Abstract
Aims
Acute pulmonary disorders are known physical triggers of takotsubo syndrome (TTS). This study aimed to investigate prevalence of acute pulmonary triggers in patients with TTS and their impact on outcomes.
Methods and results
Patients with TTS were enrolled from the International Takotsubo Registry and screened for triggering factors and comorbidities. Patients were categorized into three groups (acute pulmonary trigger, chronic lung disease, and no lung disease) to compare clinical characteristics and outcomes.
Of the 1670 included patients with TTS, 123 (7%) were identified with an acute pulmonary trigger, and 194 (12%) had a known history of chronic lung disease. The incidence of cardiogenic shock was highest in patients with an acute pulmonary trigger compared with those with chronic lung disease or without lung disease (17% vs. 10% vs. 9%, P = 0.017). In‐hospital mortality was also higher in patients with an acute pulmonary trigger than in the other two groups, although not significantly (5.7% vs. 1.5% vs. 4.2%, P = 0.13). Survival analysis demonstrated that patients with an acute pulmonary trigger had the worst long‐term outcome (P = 0.002). The presence of an acute pulmonary trigger was independently associated with worse long‐term mortality (hazard ratio 2.12, 95% confidence interval 1.33–3.38; P = 0.002).
Conclusions
The present study demonstrates that TTS is related to acute pulmonary triggers in 7% of all TTS patients, which accounts for 21% of patients with physical triggers. The presence of acute pulmonary trigger is associated with a severe in‐hospital course and a worse long‐term outcome.
Keywords: Takotsubo syndrome, Broken heart syndrome, Outcome, Acute respiratory insufficiency, Chronic obstructive pulmonary disease, InterTAK Registry
Introduction
Takotsubo syndrome (TTS) is characterized by acute left ventricular dysfunction and mainly occurs in postmenopausal women. 1 , 2 , 3 , 4 In addition, TTS is often associated with antecedent emotional or physical stressors. 4 , 5 , 6 Recent data indicate approximately one‐third of patients show identifiable physical stressful triggers, including a wide spectrum of medical conditions, such as respiratory disorders. 7 , 8 However, there is scarce information on the relationship between antecedent acute respiratory disorders and TTS.
Hypoxia during acute pulmonary decompensation may activate sympathetic drive and provoke catecholamine surge, which can trigger TTS. Acute respiratory disorders have been identified as a physical trigger for TTS in numerous case reports and systematic reviews. 9 , 10 , 11 Due to the effects of the cardio‐respiratory systems on haemodynamic status, such respiratory disorders may also affect the prognosis of TTS. 12
The aim of the present study was to investigate the prevalence of acute pulmonary triggers and history of chronic lung disease in patients with TTS and their clinical impact on in‐hospital and long‐term outcomes using the International Takotsubo Registry (InterTAK Registry; www.takotsubo‐registry.com) cohort.
Methods
Patients and inclusion criteria
Takotsubo syndrome patients were enrolled from the InterTAK Registry as previously described. 13 Data were queried from the University Hospital Zurich and 25 collaborating hospitals in nine countries (Austria, Finland, France, Germany, Italy, Poland, Switzerland, UK, and the USA) from 1 January 2011 to 31 December 2014. TTS was diagnosed according to the modified Mayo Clinic Diagnostic Criteria 13 , 14 : (i) a transient wall motion abnormality in the left ventricle beyond a single coronary artery territory; (ii) the absence of obstructive coronary artery disease or angiographic evidence of acute plaque rupture, explaining the wall motion abnormality; (iii) new electrocardiographic abnormalities or elevation in cardiac troponin values; and (iv) the absence of myocarditis. Patients matching all other criteria, in whom the wall‐motion abnormality was identical to a single coronary artery territory coincidentally, were included. TTS patients who died during the acute phase before complete recovery of wall motion were not excluded. When eligibility for inclusion was uncertain, patient charts were reviewed by several core members at the University Hospital Zurich to reach an agreement.
The investigation conforms with the principles outlined in the Declaration of Helsinki. The study protocol was reviewed by the respective local ethics committees or investigational review boards at each collaborating site. Due to the partly retrospective nature of the study, ethics committees of most study centres waived the need for informed consent. At centres in which the ethics committees or investigational review boards required informed consent or in which patients were included prospectively, formal written consent was obtained from patients or surrogates.
We screened for TTS triggering factors and comorbidities in all patients. The definition of an acute pulmonary trigger included acute exacerbation of chronic obstructive pulmonary disease (COPD) or asthma, acute respiratory infection including bacterial/viral pneumonia, bronchitis, aspiration, acute respiratory distress syndrome (ARDS), pneumothorax and acute respiratory failure from other pulmonary conditions except for apparent cardiogenic pulmonary oedema secondary to TTS. A history of chronic lung disease was defined as a clear diagnosis of COPD/asthma characterized by persistent respiratory symptoms and airflow limitation in patient charts. Follow‐up data were collected through clinical visits, clinical charts, or phone calls.
We categorized all patients with TTS into three groups: (i) those triggered by an acute pulmonary process (‘acute pulmonary trigger’), (ii) those with a known history of chronic lung disease without an acute pulmonary trigger (‘chronic lung disease’), and (iii) patients with neither an acute respiratory trigger nor a chronic lung disease (‘no lung disease’). We compared clinical characteristics, hospital course (including intensive care treatment and complications), and 5 year outcomes between the three groups.
Statistical analysis
Continuous data are presented as mean ± standard deviation, and laboratory values are given as medians and interquartile ranges. Categorical variables are provided as numbers and percentages. Comparisons of patient characteristics between different groups were performed with one‐way analysis of variance or Kruskal–Wallis test for continuous data and Pearson χ 2 test for categorical variables.
Outcome analysis was performed using Kaplan–Meier estimates and log‐rank tests. Cox‐regression analysis was conducted to determine the hazard ratio and 95% confidence intervals of an acute pulmonary trigger and past history of chronic lung disease using the no lung disease group as a reference. To adjust for potential differences between groups, a multivariable analysis (including variables that had shown a significant difference in the baseline comparison and were likely correlates of long‐term mortality) was performed via Cox‐regression analysis. Missing data on covariates were completed with multiple imputations prior to multivariable Cox‐regression. All tests were two‐sided and statistical significance was defined as P < 0.05. Statistical analyses were performed using IBM SPSS Statistics, Version 25.0 (IBM Corp., Armonk, NY, USA). Graphs were compiled with Prism 7 (GraphPad, La Jolla, CA, USA).
Results
From the InterTAK Registry, 1670 patients with complete information on triggering factors and history of lung disorders were analysed. Of these patients, TTS triggered by an acute pulmonary disease was identified in 123 patients (7%, Figure 1 ), consisting of acute exacerbation of COPD (n = 59, 48%) or asthma (n = 14, 11%), acute respiratory infection (n = 30, 24%), ARDS (n = 8, 7%), pneumothorax (n = 3, 2%), and acute respiratory failure from other pulmonary conditions (n = 9, 7%). A total of 194 patients (12%) had a known history of chronic lung disease that did not trigger TTS, including COPD (n = 151, 78%) and asthma (n = 43, 22%). The remaining 1353 patients (81%) were without an acute pulmonary disease process or known history of chronic lung disease.
Figure 1.
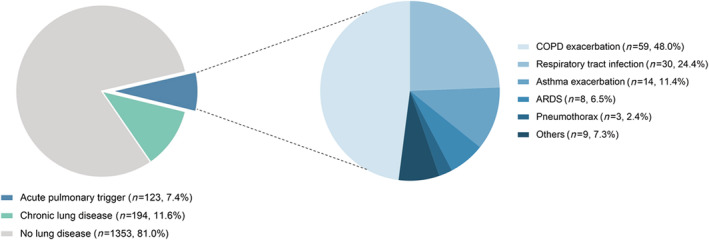
Acute pulmonary triggers and chronic lung diseases. Acute pulmonary triggers were identified in 7% of patients. In the other patients, 12% had a past history of chronic lung diseases including chronic obstructive pulmonary disease and asthma. Patients without an acute pulmonary trigger or chronic lung disease were classified into the no lung disease group (81%). ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease.
The comparison of clinical characteristics between groups is summarized in Table 1 . The proportion of female patients was significantly higher in patients with no lung disease than in the other groups (84% vs. 85% vs. 91%, P = 0.003). There was no difference in age between groups. The highest creatine kinase level on admission was shown in patients with no lung disease (factor increase of the upper limit of normal, 0.71 vs. 0.75 vs. 0.87; P = 0.016), while there was no difference in troponin or brain natriuretic peptide. White blood cell count on admission was highest in patients with an acute pulmonary trigger (11.58 vs. 10.04 vs. 9.60, ×103/μL, P < 0.001). There was no difference in electrocardiographic findings and TTS types between groups. Heart rate was significantly higher (98.3 ± 22.9 bpm vs. 91.4 ± 20.6 bpm vs. 86.1 ± 21.7 bpm, P < 0.001), and left ventricular ejection fraction (38.1 ± 12.1% vs. 39.8 ± 11.6% vs. 41.4 ± 11.7%, P = 0.005) was reduced significantly in patients with an acute pulmonary trigger. There were significantly more current smokers in the group with an acute pulmonary trigger and chronic lung disease (32% vs. 36% vs. 17%, P < 0.001), which was associated with a higher prevalence of respiratory tract cancer (4.2% vs. 6.5% vs. 1.7%, P < 0.001). Acute intensive care treatments, especially invasive or non‐invasive ventilation, were needed most frequently in patients with an acute pulmonary trigger (45% vs. 21% vs. 14%, P < 0.001). In addition, the incidence of cardiogenic shock was highest in patients with an acute pulmonary trigger (17% vs. 10% vs. 9%, P = 0.017), which was associated with the highest prevalence of catecholamine use. In‐hospital mortality was numerically higher in patients with an acute pulmonary trigger, although the difference was not statistically significant (5.7% vs. 1.5% vs. 4.2%, P = 0.13).
Table 1.
Characteristics of takotsubo patients
| Characteristic | Acute pulmonary trigger | Chronic lung disease | No lung disease | P value |
|---|---|---|---|---|
| N = 123 | N = 194 | N = 1353 | ||
| Demographics | ||||
| Female sex—no./total no. (%) | 103/123 (83.7) | 165/194 (85.1) | 1230/1353 (90.9) | 0.003 |
| Age (years) | 67.3 ± 11.5 (N = 123) | 66.6 ± 11.5 (N = 194) | 66.4 ± 13.3 (N = 1353) | 0.76 |
| Triggers—no./total no. (%) | ||||
| Physical | 123/123 (100.0) | 67/194 (34.5) | 407/1353 (30.1) | <0.001 |
| Emotional | 0/123 (0.0) | 40/194 (20.6) | 432/1353 (31.9) | <0.001 |
| No evident trigger | 0/123 (0.0) | 62/194 (32.0) | 410/1353 (30.3) | <0.001 |
| Takotsubo type—no./total no. (%) | ||||
| Typical | 92/123 (74.8) | 157/194 (80.9) | 1110/1353 (82.0) | 0.14 |
| Symptoms on admission—no./total no. (%) | ||||
| Chest pain | 56/107 (52.3) | 127/177 (71.8) | 1002/1274 (78.6) | <0.001 |
| Dyspnoea | 100/115 (87.0) | 110/177 (62.1) | 523/1265 (41.3) | <0.001 |
| Cardiac biomarkers—median (IQR) | ||||
| Troponin on admission—factor increase in ULN* | 8.67 (2.07–24.86) N = 101 | 4.90 (1.80–19.90) N = 157 | 8.00 (2.36–23.43) N = 1107 | 0.18 |
| Creatine kinase on admission—factor increase in ULN | 0.71 (0.39–1.13) N = 75 | 0.75 (0.46–1.44) N = 126 | 0.87 (0.54–1.51) N = 959 | 0.016 |
| BNP on admission—factor increase in ULN † | 4.73 (1.68–18.20) N = 40 | 6.29 (2.38–16.24) N = 66 | 6.64 (2.16–16.16) N = 324 | 0.74 |
| Inflammatory markers—median (IQR) | ||||
| CRP on admission (mg/L) | 5.80 (1.65–21.13) N = 72 | 4.00 (1.40–9.60) N = 111 | 3.80 (1.40–11.30) N = 911 | 0.33 |
| WBC on admission (103/μL) | 11.58 (8.68–15.78) N = 106 | 10.04 (8.00–12.88) N = 160 | 9.60 (7.30–12.30) N = 1150 | <0.001 |
| ECG on admission—no./total no. (%) | ||||
| ST‐segment elevation | 40/106 (37.7) | 74/172 (43.0) | 548/1238 (44.3) | 0.42 |
| T‐wave inversion | 47/106 (44.3) | 69/172 (40.1) | 509/1238 (41.1) | 0.77 |
| QTc (ms) | 453.1 ± 50.5 (N = 83) | 460.4 ± 52.9 (N = 140) | 457.4 ± 49.3 (N = 882) | 0.58 |
| Haemodynamics—mean ± SD (N) | ||||
| Heart rate (beats per minute) | 98.3 ± 22.9 (N = 99) | 91.4 ± 20.6 (N = 160) | 86.1 ± 21.7 (N = 1137) | <0.001 |
| Systolic blood pressure (mmHg) | 129.3 ± 27.1 (N = 96) | 132.2 ± 31.1 (N = 165) | 130.8 ± 28.7 (N = 1139) | 0.73 |
| Diastolic blood pressure (mm Hg) | 77.2 ± 19.0 (N = 94) | 76.4 ± 17.0 (N = 163) | 76.8 ± 16.9 (N = 1122) | 0.92 |
| Left ventricular ejection fraction (%) ‡ | 38.1 ± 12.1 (N = 110) | 39.8 ± 11.6 (N = 182) | 41.4 ± 11.7 (N = 1241) | 0.005 |
| Cardiovascular risk factors—no./total no. (%) | ||||
| Hypertension | 85/123 (69.1) | 129/193 (66.8) | 869/1343 (64.7) | 0.55 |
| Diabetes mellitus | 17/122 (13.9) | 32/193 (16.6) | 191/1348 (14.2) | 0.66 |
| Current smoking | 38/120 (31.7) | 68/190 (35.8) | 222/1309 (17.0) | <0.001 |
| Hypercholesterolemia | 42/122 (34.4) | 59/192 (30.7) | 420/1342 (31.3) | 0.76 |
| Cancer—no./total no. (%) | 16/118 (13.6) | 45/185 (24.3) | 199/1289 (15.4) | 0.007 |
| Respiratory tract | 5/118 (4.2) | 12/185 (6.5) | 22/1289 (1.7) | <0.001 |
| Medication at discharge—no. (%) | ||||
| ACE‐inhibitor or ARB | 82/109 (75.2) | 142/178 (79.8) | 952/1189 (80.1) | 0.49 |
| Beta‐blocker | 76/109 (69.7) | 124/178 (69.7) | 948/1189 (79.7) | 0.001 |
| Calcium‐channel antagonist | 13/109 (11.9) | 12/178 (6.7) | 101/1189 (8.5) | 0.31 |
| Statin | 54/109 (49.5) | 89/178 (50.0) | 616/1189 (51.8) | 0.83 |
| Aspirin | 71/109 (65.1) | 125/178 (70.2) | 794/1189 (66.8) | 0.60 |
| P2Y12 antagonist | 15/109 (13.8) | 24/178 (13.5) | 128/1189 (10.8) | 0.40 |
| Coumarin | 9/109 (8.3) | 8/178 (4.5) | 104/1189 (8.7) | 0.16 |
| Acute intensive care treatment—no./total no. (%) | 55/122 (45.1) | 48/193 (24.9) | 235/1349 (17.4) | <0.001 |
| Intra‐aortic balloon pump | 4/122 (3.3) | 4/193 (2.1) | 36/1349 (2.7) | 0.80 |
| Invasive or non‐invasive ventilation | 55/122 (45.1) | 41/193 (21.2) | 183/1349 (13.6) | <0.001 |
| Cardiopulmonary resuscitation | 12/122 (9.8) | 11/193 (5.7) | 117/1349 (8.7) | 0.32 |
| Catecholamine use | 29/122 (23.8) | 23/193 (11.9) | 147/1349 (10.9) | <0.001 |
| In‐hospital complications—no./total no. (%) | 26/122 (21.3) | 20/192 (10.4) | 148/1343 (11.0) | 0.003 |
| Cardiogenic shock | 21/122 (17.2) | 19/192 (9.9) | 123/1343 (9.2) | 0.017 |
| Death | 7/123 (5.7) | 3/194 (1.5) | 57/1353 (4.2) | 0.13 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; CRP, C‐reactive protein; ECG, electrocardiogram; IQR, interquartile range;QTc, QT time corrected for heart rate; SD, standard deviation; ULN, upper limit of normal; WBC, white blood cell count.
Including upper limits of the normal range for troponin T, high‐sensitivity troponin T, and troponin I.
Including upper limits of the normal range for brain natriuretic peptide and the N‐terminal of prohormone brain natriuretic peptide.
Data obtained during catheterization or echocardiography; if both results were available data from catheterization were used.
Five‐year survival analysis (Figure 2 ) demonstrated that patients with an acute pulmonary trigger had the worst outcome when compared with patients with chronic lung disease (P = 0.003) and patients without pulmonary disease (P = 0.001). No statistical evidence could be observed when comparing 5 year mortality between patients with chronic lung disease and patients without pulmonary disorders (P = 0.58). Cox‐regression analysis revealed that an acute pulmonary trigger was independently associated with worse 5 year mortality (hazard ratio 2.12, 95% confidence interval 1.33–3.38; P = 0.002), while chronic lung disease had no impact (P = 0.22; Figure 3 ).
Figure 2.
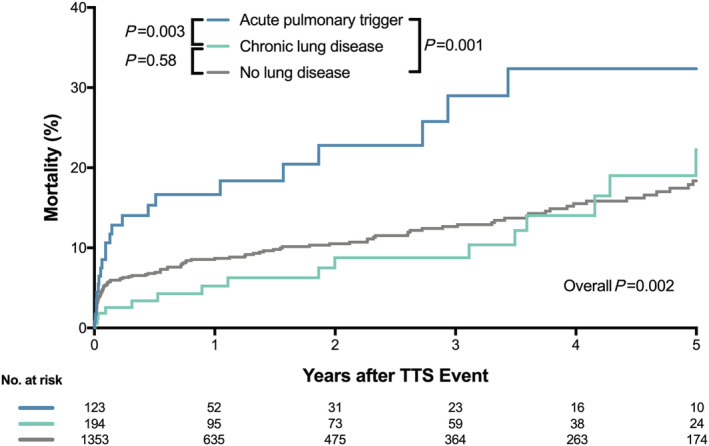
Kaplan–Meier curve for long‐term mortality. Patients with an acute pulmonary trigger had the worst long‐term prognosis, while the outcome of patients with a history of chronic lung disease was comparable with patients without lung disease. TTS, takotsubo syndrome.
Figure 3.
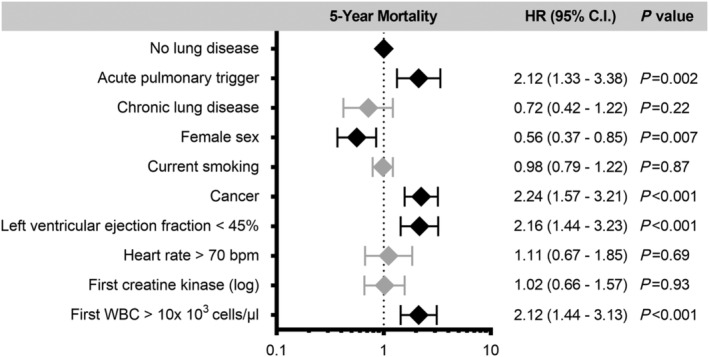
Outcome predictors. Multiple Cox‐regression analysis adjusting for potential differences between the groups using the no lung disease group as a reference. The presence of an acute pulmonary trigger was independently associated with worse long‐term mortality, while chronic lung disease had no impact. Male sex, history of cancer, left ventricular ejection fraction below 45%, and white blood cell count on admission were also independently associated with worse long‐term outcome. Bpm, beats per minute; CI, confidence interval; HR, hazard ratio; WBC, white blood cell count. The error bars indicate 95% CI.
Discussion
The principal findings of this study were as follows: (i) acute pulmonary disorders accounted for 21% of all patients with physical triggers in the InterTAK Registry; (ii) the presence of an acute pulmonary trigger in TTS was independently associated with worse long‐term outcome; (ii) the coexistence of COPD or asthma in patients who had non‐pulmonary triggers for TTS had no effects on long‐term mortality outcomes.
Although emotional stress was classically recognized a typical feature of TTS, previous studies showed that more than one‐third of patients with TTS were provoked by physical stressors. 7 , 13 Recently, significant diversity of physical stressors has been reported to trigger TTS, including acute respiratory disorders. 13 Furthermore, respiratory disorders such as ARDS may trigger a transient decrease in global systolic dysfunction, which might represent a form of TTS. Indeed, in the present study, acute pulmonary triggers were identified in 7% of all TTS patients.
While the exact role of acute pulmonary triggers in TTS has not been established, multiple factors may be involved. Hypoxia during acute respiratory dysfunction may activate sympathetic drive and cause a chemo‐reflex response that triggers TTS. 10 Moreover, hypercapnia and associated respiratory acidosis have been reported to be strong stimuli for noradrenaline and adrenaline synthesis in animal models and humans. 15 , 16 There may be potential additional effects of CO2 on catecholamine stimulation beyond systemic acidosis. 17 These cascades involve an enhanced sympathetic drive that results in elevated catecholamine levels, which is thought to have a central role in the pathophysiology of TTS. 18
In the present study, the most common pulmonary trigger for TTS was exacerbation of COPD/asthma (59%), followed by acute respiratory infections and ARDS. This strongly supports the close relationship between acute and/or chronic respiratory inflammation and cardiac diseases shown in other studies. Patients with COPD/asthma have chronically increased systemic inflammation, and additional inflammatory responses are observed during acute exacerbations. 19 Moreover, respiratory infections have been shown to increase the risk of myocardial infarction in the general population. 20 Donaldson et al. demonstrated an increased risk of secondary myocardial infarction and stroke in patients with acute exacerbations of COPD in relation to elevated inflammatory markers such as fibrinogen and C‐reactive protein. 21 Indeed, a recent experimental study revealed the importance of myocardial inflammatory activation in the pathogenesis of TTS. 22 Given that oxidative stress‐mediated inflammatory responses play a key role in pulmonary conditions such as asthma, COPD, and pneumonia, it is conceivable that the same inflammatory response may also increase the risk of developing TTS. 23 , 24
Previous observational studies imply that β2‐adrenergic agonists, which are often used as a daily treatment for obstructive lung disease, can provoke TTS. 25 Manfredini et al. reported that 72% of TTS patients with COPD/asthma were taking β2‐agonists. 9 Tornvall et al. revealed that β2‐agonist use before admission was more common in patients with TTS compared with control subjects. 26 It is difficult to determine whether acute respiratory disorders or β2‐agonist use is the primary cause of TTS in patients with exacerbation of COPD/asthma. Indeed, various factors (including hypoxia/hypercapnia, inflammation, and/or β2‐agonist use) likely have synergistic effects on the development of TTS. For example, the increased mechanical work of breathing in acute pulmonary processes based on airway resistance, lung/chest elasticity, diaphragmatic and accessory muscles use, tidal volume, and respiratory rate may all play a role in triggering TTS. 27 , 28 , 29 The catecholaminergic surge related to the psychological stress of an acute respiratory dysfunction is another important factor in TTS. 30
Exacerbation of COPD is a known precipitant of cardiac dysfunction and/or acute heart failure. 31 About 74% of patients admitted with exacerbation of COPD had elevated troponin, 32 with another study showing 23% of these patients had reduced left ventricular ejection fraction. 33 In addition, a systematic review by Hawkins et al. demonstrated that 16–60% of patients with exacerbation of COPD had elevated brain natriuretic peptide, which also predicted early adverse outcomes. 34 Therefore, COPD exacerbation may be a more common trigger for TTS than expected, particularly among intubated/sedated patients who cannot communicate their symptoms. As such, early cardiac investigation should be considered in patients admitted with exacerbated COPD/asthma, as concurrent cardiac comorbidity may significantly affect prognosis.
This is the first report demonstrating the clinical impact of acute pulmonary triggers on in‐hospital and long‐term outcomes in TTS patients. Intensive care treatment during the acute phase was more frequently required in patients with an acute pulmonary trigger than in other groups. In addition, cardiogenic shock was more often observed in patients with an acute pulmonary trigger. These results might be related to a lower left ventricular ejection fraction in patients with acute pulmonary trigger. Furthermore, multivariate analysis revealed that having an acute pulmonary trigger was independently associated with worse long‐term outcome. TTS patients with a history of COPD/asthma needed invasive or non‐invasive ventilation more frequently during the acute phase than those without lung disease, while no difference was observed in regard to long‐term mortality. These findings suggest that TTS development might reflect or affect the severity of pulmonary disorders.
Study limitations
First, differentiating primary respiratory failure triggering TTS from respiratory failure secondary to TTS is challenging. To avoid different approaches between centres, a core member of the leading hospital collected the data from every hospital that is included in this registry. All available information were carefully reviewed, and we reach the final decision by a comprehensive approach. Second, as chronically increased systemic inflammation may be related to developing TTS, it is possible that corticosteroids use has an important role for preventing TTS. However, in our registry, data on corticosteroids were only available in a minority of patients. Third, we cannot determine whether the excess of 5 year mortality is a direct consequence of acute pulmonary causes and not related to TTS. Fourth, respiratory function of patients with acute pulmonary triggers or chronic lung disease during follow‐up was not reassessed.
Conclusions
Acute respiratory disorders have previously been recognized as a possible triggering factor of TTS. However, the relationship between acute pulmonary triggers and TTS had not been evaluated until now. The present study demonstrates TTS is related to acute pulmonary triggers in 7% of all TTS patients, which accounts for 21% of patients with physical triggers. These patients more often develop cardiogenic shock and have an increased need for mechanical ventilation and catecholamine support. In addition, the presence of an acute pulmonary trigger is independently associated with worse long‐term outcome. Further research is warranted for identification of mechanisms that may be targets for prevention and management of TTS in acute pulmonary illness.
Conflict of interest
The authors declare that they have no competing interests.
Funding
C. T. has been supported by the H.H. Sheikh Khalifa bin Hamad Al‐Thani Research Programme and the Swiss Heart Foundation. The InterTAK Registry is supported by the Biss Davies Charitable Trust. L. S. M. has been supported by EU HORIZON 2020 (SILICOFCM ID777204).
Kato, K. , Cammann, V. L. , Napp, L. C. , Szawan, K. A. , Micek, J. , Dreiding, S. , Levinson, R. A. , Petkova, V. , Würdinger, M. , Patrascu, A. , Sumalinog, R. , Gili, S. , Clarenbach, C. F. , Kohler, M. , Wischnewsky, M. , Citro, R. , Vecchione, C. , Bossone, E. , Neuhaus, M. , Franke, J. , Meder, B. , Jaguszewski, M. , Noutsias, M. , Knorr, M. , Heiner, S. , D'Ascenzo, F. , Dichtl, W. , Burgdorf, C. , Kherad, B. , Tschöpe, C. , Sarcon, A. , Shinbane, J. , Rajan, L. , Michels, G. , Pfister, R. , Cuneo, A. , Jacobshagen, C. , Karakas, M. , Koenig, W. , Pott, A. , Meyer, P. , Roffi, M. , Banning, A. , Wolfrum, M. , Cuculi, F. , Kobza, R. , Fischer, T. A. , Vasankari, T. , Airaksinen, K. E. J. , Budnik, M. , Dworakowski, R. , MacCarthy, P. , Kaiser, C. , Osswald, S. , Galiuto, L. , Chan, C. , Bridgman, P. , Beug, D. , Delmas, C. , Lairez, O. , Gilyarova, E. , Shilova, A. , Gilyarov, M. , El‐Battrawy, I. , Akin, I. , Kozel, M. , Tousek, P. , Winchester, D. E. , Galuszka, J. , Ukena, C. , Poglajen, G. , Carrilho‐Ferreira, P. , Hauck, C. , Paolini, C. , Bilato, C. , Sano, M. , Ishibashi, I. , Takahara, M. , Himi, T. , Kobayashi, Y. , Prasad, A. , Rihal, C. S. , Liu, K. , Schulze, P. C. , Bianco, M. , Jörg, L. , Rickli, H. , Pestana, G. , Nguyen, T. H. , Böhm, M. , Maier, L. S. , Pinto, F. J. , Widimský, P. , Felix, S. B. , Opolski, G. , Braun‐Dullaeus, R. C. , Rottbauer, W. , Hasenfuß, G. , Pieske, B. M. , Schunkert, H. , Borggrefe, M. , Thiele, H. , Bauersachs, J. , Katus, H. A. , Horowitz, J. D. , Di Mario, C. , Münzel, T. , Crea, F. , Bax, J. J. , Lüscher, T. F. , Ruschitzka, F. , Ghadri, J. R. , and Templin, C. (2021) Prognostic impact of acute pulmonary triggers in patients with takotsubo syndrome: new insights from the International Takotsubo Registry. ESC Heart Failure, 8: 1924–1932. 10.1002/ehf2.13165
Jelena R. Ghadri and Christian Templin contributed equally to this work.
References
- 1. Tsuchihashi K, Ueshima K, Uchida T, Oh‐mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai R, Morii I. Angina pectoris‐myocardial infarction investigations in Japan: transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol 2001; 38: 11–18. [DOI] [PubMed] [Google Scholar]
- 2. Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, Mebazaa A, Omerovic E. Current state of knowledge on takotsubo syndrome: a position statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2016; 18: 8–27. [DOI] [PubMed] [Google Scholar]
- 3. Akashi YJ, Musha H, Kida K, Itoh K, Inoue K, Kawasaki K, Hashimoto N, Miyake F. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur J Heart Fail 2005; 7: 1171–1176. [DOI] [PubMed] [Google Scholar]
- 4. Citro R, Radano I, Parodi G, Di Vece D, Zito C, Novo G, Provenza G, Bellino M, Prota C, Silverio A, Antonini‐Canterin F, Rigo F, Vriz O, Galasso G, Bossone E, Salerno‐Uriarte J, Piscione F. Long‐term outcome in patients with takotsubo syndrome presenting with severely reduced left ventricular ejection fraction. Eur J Heart Fail 2019; 21: 781–789. [DOI] [PubMed] [Google Scholar]
- 5. Kato K, Lyon AR, Ghadri JR, Templin C. Takotsubo syndrome: aetiology, presentation and treatment. Heart 2017; 103: 1461–1469. [DOI] [PubMed] [Google Scholar]
- 6. Wischnewsky MB, Candreva A, Bacchi B, Cammann VL, Kato K, Szawan KA, Gili S, D'Ascenzo F, Dichtl W, Citro R, Bossone E, Neuhaus M, Franke J, Sorici‐Barb I, Jaguszewski M, Noutsias M, Knorr M, Heiner S, Burgdorf C, Kherad B, Tschope C, Sarcon A, Shinbane J, Rajan L, Michels G, Pfister R, Cuneo A, Jacobshagen C, Karakas M, Koenig W, Pott A, Meyer P, Arroja JD, Banning A, Cuculi F, Kobza R, Fischer TA, Vasankari T, Airaksinen KEJ, Napp LC, Budnik M, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Chan C, Bridgman P, Beug D, Delmas C, Lairez O, El‐Battrawy I, Akin I, Gilyarova E, Shilova A, Gilyarov M, Kozel M, Tousek P, Winchester DE, Galuszka J, Ukena C, Poglajen G, Carrilho‐Ferreira P, Hauck C, Paolini C, Bilato C, Prasad A, Rihal CS, Liu K, Schulze PC, Bianco M, Jorg L, Rickli H, Nguyen TH, Kobayashi Y, Bohm M, Maier LS, Pinto FJ, Widimsky P, Borggrefe M, Felix SB, Opolski G, Braun‐Dullaeus RC, Rottbauer W, Hasenfuss G, Pieske BM, Schunkert H, Thiele H, Bauersachs J, Katus HA, Horowitz J, Di Mario C, Munzel T, Crea F, Bax JJ, Luscher TF, Ruschitzka F, Ghadri JR, Templin C. Prediction of short‐ and long‐term mortality in takotsubo syndrome: the InterTAK Prognostic Score. Eur J Heart Fail 2019; 21: 1469–1472. [DOI] [PubMed] [Google Scholar]
- 7. Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, Haas TS, Hodges JS, Maron BJ. Natural history and expansive clinical profile of stress (tako‐tsubo) cardiomyopathy. J Am Coll Cardiol 2010; 55: 333–341. [DOI] [PubMed] [Google Scholar]
- 8. Pelliccia F, Parodi G, Greco C, Antoniucci D, Brenner R, Bossone E, Cacciotti L, Capucci A, Citro R, Delmas C, Guerra F, Ionescu CN, Lairez O, Larrauri‐Reyes M, Lee PH, Mansencal N, Marazzi G, Mihos CG, Morel O, Nef HM, Nunez Gil IJ, Passaseo I, Pineda AM, Rosano G, Santana O, Schneck F, Song BG, Song JK, Teh AW, Ungprasert P, Valbusa A, Wahl A, Yoshida T, Gaudio C, Kaski JC. Comorbidities frequency in takotsubo syndrome: an international collaborative systematic review including 1109 patients. Am J Med 2015; 128: 654 e11–654 e19. [DOI] [PubMed] [Google Scholar]
- 9. Manfredini R, Fabbian F, Giorgi AD, Pala M, Menegatti AM, Parisi C, Misurati E, Tiseo R, Gallerani M, Salmi R, Bossone E. Heart and lung, a dangerous liaison‐Tako‐tsubo cardiomyopathy and respiratory diseases: a systematic review. World J Cardiol 2014; 6: 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghadri JR, Bataisou RD, Diekmann J, Luscher TF, Templin C. First case of atypical takotsubo cardiomyopathy in a bilateral lung‐transplanted patient due to acute respiratory failure. Eur Heart J Acute Cardiovasc Care 2015; 4: 482–485. [DOI] [PubMed] [Google Scholar]
- 11. Landefeld K, Saleh Q, Sander GE. Stress cardiomyopathy in the setting of COPD exacerbation. J Investig Med High Impact Case Rep 2015; 3: 2324709615612847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hawkins NM, Petrie MC, Macdonald MR, Jhund PS, Fabbri LM, Wikstrand J, McMurray JJ. Heart failure and chronic obstructive pulmonary disease the quandary of beta‐blockers and beta‐agonists. J Am Coll Cardiol 2011; 57: 2127–2138. [DOI] [PubMed] [Google Scholar]
- 13. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschope C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Bohm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun‐Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Clinical Features LTF. Outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015; 373: 929–938. [DOI] [PubMed] [Google Scholar]
- 14. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako‐Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008; 155: 408–417. [DOI] [PubMed] [Google Scholar]
- 15. Leitner LM. Dopamine metabolism in the rabbit carotid body in vitro: effect of hypoxia and hypercapnia. Adv Exp Med Biol 1993; 337: 183–190. [DOI] [PubMed] [Google Scholar]
- 16. Low JM, Gin T, Lee TW, Fung K. Effect of respiratory acidosis and alkalosis on plasma catecholamine concentrations in anaesthetized man. Clin Sci (Lond) 1993; 84: 69–72. [DOI] [PubMed] [Google Scholar]
- 17. Offner B, Czachurski J, Konig SA, Seller H. Different effects of respiratory and metabolic acidosis on preganglionic sympathetic nerve activity. J Appl Physiol (1985) 1994; 77: 173–178. [DOI] [PubMed] [Google Scholar]
- 18. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352: 539–548. [DOI] [PubMed] [Google Scholar]
- 19. Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000; 55: 629–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004; 351: 2611–2618. [DOI] [PubMed] [Google Scholar]
- 21. Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010; 137: 1091–1097. [DOI] [PubMed] [Google Scholar]
- 22. Surikow SY, Nguyen TH, Stafford I, Chapman M, Chacko S, Singh K, Licari G, Raman B, Kelly DJ, Zhang Y, Waddingham MT, Ngo DT, Bate AP, Chua SJ, Frenneaux MP, Horowitz JD. Nitrosative stress as a modulator of inflammatory change in a model of takotsubo syndrome. JACC Basic Transl Sci 2018; 3: 213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mishra V, Banga J, Silveyra P. Oxidative stress and cellular pathways of asthma and inflammation: therapeutic strategies and pharmacological targets. Pharmacol Ther 2018; 181: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marginean C, Popescu MS, Vladaia M, Tudorascu D, Pirvu DC, Petrescu F. Involvement of oxidative stress in COPD. Curr Health Sci J 2018; 44: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salemi VM, Atik E, Kairalla RA, Queiroz EL, Rosa LV, Kalil Filho R. Takotsubo cardiomyopathy triggered by β(2) adrenergic agonist. J Bras Pneumol 2011; 37: 560–562. [DOI] [PubMed] [Google Scholar]
- 26. Tornvall P, Collste O, Ehrenborg E, Jarnbert‐Petterson H. A case‐control study of risk markers and mortality in takotsubo stress cardiomyopathy. J Am Coll Cardiol 2016; 67: 1931–1936. [DOI] [PubMed] [Google Scholar]
- 27. Chen S, Li Y, Zheng Z, Luo Q, Chen R. The analysis of components that lead to increased work of breathing in chronic obstructive pulmonary disease patients. J Thorac Dis 2016; 8: 2212–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bellani G, Pesenti A. Assessing effort and work of breathing. Curr Opin Crit Care 2014; 20: 352–358. [DOI] [PubMed] [Google Scholar]
- 29. Kallet RH, Hemphill JC 3rd, Dicker RA, Alonso JA, Campbell AR, Mackersie RC, Katz JA. The spontaneous breathing pattern and work of breathing of patients with acute respiratory distress syndrome and acute lung injury. Respir Care 2007; 52: 989–995. [PubMed] [Google Scholar]
- 30. Gift AG, Plaut SM, Jacox A. Psychologic and physiologic factors related to dyspnea in subjects with chronic obstructive pulmonary disease. Heart Lung 1986; 15: 595–601. [PubMed] [Google Scholar]
- 31. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Task Force M . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 32. Hoiseth AD, Neukamm A, Karlsson BD, Omland T, Brekke PH, Soyseth V. Elevated high‐sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax 2011; 66: 775–781. [DOI] [PubMed] [Google Scholar]
- 33. Gariani K, Delabays A, Perneger TV, Agoritsas T. Use of brain natriuretic peptide to detect previously unknown left ventricular dysfunction in patients with acute exacerbation of chronic obstructive pulmonary disease. Swiss Med Wkly 2011; 141: w13298. [DOI] [PubMed] [Google Scholar]
- 34. Hawkins NM, Khosla A, Virani SA, McMurray JJ, FitzGerald JM. B‐type natriuretic peptides in chronic obstructive pulmonary disease: a systematic review. BMC Pulm Med 2017; 17: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]


