiac rhythm. For example, regular and irregular heart rate patterns can result in the same SDNN. Importantly, only less complex heart rate patterns are considered to be a pathological condition [41]. Our comparative analysis between different AHI groups demonstrated that severe OSA patients have less complex HRV, with lower Shannon entropy and Fwshannon values and higher Forbword. The results of the symbolic dynamics display the patterning of RR interval sequences becoming more monotonous at higher AHI. This suggests that heart rate does not adequately respond and adapt to endogenous and exogenous changes in severe OSA, due to blunted cardiac autonomic modulation [42]. To date, nonlinear HRV methods are mostly applied in research; the clinical relevance of these nonlinear HRV measures in OSA patients is unknown.
Impact of impaired HRV
Impaired HRV has been previously shown in smaller studies using short-term wake HRV in patients with OSA [11–14, 35]. Moreover, lower baroreflex sensitivity and higher sympathovagal imbalance were found in OSA patients with excessive daytime sleepiness than those without [43]. This evidence highlights the long-lasting effect of OSA on automonic nervous system even during wakefulness. HRV results from the complex intergradation of brain-heart interactions, autonomic nervous system processes and physiological stimulus [44]. Current findings suggested that autonomic dysfunction in OSA may be induced by several mechanisms, such as vagal withdrawal, sympathetic hyperactivity, impaired baroreflex sensitivity, or the combination of these responses mediated by baroreflex and chemoreflex [45]. Arousal, apnea and hypoxia are the major possible mediators of baroreceptor and chemoreceptor reflex control activation, causing surges in heart rate and blood pressure, particularly during the termination of sleep apnea [46]. Chemoreflex activation in response to hypoxia contributes to elevated central sympathetic discharge via positive feedbacks in stimulating input to increase cardiovascular output [46]. Comparably, reductions in parasympathetic drive mediated baroreflex are closely associated with arousal. Consequently, baroreflex sensitivity is impaired after long-term exposure of repetitive arousals [47].
HRV combined with neuroimaging, may help understanding of how central control mechanisms regulate the adaption in cardiovascular response in order to maintain homeostasis in neurovisceral pathways [48]. Central and autonomic nervous system dysfunction may explain the regulatory mechanisms in the development of co-morbid neuropsychological dysfunction and cardiovascular diseases in OSA patients [49, 50]. Some studies indicated that continuous positive airway pressure (CPAP) treatment improves autonomic function assessed by HRV after eliminating physiological influences (e.g. arousal, hypoxia and respiratory events) in OSA population [51, 52]. Thus, further investigations on the interplay between neuro-metabolic-cardiac networking and OSA-related physiological alternations are needed.
Decreased HRV is associated with coronary heart disease, heart failure and myocardial infarction [40]. Decreased HRV is implicated in the risk and incidence of cardiovascular complications and death in different populations without heart disease [29, 30, 53]. Studies have shown that the clinical application of linear and nonlinear measures facilitates screening of OSA patients (i.e. heavy snorers with low HRV tend to have more severe sleep disordered breathing [54]). However, no studies have investigated HRV predicting the onset of cardiovascular outcomes in OSA population. This is a future direction.
Strengths and limitations
This study has several strengths. It evaluated all aspects of HRV, including time-domain, frequency and nonlinear measures, to provide a comprehensive assessment of the relationship between HRV and OSA severity. Our study is the largest on HRV in OSA to-date. and includes participants recruited from sleep clinics worldwide. Thus our results are more generalizable to real-world clinical patients throughout the world [55]. Finally, our ability to show associations between HRV during wakefulness immediately prior to the sleep study provides an easily obtainable metric of cardiovascular traits in OSA patients, facilitating clinical translation.
There are also limitations. First, since our data relies on ECG recordings during wake immediately prior to the sleep study, results may not be generalizable to daytime wakefulness. Second, while we controlled for relevant comorbidities (including hypertension, cardiovascular diseases, high cholesterol and diabetes), information on specific medications were not readily available in all patients and, thus, could not be included as covariates; this might underestimate the effect of OSA severity on HRV and reduce the sympathetic activity. Third, HRV and AHI were both measured from a single PSG. Future studies evaluating night-to-night variability in these measures [56], as well as how this may affect the observed associations, are warranted. Also, given the cross-sectional nature of our study, we cannot test the cause-effect relationship between HRV and OSA severity, or the implications of the observed lower HRV among more severe OSA patients on risk of developing new cardiac impairments. Fourth, 75% of patients were advised to avoid caffeine on the day of the sleep study or after 9 am during the day of study (all sites except the two in Australia). Fifth, we used ECGs sampled at ≥128 Hz; 250 Hz or higher may be recommended [1]. However, Voss et al. showed that there is acceptable minor error when using 128Hz ECG sampling rate both in linear and nonlinear HRV analysis in comparison with 100Hz, 200Hz, 500Hz and 1000Hz [57, 58]. Similarly, Ziemssen et al. [59] suggested ECG sampling frequency of at least 100 Hz (100 Hz and 200 Hz) has no significant influence on frequency-domain analysis, compared to 500 Hz. To control for any sample rate bias, we included ECG sampling rate as a covariate in the adjusted models. Sixth, the time from end of the 5-minute ECG recording to sleep onset varied among subjects. However, 91.8% of the sample had <30 minutes between the end of ECG and the start of sleep. To control for any potential impact of this duration on the observed results, we have added the time between the end of ECG and start of sleep as an additional covariate in adjusted analyses. Seventh, we used a 4% desaturation to define hypopneas, based on our prior studies showing excellent inter-rater agreement of scoring of the AHI in SAGIC when using a 4% criteria for hypopneas [60], while scoring agreement of the AHI degrades when arousals are included in the hypopnea definition [61]. Thus, our results may not directly generalize to disease severity based on a hypopnea definition using arousals and/or 3% desaturation. Lastly, we did not control the frequency of breathing and tidal volume for each subject (e.g. by using paced breathing) as the respiratory influences on HRV parameters are debatable [62]. However, we analyzed a standardized 5-minute window of ECG before sleep onset with the patient quiet and in a supine, relaxed position. Under these conditions, spontaneous breathing attenuates the effect of respiration on the high frequency of HRV [63]. This protocol is readily applied to different sleep laboratories and allows standardized recording of the awake HRV of patients undergoing overnight polysomnography. Further validation to identify established cardiovascular abnormalities (e.g., how well does single-channel ECG identify abnormalities compared to the full ECG exam) is still required.
Conclusion
Measures of HRV during wakefulness were significantly associated with increased OSA severity, with a decrease in the overall HRV and less complex HRV measures among more severe patients after controlling for relevant covariates. These observed differences are consistent with worse cardiovascular abnormalities in the most severe OSA group. Thus, the HRV measures studied here may provide additional information on cardiovascular physiology in the context of OSA. However, evidence regarding whether comprehensive HRV measures, in combination with other characteristics of OSA, may identify early proposed cardiac damage or improve cardiovascular risk stratification in patients with OSA is still lacking. Further research to better understand the predictive value and clinical implication of the observed differences in HRV parameters in the future risk of cardiovascular events among different OSA subgroups is necessary.
Supplementary Material
Acknowledgments
The authors represent the Sleep Apnea Global Interdisciplinary Consortium (SAGIC) (https://www.med.upenn.edu/sleepctr/sagic.html), which is a collaboration of multiple clinical sites conducting research projects worldwide on a variety of topics related to the common disorder, obstructive sleep apnea. Current and former SAGIC sites (and members) include: University of Pennsylvania (Allan I. Pack, Richard Schwab, Diane C. Lim, Greg Maislin, Brendan T. Keenan, Olivia J. Veatch, Diego Mazzotti, Mary Boland, Francis Pack, Jinyoung Kim, now at the University of Nevada, Las Vegas), The Ohio State University (Ulysses J. Magalang, Jesse Mindel, M. Melanie Lyons, Steven Holfinger, Samantha Rojas), the University of Iceland (Thorarinn Gislason, Bryndis Benediktsdottir), Instituto do Sono/AFIP, Brazil (Sergio Tufik, Lia Bittencourt), Charite Universitatsmedizin Berlin (Thomas Penzel, Bernd Sanner, Ingo Fietze, Maria Franczyk, Naima Laharnar, Hua Qin), Peking University (Fang Han, Liyue Xu, Jing Jing Guo), Shanghai University (Qing Yun Li, Yingni Lin), Chang Gung Memorial Hospital (Ning-hung Chen, Li-Pang Chuang, Yu-Sheng Lin, Shih-Wei Lin, Hung-Yu Huang), Korea University (Chol Shin, Seung Ku Lee), University of Sydney (Peter A. Cistulli, Philip deChazal, Kate Sutherland), University of Western Australia (Bhajan Singh, Nigel McArdle, Peter Eastwood). We thank all the subjects and professionals in our research teams involved in the SAGIC projects at each site. We also thank Daniel Barrett and Nicolas Steenbergen for proofreading the manuscript.
Funding
This study was supported by the National Institutes of Health (NIH) P01 HL094307 and American Academy of Sleep Medicine Foundation (AASM #194-SR-18, PI: DRM). F Vaquerizo-Villar was in receipt of a ‘Ayuda para contratos predoctorales para la Formación de Profesorado Universitario (FPU)’ grant from the Ministerio de Educación, Cultura y Deporte (FPU16/02938).
Conflict of interest statement. This was not an industry supported study. PAC has an appointment to an endowed academic Chair at the University of Sydney that was created from ResMed funding. He receives no personal fees and this relationship is managed by an Oversight Committee of the University. He has received research support from ResMed, SomnoMed, Zephyr Sleep Technologies, and Bayer. He is a consultant/adviser to Zephyr Sleep Technologies, Signifier Medical Technologies, SomnoMed, and ResMed. He has a pecuniary interest in SomnoMed related to a previous role in R&D (2004). TP received grant support from Ciedelc, Löwenstein Medical and lecturer fees from Löwenstein Medical, Neuwirth, Philips, Jazz Pharmaceuticals, and consulting fees from Bayer, Jazz Pharmaceuticals, Cerebra. He owns shares of The Siestagroup GmbH, Nukute, and Advanced Sleep Research GmbH. Other authors have declared no financial conflicts of interest and no non-financial conflicts of interest related to the work submitted for publication.
Author contributions
H.Q. and B.T.K. are co-first authors. U.J.M. and T.P. are co-supervising authors. H.Q., B.T.K., D.R.M., A.I.P., U.J.M., and T.P. were responsible for study concept and design; S.T., L.B., P.A.C., P.D.C., K.S., B.S., A.I.P., N.H.C., I.F., U.M., T.P. were responsible for data collection; H.Q., B.T.K., D.R.M., U.J.M., and T.P. prepared the manuscript draft; F.V.V. performed ECG signal processing; J.F.K. and N.W. performed ECG signal processing and HRV calculations; B.T.K. performed data analysis. All authors contributed to critical revision of the manuscript.
References
- 1. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 2. Hillebrand S, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. 2013;15(5):742–749. [DOI] [PubMed] [Google Scholar]
- 3. McNicholas WT, et al. ; Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29(1):156–178. [DOI] [PubMed] [Google Scholar]
- 4. Schlatzer C, et al. ECG risk markers for atrial fibrillation and sudden cardiac death in minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised trial. BMJ Open. 2016;6(3):e010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakamura T, et al. Corrected QT dispersion and cardiac sympathetic function in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2004;125(6):2107–2114. [DOI] [PubMed] [Google Scholar]
- 6. Sankari A, et al. Longitudinal effect of nocturnal R-R intervals changes on cardiovascular outcome in a community-based cohort. BMJ Open. 2019;9(7):e030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gami AS, et al. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–1214. [DOI] [PubMed] [Google Scholar]
- 8. The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 9. Kirkham EM, et al. Relationship between clinical and polysomnography measures corrected for CPAP Use. J Clin Sleep Med. 2015;11(11):1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lim DC, et al. ; SAGIC Investigators. Reinventing polysomnography in the age of precision medicine. Sleep Med Rev. 2020;52:101313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Narkiewicz K, et al. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98(11):1071–1077. [DOI] [PubMed] [Google Scholar]
- 12. Wang W, et al. Association of cardiac autonomic function measures with severity of sleep-disordered breathing in a community-based sample. J Sleep Res. 2008;17(3):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park DH, et al. Correlation between the severity of obstructive sleep apnea and heart rate variability indices. J Korean Med Sci. 2008;23(2):226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aydin M, et al. Cardiac autonomic activity in obstructive sleep apnea: time-dependent and spectral analysis of heart rate variability using 24-hour Holter electrocardiograms. Tex Heart Inst J. 2004;31(2):132–136. [PMC free article] [PubMed] [Google Scholar]
- 15. Dingli K, et al. Spectral oscillations of RR intervals in sleep apnoea/hypopnoea syndrome patients. Eur Respir J. 2003;22(6):943–950. [DOI] [PubMed] [Google Scholar]
- 16. Szollosi I, et al. Sleep apnea in heart failure increases heart rate variability and sympathetic dominance. Sleep. 2007;30(11):1509–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guilleminault C, et al. Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet. 1984;1(8369):126–131. [DOI] [PubMed] [Google Scholar]
- 18. Sutherland K, et al. ; SAGIC Investigators. A global comparison of anatomic risk factors and their relationship to obstructive sleep apnea severity in clinical samples. J Clin Sleep Med. 2019;15(4):629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keenan BT, et al. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis. Sleep. 2018;41(3). doi: 10.1093/sleep/zsx214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan J, et al. A real-time QRS detection algorithm. IEEE Trans Biomed Eng. 1985;32(3):230–236. [DOI] [PubMed] [Google Scholar]
- 21. Wessel N, et al. Nonlinear analysis of complex phenomena in cardiological data. Herzschrittmachertherapie und Elektrophysiologie. 2000;11(3):159–173. [Google Scholar]
- 22. Li K, et al. Spectral analysis of heart rate variability: time window matters. Front Neurol. 2019;10:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akselrod S, et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. [DOI] [PubMed] [Google Scholar]
- 24. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 25. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. [DOI] [PubMed] [Google Scholar]
- 26. Mazzotti DR, et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Penzel T, et al. Dynamics of heart rate and sleep stages in normals and patients with sleep apnea. Neuropsychopharmacology. 2003;28(Suppl 1):S48–S53. [DOI] [PubMed] [Google Scholar]
- 28. Somers VK, et al. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87(6):1953–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jarczok MN, et al.. First evaluation of an index of low vagally-mediated heart rate variability as a marker of health risks in human adults: proof of concept. J Clin Med. 2019;8(11). doi: 10.3390/jcm8111940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rautaharju PM, et al. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: the Women’s Health Initiative. Circulation. 2006;113(4):473–480. [DOI] [PubMed] [Google Scholar]
- 31. Marin JM, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. [DOI] [PubMed] [Google Scholar]
- 32. Somers VK, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carlson JT, et al. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103(6):1763–1768. [DOI] [PubMed] [Google Scholar]
- 34. Hilton MF, et al. The sleep apnoea/hypopnoea syndrome depresses waking vagal tone independent of sympathetic activation. Eur Respir J. 2001;17(6):1258–1266. [DOI] [PubMed] [Google Scholar]
- 35. Wiklund U, et al. Autonomic cardiovascular regulation in patients with obstructive sleep apnoea: a study based on spectral analysis of heart rate variability. Clin Physiol. 2000;20(3):234–241. [DOI] [PubMed] [Google Scholar]
- 36. Balachandran JS, et al. Effect of mild, asymptomatic obstructive sleep apnea on daytime heart rate variability and impedance cardiography measurements. Am J Cardiol. 2012;109(1):140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moak JP, et al. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4(12):1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noda A, et al. Circadian rhythm of autonomic activity in patients with obstructive sleep apnea syndrome. Clin Cardiol. 1998;21(4):271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kikuya M, et al. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36(5):901–906. [DOI] [PubMed] [Google Scholar]
- 40. Tsuji H, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. [DOI] [PubMed] [Google Scholar]
- 41. Stein PK, et al. Sometimes higher heart rate variability is not better heart rate variability: results of graphical and nonlinear analyses. J Cardiovasc Electrophysiol. 2005;16(9):954–959. [DOI] [PubMed] [Google Scholar]
- 42. Guzzetti S, et al. Symbolic dynamics of heart rate variability: a probe to investigate cardiac autonomic modulation. Circulation. 2005;112(4):465–470. [DOI] [PubMed] [Google Scholar]
- 43. Lombardi C, et al. Daytime sleepiness and neural cardiac modulation in sleep-related breathing disorders. J Sleep Res. 2008;17(3):263–270. [DOI] [PubMed] [Google Scholar]
- 44. Thayer JF, et al. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3):201–216. [DOI] [PubMed] [Google Scholar]
- 45. Horner RL, et al. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J Appl Physiol (1985). 1995;79(1):151–162. [DOI] [PubMed] [Google Scholar]
- 46. Dempsey JA, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khoo MC, et al. Sleep-related changes in autonomic control in obstructive sleep apnea: a model-based perspective. Respir Physiol Neurobiol. 2013;188(3):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chouchou F, et al. Heart rate variability: a tool to explore the sleeping brain? Front Neurosci. 2014;8:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alvares GA, et al. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psychiatry Neurosci. 2016;41(2):89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lombardi C, et al. Obstructive sleep apnea syndrome and autonomic dysfunction. Auton Neurosci. 2019;221:102563. [DOI] [PubMed] [Google Scholar]
- 51. Khoo MC, et al. Cardiac autonomic control in obstructive sleep apnea: effects of long-term CPAP therapy. Am J Respir Crit Care Med. 2001;164(5):807–812. [DOI] [PubMed] [Google Scholar]
- 52. Nicholl DDM, et al. CPAP therapy delays cardiovagal reactivation and decreases arterial renin-angiotensin system activity in humans with obstructive sleep apnea. J Clin Sleep Med. 2018;14(9):1509–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dekker JM, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102(11):1239–1244. [DOI] [PubMed] [Google Scholar]
- 54. Ferini-Strambi L, et al. Heart rate variability during sleep in snorers with and without obstructive sleep apnea. Chest. 1992;102(4):1023–1027. [DOI] [PubMed] [Google Scholar]
- 55. Sequeira VCC, et al. Heart rate variability in adults with obstructive sleep apnea: a systematic review. Sleep Sci. 2019;12(3):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Quan SF, et al. ; Sleep Heart Health Study (SHHS) Research Group. Short-term variability of respiration and sleep during unattended nonlaboratory polysomnography–the Sleep Heart Health Study. [corrected]. Sleep. 2002;25(8):843–849. [PubMed] [Google Scholar]
- 57. Voss A, et al. Influence of low sampling rate on heart rate variability analysis based on non-linear dynamics. In: Computers in Cardiology 1995. Vienna, Austria; 1995:689–692. [Google Scholar]
- 58. Voss A, et al. Requirements on sampling rate in Holter systems for analysis of heart rate variability. Clin Sci (Lond). 1996;91(Suppl):120–121. [DOI] [PubMed] [Google Scholar]
- 59. Ziemssen T, et al. Influence of ECG sampling frequency on spectral analysis of RR intervals and baroreflex sensitivity using the EUROBAVAR data set. J Clin Monit Comput. 2008;22(2):159–168. [DOI] [PubMed] [Google Scholar]
- 60. Magalang UJ, et al. ; SAGIC Investigators. Agreement in the scoring of respiratory events and sleep among international sleep centers. Sleep. 2013;36(4):591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kuna ST, et al. Agreement in computer-assisted manual scoring of polysomnograms across sleep centers. Sleep. 2013;36(4):583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gąsior JS, et al. Heart rate and respiratory rate influence on heart rate variability repeatability: effects of the correction for the prevailing heart rate. Front Physiol. 2016;7:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Denver JW, et al. Methodological issues in the quantification of respiratory sinus arrhythmia. Biol Psychol. 2007;74(2):286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.