Abstract
Aims
In post‐menopausal women, incidence of heart failure with preserved ejection fraction is higher than in men. Hormonal replacement therapies did not demonstrate benefits. We tested whether the non‐steroidal mineralocorticoid receptor antagonist finerenone limits the progression of heart failure in ovariectomized (OVX) mice with metabolic disorders.
Methods and results
Ovariectomy was performed in 4‐month‐old mice, treated or not at 7 months old for 1 month with finerenone (Fine) 1 mg/kg/day. Left ventricular (LV) cardiac and coronary endothelial functions were assessed by echocardiography, catheterization, and myography. Blood pressure was measured by plethysmography. Insulin and glucose tolerance tests were performed. Exercise capacity and spontaneous activity were measured on treadmill and in combined indirect calorimetric cages equipped with voluntary running wheel. OVX mice presented LV diastolic dysfunction without modification of ejection fraction compared with controls (CTL), whereas finerenone improved LV filling pressure (LV end‐diastolic pressure, mmHg: CTL 3.48 ± 0.41, OVX 6.17 ± 0.30**, OVX + Fine 3.65 ± 0.55†, **P < 0.01 vs. CTL, † P < 0.05 vs. OVX) and compliance (LV end‐diastolic pressure–volume relation, mmHg/RVU: CTL 1.65 ± 0.42, OVX 4.77 ± 0.37***, OVX + Fine 2.87 ± 0.26††, ***P < 0.001 vs. CTL, †† P < 0.01 vs. OVX). Acetylcholine‐induced endothelial‐dependent relaxation of coronary arteries was impaired in ovariectomized mice and improved by finerenone (relaxation, %: CTL 86 ± 8, OVX 38 ± 3**, OVX + Fine 83 ± 7††, **P < 0.01 vs. CTL, †† P < 0.01 vs. OVX). Finerenone improved decreased ATP production by subsarcolemmal mitochondria after ovariectomy. Weight gain, increased blood pressure, and decreased insulin and glucose tolerance in OVX mice were improved by finerenone. The exercise capacity at race was diminished in untreated OVX mice only. Spontaneous activity measurements in ovariectomized mice showed decreased horizontal movements, reduced time spent in a running wheel, and reduced VO2 and VCO2, all parameters improved by finerenone.
Conclusions
Finerenone improved cardiovascular dysfunction and exercise capacity after ovariectomy‐induced LV diastolic dysfunction with preserved ejection fraction.
Keywords: Mineralocorticoid receptor, Aldosterone, Diastolic dysfunction, Exercise, Ovariectomy, Menopause, Metabolic syndrome
Introduction
Mineralocorticoid receptor (MR) antagonists (MRAs) have been shown to reduce mortality in heart failure (HF) with reduced ejection fraction (EF) at advanced stages of the disease 1 , 2 and to slow the progression of mild symptomatic HF. 3 Concerning HF with preserved EF (HFpEF), a sub‐study from the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial performed in the Americas, Georgia, and Russia showed efficacy of the MRA spironolactone in American patients only, 4 most likely because patients from Georgia and Russia were not appropriately treated. 5
In clinical practice, the steroidal MRAs spironolactone and eplerenone remain underused because of their common risk of hyperkalemia and because of the risks of gynaecomastia, erectile dysfunction and menstrual irregularities of spironolactone that also binds to androgen and progesterone receptors. 6 The novel non‐steroidal MRA finerenone has an over 500‐fold higher selectivity vs. the androgen, progesterone, or glucocorticoid receptors. 6 When compared with spironolactone treatment in patients with chronic HF with reduced EF and mild to moderate chronic kidney disease, finerenone led to a smaller mean increase in serum potassium concentration and to a lower number of patients with hyperkalaemia or renal failure or impairment. 7 Moreover, finerenone was also shown to reduce the composite clinical endpoint of death from any cause, cardiovascular hospitalizations, or emergency presentation for worsening HF in comparison with eplerenone in patients with worsened HF and type 2 diabetes mellitus and/or chronic kidney disease. 8
Several epidemiological studies agreed to assert that women represent the majority of patients with HFpEF. Among proposed mechanisms, clinical and basic research has implicated an activation of the renin–angiotensin–aldosterone system (RAAS) in the pathogenesis of diastolic dysfunction after menopause. 9 After ovariectomy‐induced post‐menopausal conditions, increased expression of cardiac local RAAS components has been reported in rodents, including angiotensin II 10 in rats and MR in rats 11 and mice, 12 with an increase in plasma aldosterone that was evidenced in rats. 10 , 13 In obese mice, endothelial MR has been proposed as a player driving sex differences in the dysfunction of resistance arteries. Indeed, in female mice with obesity, MR deletion in endothelial cells limits endothelial dysfunction by increasing nitric oxide (NO) bioavailability, a mechanism that was not evidenced in men. 14
Ageing women tend to have more coronary microvascular endothelial dysfunction than men. 15 Endothelial dysfunction per se leads to hypertension that increases the risk of HF three‐fold in women compared with two‐fold in men. 16 Moreover, hypertension is a primary risk factor for diastolic dysfunction, and after menopause, more women than men become hypertensive. 17 The risk of HF increases with obesity and with diabetes mellitus by five‐fold in women compared with 2.4‐fold in men. 18 Interestingly, MR blockade with spironolactone prevents diet‐induced diastolic dysfunction and arterial stiffening 19 in female mice fed with a high‐fat/high‐sucrose diet that induces obesity and insulin resistance. The metabolic syndrome (MetS) whose frequency increases by 60% after menopause, 20 with central obesity as the main cause, hypertension as a criteria, and diabetes as co‐morbidity, is associated with a three‐fold increase in cardiovascular mortality. 21
To date, there is no approved treatment for HFpEF, the most frequent type of HF in women. Interestingly, it has been previously reported that MRAs are more efficacious as a cardiovascular therapy in women compared with men. 22 Emphasizing RAAS as a major target in HFpEF, a recent trial concluded that sacubitril–valsartan (combination of neprilysin inhibitor with angiotensin II receptor antagonist) compared with valsartan alone seemed to reduce the risk of HF hospitalization more in old women (aged 74 ± 8) than in old men (aged 72 ± 9). 23 Recently, a post hoc analysis of the TOPCAT trial suggested a reduction in the secondary outcome of all‐cause mortality in women, with significant interaction between sex and MRA treatment. 24 These trials highlight the need for more preclinical evaluations for the treatment of HFpEF in women. Therefore, we tested whether finerenone can improve left ventricular (LV) diastolic dysfunction with preserved EF in mice with ovariectomy and global metabolism disorders.
Methods
Detailed methods are available in the Supporting Information.
Animals
Experiments conformed to the 2010‐63 European Union Directive, the national Institutional Ethics Committee (No. 0335402), and the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health (No. 85‐23). Wild‐type (Janvier, Le Genest‐Saint‐Isle, France) mice on B6D2 background were housed five per cage on light–dark cycle 12–12 h at room temperature 22 ± 2°C, with access to food (A3 SAFE, France) and water ad libitum. Mice aged 4 months were ovariectomized (OVX), treated or not when aged 7 months for 1 month with finerenone (1 mg/kg/day) (OVX + Fine) mixed in food, and compared with age‐matched controls (CTL).
Statistics
Data are expressed as mean ± standard error of the mean and analysed with GraphPad Prism 7.04. D'Agostino–Pearson test was used to verify normal distribution. Before finerenone treatment and for comparison of the same parameters after treatment, unpaired Student's t‐test test was used. After finerenone treatment, one‐way ANOVA followed by Tukey's post hoc test was used, and repeated measures ANOVA was used for kinetic and dose–response curves. The differences between experimental groups were considered to be statistically significant from values of P < 0.05. Spontaneous activity and flow‐mediated dilatation data were assessed by multivariate analysis using generalized linear modelling with R 3.1.4 using R 3.1.4 software package (The R Foundation for Statistical Computing, http://www.r‐project.org). Differences between groups were assessed using likelihood ratio tests and Wald test. Distributions of residuals were checked as follows: (i) no outliers with the minimum and maximum values of standardized residual within [−3, +3] values, (ii) normal distribution with mean = 0 and a constant variance, and (iii) graphically by mean of QQplot. Multi‐collinearity was tested using collinearity statistics (variance inflation factor).
Results
Finerenone improves diastolic function in mice with ovariectomy
We tested the efficacy of 1 month finerenone treatment (1 mg/kg/day) after 3 months of ovariectomy in wild‐type OVX mice aged 8 months. At the cardiac level, ultrasound in OVX mice without or with finerenone treatment (OVX + Fine) compared with age‐matched controls (CTL) showed no difference in LV diameter at both end‐systole and end‐diastole nor in the resulting fractional shortening (FS) (Table 1 ), that is, the ultrasound index of LVEF. Furthermore, ovariectomy did not lead to alterations in LV stroke volume or cardiac output (Table 1 ). Concerning heart remodelling and infiltration, 3 months of ovariectomy did not induce increased LV weight (Table 1 ) despite a significant increase in cardiomyocytes mean cross‐sectional area in untreated OVX mice only (Table 1 ) and did not increased significantly the number of macrophages infiltrated in the myocardium (Supporting Information, Table S1 ). Furthermore, ovariectomy did not affect the amount of immunolabelled platelet and endothelial cellular adhesion molecule‐1 (PECAM‐1) and the related LV capillary density nor the amount of immunolabelled interstitial collagen types I and III (Table 1 and Supporting Information, Figure S1 ).
Table 1.
Cardiac parameters and arterial pressure
| CTL | OVX | OVX + Fine | |
|---|---|---|---|
| Echocardiography | |||
| LVEDD (mm) | 3.3 ± 0.1 (n = 8) | 3.5 ± 0.1 (n = 9) | 3.5 ± 0.1 (n = 10) |
| LVESD (mm) | 1.7 ± 0.1 (n = 8) | 1.9 ± 0.1 (n = 9) | 1.8 ± 0.1 (n = 10) |
| FS (%) | 49 ± 2 (n = 8) | 47 ± 3 (n = 9) | 49 ± 2 (n = 10) |
| SV (mL per beat) | 0.063 ± 0.002 (n = 8) | 0.065 ± 0.002 (n = 9) | 0.063 ± 0.003 (n = 10) |
| CO (mL/min) | 35.8 ± 1.8 (n = 8) | 37.2 ± 1.6 (n = 9) | 35.4 ± 1.8 (n = 10) |
| LV haemodynamics | |||
| dP/dtmin | 8486 ± 1464 (n = 4) | 5813 ± 723 (n = 7) | 5121 ± 735 (n = 6) |
| Tau | 6.89 ± 0.54 (n = 4) | 9.13 ± 0.65 (n = 7) | 9.68 ± 1.46 (n = 6) |
| Organ weights | |||
| HW (mg) | 119 ± 7 (n = 8) | 121 ± 5 (n = 11) | 114 ± 3 (n = 15) |
| HW/TL (mg/mm) | 6.4 ± 0.4 (n = 8) | 6.6 ± 0.3 (n = 11) | 6.2 ± 0.2 (n = 15) |
| LVW (mg) | 90 ± 5 (n = 8) | 90 ± 2 (n = 11) | 88 ± 2 (n = 15) |
| LVW/TL (mg/mm) | 4.9 ± 0.3 (n = 8) | 4.9 ± 0.1 (n = 11) | 4.74 ± 0.1 (n = 15) |
| Histology and immunolabelling | |||
| MCSA (μm2) | 294 ± 12 (n = 8) | 342 ± 8 (n = 8)* | 321 ± 11 (n = 11) |
| Capillary density | 1.20 ± 0.03 (n = 8) | 1.15 ± 0.03 (n = 10) | 1.13 ± 0.02 (n = 10) |
| PECAM‐1+ vessels (nb per field) | 86 ± 4 (n = 8) | 86 ± 4 (n = 10) | 90 ± 4 (n = 10) |
| Coll‐I (% per field) | 15.3 ± 1.0 (n = 7) | 13.8 ± 1.1 (n = 9) | 14.8 ± 0.8 (n = 10) |
| Coll‐III (% per field) | 2.9 ± 0.3 (n = 7) | 2.2 ± 0.4 (n = 9) | 2.6 ± 0.3 (n = 10) |
| Plethysmography | |||
| SBP (mmHg) | 135 ± 6 (n = 9) | 158 ± 5 (n = 7)* | 137 ± 5 (n = 8) † |
| DBP (mmHg) | 96 ± 7 (n = 9) | 120 ± 5 (n = 7)* | 97 ± 7 (n = 8) |
| Oxidative stress | |||
| LV ROS | 370.7 ± 15.8 (n = 15) | 336.5 ± 9.7 (n = 11) | 356.0 ± 18.6 (n = 9) |
| IFM ROS | 5650 ± 697 (n = 10) | 6158 ± 1467 (n = 7) | 6027 ± 645 (n = 10) |
| SSM ROS | 7047 ± 1123 (n = 10) | 7644 ± 1312 (n = 7) | 7581 ± 1052 (n = 10) |
| A/F ratio | 0.369 ± 0.038 (n = 10) | 0.358 ± 0.016 (n = 8) | 0.369 ± 0.016 (n = 6) |
Echocardiography: left ventricular (LV) end‐diastolic diameter (LVEDD); LV end‐systolic diameter (LVESD); fractional shortening (FS); stroke volume (SV); cardiac output (CO). LV haemodynamics: dP/dtmin rate of LV pressure decrease and relaxation constant Tau (Weiss method). Organ weights: heart and LV weights (HW and LVW) and ratios on tibia length (HW/TL and LVW/TL). Histology and immunolabelling: cardiomyocytes mean cross‐sectional area (MCSA) after wheat germ agglutinin (WGA) staining for contouring cardiomyocytes; capillary density as the ratio of the number of capillaries [platelet and endothelial cellular adhesion molecule‐1 (PECAM‐1) immunolabelled endothelial cells] to the number of cardiomyocytes (WGA) (×20); percentage per field of immunolabelled collagen type I and III (Coll‐I and Coll‐III) (×20). Plethysmography: systolic and diastolic blood pressure (SBP and DBP). Oxidative stress: electron paramagnetic resonance for the measurement of reactive oxygen species (ROS) (AU/mg of protein) in all left ventricles and in interfibrillar or subsarcolemmal mitochondria (IFM and SSM); ratio of aconitase/fumarase activities (A/F).
P < 0.05 vs. CTL.
P < 0.05 vs. OVX.
Providing greater precision, the recordings of pressure‐volume curves after LV catheterization allowed revealing diastolic dysfunction in OVX mice. Indeed, if ovariectomy did not alter the LV ejection pressure in systole (LV end‐systolic pressure) (Figure 1A ), it increased the LV filling pressure in diastole (LV end‐diastolic pressure), which was reduced by finerenone to the average value of CTL (Figure 1B ). The load‐independent assessment of LV systolic contractility (LV end‐systolic pressure–volume relation) indicated that it remained unchanged in OVX compared with CTL mice (Figure 1C ). However, the assessment of LV diastolic compliance (LV end‐diastolic pressure–volume relation) indicated that the LV relaxation was altered in OVX compared with CTL mice and again improved by finerenone treatment (Figure 1D ).
Figure 1.
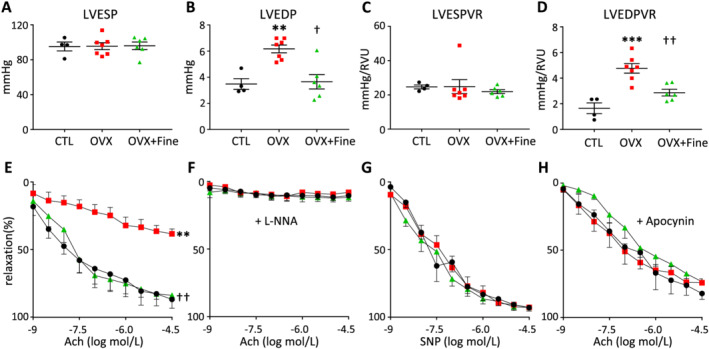
Finerenone treatment improved ovariectomy‐induced left ventricular (LV) diastolic dysfunction and improved oxidative stress‐related impairment of endothelium‐dependent relaxation of coronary arteries. (A–D) LV invasive haemodynamics in control mice (CTL, black circles, n = 4) and in mice after 4 months of ovariectomy (OVX, red squares, n = 7) without or with 1 month finerenone treatment (OVX + Fine, green triangles, n = 6). (A) LV end‐systolic pressure (LVESP). (B) LV end‐diastolic pressure (LVEDP), that is, LV filling pressure. (C) LV end‐systolic pressure–volume relation (LVESPVR), that is, LV contractility. (D) LV end‐diastolic pressure–volume relation (LVEDPVR), that is, LV compliance. (E–H) Relaxation of isolated coronary arteries from control mice (CTL, black circles, n = 5) and from mice after 4 months of ovariectomy (OVX, red squares, n = 6) without or with 1 month finerenone treatment (OVX + Fine, green triangles, n = 5). Relaxation induced (E) by acetylcholine (Ach), (F) by Ach in the presence of the NO synthase inhibitor l‐NG‐nitro‐arginine (L‐NNA) 10−4 mol/L, (G) by the NO donor sodium nitroprusside (SNP), and (H) by Ach after addition of apocynin 10−4 mol/L. Data are mean ± standard error of the mean. Statistics: one‐way ANOVA plus Tukey, **P < 0.01 and ***P < 0.001 vs. CTL; † P < 0.05 and †† P < 0.01 vs. OVX.
In summary, finerenone treatment improved LV filling pressure and LV compliance in the context of ovariectomy‐induced LV diastolic dysfunction with preserved FS/EF.
Finerenone improves peripheral and coronary arterial function in mice with ovariectomy
Compared with age‐matched CTL, only untreated OVX mice had increased blood pressure (Table 1 ). Moreover, ultrasound assessments of temperature‐induced vasodilation, and multivariate analyses adjusted to wall shear stress, indicated that the increases in blood flow induced smaller increases in diameter of peripheral femoral artery in OVX mice compared with both CTL and OVX + Fine mice (Supporting Information, Figure S2 ).
Focusing on coronary arteries, the acetylcholine (Ach)‐induced endothelium‐dependent relaxation was impaired in arteries isolated from OVX but not in those isolated from OVX + Fine mice, when compared with arteries from CTL mice (Figure 1E ). All the observed vasodilator responses relied on NO production by the endothelium, because pre‐incubation with the NO synthase inhibitor l‐NG‐nitro‐arginine abolished the Ach‐induced relaxation in all groups (Figure 1F ). The NO is known to be responsible for the relaxation of vascular smooth muscle cells, which responded similarly in all groups after pre‐incubation of isolated arteries with the NO donor sodium nitroprusside allowing bypassing its endothelial production (Figure 1G ).
To assess the hypothesis that impaired coronary function in OVX mice was at least partly related to an excess of superoxide generation, which would be responsible for an endothelial dysfunction by reducing the NO bioavailability, arteries from all groups were pre‐incubated with the NADPH oxidase inhibitor apocynin prior to Ach. Apocynin allowed improving the Ach‐induced relaxation of coronary arteries from OVX mice, which was no longer different of that of arteries from CTL mice or from OVX + Fine mice (Figure 1H ), indicating that finerenone was efficient to reduce superoxide‐mediated endothelial dysfunction. Suggesting that the oxidative stress was mostly limited to the vasculature, quantification by electron paramagnetic resonance of reactive oxygen species (ROS) in the entire LV (Table 1 ) or in isolated myocardial mitochondria (Table 1 ) did not show any difference between groups nor the evaluation of mitochondrial oxidative stress via the ratio of fumarase over aconitase enzymatic activities (Table 1 ). Thus, in this post‐menopausal ovariectomized mouse model, finerenone treatment repealed superoxide‐mediated endothelial dysfunction at the level of coronary arteries (Table 1 and Figure 1H ).
Metabolic improvements by finerenone treatment of mice with ovariectomy
Before starting treatment with finerenone, OVX mice had increased body weight compared with age‐matched CTL, encompassing increased fat and reduced lean mass as measured by echo‐magnetic resonance imaging (Table 2 ). Consistent with weight gain, plasma leptin was increased in OVX mice (Table 2 ). A 1 month finerenone treatment allowed reducing body weight of OVX mice (Table 2 ). There was no increase in total triglycerides or total cholesterol in OVX mice (Table 2 ). Nevertheless, compared with CTL mice, fasting glycaemia was increased in OVX mice before finerenone treatment and not different after treatment (Table 2 ). Furthermore, OVX mice had decreased tolerance to oral glucose compared with CTL mice (Figure 2A ), which was not the case of OVX + Fine mice (Figure 2A ). Moreover, OVX mice had decreased insulin sensitivity compared with both CTL and OVX + Fine mice (Figure 2B ). Finally, in isolated cardiac subsarcolemmal mitochondria known to be impacted by type 2 diabetes 25 and ovariectomy, 26 a decrease in ATP production was evidenced in those isolated from OVX but not from OVX + Fine mice (Figure 2C ).
Table 2.
Global metabolism
| CTL | OVX | OVX + Fine | |
|---|---|---|---|
| Before finerenone treatment | |||
| Body weight (g) | 30.2 ± 1.2 (n = 15) | 36.1 ± 0.8 (n = 21)*** | |
| Fasting glycaemia (mmol/L) | 6.5 ± 0.3 (n = 13) | 7.6 ± 0.3 (n = 19)* | |
| Echo‐MRI before finerenone | |||
| Body weight (g) | 28.2 ± 1.3 (n = 9) | 36.5 ± 0.8 (n = 18)*** | |
| Fat mass (%) | 15.5 ± 1.8 (n = 9) | 39.2 ± 1.0 (n = 18)*** | |
| Lean mass (%) | 81.7 ± 1.7 (n = 9) | 59.2 ± 0.9 (n = 18)*** | |
| Water in lean tissue (%) | 75.1 ± 1.6 (n = 9) | 53.2 ± 0.7 (n = 18)*** | |
| After finerenone treatment | |||
| Body weight (g) | 28.4 ± 0.8 (n = 15) | 33.4 ± 0.8 (n = 12)*** | 30.2 ± 1.1 (n = 9) † |
| Food consumption (g per 48 h) | 3.47 ± 0.66 (n = 8) | 2.94 ± 0.53 (n = 8) | 3.94 ± 0.53 (n = 8) |
| Water consumption (mL per 48 h) | 3.30 ± 0.94 (n = 8) | 3.43 ± 0.33 (n = 8) | 3.81 ± 0.52 (n = 8) |
| Leptinaemia (pg/mL) | 3.6 ± 1.1 (n = 7) | 48.5 ± 6.6 (n = 11)*** | 36.0 ± 7.6 (n = 11) |
| Plasma triglycerides (mmol/L) | 1.23 ± 0.14 (n = 6) | 1.12 ± 0.12 (n = 9) | 0.96 ± 0.13 (n = 7) |
| Plasma cholesterol (mmol/L) | 1.33 ± 0.28 (n = 6) | 1.54 ± 0.08 (n = 9) | 1.48 ± 0.18 (n = 8) |
| Fasting glycaemia (mmol/L) | 6.5 ± 0.5 (n = 15) | 7.7 ± 0.1 (n = 12) 1 | 6.5 ± 0.4 (n = 9) †† |
| Echo‐MRI after finerenone | |||
| Body weight (g) | 28.4 ± 0.8 (n = 14) | 33.4 ± 0.8 (n = 12)*** | 30.2 ± 1.1 (n = 9) † |
| Fat mass (%) | 18.6 ± 2.3 (n = 14) | 31.4 ± 1.4 (n = 12)*** | 26.3 ± 1.7 (n = 9) † |
| Lean mass (%) | 79.6 ± 2.0 (n = 14) | 66.2 ± 1.3 (n = 12)*** | 71.1 ± 1.6 (n = 9) † |
| Water in lean tissue (%) | 72.6 ± 1.9 (n = 14) | 60.4 ± 1.2 (n = 12)*** | 64.6 ± 1.5 (n = 9) † |
MRI, magnetic resonance imaging.
P = 0.0520.
P < 0.05.
P < 0.01.
P < 0.001 vs. CTL.
P < 0.05.
P < 0.05 vs. OVX.
Figure 2.
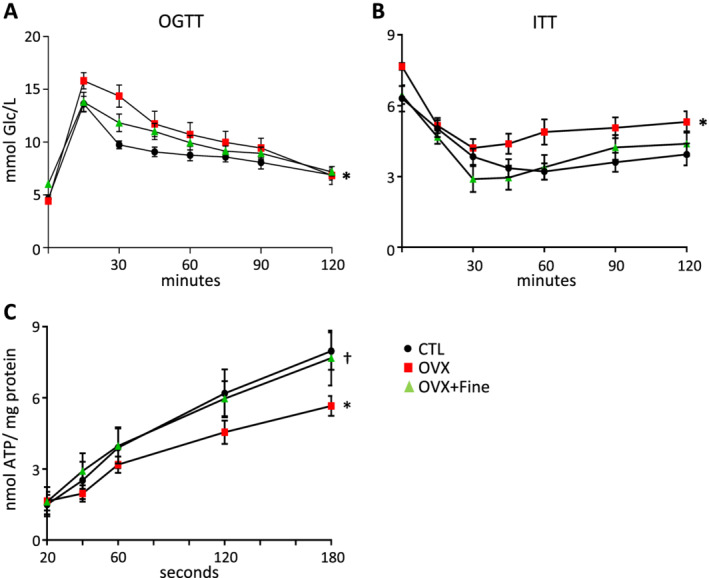
Finerenone treatment allowed improving ovariectomy‐induced reduction in glucose and insulin tolerance and reduction in myocardial ATP production. Assessments in control mice (CTL, black circles) and in mice after 4 months of ovariectomy (OVX, red squares) without or with 1 month finerenone treatment (OVX + Fine, green triangles). (A) Oral glucose tolerance test (OGTT) (n = 11, 9, 9). (B) Insulin tolerance test (ITT) (n = 13, 12, 9). (C) ATP production of subsarcolemmal mitochondria (n = 9, 9, 10). Data are mean ± standard error of the mean. Statistics: one‐way ANOVA plus Tukey, *P < 0.05 vs. CTL; † P < 0.05 vs. OVX.
Taken together, these data suggest that finerenone treatment of OVX mice improved ovariectomy‐induced weight gain, impaired insulin sensitivity and impaired glucose tolerance, and LV mitochondrial dysfunction.
Finerenone improves the exercise capacity of mice with ovariectomy
Because cardiovascular function was improved by MR blockade under post‐menopausal conditions, we tested the ability of mice to perform forced or spontaneous exercise. On two distinct protocols on treadmill, (i) a 1 h endurance running and (ii) an acceleration test at race, the mean total distance (Figure 3A and 3B ) was significantly diminished in the OVX group only but not in OVX + Fine mice compared with CTL mice. Furthermore, none of the OVX mice were able to complete the acceleration test in contrast to 50% of CTL and 21.4% of OVX + Fine mice. To refine the assessment of exercise capacity, mice were placed for 2 days in a combined indirect calorimetry system equipped with a voluntary running wheel and locomotor activity sensor frames. In OVX vs. CTL mice, data showed decreased spontaneous horizontal activity (Figure 3C ) and reduced time spent in the wheel (Figure 3D ), associated with reduced VO2 (Figure 3E ), VCO2 (Figure 3F ), and energy expenditure (Figure 3G ). In OVX + Fine vs. OVX mice, all the previously mentioned parameters were improved (Figure 3C – 3G ), demonstrating beneficial effect of finerenone treatment.
Figure 3.
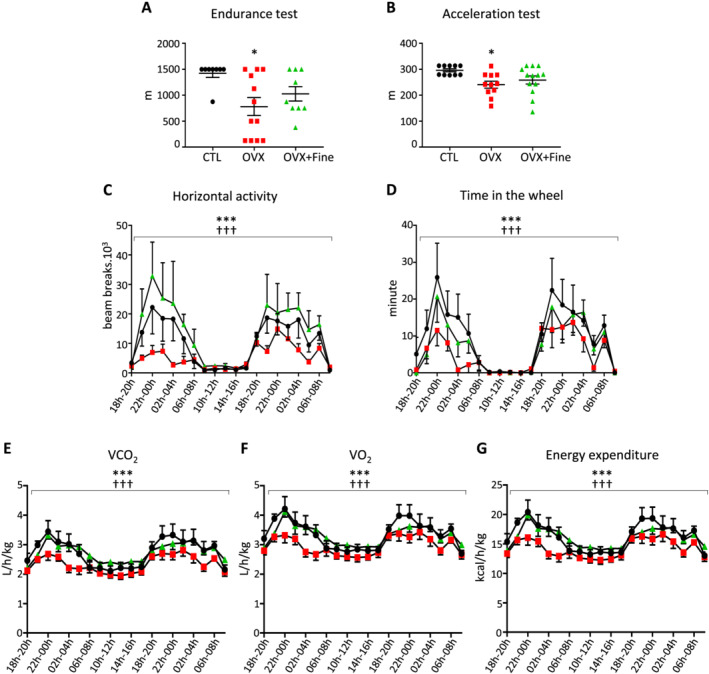
Finerenone treatment improved ovariectomy‐induced decreased ability to exercise. Assessments in control mice (CTL, black circles) and in mice after 4 months of ovariectomy (OVX, red squares) without or with 1 month finerenone treatment (OVX + Fine, green triangles). (A) Run distance during an endurance (n = 8, 12, 9) or (B) an acceleration (n = 10, 11, 13) test at race. (C–G) Spontaneous activity (n = 8, 8, 8), each x‐axis point being the integration per 2 h interval according to a 24 h clock, in cages with free access to an exercise wheel and indirect calorimetry measurements: (C) spontaneous horizontal activity, (D) time spent in the wheel, (E) VO2, (F) VCO2, and (G) energy expenditure. Data are mean ± standard error of the mean. Statistics: (A, B) one‐way ANOVA plus Tukey and (C–G) multivariate analysis, *P < 0.05 and **P < 0.01 vs. CTL; †† P < 0.01 and ††† P < 0.001 vs. OVX.
Discussion
Invasive haemodynamics indicated that OVX mice had diastolic dysfunction, as revealed by increased LV filling pressure and decreased LV compliance, which were both improved after finerenone treatment. OVX mice were considered to have isolated diastolic dysfunction because there was no reduction in LV FS as shown by echocardiography. Diastolic dysfunction in 8‐month‐old OVX mice occurred without increase in LV interstitial collagen type I and III deposits, suggesting that it mainly depended on cardiomyocyte and on coronary functions, rather than in extracellular matrix stiffening at this age. We previously showed that in mice with subtotal nephrectomy‐induced chronic kidney disease, finerenone treatment increased the phosphorylation of Ca2+–calmodulin‐dependent kinase‐II (p‐CaMKII), with subsequent increase in the phosphorylation of the phospholamban at Thr17, which allows diminishing its inhibitory effect on the sarcoendoplasmic reticulum Ca2+‐ATPase (SERCA) pump, responsible for Ca2+ recapture in the sarcoplasm in diastole. 27 In cardiomyocytes isolated from ovariectomized rats, Bupha‐Intr et al. measured reduced Ca2+ reuptake, due to reduced SERCA activity and expression, together with an increase in the supra‐inhibitory monomeric form of the SERCA‐associated protein phospholamban. 28 In mice, Rouet‐Benzineb et al. showed that preserved FS after 2 months of ovariectomy concealed increased expressions of proteins involved in Ca2+ reuptake, that is, phosphorylated Thr17 phospholamban (p‐CaMKII) and the phosphatase calcineurin. 12 Moreover, regarding myocardial stiffness, Farré et al. proposed that a reduction measured in LV strips after 6 months of ovariectomy in mice aged 9 months was due to cardiomyocytes rather than extracellular matrix, because the difference between OVX and controls disappeared in decellularized strips. 29 These publications argue in favour of the importance of the intracellular component in ovariectomy‐induced diastolic dysfunction that we showed to be ameliorated in OVX mice treated with finerenone.
Finerenone prevented the coronary endothelial dysfunction evidenced in OVX mice. Recently, a 4 week finerenone treatment has been shown to improve NO bioavailability in aortic rings and in mesenteric arteries from the Munich Wistar Frömter (MWF) rat model of chronic kidney disease. 30 , 31 Borgo et al. reported that in isolated hearts of ovariectomized spontaneously hypertensive rats, the endothelium‐dependent vasodilation in response to bradykinin was altered while it was improved in animals treated with estradiol and further improved by co‐treatment with drospirenone, a progestogen with anti‐aldosterone properties. Moreover, dihydroethidium fluorescence indicated that both treatments reduced the ovariectomy‐associated increase in oxidative stress in the coronary wall. 32 We previously reported that in chronic HF after myocardial infarction, impaired Ach‐induced endothelium‐dependent vasodilatation of isolated coronary arteries was improved in mice treated with finerenone, and we demonstrated that such improvement depended on a reduction of oxidative stress at the specific level of the arteries. 33 In the present study, ovariectomy‐induced impaired endothelium‐dependent vasodilatation was improved by finerenone, also including oxidative stress reduction in the coronary wall, because ex vivo NADPH oxidase inhibition ameliorated dilatation to the same extent in the arteries of OVX and OVX + Fine mice. These data highlight again the ability of finerenone to protect coronary function.
Several studies indicated that ovariectomy induces an increase in arterial pressure in rats and mice, 12 , 34 including in spontaneously hypertensive rats, in which the pressure delta was not reduced by oestrogen therapy but only by combining estradiol and drospirenone. 32 In our study, MR blockade with finerenone reduced the increase in blood pressure of OVX mice. Regarding vascular distensibility, DeMarco et al. reported a decrease in femoral arteries of female mice fed with a high‐fat/high‐sucrose diet while it was preserved in case of MR blockade with preventive spironolactone treatment. 19 In our hands, although under anaesthesia with a non‐evaluable degree of vasoplegia, ultrasound assessment suggested that finerenone allowed improving stiffness of femoral arteries in OVX mice.
Ovariectomy induces weight gain in which aldosterone–MR has been previously implicated. Indeed, Mook et al. showed that in ovariectomized rats, adrenalectomy attenuates weight increase 35 likely due to a decrease in aldosterone–MR interaction, because in non‐adrenalectomized ovariectomized rats, Dagnault et al. reported that 3 weeks of treatment with the MRA RU‐28318 limits fat gain unlike treatment with the glucocorticoid receptor antagonist RU‐38486. 36 In our study, a 1 month finerenone treatment limited weight gain of OVX mice. This increase in body weight of OVX mice was accompanied by increased plasma leptin. Huby et al. showed that leptin induces an increase in adrenal secretion of aldosterone 37 and participates in increased blood pressure and endothelial dysfunction of obese hyperleptinaemic female mice via aldosterone‐dependent mechanisms, 38 thus supporting the idea that MR blockade is as an interesting strategy in the context of obesity‐associated cardiovascular disease in women.
In OVX mice, the increases in body weight, blood pressure, and fasted glycaemia suggested a pre‐MetS state that was associated with decreased insulin sensitivity and decreased glucose tolerance, although we found no increase in plasmatic levels of triglycerides or total cholesterol in these animals fed with a standard diet. Ovariectomy in rats and mice has been used to evaluate many features associated with the MetS. 39 In another study, in ovariectomized rats fed with a standard diet, Olatunji et al. reported increases in plasmatic triglycerides and total cholesterol (CHO), encompassing increased low‐density and decreased high‐density lipoprotein‐CHO (LDL‐CHO and HDL‐CHO), which were improved, except HDL‐CHO, by a preventive spironolactone treatment. 13 Recently, in mice with high‐fat diet‐induced obesity and decreased insulin sensitivity, Marzolla et al. showed that a preventive finerenone treatment improved metabolism through the activation of AMP‐activated protein kinase, which, in turn, stimulated adipose triglyceride lipase activation, with subsequent increased expression of uncoupling protein‐1 in brown adipocyte. 40 Our data show that a 1 month finerenone treatment, given 3 months after the ovariectomy, allowed improving previously established parameters of the MetS.
Ovariectomy in rodents and type 2 diabetes in human and rodents have been associated with myocardial mitochondrial dysfunction. 25 , 26 In a study after 2.5 months of ovariectomy in rats, Rattanosopa et al. evidenced a decrease in complex I‐driven ATP synthesis of isolated mitochondria, while basal mitochondrial ROS production was not affected. 41 In the same extent, in the present study, no change was evidenced concerning mitochondrial ROS production, but subsarcolemmal mitochondria of OVX mice showed a decrease in ATP production that was improved after finerenone treatment.
Concerning the ability to exercise in post‐menopausal condition, Machi et al. reported a reduction of the maximal speed during a running test, 2 months after ovariectomy in rats aged 24 months, which had diastolic dysfunction without EF reduction. 34 Moreover, Mook et al. reported a rapid reduction of daily spontaneous activity of ovariectomized rats in a running wheel, during a study period of 5 to 33 post‐operative days, with an enhancement of this phenomenon during the same consecutive period. 35 In the same extent, after 2 months of ovariectomy in mice, Do‐Carmo et al. reported decreases in VO2 and energy expenditure, with clear tendency for reduced spontaneous activity. 42 In the present study, OVX mice showed reduced exercise capacity during an endurance or an acceleration test at race and reduced spontaneous activity. Unfortunately, we were unable to perform telemetry assessments, as it would be interesting to record treadmill heart rate and post‐run heart rate recovery, because HFpEF is associated with chronotropic incompetence. 43 Nevertheless, when receiving finerenone treatment, the proportion of OVX mice able to complete the acceleration test on exertion increased. Furthermore, data evidenced improvement of spontaneous activity in OVX mice with finerenone treatment, likely to participate in the improvements of global metabolism and cardiovascular function.
Our data show that MR is a relevant target in models of ovariectomy‐induced altered metabolic profile and cardiovascular dysfunction. In the same extent, Olatunji et al. reported an increase in plasma aldosterone level in OVX rats. 13 After ovariectomy in a model of MetS derived from a cross between Dahl salt‐sensitive and Zucker rats with an inactivating mutation in the leptin receptor (OVX‐DS/obese), Murase et al. measured increased LV mRNA expressions of MR and its transcriptional target Sgk‐1 (serum and glucocorticoid‐activated kinase‐1). 11 Estradiol treatment in these OVX‐DS/obese rats attenuated hypertension and obesity but did not improve heart function because of exacerbated LV fibrosis and diastolic dysfunction, probably mediated by MR activation. 11 On the other hand, in mice with ovariectomy, Rouet‐Benzineb et al. measured reduced LV protein expression of oestrogen receptor ER‐α and ER‐β while showing augmented protein expression of cardiac MR. 12
Although a theoretical enhancement of ER activity could be hypothesized at least via indirect mechanisms, for example, counter‐regulation of endogenous steroid hormone release by an unselective receptor profile as demonstrated for spironolactone 44 or recruitment of the transcriptional co‐activator steroid receptor coregulatory‐1 (Src‐1), which is shared by most steroid hormone receptors, we have no reason to believe that such indirect mechanisms apply to the benefits of finerenone in the current study. Indeed, (i) finerenone does not have any detectable affinity for both ERα and ERβ, 45 and (ii) finerenone has been demonstrated to act as an inverse agonist for Src‐1 recruitment (i.e. blocking Src‐1 recruitment even in the absence of aldosterone) in a chromatin immunoprecipitation assay in a human kidney cell line as well as in rat cardiomyocytes. 46 , 47
In summary, MR was identified as an important pharmacological target for the potential treatment of diastolic dysfunction in post‐menopausal women, and it would be prudent to investigate the novel non‐steroidal MRA finerenone in HFpEF.
Conflict of interest
P.K. is a full‐time employee of Bayer AG. The other authors report no conflicts of interest.
Funding
This work was supported by grants from the action ‘Danièle Hermann—Women Heart’ of the ‘Fondation Recherche Cardio‐Vasculaire’ of the ‘Institut de France’ and from the European COST ADMIRE Network BM1301, by Bayer Pharma AG, and by the French Inserm (Institut national pour la santé et la recherche médicale).
Supporting information
Data S1. Supporting Information
Figure S1. Left Ventricular histology. (A) Representative photographs of PECAM‐1 immunolabeled endothelial cells (lower photographs) and of Wheat‐Germ‐Agglutinin (WGA) staining for contouring cardiomyocytes (upper photographs), allowing determining: i) the cardiomyocytes mean‐crossed sectional area (MCSA) (data for n = 8 CTL, n = 8 OVX and n = 11 OVX + Fine mice in Table 1 of the article), and ii) the ratio of the number of capillaries to the number of cardiomyocytes (data for n = 9 CTL, n = 9 OVX and n = 11 OVX + Fine mice in Table 1 of the article). (B) Representative photographs of collagen type‐I (Coll‐I) (lower photographs) and collagen type‐III (coll‐III) (upper‐photographs) after co‐immunolabeling, allowing calculating the mean percentage on interstitial collagen content in each group (data for n = 7 CTL, n = 9 OVX, and n = 10 OVX + Fine mice in Table 1 of the article).
Figure S2. Finerenone treatment improved ovariectomy‐induced peripheral dysfunction. (A) Echography assessment of femoral artery diameter in function of the increase in blood flow (confident interval 95%) related to temperature‐induced vasodilatation during stepwise progressive heating of the paw (CTL, solid line, n = 10; OVX, dotted line, n = 5; OVX + Fine, dashed line, n = 12). Statistics: multivariate analysis.
Table S1. Macrophages density in the myocardium. Macrophages immuno‐labeling: number per field of immuno‐labeled macrophages (Mtotal as CD68+ cells, M1 as iNOS++CD68+ cells, M2 as CD206++CD68+ double positive cells, and M0 as Mtotal – (M1 + M2) cells) (x20). NS.
Pieronne‐Deperrois, M. , Guéret, A. , Djerada, Z. , Crochemore, C. , Harouki, N. , Henry, J.‐P. , Dumesnil, A. , Larchevêque, M. , do Rego, J.‐C. , do Rego, J.‐L. , Nicol, L. , Richard, V. , Jaisser, F. , Kolkhof, P. , Mulder, P. , Monteil, C. , and Ouvrard‐Pascaud, A. (2021) Mineralocorticoid receptor blockade with finerenone improves heart function and exercise capacity in ovariectomized mice. ESC Heart Failure, 8: 1933–1943. 10.1002/ehf2.13219
References
- 1. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J, Randomized Aldactone Evaluation Study Investigators . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 2. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 3. Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS‐HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21.21073363 [Google Scholar]
- 4. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau J‐L, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 131: 34–42. [DOI] [PubMed] [Google Scholar]
- 5. de Denus S, O'Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA, Rouleau JL. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376: 1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bärfacker L, Kuhl A, Hillisch A, Grosser R, Figueroa‐Pérez S, Heckroth H, Nitsche A, Ergüden J‐K, Gielen‐Haertwig H, Schlemmer K‐H, Mittendorf J, Paulsen H, Platzek J, Kolkhof P. Discovery of BAY 94‐8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem 2012; 7: 1385–1403. [DOI] [PubMed] [Google Scholar]
- 7. Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim S‐Y, Zannad F. Safety and tolerability of the novel non‐steroidal mineralocorticoid receptor antagonist BAY 94‐8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double‐blind trial. Eur Heart J 2013; 34: 2453–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Filippatos G, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Kim S‐Y, Nowack C, Palombo G, Kolkhof P, Kimmeskamp‐Kirschbaum N, Pieper A, Pitt B. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J 2016; 37: 2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yanes LL, Romero DG, Iliescu R, Zhang H, Davis D, Reckelhoff JF. Postmenopausal hypertension: role of the renin‐angiotensin system. Hypertension 2010; 56: 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang H, Jessup JA, Zhao Z, da Silva J, Lin M, MacNamara LM, Ahmad S, Chappell MC, Ferrario CM, Groban L. Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen‐replete mRen2.Lewis rats. PLoS ONE 2013; 8: e76992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murase T, Hattori T, Ohtake M, Nakashima C, Takatsu M, Murohara T, Nagata K. Effects of estrogen on cardiovascular injury in ovariectomized female DahlS.Z‐Lepr (fa)/Lepr (fa) rats as a new animal model of metabolic syndrome. Hypertension 2012; 59: 694–704. [DOI] [PubMed] [Google Scholar]
- 12. Rouet‐Benzineb P, Merval R, Polidano E. Effects of hypoestrogenism and/or hyperaldosteronism on myocardial remodeling in female mice. Physiol Rep 2018; 6: e13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olatunji LA, Adeyanju OA, Michael OS, Usman TO, Tostes RC, Soladoye AO. Ameliorative effect of low‐dose spironolactone on obesity and insulin resistance is through replenishment of estrogen in ovariectomized rats. Can J Physiol Pharmacol 2019; 97: 65–74. [DOI] [PubMed] [Google Scholar]
- 14. Davel AP, Lu Q, Moss ME, Rao S, Anwar IJ, DuPont JJ, Jaffe IZ. Sex‐specific mechanisms of resistance vessel endothelial dysfunction induced by cardiometabolic risk factors. J Am Heart Assoc 2018; 7: e007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merz AA, Cheng S. Sex differences in cardiovascular ageing. Heart Br Card Soc 2016; 102: 825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 1996; 275: 1557–1562. [PubMed] [Google Scholar]
- 17. Sullivan JC. Sex and the renin‐angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1220–R1226. [DOI] [PubMed] [Google Scholar]
- 18. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974; 34: 29–34. [DOI] [PubMed] [Google Scholar]
- 19. DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez‐Perez FI, Martinez‐Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley‐Connell A, Sowers JR. Low‐dose mineralocorticoid receptor blockade prevents Western diet‐induced arterial stiffening in female mice. Hypertension 2015; 66: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta‐analysis of longitudinal studies. J Am Coll Cardiol 2007; 49: 403–414. [DOI] [PubMed] [Google Scholar]
- 21. Barrett‐Connor E. Sex differences in coronary heart disease. why are women so superior? The 1995 Ancel Keys Lecture. Circulation 1997; 95: 252–264. [DOI] [PubMed] [Google Scholar]
- 22. Khosla N, Kalaitzidis R, Bakris GL. Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. Am J Nephrol 2009; 30: 418–424. [DOI] [PubMed] [Google Scholar]
- 23. McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, Lefkowitz MP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Rizkala AR, Sabarwal SV, Shah AM, Shah SJ, Shi VC, van Veldhuisen DJ, Zannad F, Zile MR, Cikes M, Goncalvesova E, Katova T, Kosztin A, Lelonek M, Sweitzer N, Vardeny O, Claggett B, Jhund PS, Solomon SD. Effects of sacubitril‐valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON‐HF. Circulation 2020; 141: 338–351. [DOI] [PubMed] [Google Scholar]
- 24. Merrill M, Sweitzer NK, Lindenfeld J, Kao DP. Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT trial. JACC Heart Fail 2019; 7: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 2008; 114: 195–210. [DOI] [PubMed] [Google Scholar]
- 26. Ribeiro Junior RF, Rodrigues PL, Morra EA, Ronconi KS, do Val Lima PR, Porto ML, Simões MR, Vassallo DV, Figueiredo SG, Stefanon I. Estrogen regulates spatially distinct cardiac mitochondrial subpopulations. Mitochondrion 2017; 35: 87–96. [DOI] [PubMed] [Google Scholar]
- 27. Bonnard B, Pieronne‐Deperrois M, Djerada Z, Elmoghrabi S, Kolkhof P, Ouvrard‐Pascaud A, Mulder P, Jaisser F, Messaoudi S. Mineralocorticoid receptor antagonism improves diastolic dysfunction in chronic kidney disease in mice. J Mol Cell Cardiol 2018; 121: 124–133. [DOI] [PubMed] [Google Scholar]
- 28. Bupha‐Intr T, Wattanapermpool J. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol 2006; 291: H1101–H1108. [DOI] [PubMed] [Google Scholar]
- 29. Farré N, Jorba I, Torres M, Falcones B, Martí‐Almor J, Farré R, Almendros I, Navajas D. Passive stiffness of left ventricular myocardial tissue is reduced by ovariectomy in a post‐menopause mouse model. Front Physiol 2018; 9: 1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. González‐Blázquez R, Somoza B, Gil‐Ortega M, Martín Ramos M, Ramiro‐Cortijo D, Vega‐Martín E, Schulz A, Ruilope LM, Kolkhof P, Kreutz R, Fernández‐Alfonso MS. Finerenone attenuates endothelial dysfunction and albuminuria in a chronic kidney disease model by a reduction in oxidative stress. Front Pharmacol 2018; 9: 1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gil‐Ortega M, Vega‐Martín E, Martín‐Ramos M, González‐Blázquez R, Pulido‐Olmo H, Ruiz‐Hurtado G, Schulz A, Ruilope LM, Kolkhof P, Somoza B, Kreutz R, Fernández‐Alfonso MS. Finerenone reduces intrinsic arterial stiffness in Munich Wistar Frömter rats, a genetic model of chronic kidney disease. Am J Nephrol 2020; 51: 294–303. [DOI] [PubMed] [Google Scholar]
- 32. Borgo MV, Claudio ERG, Silva FB, Romero WG, Gouvea SA, Moysés MR, Santos RL, Almeida SA, Podratz PL, Graceli JB, Abreu GR. Hormonal therapy with estradiol and drospirenone improves endothelium‐dependent vasodilation in the coronary bed of ovariectomized spontaneously hypertensive rats. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol 2016; 49: e4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gueret A, Harouki N, Favre J, Galmiche G, Nicol L, Henry J‐P, Besnier M, Thuillez C, Richard V, Kolkhof P, Mulder P, Jaisser F, Ouvrard‐Pascaud A. Vascular smooth muscle mineralocorticoid receptor contributes to coronary and left ventricular dysfunction after myocardial infarction. Hypertension 2016; 67: 717–723. [DOI] [PubMed] [Google Scholar]
- 34. Machi JF, da Silva Dias D, Freitas SC, de Moraes OA, da Silva MB, Cruz PL, Mostarda C, Salemi VM, Morris M, De Angelis K, Irigoyen MC. Impact of aging on cardiac function in a female rat model of menopause: role of autonomic control, inflammation, and oxidative stress. Clin Interv Aging 2016; 11: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mook DG, Kenney NJ, Roberts S, Nussbaum AI, Rodier WI. Ovarian‐adrenal interactions in regulation of body weight by female rats. J Comp Physiol Psychol 1972; 81: 198–211. [DOI] [PubMed] [Google Scholar]
- 36. Dagnault A, Deshaies Y, Richard D. The effects of RU 38486 and RU 28318 on energy balance of ovariectomized rats. Ann N Y Acad Sci 1994; 746: 418–420. [DOI] [PubMed] [Google Scholar]
- 37. Huby A‐C, Antonova G, Groenendyk J, Gomez‐Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemèle EJ. Adipocyte‐derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015; 132: 2134–2145. [DOI] [PubMed] [Google Scholar]
- 38. Huby AC, Otvos L Jr, de Belin Chantemèle EJ. Leptin induces hypertension and endothelial dysfunction via aldosterone‐dependent mechanisms in obese female mice. Hypertension 2016; 67: 1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Medina‐Contreras J, Villalobos‐Molina R, Zarain‐Herzberg A, Balderas‐Villalobos J. Ovariectomized rodents as a menopausal metabolic syndrome model. A minireview Mol Cell Biochem 2020; 475: 261–276. [DOI] [PubMed] [Google Scholar]
- 40. Marzolla V, Feraco A, Gorini S, Mammi C, Marrese C, Mularoni V, Boitani C, Lombès M, Kolkhof P, Ciriolo MR, Armani A, Caprio M. The novel non‐steroidal MR antagonist finerenone improves metabolic parameters in high‐fat diet‐fed mice and activates brown adipose tissue via AMPK‐ATGL pathway. FASEB J 2020; 34: 12450–12465. [DOI] [PubMed] [Google Scholar]
- 41. Rattanasopa C, Phungphong S, Wattanapermpool J, Bupha‐Intr T. Significant role of estrogen in maintaining cardiac mitochondrial functions. J Steroid Biochem Mol Biol 2015; 147: 1–9. [DOI] [PubMed] [Google Scholar]
- 42. Do Carmo JM, da Silva AA, Moak SP, Browning JR, Dai X, Hall JE. Increased sleep time and reduced energy expenditure contribute to obesity after ovariectomy and a high fat diet. Life Sci 2018; 212: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail 2010; 3: 29–34. [DOI] [PubMed] [Google Scholar]
- 44. Levy J, Burshell A, Marbach M, Afllalo L, Glick SM. Interaction of spironolactone with oestradiol receptors in cytosol. J Endocrinol 1980; 84: 371–379. [DOI] [PubMed] [Google Scholar]
- 45. Pitt B, Filippatos G, Gheorghiade M, Kober L, Krum H, Ponikowski P, Nowack C, Kolkhof P, Kim S‐Y, Zannad F. Rationale and design of ARTS: a randomized, double‐blind study of BAY 94‐8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. Eur J Heart Fail 2012; 14: 668–675. [DOI] [PubMed] [Google Scholar]
- 46. Amazit L, Le Billan F, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, Khan JA, Hillisch A, Lombès M, Rafestin‐Oblin M‐E, Fagart J. Finerenone impedes aldosterone‐dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator‐1. J Biol Chem 2015; 290: 21876–21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grune J, Beyhoff N, Smeir E, Chudek R, Blumrich A, Ban Z, Brix S, Betz IR, Schupp M, Foryst‐Ludwig A, Klopfleisch R, Stawowy P, Houtman R, Kolkhof P, Kintscher U. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone's antifibrotic activity. Hypertension 2018; 71: 599–608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Figure S1. Left Ventricular histology. (A) Representative photographs of PECAM‐1 immunolabeled endothelial cells (lower photographs) and of Wheat‐Germ‐Agglutinin (WGA) staining for contouring cardiomyocytes (upper photographs), allowing determining: i) the cardiomyocytes mean‐crossed sectional area (MCSA) (data for n = 8 CTL, n = 8 OVX and n = 11 OVX + Fine mice in Table 1 of the article), and ii) the ratio of the number of capillaries to the number of cardiomyocytes (data for n = 9 CTL, n = 9 OVX and n = 11 OVX + Fine mice in Table 1 of the article). (B) Representative photographs of collagen type‐I (Coll‐I) (lower photographs) and collagen type‐III (coll‐III) (upper‐photographs) after co‐immunolabeling, allowing calculating the mean percentage on interstitial collagen content in each group (data for n = 7 CTL, n = 9 OVX, and n = 10 OVX + Fine mice in Table 1 of the article).
Figure S2. Finerenone treatment improved ovariectomy‐induced peripheral dysfunction. (A) Echography assessment of femoral artery diameter in function of the increase in blood flow (confident interval 95%) related to temperature‐induced vasodilatation during stepwise progressive heating of the paw (CTL, solid line, n = 10; OVX, dotted line, n = 5; OVX + Fine, dashed line, n = 12). Statistics: multivariate analysis.
Table S1. Macrophages density in the myocardium. Macrophages immuno‐labeling: number per field of immuno‐labeled macrophages (Mtotal as CD68+ cells, M1 as iNOS++CD68+ cells, M2 as CD206++CD68+ double positive cells, and M0 as Mtotal – (M1 + M2) cells) (x20). NS.


