Abstract
Aims
This study aims to demonstrate the characteristics of late gadolinium enhancement (LGE) assessed by cardiovascular magnetic resonance (CMR) imaging in patients with giant cell myocarditis (GCM).
Methods and results
Six patients histologically diagnosed with GCM were retrospectively recruited in this study. All of them underwent CMR during hospitalization. The distribution and extent of LGE were assessed on both ventricles, and the AHA‐17 segment model was used for left ventricular (LV) analysis. Nine case reports with CMR in GCM were reviewed and summarized to investigate the features of LGE further. LGE was detected on both ventricular walls in all subjects. For a detailed analysis of LGE in the LV, the extent ranged from 21.6% to 56%. Among 70 segments (68.6%) involved by LGE, the subendocardial LGE was the most common pattern (46/102, including 24 segments located in the right‐sided septum), followed by the subepicardial pattern (23/102). The right‐sided septum, the subepicardial anterior wall, and the subendocardial right ventricular (RV) wall were observed in all subjects. To summarize the results of the present study with these case reports, the three most common patterns of LGE are the right‐sided septum (73%), the subepicardial anterior wall (60%), and the subendocardial RV wall (53%).
Conclusions
Extensive LGE seems to be common in GCM, affecting both LV and RV walls. Apart from subepicardial LGE, subendocardial LGE, which was used to be implicated in ischaemic disease, was frequently presented in GCM. The right‐sided subendocardial septum, the subepicardial anterior wall, and the subendocardial RV wall might be the vulnerable areas of LGE in GCM.
Keywords: Giant cell myocarditis, Cardiovascular magnetic resonance, Late gadolinium enhancement, Subendocardium
Background
Giant cell myocarditis (GCM), a rare but lethal myocarditis subtype, remains to be a high risk of inducing life‐threatening ventricular arrhythmia and heart failure. 1 , 2 It is crucial to identify the devastating scenario timely with a high rate of mortality and cardiac transplantation to ensure the definitive treatment and obtain better outcomes. 3 , 4
The early diagnosis of GCM is greatly dependent on the endomyocardial biopsy (EMB), an invasive manipulation not routinely performed in clinical practice on account of its risk, cost, and uneven sensitivity. Cardiovascular magnetic resonance (CMR) is represented by obtaining tissue characterization non‐invasively, which is becoming one of the essential diagnostic criteria for myocarditis. 5 However, the classical late gadolinium enhancement (LGE) pattern of myocarditis that plays a vital role in the differential diagnosis mainly reflects the vital‐related subtypes. 6 , 7 No large sample study has been published yet to investigate potential specific characteristics in CMR of GCM except for a few case reports.
Aims
This study aims to demonstrate the CMR manifestations in patients with histology‐proven GCM in our tertiary referral centre and further summarizes the LGE features of GCM combined with published case reports.
Methods
Our study retrospectively collected 12 patients with GCM proven histologically by heart transplantation or EMB during hospitalization in Fuwai Hospital (National Center for Cardiovascular Diseases of China) from August 2010 to November 2018. Only six of them underwent CMR were included in this study. The histologic diagnosis criteria for GCM were based on the inflammatory infiltrate composed of multinucleated giant cells, lymphocytes, eosinophils, and histiocytes within the myocardium associated with myocyte necrosis without granuloma formation. 3 , 4 Their relevant clinical data were retrieved from the patients' electronic medical records. Patients were followed via telephone interview or clinical visit, and the outcome measures were cardiac death, heart transplantation, appropriate implantable cardioverter defibrillation discharge, hospitalization for heart failure, or recurrent myocarditis. All of the patients excluded coronary artery disease and other heart diseases. The investigation conforms with the principles outlined in the Declaration of Helsinki. The hospital research ethics committee approved this study, and informed consent was obtained from the patients.
All CMR images were performed on a clinical 3.0 T magnetic resonance scanner (Ingenia, Philips Healthcare, Best, the Netherlands), including cine steady‐state free precession images, T2‐weighted short tau inversion recovery images, and LGE images obtained 10–15 min after I.V. gadolinium contrast (0.15 mmol/kg) administration. All images were acquired in long‐axis views of two‐chamber, four‐chamber and short‐axis planes covering left ventricle (LV). The image analyses were performed off‐line workstation with dedicated software (Circle CVI42, Calgary, Canada). The endocardial and epicardial contours were semi‐automatically drawn with manual adjustment in end‐systole and end‐diastole excluding papillary muscles in short‐axis cine images to measure conventional CMR parameters about structure and function. It indicated oedematous changes when the T2 ratio (T2 signal intensity ratio to skeletal muscle) ≥ 2. The pattern and location of LGE were visually assessed by two experienced radiologists characterized as subendocardial, subepicardial, midwall, or transmural using standard AHA‐17 segment model and biventricular analysis after consensus. LGE extent was quantified by the auto‐threshold, tracing endocardial and epicardial contours without papillary muscles and trabeculations in short‐axis LGE stack.
Results
Six patients [two men; median age 49.5 (21–56) years] with GCM based on histological grounds underwent routine CMR examinations. Table 1 presents the clinical data. The rate of cardiac death and heart transplantation was 66.7% (4/6) that indicated poor clinical outcomes. Conventional CMR and echocardiography data among patients with GCM are shown in Table 2 . All subjects had impaired biventricular function, and some of them showed the increased size of chambers. Four patients (66.7%) had elevated T2 ratios.
Table 1.
Clinical data of patients with giant cell myocarditis
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Sex | F | F | F | F | M | M |
| Age | 56 | 21 | 46 | 51 | 47 | 52 |
| Presentation | HF | Arrhythmia | Arrhythmia | HF | HF | HF |
| ECG findings | N/A | VT | VT | AV block | AV block | N/A |
| Blood pressure (mmHg) | 101/60 | 108/76 | 135/97 | 118/72 | 130/90 | 117/84 |
| Heart rate (b.p.m.) | 66 | 91 | 69 | 77 | 82 | 87 |
| NYHA class | III | IV | III | IV | III | III |
| CRP (mg/L) | 119 | 21 | 99 | 23 | 13 | 93 |
| IL‐6 (pg/mL) | N/A | 14 | 4.07 | N/A | N/A | N/A |
| PCT (ng/mL) | 3.12 | 0.04 | 0.02 | 0.15 | N/A | N/A |
| cTNI (ng/mL) | 29.15 | 0.41 | 1.09 | 53.15 | 0.04 | 96 |
| NT‐proBNP (pg/mL) | 27 917 | 11 399 | 3939 | 1806 | 4931 | 2250 |
| Leucocytes (109/L) | 25.73 | 13.55 | 7.62 | 7.17 | 8.09 | 7.37 |
| Co‐morbidities | Infection, SIRS, anaemia | Orbital inflammatory pseudotumour, reflux esophagitis, pneumonia | Reflux esophagitis, diabetes, hypertension | Hypertension, hyperlipidaemia | N/A | N/A |
| Immunosuppressive therapy | Methylprednisolone, dexamethasone | Prednisone, cyclosporine | Methylprednisolone, mycophenolate mofetil, cyclosporine | Prednisone, mycophenolate mofetil, cyclosporine | Azathioprine, prednisone, cyclosporine | Prednisone, mycophenolate mofetil, cyclosporine |
| MCS | ECMO, IABP | IABP | N/A | N/A | N/A | N/A |
| Outcome | Cardiac death | Survival with IS | ICD discharge | HT | HT | HT |
AV, atrioventricular; CRP, C‐reactive protein; cTNI, cardiac troponin I; ECG, electrocardiogram; ECMO, extracorporeal membrane oxygenation; F, female; HF, heart failure; HT, heart transplantation; IABP, intra‐aortic balloon pump; ICD, implantable cardioverter defibrillation; IL‐6, interleukin‐6; IS, immunosuppressant; M, male; MCS, mechanical circulatory support; N/A, not applicable; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PCT, procalcitonin; SIRS, system inflammatory reaction syndrome; VT, ventricular tachycardia.
Table 2.
Conventional cardiovascular magnetic resonance and echocardiography data of patients with giant cell myocarditis
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| CMR parameters | ||||||
| LVEF (%) | 29 | 28 | 42 | 23 | 13 | 23 |
| RVEF (%) | 15 | 16 | 31 | 18 | 26 | 26 |
| LVMi (g/m2) | 48.4 | 50.5 | 70.5 | 46.1 | 50.8 | 41 |
| LVEDVi (mL/m2) | 53.5 | 110 | 87 | 78.7 | 124.3 | 97.5 |
| LVESVi (mL/m2) | 37.7 | 79 | 49.8 | 60 | 107.5 | 74.7 |
| RVEDVi (mL/m2) | 97.4 | 84.7 | 63.9 | 157.3 | 68.5 | 83.1 |
| RVESVi (mL/m2) | 82 | 70.9 | 43.8 | 129 | 50.3 | 61.1 |
| T2 ratio | 2.6 | 2.3 | 3.6 | 3 | 1.8 | 1.9 |
| LGE extent (%) | 21.6 | 44.2 | 31.2 | 38.3 | 56 | 47 |
| Echocardiography | ||||||
| RWMA | POS | POS | POS | POS | POS | POS |
| RVSP (mmHg) | 26 | 39 | 37 | 24 | 44 | 56 |
| Mitral valve E/A | NA | NA | NA | >2 | NA | >2 |
| Valve abnormality | Moderate TR | Moderate MR and TR | Mild MR and TR | Severe MR | Moderate MR, mild TR | Moderate MR and TR |
| Pericardial involvement | Small PE | Small PE | NI | Small PE | NI | NI |
CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement; LVEDVi, indexed left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESVi, indexed left ventricular end‐systolic volume; LVMi, indexed left ventricular mass; MR, mitral regurgitation; NA, not available; NI, not involved; PE, pericardial effusion; POS, positive: hypokinesia in one or more segments; RVEDVi, indexed right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; RVESVi, indexed right ventricular end‐systolic volume; RVSP, right ventricular systolic pressure; RWMA, regional wall motion abnormality; TR, tricuspid regurgitation.
Both ventricular walls showed LGE in all subjects. Multiple layers of myocardium could be implicated by LGE. The representative case is shown in Figure 1 . For a detailed analysis of LGE in the LV, the extent of LGE ranged from 21.6% to 56%, and 70 segments (68.6%) were involved. The most common pattern was subendocardial (46/102, including 24 segments located in the right‐sided septum, as shown in Figure 2 ), followed by the subepicardial LGE (23/102) and transmural LGE (14/102). Figure 3 presents the spatial distribution of the prevalence of LGE segments. The right‐sided septum (100%) and the anterior wall (100%) were frequently involved by LGE for subendocardial and subepicardial patterns, respectively. For the right ventricular (RV) wall, the subendocardial LGE was also presented in all subjects, two of which showed an additional pattern—transmural LGE. Half of them were mainly located in the distal portion of RV, and the remaining three showed multiple, extensive enhancing areas.
Figure 1.
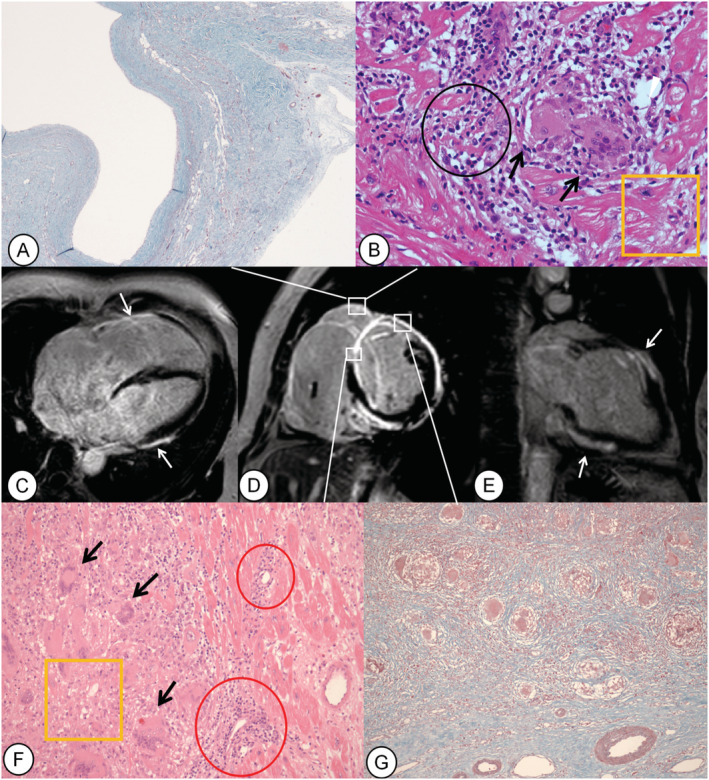
Female, 51 years of age, presenting chest tightness for more than 1 year and syncope twice in 2 days (Case 4). Late gadolinium enhancement (LGE) images of the short‐axis (D), four‐chamber (C), and two‐chamber (E) views show enhancing area in the right ventricular (RV) wall, transmural and both‐sided LGE in the septum, transmural and epicardial LGE in the anterior wall, and epicardial LGE in the lateral and inferior walls (white arrows). Histopathologic findings show the transmural fibrosis in the RV free wall (A: Masson stain, ×40), anterior septum and anterior wall of left ventricle (G, Masson stain, ×100), and multinucleated giant cells in the anterior wall of left ventricle, septum (F: haematoxylin–eosin stain, ×200), and RV wall (B: haematoxylin–eosin stain, ×400). Black arrows indicate multinucleated giant cells, black circle indicates lymphocytic infiltrate, yellow rectangles indicate damaged myocardium, and red circles represent capillaries surrounded with lymphocytic infiltrates.
Figure 2.
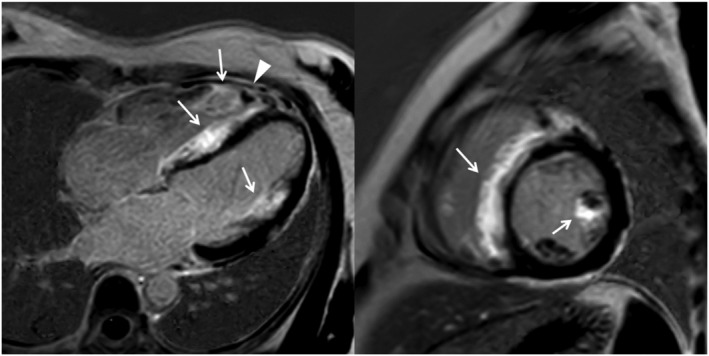
Four‐chamber view (left panel) and short‐axis view at the mid‐ventricular level (right panel) of late gadolinium enhancement images show the extensive enhancement predominantly involving the right‐sided septum and the anterior papillary muscle (arrows). Besides, there is a right ventricular apical thrombus (arrowheads).
Figure 3.
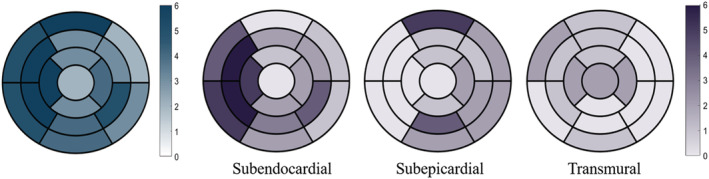
Spatial distribution of the prevalence of segments involved by late gadolinium enhancement (blue), as well as subendocardial, subepicardial, and transmural late gadolinium enhancement (purple) in 17 segments of left ventricular myocardium, represented as bull's eye map.
Nine case reports related to LGE in patients with GCM who were proven histologically and excluded coronary disease between 2005 and 2019 (Table 3 ) were reviewed. It revealed that multilayer infiltrations were also depicted by LGE. The most common pattern was subendocardial LGE (7/9), but less often in subepicardial (6/9). The septum (8/9) and the anterior wall (6/9) of LV were the most vulnerable regions. Two of all cases had LGE in the RV wall. To summarize the results of the present study with these case reports (Figure 4 ), the three most common patterns of LGE were distributed in the right‐sided septum (73%), the subepicardial anterior wall (60%), and the subendocardial RV wall (53%).
Table 3.
Giant cell myocarditis case reports included in the literature review
| Author | Published date | Age | Sex | Presentation | ECG findings | Outcome | LVEF (%) | RVEF (%) | LGE in CMR | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LGE location | LGE pattern | |||||||||||
| LV | Septum | RV | ||||||||||
| Shonk et al. | 2005 | 33 | F | Palpitation, syncope | VT | HT | 49 | 25 | Anterior | Left side | Free wall | SED |
| Azarine et al. | 2009 | 17 | M | Fatigue, dyspnoea | Sinus tachycardia | HT | 12 | 10 | Lateral | Right side | NI | SED, SEP, MW |
| Ali et al. | 2011 | 57 | M | Breathlessness, pre‐syncope | VT, RBBB, ST‐segment changes | HT | 40–45 | Impaired | NI | Right side | Diffuse | SED, TM |
| Ashikaga et al. | 2013 | 67 | M | Nausea, fever | VT, AV block | Survival with IS | 35 | NA | Anterior | Right side | NI | SED, SEP |
| Sujino et al. | 2014 | 73 | F | Chest pain, dyspnoea | VT, ST changes | Death | 20.8 | NA | Inferior, anterolateral | Both sides | NI | SED, SEP, TM |
| Hayase et al. | 2015 | 28 | F | Breathlessness, palpitation | Q waves, ST‐segment changes, T‐wave changes | Survival | 63 | NA | Inferior | Both sides | NI | SED |
| Ammirati et al. | 2016 | 31 | M | Dyspnoea | VT, ST‐segment changes | Survival with IS | 30 | NA | Anterior, inferior | NI | NI | SEP |
| Ziperstein et al. | 2018 | 53 | M | Chest pain, palpitations, headedness | VT, bifascicular block | HT | 43 | 36 | Anterior, inferolateral | Right side | NI | SED, SEP, MW |
| Fallon et al. | 2019 | 54 | F | Chest tightness, palpitations | VT | HT | 32.8 | NA | Anterior | Both sides | NI | SEP, TM |
AV, atrioventricular; ECG, electrocardiogram; F, female; HT, heart transplantation; IS, immunosuppressant; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; M, male; MW, midwall; NA, not available; NI, not involved; RBBB, right bundle branch block pattern; RV, right ventricle; RVEF, right ventricular ejection fraction; SED, subendocardial; SEP, subepicardial; TM, transmural; VT, ventricular tachycardia.
Figure 4.
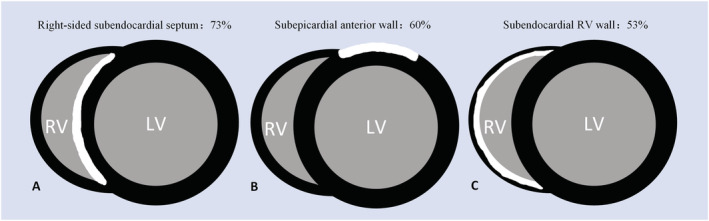
The three most common late gadolinium enhancement patterns of giant cell myocarditis combined the present study with the case reports available: the right‐sided subendocardial septum (A), subepicardial anterior wall (B), and subendocardial RV wall (C). LV, left ventricle; RV, right ventricle.
Discussion
In the present study, we considered a mini‐series of patients with GCM evaluated with CMR focusing on the LGE presentations. Consistent with the results derived from the nine case reports, we found that subendocardial LGE was frequently presented in GCM despite subepicardial LGE. Additionally, LGE in the right‐sided septum and the subepicardial anterior wall were common in all cases, but more RV involvement in our group. A previous study also reported that RV is usually involved in the process of GCM. 8
Many experimental animal models have proposed that GCM is induced by auto immunization with cardiac myosin, 9 , 10 a main structural protein distributed in the myocardium. Thus, it was reasonable to find multilayer LGE with extensive lesions. Notably, despite subepicardial enhancement, which is considered the most frequent pattern in myocarditis, 6 , 7 most aforementioned cases of GCM had subendocardial or transmural LGE not following the distribution of coronary arterial territory. Nevertheless, the mechanism of this atypical LGE pattern used to be regarded as ischaemia‐mediated injury is still unclear for GCM. To some extent, one of the possible mechanisms may be related to ischaemia factors. Klein et al. 11 found that patients with biopsy‐proven inflammatory infiltrate with a diminished coronary reserve due to reduced coronary vasodilator capacity. Fallon et al. 12 showed a histologic image of GCM with a capillary surrounded by the lymphocytic infiltrates, which is also found in our patients (Figure 1 F ). Additionally, previous investigations have shown the infiltrate mixed with multinucleated giant cells in the subendocardial area in GCM by autopsy. 13 , 14 , 15 Therefore, we would like to assume that the subendocardial LGE is a relatively specific LGE distribution in GCM caused by the extensive myocardium damage itself and coronary microvascular involvement. Further work on this direction is warranted.
Our results have found some patients with an unmatched relationship between LGE and T2 ratio. The LGE is limited to depict diffuse lesions accurately because of the mistakes in myocardial nulling, such as oedema and fibrosis that lead to increased interstitial space. Besides, the T2 ratio may be influenced by a long duration of disease, the therapy, and the inherently low signal‐to‐noise ratio of T2‐weighted imaging.
The sensitivity of EMB for GCM is variable but generally higher than lymphocytic myocarditis. 16 Firstly, the right‐sided septum was noted to be the targeted sampling area in most cases, 4 which proposed the common LGE location in our study so that it might be one reason for the relatively high biopsy‐positive rate. Besides, GCM usually has extensive infiltrates of the myocardium, 16 as depicted by LGE in our results, so it may be easier to capture the lesions for EMB.
Our study was limited by the mini amount of patients with GCM because of its rare morbidity and the intolerance to CMR scanning. The T1 and T2 mapping sequences were not applied in this retrospective study, which is essential to the diagnosis of myocarditis, especially with a diffuse myocardial injury such as GCM.
Conclusions
Extensive LGE seems to be common in GCM, affecting both LV and RV walls. Apart from subepicardial LGE, subendocardial LGE used to be implicated in ischaemia disease was frequently presented in GCM. The right‐sided subendocardial septum, the subepicardial anterior wall, and the subendocardial RV wall were the primary LGE areas, which might be the potential specific LGE features in GCM. These LGE patterns may be suggestive of GCM and guide to utilizing EMB earlier, which is necessary for suspected patients with GCM.
Conflict of interest
The authors report no relationship that could be construed as a conflict of interest.
Acknowledgements
The study was supported by grant numbers 81930044 and 81620108015 from the key projects of the National Natural Science Foundation of China.
Yang, S. , Chen, X. , Li, J. , Sun, Y. , Song, J. , Wang, H. , and Zhao, S. (2021) Late gadolinium enhancement characteristics in giant cell myocarditis. ESC Heart Failure, 8: 2320–2327. 10.1002/ehf2.13276
References
- 1. Ekstrom K, Lehtonen J, Kandolin R, Raisanen‐Sokolowski A, Salmenkivi K, Kupari M. Incidence, risk factors, and outcome of life‐threatening ventricular arrhythmias in giant cell myocarditis. Circ Arrhythm Electrophysiol 2016; 9: e004559. [DOI] [PubMed] [Google Scholar]
- 2. Ammirati E, Veronese G, Brambatti M, Merlo M, Cipriani M, Potena L, Sormani P, Aoki T, Sugimura K, Sawamura A, Okumura T, Pinney S, Hong K, Shah P, Braun O, Van de Heyning CM, Montero S, Petrella D, Huang F, Schmidt M, Raineri C, Lala A, Varrenti M, Foa A, Leone O, Gentile P, Artico J, Agostini V, Patel R, Garascia A, Van Craenenbroeck EM, Hirose K, Isotani A, Murohara T, Arita Y, Sionis A, Fabris E, Hashem S, Garcia‐Hernando V, Oliva F, Greenberg B, Shimokawa H, Sinagra G, Adler ED, Frigerio M, Camici PG. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2019; 74: 299–311. [DOI] [PubMed] [Google Scholar]
- 3. Ekström K, Lehtonen J, Kandolin R, Räisänen‐Sokolowski A, Salmenkivi K, Kupari M. Long‐term outcome and its predictors in giant cell myocarditis. Eur J Heart Fail 2016; 18: 1452–1458. [DOI] [PubMed] [Google Scholar]
- 4. Kandolin R, Lehtonen J, Salmenkivi K, Raisanen‐Sokolowski A, Lommi J, Kupari M. Diagnosis, treatment, and outcome of giant‐cell myocarditis in the era of combined immunosuppression. Circ Heart Fail 2013; 6: 15–22. [DOI] [PubMed] [Google Scholar]
- 5. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648 2648a‐2648d. [DOI] [PubMed] [Google Scholar]
- 6. Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006; 114: 1581–1590. [DOI] [PubMed] [Google Scholar]
- 7. Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, Di Bella G, Cardiac Magnetic Resonance Working Group of the Italian Society of C . Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol 2017; 70: 1977–1987. [DOI] [PubMed] [Google Scholar]
- 8. Murray LK, Gonzalez‐Costello J, Jonas SN, Sims DB, Morrison KA, Colombo PC, Mancini DM, Restaino SW, Joye E, Horn E, Takayama H, Marboe CC, Naka Y, Jorde UP, Uriel N. Ventricular assist device support as a bridge to heart transplantation in patients with giant cell myocarditis. Eur J Heart Fail 2012; 14: 312–318. [DOI] [PubMed] [Google Scholar]
- 9. Shioji K, Kishimoto C, Nakayama Y, Sasayama S. Strain difference in rats with experimental giant cell myocarditis. Jpn Circ J 2000; 64: 283–286. [DOI] [PubMed] [Google Scholar]
- 10. Kodama M, Matsumoto Y, Fujiwara M, Masani F, Izumi T, Shibata A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol 1990; 57: 250–262. [DOI] [PubMed] [Google Scholar]
- 11. Klein RM, Schwartzkopff B, Gabbert HE, Strauer BE. Diminished coronary reserve in patients with biopsy‐proven inflammatory infiltrates. Cardiology 2003; 100: 120–128. [DOI] [PubMed] [Google Scholar]
- 12. Fallon JM, Parker AM, Dunn SP, Kennedy JLW. A giant mystery in giant cell myocarditis: navigating diagnosis, immunosuppression, and mechanical circulatory support. ESC Heart Fail 2019; 7: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chow LH, Radio SJ, Sears TD, McManus BM. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol 1989; 14: 915–920. [DOI] [PubMed] [Google Scholar]
- 14. Sujino Y, Kimura F, Tanno J, Nakano S, Yamaguchi E, Shimizu M, Okano N, Tamura Y, Fujita J, Cooper LT, Senbonmatsu T, Muramatsu T, Nishimura S. Cardiac magnetic resonance imaging in giant cell myocarditis: intriguing associations with clinical and pathological features. Circulation 2014; 129: e467–e469. [DOI] [PubMed] [Google Scholar]
- 15. Litovsky SH, Burke AP, Virmani R. Giant cell myocarditis: an entity distinct from sarcoidosis characterized by multiphasic myocyte destruction by cytotoxic T cells and histiocytic giant cells. Mod Pathol 1996; 9: 1126–1134. [PubMed] [Google Scholar]
- 16. Shields RC, Tazelaar HD, Berry GJ, Cooper LT Jr. The role of right ventricular endomyocardial biopsy for idiopathic giant cell myocarditis. J Card Fail 2002; 8: 74–78. [DOI] [PubMed] [Google Scholar]


