Abstract
Aims
Differences between female and male patients in clinical presentation, causes and treatment of cardiogenic shock (CS) are poorly understood. We aimed to investigate sex differences in presentation with and treatment of CS.
Methods and results
We analysed data of 978 patients presenting with CS to a tertiary care hospital between October 2009 and October 2017. Multivariable adjusted logistic/Cox regression models were fitted to investigate the association between sex and clinical presentation, use of treatments and 30 day mortality.
Median age was 70 years (interquartile range 58–79 years), and 295 (30.2%) patients were female. After adjustment for multiple relevant confounders, female patients were more likely to be older [odds ratio (OR) 1.21, 95% confidence interval (CI) 1.02–1.42, P = 0.027], but other relevant presentation characteristics did not differ between both sexes. Despite the similar presentation, female patients were less likely to be treated with percutaneous left ventricular assist devices (OR 0.78, 95% CI 0.64–0.94, P = 0.010), but more likely to be treated with catecholamines (OR 1.21, 95% CI 1.02–1.44, P = 0.033) or vasopressors (OR 1.26, 95% CI 1.05–1.50, P = 0.012). A 30 day mortality risk in female patients was as high as in male patients (hazard ratio 1.08, 95% CI 1.00–1.18, P = 0.091).
Conclusions
In this large, contemporary cohort, clinical presentation was comparable in female and male patients, and both sexes were associated with a comparably high mortality risk. Nevertheless, female patients received different treatment for CS and were most importantly less likely to be treated with percutaneous left ventricular assist devices.
Keywords: Cardiogenic shock, Sex‐related differences, Heart failure
Introduction
Cardiogenic shock (CS) is the most severe form of acute heart failure. The main pathophysiology of CS is low cardiac output, which leads to tissue hypoperfusion, multi‐organ failure and ultimately death. Although several attempts have been made in the last years to develop new treatments for CS, the mortality rate in this patient population is still high, 50–60%. 1 Inotropes, once considered to be the cornerstone of CS treatment, increase myocardial oxygen demand and are therefore associated with poor outcomes in CS. 2 As a consequence, active mechanical circulatory support with percutaneous left ventricular assist devices (pLVADs) and veno‐arterial extracorporeal membrane oxygenation (va‐ECMO) have been increasingly used to treat CS in the past years. However, observational data indicate that these devices might not improve outcomes and could even increase risk of severe complications. 3 , 4 , 5 , 6
The high mortality risk of CS requires research efforts to understand the disease and its risk factors, with the ultimate aim to improve prognosis. Based on the overall observation of sex differences in clinical presentation, use of treatments, and outcomes in other cardiovascular diseases such as coronary artery disease, myocardial infarction, and heart failure, 7 , 8 we hypothesized that there are relevant sex differences in CS, too. Improving the understanding of sex differences might facilitate the development of successful treatment strategies and conduction of clinical trials in this field.
Hence, the primary aim of this study was to investigate sex differences in clinical presentation, use of treatments, and outcomes between female and male patients in CS.
Material and methods
Study population
The present study considered patients with CS treated at the University Heart and Vascular Center Hamburg/University Clinic Hamburg‐Eppendorf between October 2009 and October 2017. To identify eligible patients, the claims database was scanned for the International Classification of Diseases‐10 code of CS (R.57). Hereafter, every identified case was checked based on electronic case records to validate the diagnosis of CS. Baseline data including known comorbidities upon presentation, laboratory analyses directly after presentation, use of treatments during hospital stay and in‐hospital follow‐up, and all‐cause mortality were collected in a dedicated database.
In regard to va‐ECMO use, standard cannulation included an arterial cannula size of 15–17 French and a venous cannula size of 21–23 French, although exact cannula size per patient was not recorded in this database. In regard to pLVAD use, the Impella CP® (Abiomed, Danvers, MA, USA) was predominantly used per standard operating procedure, with few exceptions of Impella 2.5® use (exact device type not recorded in this database).
This study was performed in accordance with the Declaration of Helsinki and was approved by the local ethics committee. As this was a retrospective analysis of anonymized data, the need for an informed consent was waived by the local ethics committee.
Statistical analysis
Continuous variables are shown as median (25th percentile, 75th percentile); binary variables are shown as absolute and relative frequencies. Mann–Whitney test was used to compare continuous variables and χ 2 test was used to compare binary variables.
To enable multivariable analyses including all observations, missing data were handled by chained‐equation multiple imputation (50 imputed data sets; R package mice). Except acute heart failure, other cause of CS, C‐reactive protein, diastolic blood pressure, creatinine kinase, high‐sensitive troponin T, all the variables shown in Table 1 , as well as use of catecholamines, vasopressors, va‐ECMO, and pLVAD were used for the multiple imputation.
Table 1.
Baseline characteristics of all patients and divided by sex
| All (N = 978) | Missing (%) | Female patients (N = 295) | Male patients (N = 683) | P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 70.0 (58.0, 79.0) | 0 | 73.0 (61.0, 80.0) | 69.0 (57.0, 78.0) | <0.001 |
| Cardiovascular risk factors | |||||
| BMI (kg/m2) | 24.7 (24.2, 28.3) | 23.0 | 24.6 (23.0, 28.8) | 24.7 (24.2, 28.2) | 0.003 |
| BSA (m2) | 1.96 (0.23) | 23.0 | 1.80 (0.22) | 2.03 (0.20) | <0.001 |
| Smoking No. (%) | 295 (30.3) | <0.01 | 59 (20.1) | 236 (34.8) | <0.001 |
| Arterial hypertension No. (%) | 484 (49.8) | <0.01 | 146 (49.8) | 338 (49.8) | 1.000 |
| Hypercholesterinaemia No. (%) | 98 (10.1) | <0.01 | 25 (8.5) | 73 (10.8) | 0.350 |
| Diabetes mellitus No. (%) | 260 (26.7) | <0.01 | 78 (26.6) | 182 (26.8) | 1.000 |
| Chronic kidney disease No. (%) | 174 (17.9) | <0.01 | 53 (18.1) | 121 (17.8) | 0.990 |
| Prior myocardial infarction No. (%) | 237 (24.4) | <0.01 | 54 (18.4) | 183 (27.0) | 0.006 |
| Prior stroke No. (%) | 95 (9.8) | <0.01 | 36 (12.3) | 59 (8.7) | 0.110 |
| Presentation | |||||
| Cause of cardiogenic shock | 0.0 | ||||
| Acute myocardial infarction No. (%) | 473 (48.4) | 123 (41.7) | 350 (51.2) | 0.008 | |
| Acute heart failure No. (%) | 269 (27.5) | 95 (32.2) | 174 (25.5) | 0.037 | |
| Other No. (%) | 242 (24.7) | 82 (27.8) | 160 (23.4) | 0.170 | |
| CPR No. (%) | 567 (58.0) | <0.01 | 157 (53.2) | 410 (60.1) | 0.053 |
| Time until ROSC (min) | 20.0 (10.0, 45.0) | 18.3 | 20.0 (8.0, 36.2) | 21.5 (10.0, 45.0) | 0.110 |
| Refractory cardiac arrest No. (%) | 116 (28) | 15.8 | 32 (27.1) | 84 (28.4) | 0.890 |
| Mechanical ventilation No. (%) | 664 (68.2) | <0.01 | 193 (65.4) | 471 (69.5) | 0.240 |
| Ejection fraction | 20.1 | ||||
| Preserved No. (%) | 134 (17.2) | 53 (22.7) | 81 (14.8) | 0.009 | |
| Mid‐ranged No. (%) | 127 (16.3) | 34 (14.6) | 93 (17.0) | 0.470 | |
| Reduced No. (%) | 520 (66.5) | 146 (62.7) | 374 (68.2) | 0.150 | |
| SCAI CS classification | 2.0 | ||||
| SCAI class B | 31 (3.2) | 22 (3.3) | 9 (3.1) | 0.999 | |
| SCAI class C | 415 (43.3) | 286 (42.7) | 129 (44.8) | 0.590 | |
| SCAI class D | 327 (34.1) | 218 (32.5) | 109 (37.8) | 0.130 | |
| SCAI class E | 185 (19.3) | 144 (21.5) | 41 (14.2) | 0.012 | |
| Haemodynamics | |||||
| Systolic blood pressure (mmHg) | 104.0 (85.0, 126.0) | 8.0 | 100.0 (83.0, 126.1) | 105.0 (85.0, 126.0) | 0.650 |
| Diastolic blood pressure (mmHg) | 60.0 (47.7, 78.0) | 8.3 | 60.0 (47.9, 77.1) | 61.0 (47.2, 78.0) | 0.510 |
| Shock index ≥1 No. (%) | 307 (35.1) | 10.5 | 105 (39.2) | 202 (33.3) | 0.110 |
| Heart rate (bpm) | 90.0 (71.0, 110.0) | 7.0 | 90.0 (73.0, 113.0) | 89.0 (70.0, 109.0) | 0.130 |
| Laboratory | |||||
| Lactate (mmol/L) | 4.0 (2.0, 8.0) | 10.0 | 4.0 (2.0, 8.3) | 4.0 (2.0, 8.0) | 0.620 |
| pH | 7.3 (7.2, 7.4) | 6.5 | 7.3 (7.2, 7.4) | 7.3 (7.1, 7.4) | 0.710 |
| calculated GFR (ml/min) | 42.8 (28.5, 58.9) | 2.9 | 38.4 (25.5, 57.8) | 43.9 (28.9, 59.7) | 0.063 |
| ASAT (U/I) | 122.0 (47.0, 381.3) | 14.4 | 124.0 (49.8, 328.8) | 120.0 (46.0, 411.3) | 0.720 |
| CK total (U/I) | 207.5 (101.9, 681.2) | 7.0 | 198.0 (89.0, 721.3) | 214.0 (109.0, 672.7) | 0.180 |
| Troponin T (pg/ml) | 221.5 (54.9, 1410.0) | 16.4 | 246.0 (58.0, 1379.3) | 211.0 (53.0, 1450.3) | 0.750 |
| CRP (mg/l) | 16.0 (5.0, 72.0) | 7.0 | 21.0 (6.0, 77.7) | 15.0 (5.0, 69.7) | 0.057 |
ASAT, aspartate aminotransferase; BMI, body mass index; BSA, body surface area; CK, creatine kinase; CPR, cardiopulmonary resuscitation; CRP, C‐reactive protein; ROSC, return of spontaneous circulation; SCAI CS classification, Society for Cardiovascular Angiography and Intervention cardiogenic shock classification; calculated GFR, calculated glomerular filtration rate.
Continuous FV (25th, 75th percentile), binary variables as absolute and relative frequencies. The P value given is for the Mann–Whitney test or the χ 2 test.
To analyse differences in presentation characteristics between both sexes, a multivariable‐adjusted logistic regression model with sex as the dependent variable and selected presentation characteristics as independent variables was fitted (variables are shown in Figure 1 ).
Figure 1.
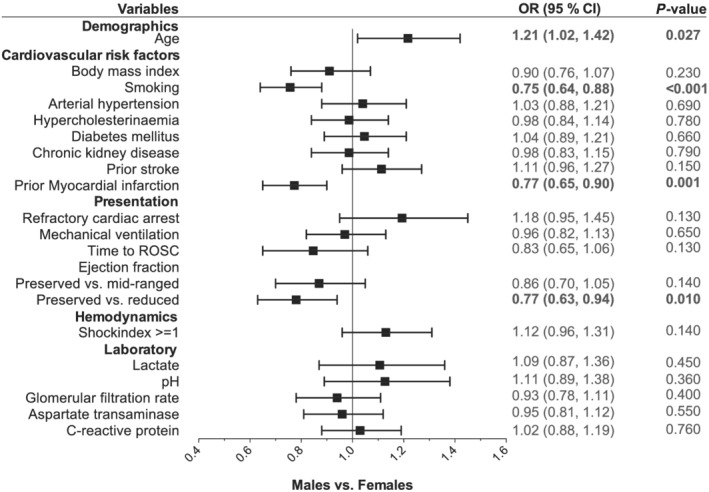
Presentation characteristics of patients divided by sex. Multivariable adjusted logistic regression model for sex (female vs. male) as the dependent variable and all shown baseline characteristics as independent variables. Odds ratios (ORs) are shown on the x‐axis. CI, confidence interval; ROSC, return of spontaneous circulation.
To analyse the association between sex and use of selected treatments (catecholamines, vasopressors, va‐ECMO, or pLVAD), a logistic regression model with the respective treatment as the dependent variable and sex as the independent variable was fitted, adjusted for age, prior cardiac arrest and time to return of spontaneous circulation, shock index (heart rate divided by systolic blood pressure) ≥1, baseline lactate, and baseline pH. These variables were selected based on clinical and scientific knowledge. 9 , 10 , 11 , 12
To analyse the association between sex and outcome, a Cox regression model with 30 day in‐hospital mortality as the dependent variable and sex as the independent variable was fitted, adjusted for the same variables as mentioned earlier. Median follow‐up time was estimated by the reverse Kaplan–Meier estimator. Survival curves were produced using the Kaplan–Meier method. Additionally, to investigate the interaction between sex and the Society for Cardiovascular Angiography and Intervention CS classification (SCAI CS classification) as well as with the cause of CS, an interaction term was added to the Cox regression model described earlier. Variables used for the adjustment of the Cox and the logistic regression models described earlier were selected based on clinical and scientific knowledge.
All analyses were performed with R statistical software version 3.6.0; a P value below 0.05 was considered statistically significant. Change in continuous variables was modelled as change per standard deviation for all regression models.
Results
Overall study cohort
Baseline characteristics for the overall study cohort and divided by sex are shown in Table 1 . In this cohort, 978 patients with CS were included. Less than one‐third of the cohort were female (N = 293, 30.2%). In the unadjusted analysis, several baseline characteristics were distributed unequally between female patients and male patients; e.g. female patients were older (73.0 years vs. 69.0 years, P < 0.001), were less frequent smokers (20.1% vs. 34.8%, P < 0.001), presented less frequently with acute myocardial infarction (18.4% vs. 27%, P = 0.006), but more frequently with acute heart failure (32.2% vs. 25.5%, P = 0.037). Additionally, female patients presented more frequently in SCAI Class E, without significant differences in regard to the other classes of the SCAI CS classification.
Presentation characteristics
As baseline characteristics are unadjusted, we used a multivariable adjusted logistic regression model to identify differences in clinical presentation between female and male patients (Figure 1 ). In the adjusted model, female patients presenting with CS were more likely to be older than males [odds ratio 1.21, 95% confidence interval (CI) 1.02, 1.42; P = 0.027], were less likely to be smokers (odds ratio 0.75, 95% CI 0.64, 0.88; P < 0.001), less likely to have had a prior myocardial infarction (odds ratio 0.77, 95% CI 0.65, 0.90; P = 0.001), and less likely to present with a reduced ejection fraction (odds ratio 0.77, 95% CI 0.63, 0.94; P = 0.01). However, other cardiovascular risk factors and factors related to CS severity (e.g. lactate, pH, cardiac arrest, and shock index) were not significantly different between both sexes.
Use of treatments during cardiogenic shock
Patients with CS can receive both pharmacological therapy and mechanical circulatory support. In total, more pharmacological therapy in the form of catecholamines and vasopressors was applied in both sexes compared with device therapy. In our analysis, 764 (78.3%) patients were treated with catecholamines, 701 patients (74.3%) with vasopressors. Of the patients, 256 (26.2%) received va‐ECMO, and 162 patients (16.6%) were treated with pLVAD. In both sexes, the use of va‐ECMO in patients with CS increased from 2010 on and peaked in 2016, whereas the use of pLVAD started to increase later, in 2012, but also peaked in 2016 (Figure 3 ). In the multivariable adjusted logistic regression analysis, female patients were more likely to be treated with catecholamines (odds ratio 1.21, 95% CI 1.02, 1.44; P = 0.033) and vasopressors (odds ratio 1.26, 95% CI 1.05, 1.50; P = 0.012), but less likely to be treated with pLVAD (odds ratio 0.78, 95% CI 0.64, 0.94; P = 0.01) (Figure 2). The likelihood of treatment with va‐ECMO was not significantly different between both sexes.
Figure 3.
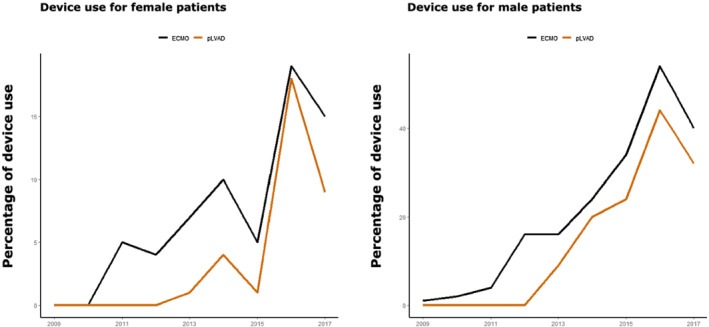
Device use in female and male patients over time. pLVAD: percutaneous left ventricular assist device; va‐ECMO: veno‐arterial extracorporeal membrane oxygenation.
Figure 2.
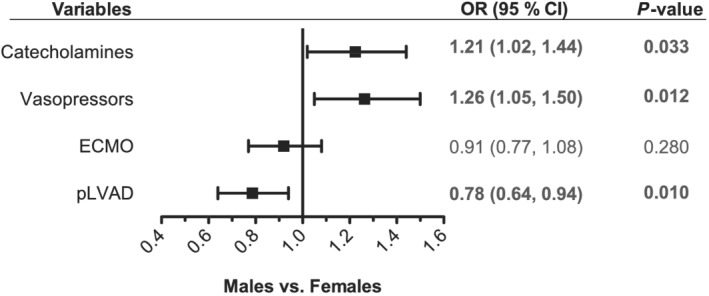
Use of therapies during cardiogenic shock in female and male patients. Logistic regression model for sex (female vs. male patients) as the dependent variable and all shown therapies as independent variables. Additionally, all models were adjusted for age, sex, prior cardiac arrest, time to return of spontaneous circulation, shock index ≥1, baseline lactate, and baseline pH. Odds ratios (ORs) are shown on the x‐axis. CI, confidence interval; ECMO, veno‐arterial extracorporeal membrane oxygenation; pLVAD, percutaneous left ventricular assist device.
Outcome
Cumulative incidence curves for mortality are shown in Figure 4 . During a median follow‐up time of 26 days (95% CI 24; 28 days), 547 deaths (55.9%) occurred in the overall cohort, which corresponds to a survival probability of 38% (95% CI 35–42%) after 30 days. Survival probability in female patients only was 36% (95% CI 30–43%) and the survival probability in male patients only was 39% (95% CI 35–43%; P = 0.091).
Figure 4.
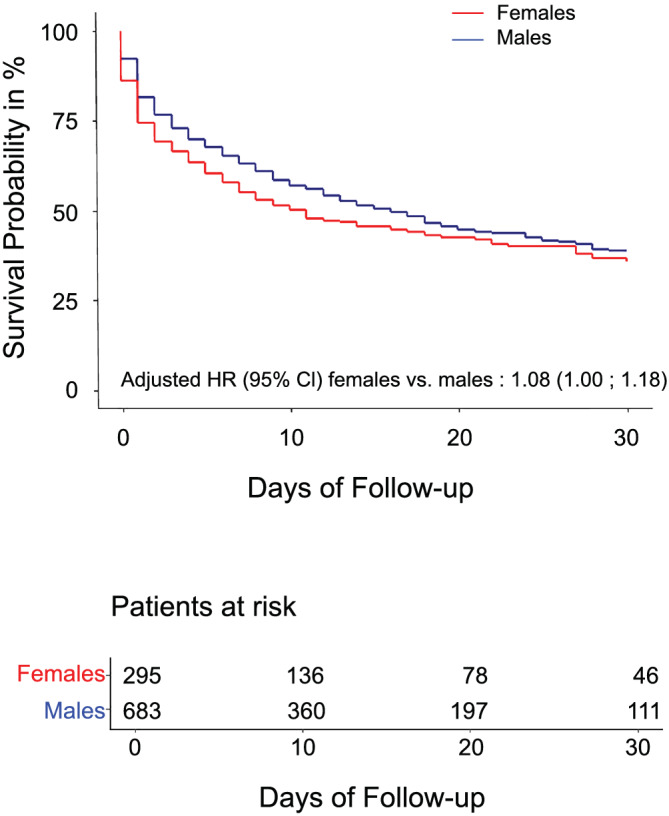
Survival curves divided by sex. Survival curves for 30 day mortality in female and male patients presenting with cardiogenic shock. The numbers of patients at risk are provided under the figure. HR, hazard ratio.
In the multivariable adjusted Cox regression model, female sex was associated with a similar risk of 30 day all‐cause mortality as male sex, although there was a trend towards a higher mortality risk in female patients [hazard ratio (HR) 1.08, 95% CI 1.00–1.18; P = 0.065].
Mortality risk was comparable between female and male patients across the strata of the SCAI CS classification: SCAI Class C vs. B HR 1.03 (95% CI 0.54–1.96) in female patients vs. HR 1.99 (95% CI 0.62–6.34) in male patients (interaction P = 0.33); SCAI Class D vs. B HR 1.10 (95% CI 0.58–2.11) in female patients vs. HR 3.24 (95% CI 1.02–10.31) in male patients (interaction P = 0.11); and SCAI Class E vs. B HR 2.06 (95% CI 1.07–3.94) in female patients vs. HR 4.95 (95% CI 1.51–16.22) in male patients (interaction P = 0.20).
Similarly, the mortality risk was not different between female and male patients in regard to the underlying cause of CS: ischaemic CS (with acute myocardial infarction) vs. non‐ischaemic CS (without acute myocardial infarction) HR 0.82 (95% CI 0.66–1.01) in female patients vs. HR 0.65 (95% CI 0.47–0.90) in male patients (interaction P = 0.24).
Discussion
The main findings of this study are as follows:
Female patients presenting with CS were older than male patients, but there were no relevant differences in factors relating to shock severity;
Mortality risk in CS was comparably high in female and male patients, even after adjustment for relevant confounders;
Treatment of CS differed significantly between both sexes, as female patients were less likely to be treated with pLVAD and more likely to be treated with catecholamines or vasopressors.
These findings are of major importance, as they reveal marked sex differences in the use of treatment for CS, despite a comparably high‐risk profile.
Sex‐differences in clinical presentation and outcome
A substudy of the IABP‐SHOCK II‐trial in patients with CS complicating myocardial infarction showed that female patients were older than male patients and revealed a worse cardiovascular risk profile compared with male patients. Significant sex‐related differences in therapy and short‐term outcome could not be observed. 13 In our study, female patients were also older than male patients; they were less likely to be active smokers, but other cardiovascular risk factors did not differ significantly. We also observed a similar outcome with a high risk of 30 day mortality in both sexes, which was consistent across all classes of the SCAI CS classification. In another study of CS caused by acute myocardial infarction, Abdel‐Qadir et al. also showed that female patients presenting with CS tend to have a higher age than male patients and also reported comparable mortality rates for both sexes. 8
In a substudy of the CULPRIT‐SHOCK trial (The Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock), sex did not influence mortality and renal failure, although female patients presented with a different risk factor profile. 14 Importantly, these three studies focused exclusively on patients with CS caused by acute myocardial infarction. However, acute myocardial infarction only accounts for about 50% of CS cases, and the other 50% is caused by non‐ischaemic causes such as acute decompensated heart failure. 15 This is of major importance, as non‐ischaemic causes of CS call for a different treatment approach and are associated with a worse prognosis than CS caused by acute myocardial infarction. 15 Our study enrolled an unselected cohort of CS patients, including cases caused by acute myocardial infarction as well as non‐ischaemic CS and thus allows us to generalize the observation on sex differences in CS to the overall CS population.
Use of treatments
It is known that there are differences between female and male patients in therapy of cardiovascular diseases. Female patients are less likely to be treated with device therapy or interventional therapy, for example, cardiac resynchronization therapy, 16 although female patients were demonstrated to derive greater therapeutic benefit from cardiac resynchronization therapy compared with male patients. 17 Similarly, female patients are less likely to be treated with implantable cardioverter defibrillators. 18
Our study identified a similar pattern of underuse of device therapies in female patients presenting with CS. Although female patients had a similar risk profile as male patients presenting with CS, and although mortality risk was comparable between both sexes, female patients received a different therapy for CS. They were more likely to be treated with catecholamines or vasopressors, but were less likely to undergo pLVAD support. Pharmacological therapies are important in treatment of CS. Catecholamines and vasopressors such as dobutamine and norepinephrine show a positive effect on cardiac inotropy and can help to maintain sufficient blood pressure. However, they also increase myocardial oxygen demand, increase cardiac afterload, but seem not to improve outcomes in patients with CS. 19 , 20 Recently, active mechanical circulatory support with pLVAD and va‐ECMO has been proposed as an alternative to catecholamines/vasopressors for the treatment of CS. This has led to an increasing use of these devices over the past years. 2 , 3 , 21 Initial observational studies did not show a survival benefit associated with the use of pLVAD/va‐ECMO. 4 , 5 , 6 , 22 However, these devices are still considered as potentially beneficial for the treatment of CS from a pathophysiological point of view. Hence, several randomized controlled trials are currently ongoing, aiming to clarify the efficacy and safety of these devices. 23 , 24 These trials might also help to identify relevant selection criteria for these devices, for example, help physicians to identify patients most likely to benefit from device‐based treatment.
A potential reason for the underuse of pLVAD in female patients might be their smaller body and thus vessel size, as indicated by a significant difference in body surface area in this analysis. As pLVAD require a large bore vessel access, physicians might be reluctant to implant such devices in female patients with smaller arteries; being worried about a higher complication risk. Additionally, the higher likelihood of heart failure with preserved ejection fraction with presumably smaller left ventricular cavities in female patients might be another explanation for the less frequent use of pLVAD; as physicians might be less inclined to use these devices for concerns regarding their efficacy in this setting (e.g. suction phenomena due to smaller left ventricular cavities). Importantly, this does not indicate that female patients received less ideal treatment than male patients, especially as the mortality risk was comparable between both sexes. However, this highlights a potential sex‐specific selection bias in the use of pLVADs for the treatment of CS and indicates that these devices might not be optimized for the use in female patients. Currently, the available data on mechanical circulatory support in CS do not indicate any sex‐specific difference in a potential benefit from pLVAD. However, the one‐size‐fits‐all design of current device platforms might prevent detection of such differences. As an example, optimized device platforms with cannulas specifically designed for female patients might reduce device‐associated complications. As a consequence, this could unmask a potential benefit from mechanical circulatory support in females. Ultimately, concerns regarding anatomical disadvantages of female patients might be met by adapting the design of these devices, for example, by reducing the size of the required bore vessel access or by adjusting the inflow cannula design, which could then improve its overall use.
Limitations
Strengths of this study include the large, well‐characterized and contemporary CS cohort with data on clinical presentation, use of treatment, and outcomes. The cause of CS was not limited to ischaemic aetiology, as patients with non‐ischaemic aetiology were included, too.
Its main limitation is its retrospective, observational design, which does not allow us to rule out residual or unmeasured confounding. The use of International Classification of Diseases‐10 codes for patients with CS might have changed over time and could have impacted the results. The study is based on a cohort from a single hospital, which might limit generalizability to other hospitals or health care systems. Additionally, haemodynamic data (e.g. right heart catheterization) were not available for this study and could thus not be analysed; and its absence might have also influenced the treating physicians choice of therapies.
Conclusions
In this large, contemporary cohort of patients presenting with CS, there were no relevant differences in clinical presentation and shock severity between female and male patients, and mortality risk was comparable for both sexes. Despite these similarities, female patients received different treatment for CS, for example, they were more likely to be treated with catecholamines or vasopressors, but less likely to be treated with pLVAD. Smaller vessel size and a higher prevalence of preserved ejection fraction might have contributed to this sex‐specific treatment selection; and concerns regarding anatomical disadvantages of female patients might be met by adapting the design of these devices.
Conflict of interest
Dr Beer reports honoraria from Siemens Healthineers, outside the submitted work.
Dr Seiffert reports non‐financial support from Abbott Vascular, personal fees from Abiomed, personal fees from AstraZeneca, personal fees from Bayer Healthcare, non‐financial support from Biotronik, personal fees from Boehringer Ingelheim, personal fees and non‐financial support from Boston Scientific, personal fees from Bristol‐Myers Squibb, non‐financial support from Edwards Lifesciences, non‐financial support from Nicolai Medizintechnik, non‐financial support from OrbusNeich Medical, grants and personal fees from Philips, personal fees from Medtronic, and personal fees from Amgen, outside the submitted work.
Prof Schnabel reports personal fees from BMS/Pfizer outside the submitted work.
Prof Blankenberg reports grants and personal fees from Abbott Diagnostics, grants and personal fees from Bayer, grants and personal fees from Siemens, grants from Singulex, grants and personal fees from Thermo Fisher, personal fees from Abbott, personal fees from AstraZeneca, personal fees from Amgen, personal fees from Medtronic, personal fees from Pfizer, personal fees from Roche, personal fees from Siemens Diagnostics, and personal fees from Novartis, outside the submitted work.
Prof Kirchhof receives research support for basic, translational, and clinical research projects from the European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last 3 years. He is also listed as an inventor on two patents held by the University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783).
Dr Schrage reports personal fees from AstraZeneca, outside the submitted work.
Prof Westermann reports personal fees from AstraZeneca, personal fees from Bayer, personal fees from Novartis, personal fees from Berlin‐Chemie, and personal fees from Medtronic, outside the submitted work.
The other authors have nothing to disclose.
Funding
This study was funded by the University Heart and Vascular Center Hamburg.
Yan, I. , Schrage, B. , Weimann, J. , Dabboura, S. , Hilal, R. , Beer, B. N. , Becher, P. M. , Seiffert, M. , Magnussen, C. , B. Schnabel, R. , Kirchhof, P. , Blankenberg, S. , and Westermann, D. (2021) Sex differences in patients with cardiogenic shock. ESC Heart Failure, 8: 1775–1783. 10.1002/ehf2.13303
Isabell Yan and Benedikt Schrage contributed equally to this study.
References
- 1. Buerke M, Lemm H, Dietz S, Werdan K. Pathophysiology, diagnosis, and treatment of infarction‐related cardiogenic shock. Herz 2011; 36: 73–83. [DOI] [PubMed] [Google Scholar]
- 2. Schrage B, Westermann D. Mechanical circulatory support devices in cardiogenic shock and acute heart failure: current evidence. Curr Opin Crit Care 2019; 25: 391–396. [DOI] [PubMed] [Google Scholar]
- 3. Becher PM, Schrage B, Sinning CR, Schmack B, Fluschnik N, Schwarzl M, Waldeyer C, Lindner D, Seiffert M, Neumann JT, Bernhardt AM, Zeymer U, Thiele H, Reichenspurner H, Blankenberg S, Twerenbold R, Westermann D. Venoarterial extracorporeal membrane oxygenation for cardiopulmonary support. Circulation 2018; 138: 2298–2300. [DOI] [PubMed] [Google Scholar]
- 4. Schrage B, Ibrahim K, Loehn T, Werner N, Sinning JM, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, Westenfeld R, Horn P, Pauschinger M, Eckner D, Twerenbold R, Nordbeck P, Salinger T, Abel P, Empen K, Busch MC, Felix SB, Sieweke JT, Møller JE, Pareek N, Hill J, MacCarthy P, Bergmann MW, Henriques JPS, Möbius‐Winkler S, Schulze PC, Ouarrak T, Zeymer U, Schneider S, Blankenberg S, Thiele H, Schäfer A, Westermann D. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation 2019; 139: 1249–1258. [DOI] [PubMed] [Google Scholar]
- 5. Dhruva SS, Ross JS, Mortazavi BJ, Hurley NC, Krumholz HM, Curtis JP, Berkowitz A, Masoudi FA, Messenger JC, Parzynski CS, Ngufor C, Girotra S, Amin AP, Shah ND, Desai NR. Association of use of an intravascular microaxial left ventricular assist device vs intra‐aortic balloon pump with in‐hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA ‐ J Amer Chem Soc 2020; 323: 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amin AP, Spertus JA, Curtis JP, Desai N, Masoudi FA, Bach RG, McNeely C, Al‐Badarin F, House JA, Kulkarni H, Rao SV. The evolving landscape of impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation 2020; 141: 273–284. [DOI] [PubMed] [Google Scholar]
- 7. Cenko E, van der Schaar M, Yoon J, Manfrini O, Vasiljevic Z, Vavlukis M, Kedev S, Miličić D, Badimon L, Bugiardini R. Sex‐related differences in heart failure after ST‐segment elevation myocardial infarction. J Am Coll Cardiol 2019; 74: 2379–2389. [DOI] [PubMed] [Google Scholar]
- 8. Abdel‐Qadir HM, Ivanov J, Austin PC, Tu JV, Džavík V. Sex differences in the management and outcomes of ontario patients with cardiogenic shock complicating acute myocardial infarction. Can J Cardiol 2013; 29: 691–696. [DOI] [PubMed] [Google Scholar]
- 9. Hongisto M, Lassus J, Tarvasmäki T, Sionis A, Roselló JS, Tolppanen H, Kataja A, Jäntti T, Sabell T, Lindholm MG, Banaszewski M. Mortality risk prediction in elderly patients with cardiogenic shock: results from the CardShock study. ESC Heart Fail 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, Naidu SS, Baran DA. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol 2019; 74: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 11. El‐Menyar A, Al Habib KF, Zubaid M, Alsheikh‐Ali AA, Sulaiman K, Almahmeed W, Amin H, AlMotarreb A, Ullah A, Suwaidi JA. Utility of shock index in 24,636 patients presenting with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2020; 9: 546–556. [DOI] [PubMed] [Google Scholar]
- 12. Fuernau G, Desch S, de Waha‐Thiele S, Eitel I, Neumann FJ, Hennersdorf M, Felix SB, Fach A, Böhm M, Pöss J, Jung C, Ouarrak T, Schneider S, Werdan K, Zeymer U, Thiele H. Arterial lactate in cardiogenic shock: prognostic value of clearance versus single values. JACC Cardiovasc Interv 2020; 13: 2208–2216. [DOI] [PubMed] [Google Scholar]
- 13. Fengler K, Fuernau G, Desch S, Eitel I, Neumann FJ, Olbrich HG, de Waha A, de Waha S, Richardt G, Hennersdorf M, Empen K. Gender differences in patients with cardiogenic shock complicating myocardial infarction: a substudy of the IABP‐SHOCK II‐trial. Clin Res Cardiol 2014; 104: 71–78. [DOI] [PubMed] [Google Scholar]
- 14. Rubini Gimenez M, Zeymer U, Desch S, de Waha‐Thiele S, Ouarrak T, Poess J, Meyer‐Saraei R, Schneider S, Fuernau G, Stepinska J, Huber K. Sex‐specific management in patients with acute myocardial infarction and cardiogenic shock: a substudy of the CULPRIT‐SHOCK trial. Circ Cardiovasc Interv 2020; 13: e008537. [DOI] [PubMed] [Google Scholar]
- 15. Schrage B, Weimann J, Dabboura S, Yan I, Hilal R, Becher PM, Seiffert M, Bernhardt AM, Kluge S, Reichenspurner H, Blankenberg S. Patient characteristics, treatment and outcome in non‐ischemic vs ischemic cardiogenic shock. J Clin Med 2020; 9: 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nevzorov R, Porter A, Mostov S, Kazum S, Eisen A, Goldenberg G, Iakobishvili Z, Kusniec J, Golovchiner G, Strasberg B, Haim M. Gender‐related differences in outcomes of patients with cardiac resynchronization therapy. Isr Med Assoc J 2018; 20: 311–315. [PubMed] [Google Scholar]
- 17. Nishimura M, Birgersdotter‐Green U. Gender‐based differences in cardiac resynchronization therapy response. Card Electrophysiol Clin Elsevier Inc 2019; 11: 115–122. [DOI] [PubMed] [Google Scholar]
- 18. Schrage B, Uijl A, Benson L, Westermann D, Ståhlberg M, Stolfo D, Dahlström U, Linde C, Braunschweig F, Savarese G. Association between use of primary‐prevention implantable cardioverter‐defibrillators and mortality in patients with heart failure: a prospective propensity score‐matched analysis from the Swedish Heart Failure Registry. Circulation 2019; 140: 1530–1539. [DOI] [PubMed] [Google Scholar]
- 19. Léopold V, Gayat E, Pirracchio R, Spinar J, Parenica J, Tarvasmäki T, Lassus J, Harjola VP, Champion S, Zannad F, Valente S. Epinephrine and short‐term survival in cardiogenic shock: an individual data meta‐analysis of 2583 patients. Intensive Care Med Springer Berlin Heidelberg 2018; 44: 847–856. [DOI] [PubMed] [Google Scholar]
- 20. Tarvasmäki T, Lassus J, Varpula M, Sionis A, Sund R, Køber L, Spinar J, Parissis J, Banaszewski M, Cardoso JS, Carubelli V. Current real‐life use of vasopressors and inotropes in cardiogenic shock—adrenaline use is associated with excess organ injury and mortality. Crit Care Critical Care 2016; 20: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shah M, Patnaik S, Patel B, Ram P, Garg L, Agarwal M, Agrawal S, Arora S, Patel N, Wald J, Jorde UP. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non‐infarction related cardiogenic shock in the United States. Clin Res Cardiol Springer Berlin Heidelberg 2018; 107: 287–303. [DOI] [PubMed] [Google Scholar]
- 22. Brunner S, Guenther SPW, Lackermair K, Peterss S, Orban M, Boulesteix AL, Michel S, Hausleiter J, Massberg S, Hagl C. Extracorporeal life support in cardiogenic shock complicating acute myocardial infarction. J Am Coll Cardiol 2019; 73: 2355–2357. [DOI] [PubMed] [Google Scholar]
- 23. Udesen NJ, Møller JE, Lindholm MG, Eiskjær H, Schäfer A, Werner N, Holmvang L, Terkelsen CJ, Jensen LO, Junker A, Schmidt H. Rationale and design of DanGer shock: Danish–German cardiogenic shock trial. Am Heart J The Author(s) 2019; 214: 60–68. [DOI] [PubMed] [Google Scholar]
- 24. Ostadal P, Rokyta R, Kruger A, Vondrakova D, Janotka M, Smíd O, Smalcova J, Hromadka M, Linhart A, Bělohlávek J. Extra corporeal membrane oxygenation in the therapy of cardiogenic shock (ECMO‐CS): rationale and design of the multicenter randomized trial. Eur J Heart Fail 2017; 19: 124–127. [DOI] [PubMed] [Google Scholar]


